Abstract
The relationships between commitments of dendritic cells (DCs) and T cells in human hematopoietic stem cells are not well-understood. In this study, we enumerate and characterize conventional-DC (cDC) and plasmacytoid-DC (pDC) precursors in association with T-cell and thymus-derived types of NK-cell precursors among CD34+ hematopoietic progenitor cells (HPCs) circulating in human peripheral blood (PB). By limiting-dilution analyses using co-culture with stroma cells expressing Notch1 ligand, the precursor frequencies (PFs) of DCs in HPCs were found to significantly correlate with T-cell PFs, but not with NK-cell PFs, among healthy donors. Clonal analyses showed that the majority of T/NK-dual and T-single lineage precursors - but only a minority of NK-single lineage precursors - were associated with the generation of DC progenies. All clones producing both DC and T-cell progenies were found with monocyte and/or granulocyte progenies, suggesting DC differentiation via myeloid DC pathways. Analyses of PB HPC subpopulations revealed that the lineage split between DC and T/NK-cell progenitor occurs at the stage prior to bifurcation into T- and NK-cell lineages. The findings suggest a strong linkage between DC and T-cell commitments, which may be imprinted in circulating lymphoid-primed multipotent progenitors or in more upstream HPCs.
INTRODUCTION
Dendritic cells (DCs) are antigen-presenting cells crucial for initiating adaptive immune responses as well as maintaining immune tolerance to self-antigens (1). Two DC subsets, conventional dendritic cells (cDC) and plasmacytoid dendritic cells (pDC), have been identified in both mouse and human hematolymphoid organs (2). Non-migratory DCs in those organs are subdivided into pDCs and two subsets of cDCs: CD8+ and CD11b+ cDCs in mice, and BDCA1+ (CD1c) and BDCA3+ (CD141) cDC in humans (3). Those DC subsets have all been shown to develop via either common myeloid progenitors (CMP) or common lymphoid progenitors(CLP) (4, 5), although the lymphoid- and myeloid-derived DC subsets possessed similar expression profiles of proteins and genes related to DC development and functions in both mice and humans (6–8). A recent report using a barcoding technique for single lymphoid-primed multipotent progenitors (LMPPs) suggested that DCs are considered a distinct lineage from myeloid and B-cell lineages (9), although the relationships between DC and T-cell lineages could not be examined using this technique.
Since DCs contribute to the deletion of autoreactive T-cell precursors in the process of negative selection in the thymus, the developmental origin and pathway of murine thymic DCs have been extensively studied in relation to T-cell commitment. The CD11b+ cDCs arise from blood precursors that continuously enter the thymus (10, 11). That DC subset derives from bone marrow DC progenitors which are composed of common macrophage-DC progenitors (MDP), common DC progenitors (CDP) and pre-cDC (3, 12, 13). In contrast, the CD8+ cDCs develop intra-thymically and originate from early T-cell progenitors (11, 14, 15). However, contradictory findings have suggested that the thymic CD8+ cDCs are also derived from myeloid precursors (4, 16), or from precursors unrelated to T-cell lineage (17). Thymic pDCs were thought to differentiate from lymphoid progenitors (15), but it has recently been reported in a parabiotic study that thymic pDCs originate extrathymically and continually migrate to the thymus (11).
In humans, developmental origin and pathways of thymic DCs were mainly studied in culture (18–20) or in immunodeficient mouse-human chimeras (21) using cord blood (CB), and fetal or newborn thymus for a progenitor source. Results of all those human experiments suggested the presence of common progenitors for T cells and DCs in the thymus, although clonal analyses to confirm a common origin were not conducted. However, due to the lack of human in vivo experimental systems in a physiological setting, a definitive conclusion is thought to be currently unobtainable.
Regardless of whether thymic DCs are derived intra-thymically from common progenitors for T cells and DCs or from extra-thymically from discrete DC lineage progenitors, we assume that possible regulatory mechanisms maintain appropriate numbers of pre-T cells and DCs for normal progression of the negative selection in the thymus. In fact, murine thymic DCs displayed kinetics of both generation and decay similar to thymocytes, suggesting a coordinated development of DCs and T-cells (22–24). Our hypothesis is that the proportion of DC to T-cell precursors entering into the thymus from blood is maintained at a constant level by linkage of commitments between the two lineages at some stage prior to the DC/T split. To test this hypothesis, we sought to establish in vitro functional and quantitative assays of human cDC and pDC progenitors in association with T- and NK-cell progenitors for the present study. Human peripheral blood (PB) was used as a source of progenitors since these progenitors are assumed to migrate from bone marrow (BM) to the thymus through the blood stream (25).
In our previous study we developed a cell-sorting based limiting-dilution assay (LDA) and clonal analyses using a 384-well plate for quantification and characterization of T/NK progenitors among CD34-positive/lineage marker-negative (CD34+Lin−) hematopoietic progenitor cell (HPC) populations circulating in PB of healthy adult humans (26). The surface phenotype of NK-cell progenies that developed in the culture represented CD56hi CD161+CD16− thymus-derived type (thymic) NK cells. Using single-cell analyses we classified HPCs into T/NK-dual and T- or NK-single lineage precursors. The vast majority of these T- and/or NK-cell precursor clones were found to be derived from LMPP or more up-stream progenitors that co-produce myeloid cells. The assays used co-cultures with OP9-DL 1 stroma cells expressing the Notch1 ligand, Delta-like 1 in the presence of Kit ligand (KL), IL-7 and Flt-3 ligand (FL). Some reports described the involvement of Notch 1 signaling in DC development (27, 28), and FL is known to be an essential cytokine for DC development (29). Therefore, it was expected that both DC and T/NK-cell commitments would be simultaneously observed in identical culture conditions.
Using the assays, we measured cDC and pDC PFs of PB HPCs from healthy adult human donors in conjunction with T and NK PFs. We also examined CB HPCs for their DC/T potentials as a positive control that is thought to extensively supply progenitors to the thymus. We found that DC PFs correlated with T-cell but not with NK-cell PFs, suggesting a linkage between DC and T-cell potentials in circulating HPCs. Single HPC analyses also indicated a strong linkage between commitments of the two lineages, which may be primed in HPCs prior to bifurcation into T- or NK-cell lineages.
MATERIALS AND METHODS
Cytokines and Antibodies
Recombinant human KL, FL, IL-3, IL-7, and GM-CSF were purchased from PeproTech. Anti- CD3 (SK7), cytoplasmic CD3 (UCHT1), CD4 (SK-3), CD5 (UCHT2), CD7 (M-T701), CD8 (SK1), CD16 (3G8), CD19 (HIB19), CD20 (2H7), CD117 (104D2), HLA-DR (L243) and glycophorin A (GA-R2) Abs were purchased from BD Biosciences. Anti- CD16a (3G8), CD34 (581), and CD56 (N901) Abs were purchased from Beckman Coulter. Anti-CD14 (TÜK4), T-cell receptor (TCR) αβ (BMA031), and TCRγδ (5A6.E9) Abs were purchased from Invitrogen. CD1c (L161), CD11c (Bu15), CD15 (W6D3), CD34 (581), CD141(M80), CD303(201A) and CD304(12C2) Abs were purchased from BioLegend. CD10 (CB-CALLA), CD14 (61D3), and CD123 (6H6) were obtained from eBio. CD127 (R34-34) Ab was purchased from Tombo.
Cell preparation
Human PB samples were collected from 20 healthy in-house volunteer donors (Japanese) with informed consent, following the guidance of the institutional review board (Human Investigation Committee of the Radiation Effects Research Foundation), which approved this study. PB mononuclear cells (PBMCs) were separated from 10 ml PB samples by Ficoll density gradient centrifugation (Lymphocyte Separation Medium 1077, Wako Pure Chemical Industry). CB mononuclear cells (CDMNCs) and BM mononuclear cells were purchased from Lonza Walkersville.
Stroma cells
Generation of the mouse OP9-DL1 stroma cells engineered to express the green fluorescent protein (GFP) and the mouse Delta-like 1 gene has been described previously (30). The OP9-DL1 and the OP9 (31) parental stroma cells were maintained by culturing in alpha MEM (Gibco) supplemented with 20% FBS (Hyclone), 4 × 10−6 M 2-mercaptoethanol, and penicillin-streptomycin at 37°C in a humidified atmosphere flushed with 5% CO2.
LDA of DC/T/NK precursors
Procedures and culture conditions for the LDA were described previously (26). Briefly, for progenitor cell culture, OP9-DL1 stroma cells were seeded in wells (50 to 80% confluence) of a 384-well flat-bottom black plate (BD Bioscience). At least 4 hrs prior to progenitor cell sorting, the culture medium in each well was replaced by 50 μl phenol red-free alpha MEM containing 20% knock-out serum replacement (Gibco), 10−4 M monothioglycerol (Sigma), 50 μg/ml gentamycin (Sigma), 10 ng/ml KL, 10 ng/ml FL and 10 ng/ml IL-7 (DC/T/NK-medium). For progenitor cell sorting, PBMCs and CBMNs were stained with allophycocyanin-conjugated anti-CD34 Ab and PE-conjugated anti-lineage markers (anti-CD3, CD14, CD16, CD19, CD20, CD56, and glycophorin A Abs) for 30 min on ice, and dead cells were excluded by 1μg/ml propidium iodide staining (BD Bioscience), as shown in Fig.1A. One thousand CD34+Lin− cells were sorted into 80 wells of a 384-well plate at a frequency of 20, 15, 10, or 5 cells per well (20 wells for each cell frequency) by FACS Aria II (BD Biosciences). LDA culture was maintained at 37°C in a humidified atmosphere flushed with 5% CO2, and half of the culture medium (25 μl) was changed every week.
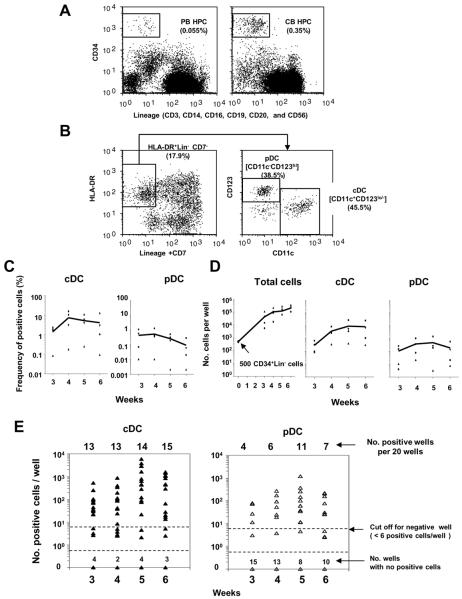
Figure 1. Growth and differentiation kinetics of DC-lineage progenies generated from PB HPCs.
(A) Representative flow cytograms of CD34+Lin− cells (HPCs) in PB (left panel) and CB (right panel) mononuclear cells.
(B) Representative flow cytograms of HLA-DR+Lin−CD11c+ CD123lo-cDC lineage and HLA-DR+Lin−CD11c− CD123hi - pDC -lineage progenies generated from PB CD34+Lin− cells in 5-week OP9-DL1 co-culture in the presence of KL, IL-7, and FL.
(C and D) Time courses of cDC- and pDC-lineage differentiation from PB CD34+Lin− cells. PB CD34+Lin− cells from four donors were cultured at a concentration of 500 cells per well in a 24-well plate where OP9-DL1 stroma cells had been pre-seeded in the presence of KL, IL-7, and FL. Percentage (C) and absolute number (D) of cDC- and pDC-lineage progenies generated from CD34+Lin− cells are plotted against time (weeks). Solid lines connect the average values.
(E) Time course of cDC- and pDC-lineage differentiation from CD34+Lin− cells of one donor. Each dot represents the total number of cDC (left panel) or pDC (right panel) generated from twenty CD34+Lin− cells in a well of 384-well plate. Twenty wells were analyzed for each of 3, 4, 5, and 6 weeks. A well exhibiting 6 or greater positive events was designated a positive well for LDA. Similar results were obtained from two experiments for two other donors.
After 5 weeks of culture, all cells grown in wells were harvested by vigorous pipetting and washed with PBS containing 2 mM EDTA, 0.01% NaN3 and 1% FBS (washing buffer, abbreviated as WB). For detection of cDC- and pDC-lineage progenies by Cyan (Beckman Coulter), cells were stained with a PE-conjugated mixture of the lineage markers and CD7, PE-Cy7-conjugated HLA-DR, allophycocyanin-conjugated CD11c, and PerCPCy5.5-conjugated CD123 Abs in WB. Stained cells were resuspended in 200 μl of WB containing 1 μg/ml DAPI (Invitrogen) to exclude dead cells by flow cytometry. OP9-DL1 cells and their cell debris were gated out using GFP fluorescence. After gating on DR+ CD7−Lin− cells, CD11c+CD123lo and CD11c−CD123hi cells remaining in the constant gated-fluorescence region were defined as cDC- and pDC-lineage progenies, respectively. For detection of T/NK- and myeloid lineage progenies, cells were stained with allophycocyanin -conjugated CD5, PE-conjugated CD7, PE-Cy7-conjugated CD56, APC-AlexaFluor750-conjugated CD14, and PerCPCy5.5-conjugated CD15 Abs in WB, as described previously. The absolute number of each progeny per well was calculated from the number of events on a flow cytogram in a volume (160 μl) of cell suspension. A well exhibiting six or more positive events was designated as positive. PFs of cDC-, pDC, T-, and NK-cell were calculated by online analysis using ELDA software (32), available on the home page of Walter Elisa Hall Institute Bioinformatics Division (http://bioinf.wehi.edu.au/software/elda/index.html).
For LDA of PB HPC subpopulations, PBMCs were stained with APC-conjugated CD34, PE-conjugated lineage markers, PE-Cy7-conjugated cKit (CD117), and FITC-conjugated CD10 or CD7 Abs. For LDA of in vitro-generated HPC subpopulations, total progenies generated from PB HPCs in OP9-DL1 culture were stained with APC-Cy7-conjugated CD34, PE-conjugated Lin, and allophycocyanin-conjugated CD7 Abs.
For differentiation kinetics and phenotype analyses, CD34+Lin− cells were cultured in some experiments at 500 cells per well using a 24-well plate in the same conditions as those used for the 384-well plates.
Clonal analysis
Procedures for single HPC analysis were previously described (26). Briefly, CD34+Lin−, CD34+Lin−cKithi/loCD7+, and CD34+Lin−cKitloCD10+cells in PB were sorted into a total of more than 280 wells for each experiment, using 384-well plates with a single cell per well. CD34+Lin−CD7+ cells generated in co-culture with OP9-DL1 cells were also used for the single cell sorting. The conditions of OP9-DL1 co-culture for single HPC culture were the same as those for LDA, described above. All cells in a well of a 384-well plate exhibiting generation of progenies after a five-week culture were transferred to one well of a 96-well plate, where OP9-DL1 cells were pre-seeded. After culture for 5 –10 days in the presence of KL, IL-7, and FL, cells were split into two wells of a 96-well plate. Cells in each of the two wells were analyzed for cDC/pDC or T/NK/myeloid-lineage markers by flow cytometry, as described above. A well exhibiting three or more positive events was designated as positive. This cut-off value for clonal analysis was one-half of that for LDA because progeny cells in one well were divided into two equal parts for immunofluorescence staining, as described above.
Surface phenotyping of DC/ T/NK progenies
For analyses of DC subsets, progeny cells from PB HPCs in co-culture with OP9-DL1 cells were stained with PerCPCy5.5-conjugated CD141, CD303 or CD304, and allophycocyanin-Cy7-conjugated CD1c, in combination with a mixture of lineage markers and CD7, HLA-DR, CD11c, and CD123 Abs, as described above. In order to determine the stage of T-cell maturation, progeny cells were stained with PECy7-conjugated CD3, PerCPCy5.5-conjugated CD4, and allophycocyanin-Cy7-conjugated CD8 Abs, in combination with CD5 and CD7 Abs. For T-cell receptor (TCR) expression, cells were stained with allophycocyanin-conjugated CD7, PE-Cy7-conjugated CD5, allophycocyanin-Cy7-conjugated CD3, PE-conjugated TCRαβ, or TCRγδ Abs. To allow us to detect CD127 expressions in progeny cells, progenies were pre-cultured with DC/T/NK medium in the absence of IL-7 for 3 days. For CD127 expressions in CD34+CD7−, CD34+CD7+, and CD34−CD7+ cells, progenies were stained with PE-conjugated CD7, allophycocyanin-Cy7-conjugated CD34, and allophycocyanin-conjugated CD127 Abs. For characterization of NK-lineage progenies and PB NK-cell subsets, cells were stained with PerCPCy5.5-conjugated CD16 and allophycocyanin -conjugated CD127 Abs, in combination with CD7 and CD56 Abs.
Methylcellulose colony assay
CFU-granulocyte/macrophage (GM) and burst-forming unit erythroid (BFU-E) in CD34+CD7+ CD5−CD56− cell populations generated from CD34+Lin− cells in 4-weeks OP9-DL1 co-cultures were assayed in methylcellulose cultures using 96-well plates. Briefly, CD34+CD7+ CD5−CD56− were sorted into wells at one cell per well in 50-μl culture containing 1.2% methylcellulose (Stem Cell Technology) with erythropoietin (6u/ml), KL(20ng/ml), G-CSF(20ng/ml), and IL-3(20ng/ml). The methylcellulose cultures were microscopically observed after 14 days to look for the presence of a colony in each well.
Cytokine measurement
At the 4th week of OP9-DL1 co-culture using a 24-well plate, cDC and pDC fractions were sorted and cultured with 50μl DC/T/NK-medium in the presence of GM-CSF (10 μg/ml) and IL-3(10 μg/ml) for 5 days,respectively using a 384-well plate (5,000 cells per well). Poly I:C (0.2μg/ml) and R848 (2μM) for TLR stimuli were added to cDC and pDC culture, respectively, at day 3 after the initiation of the culture. After 5 days culture, supernatants were collected and assayed for IL-6 and interferon alpha (IFNα) production. IL-6 and IFNα assays were conducted using Quantikine HS ELISA (R&D Systems) and VeriKine ELISA (PBL Interferon Source) kits, respectively, according to the manufacturers' instructions.
Statistics
Spearman's rank correlation analysis, Wilcoxon's signed rank sum test, Mann-Whitney U test and chi-square test were conducted using SPSS 16.01 software (SPSS Inc.).
RESULTS
LDA of cDC/pDC precursors in HPCs
PB and CB HPCs (Figure 1A) were cultured with OP9-DL1 stroma cells in the presence of KL, FL, and IL-7. Generation of cDC- and pDC-lineage progenies in OP9-DL1 cultures was determined by flow-cytometric detection of CD123loCD11c+ and CD123hiCD11c− cells in HLA-DR+ CD7−Lin− cell populations, respectively (Figure 1B). Although variations among individual donors were numerous, the absolute numbers of both progenies-per-well on average were found to reach plateau levels after 4 to 5 weeks of culture in both 24- and 384-well plates (Figures 1 C, D, and E). When we seeded 20 CD34+Lin− cells into wells of 384-well plates, we observed the development of cDC or pDC in approximately half of the wells, but the numbers of DC progenies could differ by more than 100-fold from well to well (Figure 1E). Based on our previous report describing LDA for T/NK-cell precursors (26), we decided on six or more positive events per well as the cut-off value for a positive well in the LDA for cDC and pDC precursors. Although this cut-off value was somewhat arbitrary, regression curves of negative fractions for both cDC- and pDC-precursors fit well with this model (Figure 2A). Furthermore, this PF assay showed high reproducibility in nonparametric correlation analysis between the first and second measurements among seven adults (cDC: ρ = 0.89, P = 0.007; pDC: ρ = 0.82, P = 0.023). The PFs in HSCs were converted to the percentages of precursors in the CD34+Lin− population to more easily assess the population size of progenitors (Figure 2A). We simultaneously enumerated CD7+CD5+ T-cell and CD7+CD56+ NK-cell PFs for each donor (Fig. S1), as described previously (26).
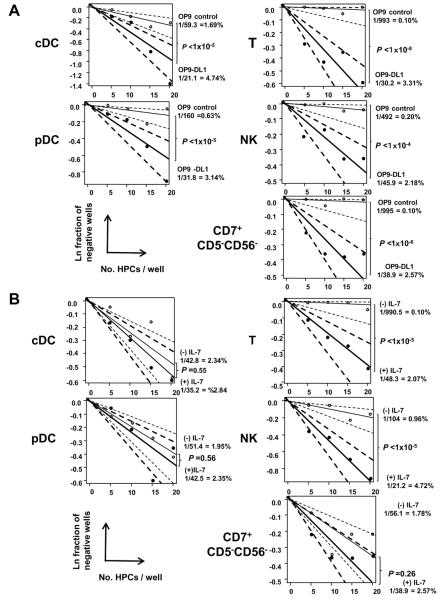
Figure 2. LDA for determination of cDC, pDC, T-, and NK-cell PFs in PB HPCs in the presence or absence of Notch 1 signaling and IL-7.
LDA for determination of cDC,pDC, CD7+CD5+ T-, CD7+56+ NK-, and CD7+CD5−CD56− cell PFs in HPCs by co-culture with OP9-DL1 cells (closed circle and thick line) or OP9 control cells (open circle and thin line) (A) and with OP9-DL1 cells in the presence (closed circle and thick line) and absence (open circle and thin line) of IL-7 (B). One thousand PB CD34+Lin− cells from one donor were sorted into wells of 384-well plates at frequency of 20, 15, 10, or 5 cells per well (20 wells for each cell frequency) and co-cultured with OP9-DL1 or OP9 control cells for five weeks. Natural logs of negative-well fractions were plotted against the number of CD34+Lin− cells plated per well for cDC and pDC (left panel), and T-, NK-, and CD7+CD5−CD56−-cell (right panel) precursors using ELDA software. Dotted lines represent 95% confidence intervals. P-values of the differences of PFs between co-culture with OP9-DL1 and OP9 controls, and between presence and absence of IL-7, are shown in each LDA plot. The PFs were converted to the percentage in CD34+Lin− cells. Similar results were obtained from three experiments for three other donors.
Both cDC and pDC precursor assays using OP9 controls showed that about 70 % of precursor-generated DC progenies depended on Notch 1 signaling, but significant parts of the precursor population, especially cDC precursors, were able to produce DC progenies in the absence of Notch 1 signaling (Fig. 2A). Notch1-independent DC precursors may be myeloid-derived DC progenitors: only a few T- or NK-cell precursors were observed in OP9 controls (Fig. 2A). In fact, about 90% of DC-positive wells in OP9 control co-cultures for LDA (31 wells out of 35 wells among four donors) exhibited co-existence of CD14+CD15− monocyte- and/or CD14+CD15+ granulocyte-lineage cells (Fig. S1). This result was confirmed by the presence of PB HPC clones producing both DCs and myeloid cells, but was not associated with lymphoid progenies, as shown below. As expected, generation of T/NK-lineage cells was IL-7-dependent, but generation of DCs was IL-7-independent in the OP9-DL1 co-cultures (Fig. 2B). It is noteworthy that generation of CD7+CD5−CD56− cells (Fig. S1) was Notch 1-dependent and IL-7-independent (Fig. 2). Since more than 50% of these
CD7+CD5−CD56− cells were found to express CD34 (Fig. S1), this cell population should have comprised lymphoid progenitors at the stage prior to bifurcation into T- and NK-cell lineages. Single-cell methylcellulose colony assays confirmed that CD34+CD7+ CD5−CD56−cells retained monocyte-granulocyte potential but lacked erythroid potential [% CFU in total sorted cells, median (range), CFU-GM: 14% (7 – 27), BFU-E: 0% (0-0) (No. donors=3)], whereas CD34+Lin−CD7− cell population retained both myeloid and erythroid potentials [CFU-GM: 20% (14 –26), BFU-E: 13% (9–25) (No. donors=3)].
Characterization of DC, T-cell, and NK-cell lineage progenies
The major population of PB HPC-derived cDC progenies comprised CD1c+CD141− cells, and the minority was composed of CD1c−CD141+ or CD1c+CD141+ (Fig. 3A). In contrast, pDC progenies did not express CD1c at all, but did express CD303 and CD304. The surface phenotypes of both cDC and pDC progenies were similar to those of mature cDCs and pDCs in adult PB (33, 34), although expression levels of CD141 and CD304 in cDC and pDC progenies, respectively, were somewhat lower than PB mature DCs (Figure S2). cDCs isolated from OP9-DL1 co-cultures were found to produce IL-6 with poly I:C, Toll-like receptor (TLR)-3 ligand-stimulation only after additional culture with GM-CSF (Fig. 3A). Similarly, pDCs showed R848 (TLR-7 and-8 ligand)-stimulated IFNα production that also required additional culture in the presence of IL-3. These results indicate that both DC-lineage progenies in the present culture conditions are functionally immature, and that their maturation is induced by appropriate cytokine signals.
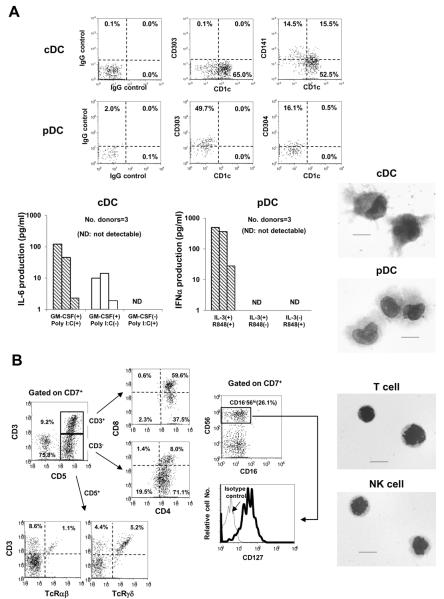
Figure 3. Characterization of cDC, pDC, T-cell, and NK-cell lineage progenies generated from PB HPCs.
(A) Representative flow cytograms of BDCA1 (CD1c), BDCA2(CD303), BDCA3 (CD141), and BDCA4 (CD304) expressions in HLA-DR+Lin−CD11c+ cDC- and HLA-DR+Lin−CD123+ pDC-lineage progenies generated from four-week mass-cultures of HPCs with OP9-DL1 cells in the presence of KL, FL, and IL-7 (upper panel). Cytokine production of cDC- and pDC-lineage progenies generated from four-week mass-cultures from 3 healthy donors, as described above (lower left panels). Productions of IL-6 and IFNα by cDC and pDC progenies were measured after five-day culture with or without GM-CSF and IL-3 (10 ng/ml), respectively, in the absence of stroma cells. Poly I:C and R848 were added to the cultures of cDC and pDC, respectively, at day 3. Cytokine measurement for each donor was independently performed. May-Giemsa staining confirmed that GM-CSF-stimulated HLA-DR+Lin−CD11c+ and IL-3-stimulated HLA-DR+Lin−CD123+ were mature cDCs and pDCs, respectively (lower right panels). The scale bars represent 10μm. Images were acquired by BIOREVO model BZ-9000 (Keyence Co.) with Plan Apo 100×/1.40 numerical aperture objective lens (Nikon) using BZ-II Viewer and BZ-II Analyzer software (right panels).
(B) Representative flow analyses and morphology of CD7+CD5+ T- and CD7+CD56+ NK- lineage cells generated in OP9-DL1 co-cultures. Flow cytograms represent CD4 and CD8 expressions in CD7+CD5+CD3+ and CD7+CD5+CD3− cells generated in ten-week co-culture (upper left panels). Expressions of T-cell receptor αβ and γδ in CD7+CD5+ CD3+ cells was also confirmed (lower panels). Expressions of CD16 and CD127 were analyzed for CD7+CD56+ cells generated in five-week co-culture (upper and lower middle panel, respectively). May-Giemsa staining of CD7+CD5+ T- (upper right panel) and CD7+CD56+ NK- (lower right panel) lineage cells represents cycling lymphoid cell features.
We previously reported that nearly all CD7+5+ T-lineage progenies produced cytoplasmic CD3ε but did not exhibit surface CD3 expression after a 5-week co-culture with OP9-DL1(26). When the CD7+5+ cells were cultured with fresh OP9-DL1 cells for an additional 5 weeks, about 10 to 50% of the cells expressed CD3 at the stages of CD4/CD8-double positive and CD4-single positive (Fig. 3B). The majority of surface CD3− cells coexisting in the culture revealed immature CD4-single positive or CD4/CD8-double positive phenotypes, and the surface CD3+ T cells were found to express TCRαβ or TCRγδ. These observations agreed with a previous report by other researchers describing T-cell differentiation of human adult BM HPCs in OP9-DL1 co-culture (35).
As also shown in our previous report (26), CD7+56+ NK-lineage progenies expressed CD161 but not CD16, suggesting that these cells are similar to the thymic NK cells observed in PB (Fig. S2). We confirmed this consideration by finding the expression of CD127 (Fig 3B), which is known to be expressed in thymic NK cells but lacking in BM-derived CD16+ NK-cells (36, 37).
Correlation of PFs between cDC/pDC and T/NK-cell lineages in PB HPCs
We obtained the frequencies of cDC and pDC precursors in HPCs from PB (n = 20) and CB (n = 3) (Table I). Frequencies of cDC and pDC precursors in PB HPCs ranged from 1 to 10% and from 0.1 to 5% among 20 healthy donors, respectively. Differences between cDC and pDC PFs were highly significant (P = 8.8 × 10−5 in a paired Wilcoxon's signed rank sum test). PFs of cDCs and pDCs in CB HPCs were much higher than those in PB HPCs (P= 0.006 and P = 0.006, respectively, in Mann-Whitney U tests). Similarly, T and NK PFs were 5 to 10 fold higher for CB HPCs than for PB HPCs (Table I). Since we previously showed that PB HPCs had levels of T- and NK-precursor activity similar to BM HPCs (26), the differences of both DC PFs between PB and BM HPCs were not significant (data not shown).
Table I.
Frequencies of cDC- and pDC precursors in PB and CB HPCs
| Cell lineage | Median PF (range) in HPCs (%) |
|
|---|---|---|
| PB (n=20) | CB (n=3) | |
| cDC | 5.14 (1.57 – 9.12) | 25.6 (17.3 – 47.2) |
| pDC | 1.85 (0.02 – 4.59) | 20.3 (13.7 – 23.6) |
| T cell | 3.13 (0.97 – 4.52) | 29.2 (17.3 – 37.3) |
| NK cell | 3.62 (2.44 – 6.45) | 18.0 (14.1 – 19.7) |
PFs of each cell lineage were obtained for PB and CB samples from 20 adults (age range: 28 to 64) and 3 neonates, respectively, by LDA. Most T- and NK-cell PFs from adults were obtained in our previous study.
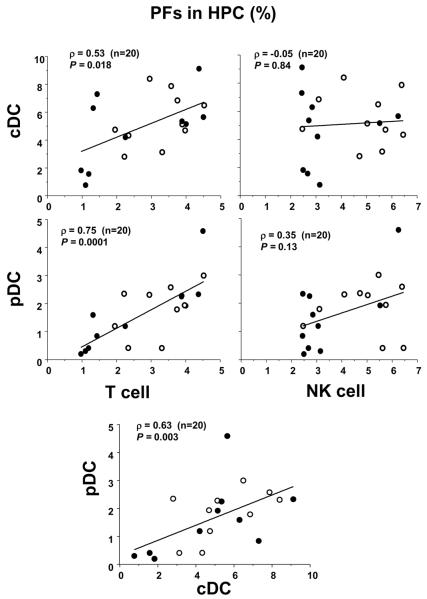
cDC PFs in adult PB HPC were found to weakly but significantly correlate with T cell PFs (ρ = 0.53) (Fig. 4). Interestingly, pDC PFs showed strong correlation with T-cell PFs (ρ = 0.75) for the same donors. In contrast, neither cDC nor pDC PFs showed any significant correlation with NK-cell PF (Fig. 4). As expected, the correlation between cDC and pDC PFs was found to be significant (ρ = 0.63) (Fig. 4).
Figure 4. Correlation of PF between DC and T- or NK-cell lineages in PB HPCs from healthy donors.
Correlation of PF between cDC and T- or NK-cell lineages (upper panel) and between pDC and T- or NK-cell lineages (lower panel) in PB from 20 donors (10 females: open circles, 10 males: closed circles, age range: 28 to 64). Spearman's rank correlation coefficients (ρ) are shown in each panel with P-values.
Clonal analyses of DC/T/NK differentiation from a single PB HPC
To examine whether the significant correlations between T-cell and cDC or pDC PFs might be due to the high prevalence of common DC/T precursors, we performed clonal analyses of T-, NK-, cDC-, pDC- and myeloid-lineage progenies generated from a single HPC in PB and CB by flow cytometry (Fig. S3). The number of progenies per well (clone) was distributed largely in both PB and CB (Fig. S4), but cloning efficiency did not differ greatly among the four different progenitors (about 60 to 70%). In that analysis, lymphoid precursors were classified as T/NK dual-, T single-, and NK single-lineage precursors based on the surface phenotype of their progenies (Table II), as described previously (26). DC precursors also were classified as cDC/pDC dual-, cDC single-, and pDC single-lineage precursors (Fig. 5.A) in combination with T/NK classification. In addition, three other categories of the HPC clones that generated CD7+CD5−CD56− cells without the presence of CD7+CD5+ T- or CD7+CD56+ NK-cell progenies (Fig. S1), myeloid cells in association with no CD7+ lymphoid progenies, and DC progeny alone were included in the classification (Table II). Findings obtained from the clonal analyses are summarized below.
Table II.
Classification of T/NK-cell and DC potential of PB and CB HPC clones
| PB HPC precursor clone | Number of clones producing DC |
||||
|---|---|---|---|---|---|
| cDC and pDC | cDC | pDC | No DC | Total | |
| T and NK | 29b (49%) | 22b (24%) | 0 | 11(10)c | 62(61)c |
| T | 6b (10%) | 13b (14%) | 0 | 4(3)c | 23(22)c |
| NK | 9b (15%) | 10b (11%) | 0 | 67(63)c | 86(82)c |
|
| |||||
| T/NK subtotal | 44b (75%) | 45b (48%) | 0 | 82(76)c | 171(165)c |
| CD7+CD5−CD56− | 9b (16%) | 15b (16%) | 0 | 11(8)c | 35(32)c |
| Myeloid w/o CD7+ cellsa | 5 (9%) | 27 (29%) | 0 | 184 | 216 |
| DC alone | 0 | 8 (9%) | 0 | - | 8 |
|
| |||||
| Total | 58 (100%) | 95(100%) | 0 | 277 | 430 |
| CB HPC precursor clones | Number of clones producing DC |
||||
|---|---|---|---|---|---|
| cDC and pDC | cDC | pDC | No DC | Total | |
| T and NK | 134b (92%) | 63b (71%) | 0 | 3(3)c | 200(200)c |
| T | 7b (5%) | 16b (18%) | 1 | 13(12)c | 37(36)c |
| NK | 2b (1%) | 0 | 0 | 0 | 2(2)c |
|
| |||||
| T/NK subtotal | 143b (99%) | 79b (89%) | b1 | 16(15)c | 239(238)c |
| CD7+CD5−CD56− | 2b (1%) | 8b (9%) | 0 | 4(4)c | 14(14)c |
| Myeloid w/o CD7+ cellsa | 0 | 2 (2%) | 0 | 26 | 28 |
| DC alone | 0 | 0 | 0 | - | 0 |
|
| |||||
| Total | 145 (100%) | 89 (100%) | 1 | 46 | 281 |
Progenies from 430 and 281 clones in PB and CB HPCs were obtained by single cell sorting of CD34+Lin− cells from 9 and 3 donors, respectively. The DC potential was classified as cDC and/or pDC in each T and/or NK precursor clone.
The presence of myeloid cells including CD14+CD15− monocytic and/or CD14+CD15+ granulocytic cells but not CD7+ lymphoid cells (Fig. S3).
All clones producing both DCs and lymphoid cells gave rise to myeloid cells.
Number of clones accompanying monocyte and/or granulocyte progenies in parentheses.
Figure 5. Analyses of cDC/pDC and T/NK-lineage progenies derived from a single PB and CB HPC.
PB and CB HPCs from each donor were sorted into more than 560 wells with 384-well plates in OP9-DL1 co-cultures at a frequency of one cell per well. After five-week culture, wells exhibiting cell growth were analyzed for surface phenotypes of cDC/pDC and T/NK lineages.
(A) Representative flow cytograms of CD11c and CD123 expressions in HLA-DR+Lin−-gated progenies from a single CD34+Lin− cell. Each precursor was classified as cDC/pDC dual-lineage (upper panel) or cDC single-lineage (lower panel).
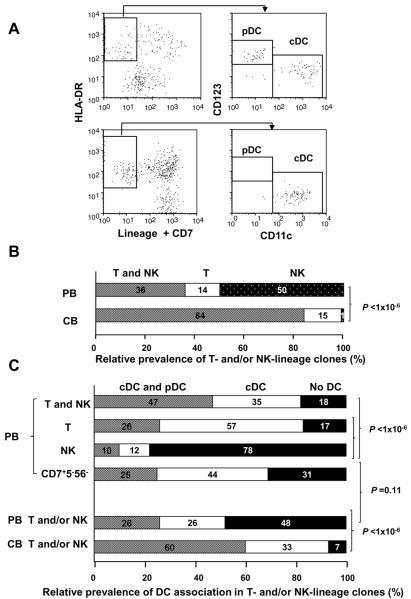
(B) Different distributions of precursor clones producing T and/or NK-lineage progenies between PB and CB CD34+Lin− cells. Classifications of T/NK dual-lineage, T single-lineage, and NK single-lineage progenies were noted in the previous report (26). Distribution of each lineage is expressed as the relative prevalence calculated from the data listed in Table II. The difference in distribution of the T/NK-precursor clones between PB and CB was highly significant by chi-square test based on the number of clones (P<1×10−6).
(C) Classification of precursor clones producing cDC/pDC-dual or pDC-single lineage in combination with T/NK-dual lineage, T-single lineage, NK-single lineage, and CD7+CD5−CD56− phenotypes in PB and CB HPCs. Distribution of each lineage is expressed as a relative prevalence obtained from the data listed in Table II. The difference in distribution of the cDC/pDC-precursor clones between T/NK dual- and NK single-, as well as between T single- and NK single-lineage phenotypes in PB (upper), was highly significant by chi-square test based on the number of clones (P<1×10−6). The difference in distribution of the cDC/pDC-precursor clones in association with T and/or NK lineage between PB and CB (lower) was highly significant by chi-square test (P<1×10−6). Distribution of the cDC/pDC-precursor clones in association with CD7+CD5−CD56− phenotype did not differ from that with T and/or NK phenotype in PB HPCs (P = 0.11)
Nearly all pDC-producing clones except for one CB clone were found to be associated with cDC progenies, whereas about 60% and 40% of cDC-producer clones were not associated with pDC in PB or CB HPCs, respectively (Table II). Among PB HSC clones, the majority (91%:75+16) of pDC-producing clones (or cDC/pDC-dual producers) were associated with lymphoid lineage, including CD7+CD5−CD56− cells. In contrast, only 64% (48+16) of cDC single lineage clones revealed lymphoid association, and 29% of those were myeloid-associated DCs (Table II). Viewing DC potential data (Table II) from the lymphoid side, more than 80% of PB T/NK-dual and T-single precursor clones were associated with generation of DC progenies (Fig. 5C), while only about 20% of PB NK-single lineage producers were associated with DC-progenies. Furthermore, distribution of relative prevalence of cDC and pDC association in CD7+CD5−CD56− clones did not differ from that in total T- and/or NK-cell clones (Fig. 5C), suggesting that DC commitment may have mainly been determined at the stage prior to T/NK bifurcation. Among CB, almost no NK-single lineage clones were observed (Fig. 5B and Table II), and nearly all T/NK-dual and T-single lineage clones produced DC progenies (Fig.5C).
More than 95% of T and/or NK clones from both PB and CB were associated with monocytes and/or granulocytes (PB: 165/171; CB: 238/239)(Table II), suggesting that the majority of T/NK-lineage clones are derived from LMPP or more upstream HPC, as described previously (26) It is noteworthy that all the clones producing both T/NK and DC progenies gave rise to myeloid cells (PB: 89/89; CB: 222/222) (Table II).
DC/T/NK potentials in different PB HPC subpopulations
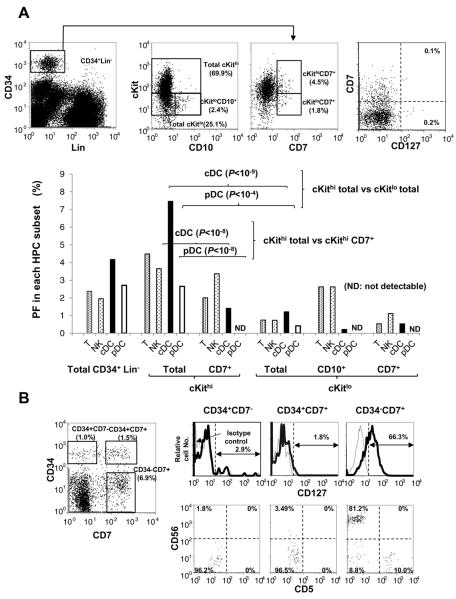
To more precisely assess which subset of PB HPC is an origin of cDC, pDC, T-, and NK-cell precursors detected by OP9-DL1 co-culture, we conducted LDA for cKithiCD7+, cKitloCD7+, and cKitloCD10+ subsets in addition to the total cKithi and cKitlo subsets among an PB HPC population (Fig.6A). The proportions of total cKit hi and cKitlo cells in HPCs from three donors were about 60% (median: 55.9; range: 55.5–63.2) and 40% (median: 37.7; range: 27.4–38.3), respectively. Because PFs of all the four different lineages in the total cKithi population were approximately 5-fold higher than the PFs of cKitlo, nearly 90% of each lineage precursor is thought to exist in the cKithi population. cDC potentials of CD7+ HPCs in both cKithi and cKitlo subsets decreased considerably but were remained at significant levels. In both subsets pDC potential was very low or absent.. cKitloCD10+ cells, which may be a human counterpart of the murine common lymphoid progenitor (CLP), showed relatively high lymphoid potentials among cKitlo HPC populations, but very low DC potentials. It is noteworthy that both cKitloCD7+ and cKitloCD10+ subsets retained myeloid potentials by single cell analyses, as shown below. Furthermore, few CD127+ cells were detected in any PB HPC subsets (Fig.6A).
Figure 6. Frequencies of cDC, pDC, T- and NK-cell precursors in different subsets of PB HPCs and CD127 expression in subsets of in vitro-generated HPC.
(A) Representative flow cytograms and gating strategy for sorting cKithi, cKitlo, cKit+/hiCD7+, cKitloCD7+, and cKitloCD10+ subsets of PB HPCs and for CD127 expression in CD7+ PB HPCs (upper panels). PFs of cDCs, pDCs, T cells and NK cells were obtained by LDA of each HPC subsets using OP9-DL1 co-culture (lower panels).
(B) Flow cytometric analyses of CD127 expression (upper) in CD34+CD7−, CD34+CD7+, and CD34−CD7+ cells generated in four-week-bulk-culture of PB HPCs. CD5 and CD56 expressions were analyzed for the three HPC subsets (lower).
Interestingly, single-cell analyses of CD34+CD7+ and CD34+CD10+ progenitors showed that more than 20% of T/NK-dual and T-single lineage precursors were associated with generation of DC progenies, while only 4% of NK-single lineage precursors were associated with DC progenies (Table III). Moreover, only about 30% of T and/or NK-producing clones were accompanied by myeloid cells, whereas all the clones producing both T cells and cDCs gave rise to myeloid cells (Table III).
Table III.
Classification of T/NK-cell and DC potential in CD34+CD7+ and CD34+CD10+clones
| In vivo CD34+CD7+ and CD34+CD10+ clones | Number of clones producing DC |
||||
|---|---|---|---|---|---|
| cDC and pDC | cDC | pDC | No DC | Total | |
| T and NK | 0 | 4 (4)a | 0 | 15 (3) | 19 (7) |
| T | 0 | 4 (4) | 0 | 10 (0) | 14 (4) |
| NK | 2 (1) | 1 (1) | 0 | 79 (17) | 82 (19) |
|
| |||||
| T/NK subtotal | 2 (1) | 9 (9) | 0 | 104 (20) | 115 (30) |
| CD7+CD5−CD56− | 0 | 3(3) | 0 | 5 (1) | 8 (4) |
| Myeloid w/o CD7+ cells | 0 | 6 | 0 | 172 | 178 |
| DC alone | 0 | 14 | 0 | - | 14 |
|
| |||||
| Total | 2 | 32 | 0 | 281 | 315 |
CD34+Lin− CD7+ or CD34+Lin− CD10+ were sorted from 5 donors at single cell per well and cultured with OP9-DL1 cells for 4 to 5 weeks. The presence of T/NK/DC progenies was analyzed and classified, as noted in Table II. The difference in distribution of total DC-producer clones were significant by chi-square test (Yates' correction) (P = 0.002), based on the number of clones between the total T lineage-(T/NK dual lineage- plus T single lineage-phenotypes) and NK single lineage-phenotype (T: 8 producer clones vs 25 non-producer clones, NK: 3 producer clones vs 79 non-producer clones).
Number of clones accompanying monocyte and/or granulocyte progenies in parentheses.
We also examined CD127 expression in progenitor subpopulations generated in vitro from PB HPCs by co-culture with OP9-DL1 cells. During 3 to 4 weeks of co-culture, PB HPCs were found to generate and retain CD34+CD7−, CD34+CD7+ and CD34−CD7+cell populations (Fig. 6B). As noted with in vivo HPC, in vitro-generated CD34+CD7− and CD34+CD7+cells both lacked CD127 expression. The CD34−CD7+cell population mainly contains CD5+ T- and CD56+ NK-lineage-committed progenies expressing CD127.
DISCUSSION
In the present study we developed assays for the functional enumeration and characterization of rare PB cDC/pDC precursors, using cell sorting-based limiting-dilution and clonal analyses. Since the conditions of co-culture with OP9-DL1 stroma cells in the presence of KL, FL, and IL-7 are identical to the precursor assay for T/NK cells (26), we were able to analyze the relationship between cDC/pDC and T/NK-cell commitment at the single-cell level. Using these assays, we tested our hypothesis that the proportion of DC to T-cell precursors circulating in PB is maintained through linkage of lineage commitments. To determine the lineage commitment, we used surface phenotyping of the progenies generated in culture. Although the progenies in each lineage as defined by their surface phenotypes remained in an immature state, we demonstrated that these progenies could mature in appropriate conditions. cDC and pDC progenies did not produce TLR agonists-stimulated cytokines, but did mature into IL-6- or IFNα-producing cells by culturing with GM-CSF or IL-3, respectively. Similarly, NK-lineage progenies were at first immature but became NKG2A-, granzyme B- and IFNγ-expressing cells after short-term culture with IL-15, as seen in our previous study (26). T-lineage progenies also exhibited differentiation up to the surface TCR+cell stage after prolonged culture with OP9-DL1 cells. Thus, lineage classification by flow analyses of surface phenotypes is apparently valid for testing the hypothesis.
LDA showed that the frequencies of DC precursors in PB HPC are significantly correlated with the frequencies of T-cell precursors, but not with those of NK-cell precursors. This finding was corroborated by results obtained from single cell analyses of HPC: More than 80% of PB T/NK-dual and T-single lineage clones co-produced cDCs and/or pDCs, whereas only about 20% of PB NK-single lineage producers were associated with DC differentiation. Furthermore, the difference of correlation coefficients with T-cell PF between cDC and pDC precursors (ρ= 0.53 and 0.75, respectively) can also be explained by the results of clonal analyses: About 60% of precursor clones producing pDC were associated with T-cell progenies, whereas less than half of cDC-producing clones were associated with T-cell commitment. In addition, a larger prevalence of myeloid cells-associated cDC-single lineage clone is thought to contribute to the lower correlation coefficient between T and cDC PF.
Interestingly, clonal analyses of PB HPCs showed that almost all pDC-producing clones were associated with cDC progenies; these findings strongly suggest that HPC-derived pDC are generated via CDPs. This result agrees with that of mouse CDP, which at clonal levels give rise to cDCs and pDCs in FL-supplemented cultures (38, 39). Therefore, although we did not directly examined the PF of CDPs for the LDA in the present study, it can be inferred that the PF of human putative CDPs also correlates with that of T cell. LDA found that CD34+CD7+ cells had only low pDC potential, irrespective of their cKit expression levels, indicating that in conjunction with the expression of CD7, HPCs lose CDP potentials. Thus, the lineage split between the majority of putative CDPs and T/NK precursors occurs prior to CD7 expression. Similar findings on divarication of DC/T-NK lineages have been reported by the analysis of progenies differentiated from human fetal liver CD34+CD38− cells in mouse fetal thymus organ culture (19), although cDCs and pDCs were not discriminated in these studies.
It is important to note that clonal analyses of PB HPCs showed that all clones producing both DC and T-cell progenies were accompanied by granulocyte and/or monocyte progenies. Similar results have been obtained from clonal analyses of CD7+ or CD10+ HPC. These findings lead to our inference that DC progenies of T/DC clones may be generated via common granulocyte-monocyte progenitors (GMPs). If this is the case, GMPs give rise to the above-mentioned human putative CDPs via MDPs according to the well-accepted model of the myeloid DC differentiation pathway (3, 12, 13), as discussed below.
Although data are not shown in the present study, human PB HPCs were found to retain B-cell potential at very low levels in comparison with T/NK-cell potentials (S. Kyoizumi, Y. Kubo, J. Kajimura, K. Yoshida, T. Hayashi, K. Nakachi, L. F. Young, M.A. Moore, M. R.M. van den Brink and Y. Kusunoki, manuscript in preparation). Furthermore, because PB-derived CD34+CD7+ and cKitloCD10+ cells have T- and/or NK-cell potentials but only low levels of B-cell potential, these HPC subpopulations are thought to mainly consist of T/NK-oriented lymphoid progenitors. These progenitor populations may be the human PB counterpart of murine PB CLPs (40), based on their expression of CD7 or CD10, but human PB CD7+ or CD10+ progenitors in the present study did not express CD127, which is detected in murine CLPs. Nevertheless, both human PB CD7+ or CD10+ progenitors and murine PB CLPs (40) retained DC and myeloid potential in vitro at low but significant levels.
CB HPCs have a high potential for both T/NK and cDC/pDC in comparison with adult PB HPCs. This may be related to extensive development of the thymus required for the establishment of a total immune system in the early stages of human life. Nearly all T/NK-cell progenitor clones were T/NK-dual or T-single lineage producers while only 1% of T/NK-lineage clones were NK-single lineage, which is in contrast to the proportions of NK-single lineage producers in adult PB-derived clones. More than 90% of CB T and/or NK-cell progenitor clones gave rise to cDCs and/or pDCs. Therefore, these findings are in accord with the linkage between DC and T-cell commitments. As observed in PB HPCs, all CB HPC clones producing both T cells and DCs also gave rise to myeloid cells.
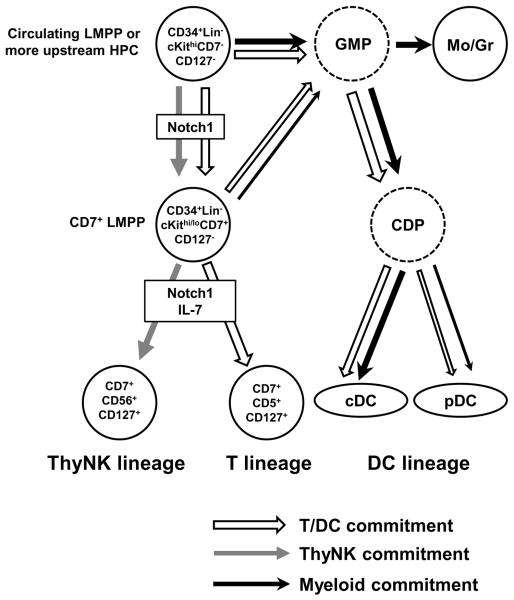
Taking account of all the findings obtained in the present study, we propose a model for differentiation pathways and linkage of cDC/pDC/T/NK-cell lineages from human PB HPCs (Fig. 7). PB CD34+Lin−CD7− cells expressing high levels of cKit give rise to both cDCs and pDCs, most probably via GMPs/CDPs, as mentioned above. Since this population also produces CD34+CD7 +CD5−CD56− cells, a GMP-CDP/CD34+CD7+ split occurs mainly at the CD34+CD7− stage. CD34+CD7+ cells retain T and/or NK-cell potentials as well as myeloid/DC potential at a low but significant level, indicating that CD34+CD7+ cells branch off into T/NK-lineage cells and GMPs/CDPs. Because CD34+Lin−CD7+ cells retain both lymphoid and myeloid potential, we define these cells as CD7+LMPP in the model. Our model agrees with recent findings that all subtypes of murine thymic DCs, including CD11b+cDCs, CD8+cDCs, and pDCs, are derived from myeloid precursors (4, 16) or from precursors with no history of CD127 expression (17). All PB HPC clones producing both T-cell and DC progenies gave rise to myeloid cells, so DC precursors linked with T-cell commitment may differentiate to cDCs/pDCs through a pathway common to myeloid DC differentiation, as shown in Fig. 7. Our model does not necessarily assume that T-lineage-linked DC commitment and differentiation occur in the thymus: DCs generated from T-linked myeloid progenitors in BM may migrate to thymus. Furthermore, no PB HPCs, including CD7+LMPPs, expressed CD127 at a significant level. Neither cDC nor pDC generation required IL-7 in OP9-DL1 co-culture.
Figure 7. Proposed model for differentiation pathways and commitments of cDC, pDC, T-cell, and thymic NK-cell lineages.
Open, gray, and closed lines indicate the linkage of cDC/pDC/T-cell commitments, thymic NK-cell commitment, and myeloid commitment, respectively. Mo and Gr represent monocyte and granulocyte lineages, respectively. Dashed circles represent putative common granulocyte-macrophage progenitors (GMP) and common dendritic cell progenitors (CDP).
In the present culture conditions, Notch 1 is required for differentiation of T- and NK-lineage cell from PB HPCs (Fig.7). Both processes, from CD34+CD7− to CD34+CD7+ and from CD34+CD7+ to T- or NK-cell lineage, were found to be Notch-dependent. In the absence of Notch 1, nearly all CD34+ cells are committed to myeloid lineages, including monocytes, granulocytes, and DCs. It is well known that Notch 1 signaling is essential for generation of mature naïve T-cells in the mouse thymus (41), although the precise role of Notch 1 in thymic DC commitment in vivo is somewhat controversial (42). An essential role for Notch signaling in DC development has been demonstrated only for splenic and small intestine CD11b+cDCs using mice lacking RBP-J gene transcript (43) and Notch 2 receptor (44), respectively. Conversely, deletion of the Notch 1 gene converted pro-T cells to cDCs and pDCs in the thymus (45). For humans, it has been reported that Delta-1 stimulated pDC differentiation from CB and BM CD34+ cells (27). We also demonstrated that OP9-DL1 cells promoted differentiation of cDC, in addition to pDC, from PB and CB HPCs, but the role of Notch 1 signaling in human DC development in vivo is still unknown. We assume that T-cell commitment of DC-linked T-cell progenitors may essentially require Notch signaling, but DC commitment of these progenitors might be indirectly induced by crosstalk between myeloid DC-inducing signal and Notch 1 downstream signal. If this is the case, DC-linked T-cell progenitors might differentiate to DC lineage by myeloid DC-inducing signal in the absence of Notch 1 stimulation. In our previous study, Notch 1 signaling induced thymic NK-cell commitment much more effectively than IL-15 did in culture, although IL-15 can induce NK-cell commitment in the absence of Notch 1 and it is essential for functional maturation of thymic NK cells (26).
As discussed above, the strong correlation of PFs between T-cell and pDC or putative CDP suggests a linkage between T-cell and DC commitments (Fig.7). The high prevalence of HPC clones retaining both T and DC potentials also support this assumption. If our model of the differentiation pathway (Fig. 7) is correct, this linkage is primed in progenitors at the stage of CD34+Lin−cKithi CD7− population. In other words, the dichotomy fate of downstream T/NK bipotent progenitor is determined by the upstream event of DC/T-NK split. If a T/NK bipotent progenitor experiences the DC commitment at the stage of DC/T-NK split, the bipotent progenitor will be directed to T-cell lineage with high probability. It is important to note that analyses of HPC clones generated from PB CD34+Lin−CD7+ or CD10+ showed that DC progenies generated from these progenitors are more frequently associated with T/NK dual or T-single lineage precursor clones than with NK-single lineage clones. These findings support the concept that linkage between T and DC commitment is retained at the downstream CD34+CD7+ or CD10+stage (Fig. 7). On the other hand, commitment of human PB HPCs to thymic NK-cell lineage is thought to be independent of the T/DC-linkage (Fig. 7). In this context, our previous study showed that the ratios of T-to NK-cell PF varied among individuals but were fairly constant in each individual for as long as 6 months (26). This observation suggests that the bifurcation of co-progenitors to T- and/or NK-cell lineages is not stochastic, but is presumed to be also primed in HPCs at some upstream stage.
Molecular mechanisms involved in the linkage between T- and DC-lineage commitments remain to be determined. Transcription networks for commitment of each lineage might be cross-linked by common transcription factors between the two lineages, and we think that the linkage signal might be intrinsically imprinted in long-lived HSCs, but not in CLPs, LMPPs or MPPs with transient life spans, since such linkages would be stably integrated in the long-term throughout human life. Extrinsic signals from niche including cytokines may be instructive for DC development (29), but they also seem to be transient. Recent mouse studies using a barcoding technique for a single LMPP suggested that the fate of the progenitors is imprinted in an early stage of hematopoiesis (9). Thus, intrinsic signals linking the two lineages may be generated in HSCs and propagated through intermediate stages to a tail branch on the differentiation tree.
Supplementary Material
Acknowledgments
We thank M. Yamaoka for her excellent assistance with FACS analyses and E. Douple for his critical review of the manuscript.
The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a private, non-profit foundation funded by the Japanese Ministry of Health, Labor and Welfare and the U.S. Department of Energy, the latter in part through DOE Award DE-HS0000031 to the National Academy of Sciences. This study was based on RERF Research Protocol RP#5-09 and was supported by the U.S. National Institute of Allergy and Infectious Diseases (NIAID Contract HHSN272200900059C). The views of the authors do not necessarily reflect those of the two governments.
Abbreviations used in this paper
- CB
cord blood
- cDC
conventional DC
- CDP
common DC progenitor
- CLP
common lymphoid progenitor
- CMP
common myeloid progenitor
- FL
Flt-3 ligand
- GMP
common granulocyte-macrophage progenitor
- HPC
hematopoietic progenitor cell
- HSC
hematopoietic stem cell
- KL
Kit ligand
- LMPP
lymphoid-primed multipotent progenitor
- LDA
limiting-dilution assay
- MC
mononuclear cell
- MPP
multipotent progenitor
- PB
peripheral blood
- pDC
plasmacytoid DC
- PF
precursor frequency
Footnotes
Disclosures The authors declare they have no financial conflicts of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 3.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 5.Chicha L, Jarrossay D, Manz MG. Clonal type I interferon-producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations. J. Exp. Med. 2004;200:1519–1524. doi: 10.1084/jem.20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 7.Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, Traver D, Leder P, Sakaguchi N, Akashi K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa F, Niiro H, Iino T, Yoshida S, Saito N, Onohara S, Miyamoto T, Minagawa H, Fujii S, Shultz LD, Harada M, Akashi K. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110:3591–3660. doi: 10.1182/blood-2007-02-071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik SH, Perie L, Swart E, Gerlach C, van Rooij N, de Boer RJ, Schumacher TN. Diverse and heritable lineage imprinting of early haematopoietic progenitors. Nature. 2013;496:229–232. doi: 10.1038/nature12013. [DOI] [PubMed] [Google Scholar]
- 10.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J. Exp. Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O'Keeffe M, Wu L, Wilson A, Shortman K. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 16.Luche H, Ardouin L, Teo P, See P, Henri S, Merad M, Ginhoux F, Malissen B. The earliest intrathymic precursors of CD8alpha(+) thymic dendritic cells correspond to myeloid-type double-negative 1c cells. Eur. J. Immunol. 2011;41:2165–2175. doi: 10.1002/eji.201141728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, Fehling HJ, Rodewald HR. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Res P, Martinez-Caceres E, Cristina Jaleco A, Staal F, Noteboom E, Weijer K, Spits H. CD34+CD38dim cells in the human thymus can differentiate into T, natural killer, and dendritic cells but are distinct from pluripotent stem cells. Blood. 1996;87:5196–5206. [PubMed] [Google Scholar]
- 19.Plum J, De Smedt M, Verhasselt B, Offner F, Kerre T, Vanhecke D, Leclercq G, Vandekerckhove B. In vitro intrathymic differentiation kinetics of human fetal liver CD34+CD38- progenitors reveals a phenotypically defined dendritic/T-NK precursor split. J. Immunol. 1999;162:60–68. [PubMed] [Google Scholar]
- 20.Dalloul AH, Patry C, Salamero J, Canque B, Grassi F, Schmitt C. Functional and phenotypic analysis of thymic CD34+CD1a- progenitor-derived dendritic cells: predominance of CD1a+ differentiation pathway. J. Immunol. 1999;162:5821–5828. [PubMed] [Google Scholar]
- 21.Weijer K, Uittenbogaart CH, Voordouw A, Couwenberg F, Seppen J, Blom B, Vyth-Dreese FA, Spits H. Intrathymic and extrathymic development of human plasmacytoid dendritic cell precursors in vivo. Blood. 2002;99:2752–2759. doi: 10.1182/blood.v99.8.2752. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Vremec D, Ardavin C, Winkel K, Suss G, Georgiou H, Maraskovsky E, Cook W, Shortman K. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur. J. Immunol. 1995;25:418–425. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]
- 23.Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J. Immunol. 2003;170:3514–3521. doi: 10.4049/jimmunol.170.7.3514. [DOI] [PubMed] [Google Scholar]
- 24.Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J. Immunol. 2003;171:3568–3575. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- 25.Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Annals NY Acad. Sci. 2011;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyoizumi S, Kubo Y, Kajimura J, Yoshida K, Imai K, Hayashi T, Nakachi K, Young LF, Moore MA, van den Brink MR, Kusunoki Y. Age-Associated Changes in the Differentiation Potentials of Human Circulating Hematopoietic Progenitors to T- or NK-Lineage Cells. J. Immunol. 2013;190:6164–6172. doi: 10.4049/jimmunol.1203189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivier A, Lauret E, Gonin P, Galy A. The Notch ligand delta-1 is a hematopoietic development cofactor for plasmacytoid dendritic cells. Blood. 2006;107:2694–2701. doi: 10.1182/blood-2005-03-0970. [DOI] [PubMed] [Google Scholar]
- 28.Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109:507–515. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol. Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 31.Kodama H, Nose M, Niida S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp. Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 32.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Meth. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 35.De Smedt M, Leclercq G, Vandekerckhove B, Kerre T, Taghon T, Plum J. T-lymphoid differentiation potential measured in vitro is higher in CD34+CD38-/lo hematopoietic stem cells from umbilical cord blood than from bone marrow and is an intrinsic property of the cells. Haematologica. 2011;96:646–654. doi: 10.3324/haematol.2010.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 37.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 38.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 39.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 40.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Ann. Rev. Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 42.Cheng P, Gabrilovich D. Notch signaling in differentiation and function of dendritic cells. Immunol. Res. 2008;41:1–14. doi: 10.1007/s12026-007-8011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, Gossler A, Gale NW, Radtke F, Fehling HJ, Rodewald HR. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.