Abstract
Fas ligand expression in certain tumors has been proposed to contribute to immunosuppression and poor prognosis. However, immunotherapeutic approaches may elicit the Fas-mediated elimination of immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) within tumors that represent major obstacles for cancer immunotherapy. Previously, we showed that IL-2 and agonistic CD40 Ab (αCD40) elicited synergistic antitumor responses coincident with the efficient removal of Tregs and MDSCs. We demonstrate in this study in two murine tumor models that Treg and MDSC loss within the tumor microenvironment after IL-2/αCD40 occurs through a Fas-dependent cell death pathway. Among tumor-infiltrating leukocytes, CD8+ T cells, neutrophils, and immature myeloid cells expressed Fas ligand after treatment. Fas was expressed by tumor-associated Tregs and immature myeloid cells, including MDSCs. Tregs and MDSCs in the tumor microenvironment expressed active caspases after IL-2/αCD40 therapy and, in contrast with effector T cells, Tregs significantly downregulated Bcl-2 expression. In contrast, Tregs and MDSCs proliferated and expanded in the spleen after treatment. Adoptive transfer of Fas-deficient Tregs or MDSCs into wild-type, Treg-, or MDSC-depleted hosts resulted in the persistence of Tregs or MDSCs and the loss of antitumor efficacy in response to IL-2/αCD40. These results demonstrate the importance of Fas-mediated Treg/MDSC removal for successful antitumor immunotherapy. Our results suggest that immunotherapeutic strategies that include exploiting Treg and MDSC susceptibility to Fas-mediated apoptosis hold promise for treatment of cancer.
Introduction
The accumulation of immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment represents a major obstacle for the development of effective antitumor immunotherapies. Treg removal using either cyclophosphamide (1) or CD25 Abs (2), or MDSC removal by sunitinib (3) restored tumor-specific T cell responses and represent clinically feasible approaches for inducing therapeutic responses. As we gain better understanding of the mediators responsible for the development, recruitment, and expansion of Tregs or MDSCs within tumors, more effective strategies aimed at controlling them can be exploited.
Activated lymphocytes frequently express increased levels of death receptors, rendering them susceptible to apoptosis (4, 5). Interactions between the Fas death receptor and its ligand activate cysteine-aspartic proteases (caspases) and induce lymphocyte apoptosis (5–8). The elimination of clonally expanded, activated immune cells balances immune responses by controlling the ratio between effector T cells (Teffs) and Tregs (9, 10). In contrast with Teffs, Tregs frequently display activation markers (e.g., CD25), have faster basal turnover rates, and possess suppressor function independent of their proliferation status (11). In contrast with conventional T cells, freshly isolated Tregs express high levels of Fas and are prone to Fas ligand (FasL)–mediated apoptosis (12, 13). Antitumor strategies that target Tregs, including the intratumoral administration of FasL (14), are in development. However, some naive Tregs remain resistant to Fas-mediated apoptosis (11, 13), and Treg sensitivity to Fas-induced cell death is regulated by TCR ligation and Treg stimulation (12, 13). Under certain inflammatory conditions, MDSCs also express Fas and have similarly been shown to undergo apoptosis in response to T cell–derived Fas ligand (15, 16). As such, there is considerable potential for exploiting the sensitivities of these cells to Fas-mediated apoptosis as part of an overall strategy to treat cancer.
The Fas pathway is a critical mechanism by which activated leukocytes lyse tumor cells (17). However, Fas ligand expression by tumors, including renal cell carcinoma (RCC) (18, 19), can contribute to tumor escape through a process referred to as “tumor counterattack,” whereby Fas+ immune cells are killed (reviewed in Ref. 20). We hypothesized that immunotherapy would alter leukocyte sensitivity to counterattack within the tumor microenvironment and therefore tip the balance toward tumor killing. We showed previously that treatment of mice bearing metastatic RCC with the combination of IL-2 and agonistic CD40 Ab (αCD40) elicits synergistic antitumor responses in association with removal of Tregs and MDSCs from primary tumors. In this article, we show for the first time, to our knowledge, that the loss of these suppressor cell populations in two different tumor models occurs via Fas-mediated apoptosis. Our data highlight the ability of combination immunotherapies, such as IL-2/αCD40, to therapeutically exploit the preferential susceptibility in the tumor microenvironment of Tregs and MDSCs to active cell death.
Materials and Methods
Mice
BALB/cJ wild-type (WT) and IFN-γ−/− mice were obtained from the Animal Production Area of National Cancer Institute (Frederick, MD). BALB/c CD45.1 congenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL6 MRL-Fas(lpr) and mice expressing enhanced GFP (eGFP) under control of the β-actin promoter were from Jackson Laboratory and backcrossed onto a BALB/c background at least 10 generations. All mice were genotyped before use.
Cells and reagents
Renal adenocarcinoma of BALB/c origin (Renca) was passaged i.p. as described previously (21). The 4T1 cell line was obtained from American Type Culture Collection. Recombinant human IL-2 (Teceleukin) was obtained from the Biological Resources Branch, Division of Cancer Treatment and Diagnosis, National Cancer Institute. Agonist rat anti-mouse CD40 (clone FGK115B3) was purified from ascites as described previously (22, 23). Fas ligand blocking Ab (clone MFL4) was generated as described previously (24). The isotype-matched control Ab was Syrian hamster γ-globulin (Jackson Immunoresearch Laboratories, West Grove, PA). CD8+ cells were depleted in vivo by i.p. injection of rat anti-mouse CD8 (clone Ly2.2) on days −1, 4, 11, and 18. NK cells were depleted in vivo by i.p. injection of rabbit anti–asialo-GM1 (10 μl in 90 μl PBS/dose; Wako Chemicals, Richmond, VA) on days −1, 4, and 11.
Immunohistochemistry
Murine primary tumors (formalin fixed, paraffin embedded) were harvested on day 21 and sectioned at 5 μm. Kidney tissue microarrays consisting of TNM-staged human RCC and adjacent normal tissue were purchased from US Biomax (Rockville, MD). Each slide consisted of duplicated cores and matched normal adjacent tissue collected at time of patient surgery. Patients had not received any therapy before surgery. Slides were blocked using 10% goat serum followed by overnight incubation at 4°C with anti-Fas and anti–Fas ligand Abs (Abcam, Cambridge, MA). Slides were then incubated for 1 h at room temperature using biotinylated goat polyclonal anti-rabbit IgG and ABComplex (Vector Laboratories, Burlingame, CA).
In vivo tumor models and treatments
A total of 1 × 105 Renca or 4T1 tumor cells was injected under the kidney capsule or mammary fat pad, respectively. Mice treated with IL-2 received 300,000 IU i.p. twice a day on days 11, 15, 18, and 21 posttumor injection. Mice treated with anti-CD40 received 65 μg i.p. once on days 11–15 and 18–21 posttumor injection. In some experiments, mice were treated with 0.5 mg Fas blocking Ab or isotype control on days 11, 13, 15, 18, and 21.
Adoptive transfer of Tregs and MDSCs
Mice transgenic for eGFP under the control of the β-actin promoter were used as recipient mice for adoptively transferred Tregs. The mice were depleted of endogenous Tregs (>90% peripheral depletion) by three sequential injections of PC61 ascites (1:5 dilutions given over 4 d). Mice received adoptively transferred Tregs 2 d after the final injection of PC61. For each adoptive transfer experiment, the splenocytes from 30 WT or MRL-Fas(lpr) mice were pooled. RBCs were lysed in ACK buffer (Lonza, Walkersville, MD). Splenocytes were incubated with CD4 T cell biotin Ab mixture and anti-biotin microbeads (Miltenyi Biotec, Auburn, CA) to enrich for CD4+ T cells. T cells were incubated with fluorescently conjugated Abs for CD4 and CD25, and the CD4+CD25+ population representing Tregs was purified on a FACSAria cell sorter (BD Biosciences, Franklin Lakes, NJ) to >95% purity. Sorted cells were washed with cold saline and injected (5 × 105 cells) into the tail veins of recipient mice. The next day, mice were inoculated orthotopically with Renca tumor cells.
For the adoptive transfer of MDSCs, the earlier procedure was followed except CD45.1 congenic mice were used as recipient mice for adoptively transferred MDSCs and the mice were depleted of endogenous MDSCs (>90% peripheral depletion) by three sequential injections of RB68C5 ascites (1:5 dilutions given over 4 d). To enrich for MDSCs, we inoculated donor mice with 1 × 105 4T1 tumor cells s.c., which grew untreated for 12 d. Splenocytes from three WT or MRL-Fas mice were pooled and the CD11b+Gr1LoNKp46− population representing MDSCs was purified to >95% purity. Sorted cells were washed and injected (1 × 106) into recipient mice. The next day, mice were inoculated s.c. with 4T1 tumor cells.
Isolation of splenic and tumor-associated leukocytes
Leukocytes were isolated from the tumors and spleens of mice as described previously (23).
Assay of Treg suppressor function
Single-cell suspensions of splenocytes were prepared as described previously (23). CD4 cells were purified using magnetic beads and positive selection (Miltenyi Biotec). Washed CD4+ cells were incubated with fluorescently conjugated CD4 and CD25 Abs and sorted into CD4+CD25− Teffs and CD4+CD25+ Treg populations. CD4-depleted splenocytes from WT mice were CFSE labeled and used as stimulator APCs. Teffs (50,000 cells/well) were stimulated with 200,000 APCs and 1 μg/ml functional grade anti-CD3 Ab for 72 h. Tregs were added at various Teff/Treg ratios. Cell proliferation was assessed after gating on live cells (Invitrogen).
Quantitative PCR
Total RNA was isolated from spleen and tumor tissue as described previously (23). Samples were analyzed using the ΔΔCT method (25). Gene expression was normalized to the level of GAPDH housekeeping gene.
Flow cytometry
Cells (1 × 106) were incubated in cell staining buffer (0.1% BSA, 0.1% sodium azide) containing 250 μg/ml 2.4G2 ascites for 15 min. Cells were stained with diluted fluorescently conjugated Abs (BD Pharmingen, San Jose, CA) for 20 min followed by two washes in staining buffer. For intracellular staining of BrdU, Bcl-2, and Foxp3, cells were fixed and permeabilized according to the manufacturer’s instructions (BD Pharmingen for BrdU and Bcl-2; eBioscience, San Diego, CA, for Foxp3). Labeled cells were analyzed on an LSR-II flow cytometer (Becton Dickinson, Mountain View, CA). Tregs were identified as CD4+CD25+ cells; CD4 “non-Tregs” were CD4+CD25−, and CD8 T cells were CD4−CD8+. MDSCs were identified as CD11b+Gr1LoCD124+ cells.
Statistical analysis
Statistical differences were analyzed using Mann–Whitney U test (GraphPad Prism; GraphPad Software, San Diego, CA). Significance was indicated by p values <0.05.
Results
Fas ligand is associated with tumor stage in human RCC
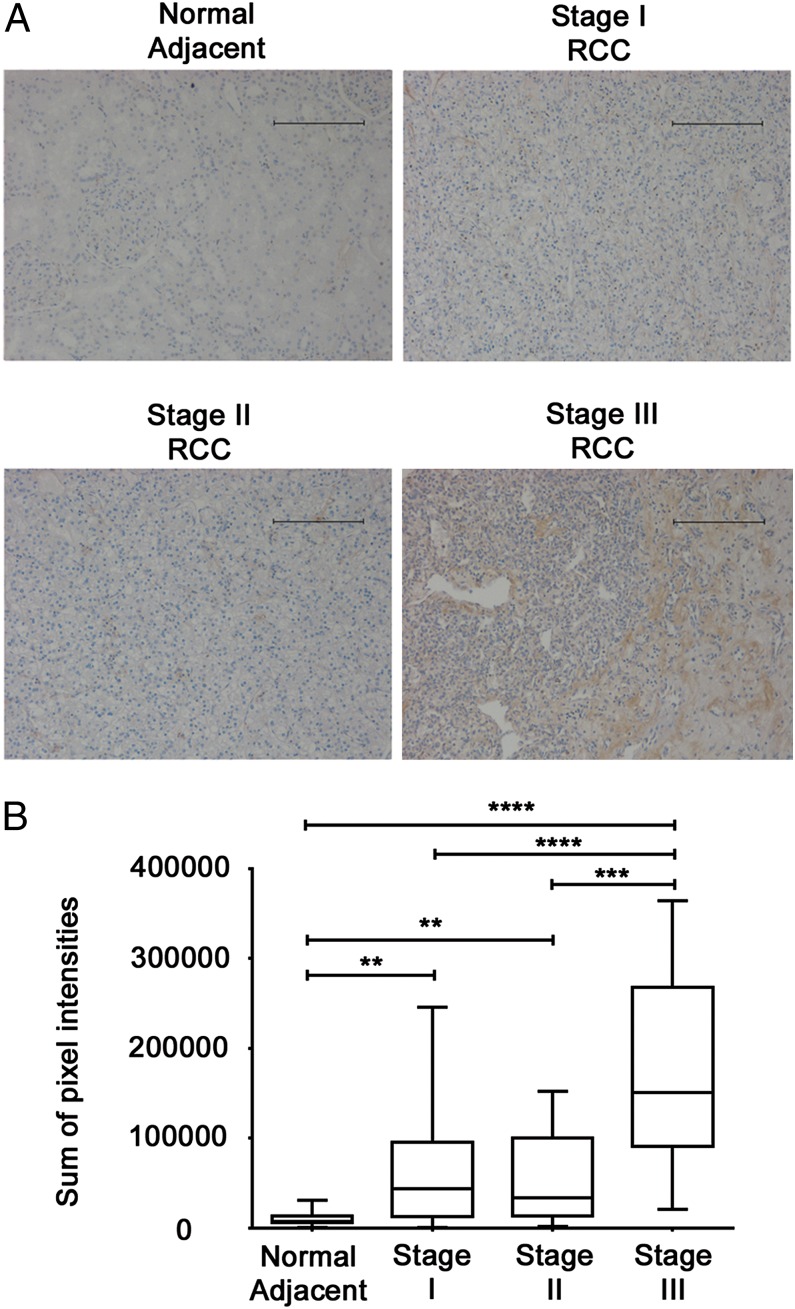
Although tumor-derived Fas expression associates with poorer outcome in RCC patients (19), the contributions of tumor- versus leukocyte-associated Fas ligand expression remains incompletely defined, particularly in the context of immunotherapy. By immunohistochemistry, we found that increased Fas ligand expression was associated with advanced stage RCC (Fig. 1). Fas ligand was minimally expressed by normal adjacent tissues, as well as stages I or II RCC, but was uniformly upregulated among stage III RCC from patients (Fig. 1A). By quantitating the sum of Fas ligand staining intensities, we found significant upregulation of Fas ligand immunoreactivity for stage III RCC, as compared with all other samples evaluated (Fig. 1B). Fas ligand–positive, tumor-associated lymphocytes in RCC have been described previously (17), and although we do not rule out leukocyte contributions, the staining pattern for Fas ligand was homogeneous throughout tumor sections and most evidently associated with tumor cells.
FIGURE 1.
Fas ligand is associated with tumor stage in human RCC. Human clear-cell RCC and adjacent normal tissue were analyzed for Fas and Fas ligand expression by immunohistochemistry. (A) Reactivity is denoted by brown DAB staining (original magnification ×200). Scale bars, 100 μM. Results are representative of duplicate core sample from 9 normal adjacent controls, 74 stage I RCC, 20 stage II RCC, and 7 stage III RCC samples. (B) Immunostaining was quantitated using Cell Profiler and represented as the sum of pixel intensities (**p < 0.005, ***p < 0.0005, ****p < 0.0001 for the indicated comparisons).
Next, we analyzed Fas and Fas ligand expression in murine Renca tumors from vehicle control (VC)– and IL-2/αCD40–treated mice. Fas expression was expressed weakly in tumors from VC-treated mice and upregulated within tumors from IL-2/αCD40–treated mice (Supplemental Fig. 1). Fas ligand expression was essentially undetectable in tumors from VC-treated mice, yet robustly expressed by both tumor and tumor-infiltrating leukocytes from IL-2/αCD40–treated mice (Supplemental Fig. 1). These findings indicate that IL-2/αCD40 treatment resulted in the accumulation of Fas ligand–positive leukocytes within tumors.
Loss of Tregs and MDSCs in the tumor microenvironment after IL-2/αCD40 therapy is dependent on Fas
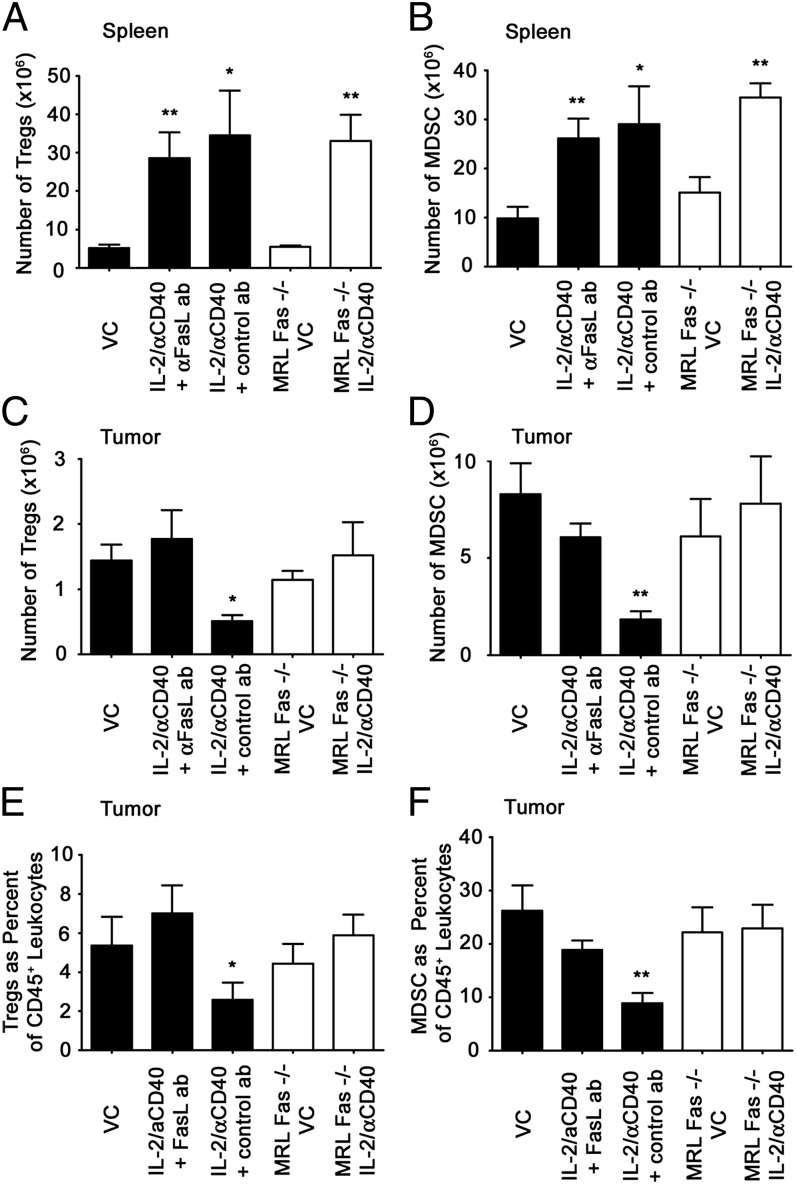
Although Fas ligand expression was positively associated with increasing RCC tumor stage, we hypothesized that immunotherapy may target immunoregulatory cells for apoptosis. Previously, we showed synergistic antitumor responses achieved by IL-2/αCD40 therapy were accompanied by the removal of Tregs and MDSCs within the tumor microenvironment (23). Although host Fas signaling is necessary for the antitumor efficacy of this immunotherapy (22), its precise contributions remain unclear. We therefore evaluated IL-2/αCD40 therapy in MRL-Fas(lpr) mice that are deficient in functional Fas. Because these mice have well-characterized immune dysregulation, we also used a Fas ligand neutralizing Ab. IL-2/αCD40 induced the expansion of Tregs and MDSCs in the spleen, and anti-Fas ligand had no effect on this increase (Fig. 2A and 2B). A similar expansion of splenic Tregs and MDSCs was observed using MRL-Fas(lpr) mice. In contrast, IL-2/αCD40 failed to reduce tumor-associated Tregs when Fas ligand was blocked or in MRL-Fas(lpr) mice (Fig. 2C). Similarly, the loss of tumor-associated MDSCs in IL-2/αCD40–treated mice was abrogated when Fas/Fas ligand was blocked (Fig. 2D). The frequency of Tregs and MDSCs among all CD45+ leukocytes was similarly reduced (Fig. 2E and 2F), indicating that reduced Tregs and MDSC numbers were not due to smaller tumor size after IL-2/αCD40 treatment. Thus, Fas is required for loss of Tregs and MDSCs within tumors after IL-2/αCD40. The depletion of CD8+ cells, but not NK cells, during immunotherapy also abrogated the loss of tumor-associated Tregs and MDSCs (data not shown).
FIGURE 2.
IL-2/αCD40 induces the Fas-dependent loss of Tregs and MDSCs in the tumor microenvironment. Renca tumor-bearing mice were treated with VC or IL-2/αCD40. Black bars denote mice that were treated with either blocking Ab against Fas ligand or isotype-matched control Ab. White bars indicate VC- or IL-2/αCD40–treated MRL-Fas(lpr) mice. On day 22, mice were euthanized, and the spleens and primary tumors were harvested. The total number of (A) Tregs and (B) MDSCs in the spleen was determined by multiplying the number of CD45+ leukocytes by the percentage in each sample. The number of tumor-associated (C) Tregs and (D) MDSCs were quantitated by multiplying the number of CD45+ leukocytes by the percentage in each sample. The percentage of tumor-associated (E) Tregs and (F) MDSCs among CD45+ tumor leukocytes is shown. *p < 0.02, **p < 0.005, compared with VC. Data are derived from five mice per treatment group in two separate experiments.
To corroborate these findings in another tumor model, we analyzed Treg and MDSC frequencies in the spleens and tumors of mice bearing 4T1 breast cancer cells. IL-2/αCD40 caused a significant reduction in Treg and MDSC frequency in primary tumors (Supplemental Fig. 2C–F). In this model, αCD40 as a single agent was also capable of reducing tumor-associated Tregs (Supplemental Fig. 2C and 2E), but not MDSCs. In 4T1-bearing mice, IL-2 and αCD40 expanded splenic Tregs (Supplemental Fig. 2A) but had no effect on MDSCs (Supplemental Fig. 2B).
Fas and Fas ligand are expressed by tumor-associated leukocytes after IL-2/αCD40 treatment
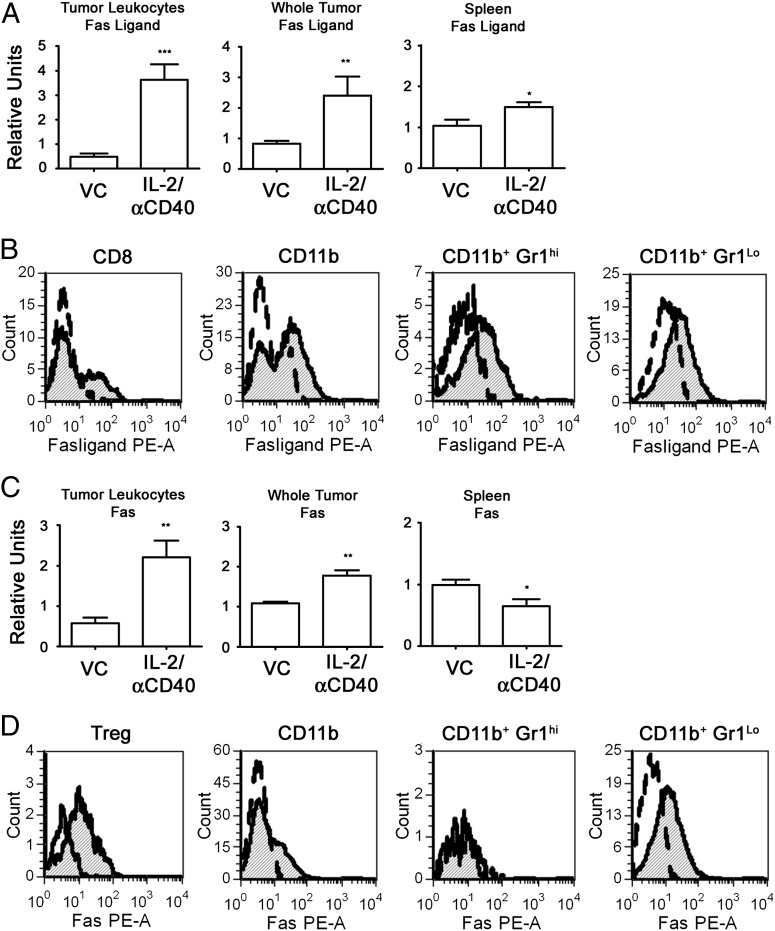
By quantitative PCR (qPCR), we found that IL-2/αCD40 significantly increased Fas ligand mRNA expression from tumor-associated leukocytes, as compared with control-treated mice (Fig. 3A). Fas ligand mRNA expression was also significantly increased in splenocytes in response to IL-2/αCD40 treatment.
FIGURE 3.
IL-2/αCD40 induces Fas- and Fas ligand–positive leukocytes within tumors and spleens. Tumor-bearing mice were treated with VC or IL-2/αCD40. On day 15, tumors and spleens were either homogenized directly in TRIzol or leukocytes were isolated by Percoll gradient before lysis. Total mRNA was reverse-transcribed and analyzed by qPCR for (A) Fas ligand and (C) Fas gene expression. Results from VC-treated samples were normalized to 1 (*p < 0.04, **p < 0.002, ***p < 0.0002). Results are derived from at least nine samples per group. Percoll-purified leukocytes isolated from primary tumors were gated by CD45 and analyzed for Fas ligand (B) and Fas (D) protein expression by flow cytometry. Histograms denote the fluorescence intensity among cell populations for Fas ligand (B) and Fas (D) expression. Open curves represent VC-treated groups; shaded curves represent IL-2/αCD40–treated groups. Flow results are representative of five mice per treatment group in two separate experiments.
We showed previously that IL-2/αCD40 induces significant accumulation of CD8+ T cells, NK cells, and myeloid cells in primary tumors (23). We therefore sought to identify the cellular source of Fas ligand and Fas within tumors after IL-2/αCD40 treatment. Fas ligand was expressed by a subpopulation of CD8+ T cells, as well as by a considerable number of CD11b+ myeloid cells (Fig. 3B). When we further analyzed the CD11b+ cells, we found that Fas ligand was expressed by Gr1hi granulocytic and by Gr1lo monocytic MDSCs. The induction of Fas ligand expression was dependent on IFN-γ, because no upregulation was detected in IL-2/αCD40–treated IFN-γ−/− mice (data not shown).
Fas expression in tumors and spleens was similarly examined by qPCR. As shown in Fig. 3C, significant increases in Fas mRNA were observed in leukocytes isolated from the tumor, as well as whole tumor tissue after IL-2/αCD40 treatment. In contrast, splenocytes had reduced Fas expression after IL-2/αCD40 treatment.
Next, we analyzed Fas protein expression on tumor-associated leukocytes. Fas expression was observed on Tregs, as well as CD11b+Gr1Lo cells, which include MDSCs (Fig. 2D). Fas was undetectable among CD11b+Gr1hi granulocytes (Fig. 3D) and CD8+ T cells (Supplemental Fig. 3). The induction of Fas receptor expression was also dependent upon IFN-γ, because no upregulation was detected in IL-2/αCD40–treated IFN-γ−/− mice (data not shown). IFN-γ mRNA expression was significantly higher among tumor-associated leukocytes from IL-2/αCD40–treated mice, as compared with control-treated mice (Fig. 4). In contrast, no significant increase in IFN-γ mRNA expression was observed for splenocytes from IL-2/αCD40–treated mice. Thus, the selective upregulation of IFN-γ expression in the tumor microenvironment after immunotherapy directly correlates with the tumor microenvironment-specific upregulation of Fas and Fas ligand expression.
FIGURE 4.

IL-2/αCD40 induces IFN-γ expression in the tumor microenvironment but not in the spleen. Tumor-bearing mice were treated with VC or IL-2/αCD40. On day 15, (A) tumor leukocytes and (B) splenocytes were isolated as described previously and lysed in TRIzol reagent. The total mRNA was reverse transcribed into cDNA and analyzed by qPCR for IFN-γ gene expression. Results from VC-treated samples were normalized to 1 (*p < 0.03; ns, not significant). Data are derived from four mice per treatment group in two separate experiments.
Tregs and MDSCs preferentially undergo apoptosis within the tumor microenvironment after IL-2/αCD40 therapy
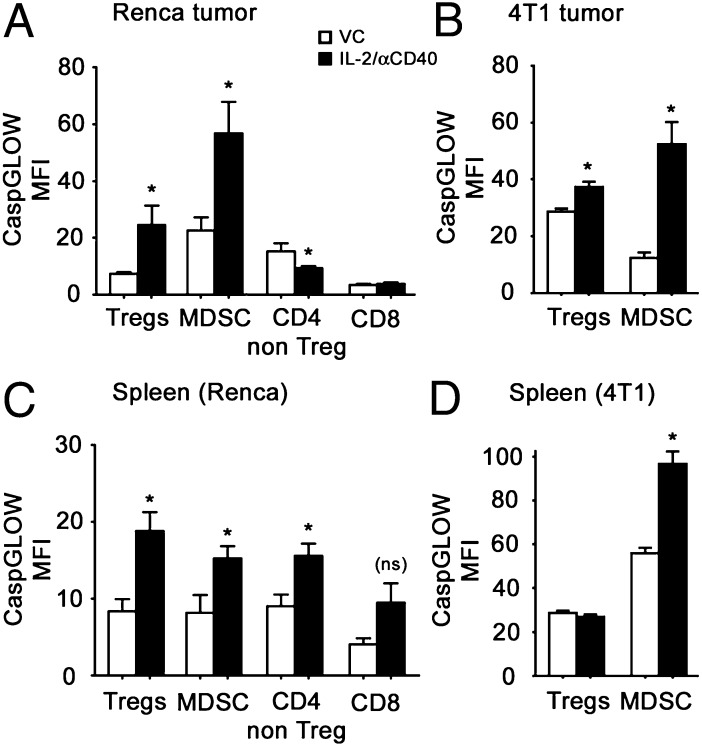
To determine whether the reduced numbers of Tregs and MDSCs were associated with active cell death, we analyzed active caspase levels in leukocytes isolated from tumors and spleens of VC- and IL-2/αCD40–treated mice. Among Renca tumor-associated leukocytes, Tregs and MDSCs significantly upregulated active caspase levels after IL-2/αCD40 treatment (Fig. 5A). No upregulation in activated caspases was observed in IL-2/αCD40–treated IFN-γ−/− mice or in mice depleted of CD8+ cells (data not shown). In contrast, CD4+CD25− non-Tregs (effector) modestly, yet significantly, downregulated active caspases, whereas no change was observed for CD8+ T cells, indicating the preferential activation of this pathway in tumor-associated Tregs and MDSCs after therapy. Similar increases in Treg- and MDSC-associated caspase activation were observed in the 4T1 tumor model (Fig. 5B).
FIGURE 5.
IL-2/αCD40 activates caspases on Tregs and MDSCs in the tumor and spleen. Renca or 4T1 tumor-bearing mice were treated with VC or IL-2/αCD40. On day 15, the primary tumors and spleens were harvested. Activated caspases were analyzed on day 15 using the cell-permeable CaspGLOW active staining kit and flow cytometry. (A and C) Results from tumor-associated leukocytes and splenocytes from Renca tumor-bearing mice, respectively. (B and D) Results from tumor-associated leukocytes and splenocytes from 4T1-tumor bearing mice, respectively. Statistics were computed in comparison with the corresponding VC (*p < 0.05; ns, not significant). Data are derived from five mice per treatment group in two separate experiments.
In the spleens of Renca-bearing mice, upregulated expression of active caspase was detected for all leukocyte populations analyzed, although this increase did not reach significance for CD8+ T cells (Fig. 5C). In the spleens of 4T1-tumor bearing mice, Tregs had no increased caspase activation, whereas MDSCs did upregulate active caspases (Fig. 5D). Thus, there was no selectivity for caspase activation among splenocytes in mice receiving immunotherapy.
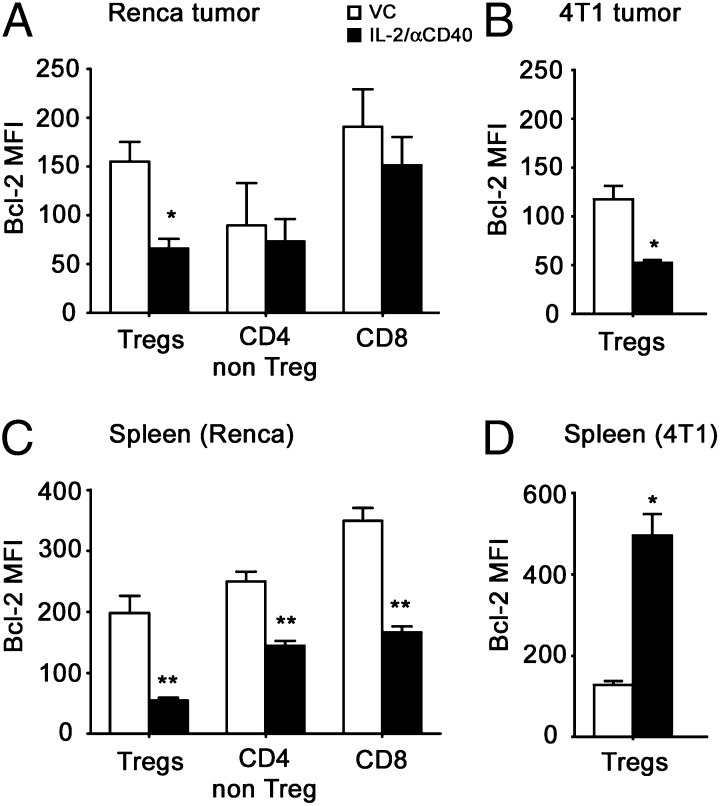
Treg loss was accompanied by reduced expression of the antiapoptotic protein, Bcl-2
Bcl-2 downregulation occurs at the early stages of apoptosis and has been shown to be a marker of Treg apoptosis in vivo (26). By flow cytometry, we observed a significant decrease in Bcl-2 expression among Renca tumor-associated Tregs after IL-2/αCD40 therapy (Fig. 6A), which was not observed for CD4+CD25− non-Tregs or CD8+ T cells. A similar decrease in Treg-associated Bcl-2 levels was observed among 4T1 tumor-associated Tregs (Fig. 6B). In the spleens of IL-2/αCD40–treated mice, all three lymphocyte populations had significantly reduced Bcl-2 expression, as compared with VC-treated mice (Fig. 6C). Thus, there did not appear to be selectivity for Bcl-2 downmodulation among splenocytes in mice receiving immunotherapy. Interestingly, splenic Tregs in the 4T1 model exhibited significantly increased Bcl-2 levels after IL-2/αCD40 treatment (Fig. 6D).
FIGURE 6.
Tregs downregulate Bcl-2 expression in the tumor and spleen after IL-2/αCD40 treatment. Renca or 4T1 tumor-bearing mice were treated with VC or IL-2/αCD40. On day 15, the primary tumors and spleens were harvested. Bcl-2 expression was determined on day 15 by intracellular flow cytometric cell staining. (A and C) Results from tumor-associated leukocytes and splenocytes from Renca tumor-bearing mice, respectively. (B and D) Results from tumor-associated leukocytes and splenocytes from 4T1-tumor bearing mice, respectively. Statistics were computed in comparison with the corresponding VC (*p < 0.02, **p < 0.008). Data are derived from five mice per treatment group in two separate experiments.
Tregs and MDSCs expand in the spleens of IL-2/αCD40–treated mice via proliferation
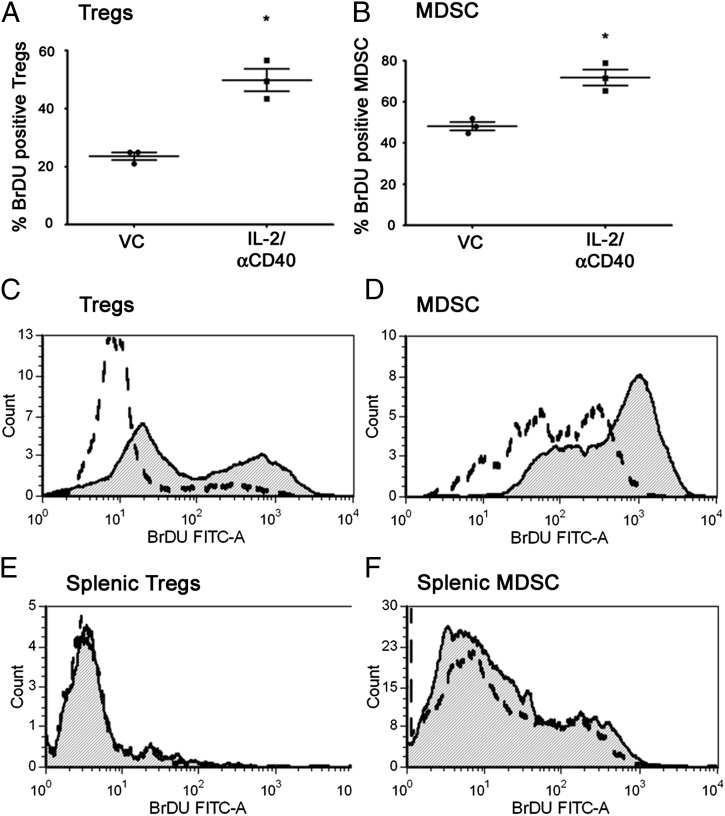
Our data indicated that Tregs and MDSCs expanded in the spleens of IL-2/αCD40–treated mice, despite reduced Bcl-2 expression and elevated Fas ligand and active caspase expression after therapy. To reconcile these findings, we hypothesized that Tregs and MDSC apoptosis in the spleen might be caused by overcompensation via proliferation (27). Consistently, we found that IL-2/αCD40 caused Treg proliferation in the spleen, as evidenced by BrdU uptake (Fig. 7A and 7C). We also show that MDSCs proliferate significantly in the spleens after IL-2/αCD40 treatment (Fig. 7B and 7D). In contrast, IL-2/αCD40 did not induce significant proliferation of tumor-associated Tregs or MDSCs (Fig. 7E and 7F).
FIGURE 7.
Tregs and MDSCs proliferate in the spleens, but not tumors, of IL-2/αCD40–treated mice. Tumor-bearing mice were treated with VC or IL-2/αCD40. On day 15, the spleens were harvested and the percentage of BrdU+ (A) Tregs and (B) MDSCs were quantified (*p < 0.01; n = 3). The flow histograms depict the shift in BrdU mean fluorescence intensity in one sample representative of three total samples for (C) splenic Tregs, (D) splenic MDSCs, (E) tumor-associated Tregs, and (F) tumor-associated MDSCs. Open dashed curve represents VC-treated groups; shaded curves represent IL-2/αCD40–treated groups. Data are derived from five mice per treatment group in two separate experiments.
The Fas-mediated loss of Tregs in the Renca tumor microenvironment underlies the antitumor efficacy of IL-2/αCD40 therapy
To demonstrate that the Fas-mediated loss of Tregs is central to the success of IL-2/αCD40 therapy of Renca, we adoptively transferred Tregs from MRL-Fas(lpr) mice into Treg-depleted WT hosts. If the principal contribution of Fas expression to IL-2/αCD40 antitumor responses is the loss of Tregs, then the antitumor effects of this regimen should be abrogated in mice receiving MRL-Fas(lpr) Tregs.
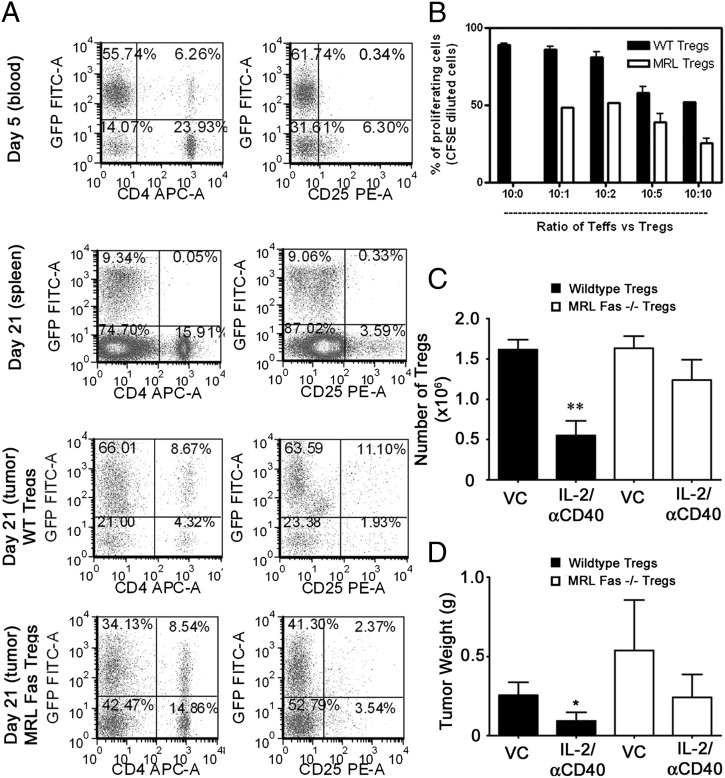
As early as day 5, adoptively transferred Tregs persisted in recipient hosts and represented the majority of CD4+ and CD4+CD25+ cells in the peripheral blood (Fig. 8A). We used mice transgenic for eGFP under the β-actin promoter, to distinguish between recipient (eGFP+) and transferred (eGFP−) cells. By day 21, the adoptively transferred (eGFP−) cells expanded in the spleens, such that >99% of the CD4+ T cells were eGFP− (Fig. 8A). At day 21, both host and transferred Tregs were detectable in primary tumors of mice that received IL-2/αCD40 (Fig. 8A). Whereas the majority of tumor-associated Tregs in mice receiving WT Tregs were GFP+ endogenous cells, the situation was reversed in mice receiving Tregs from MRL-Fas(lpr) mice. The inability of MRL-Fas(lpr) Tregs to undergo Fas-mediated apoptosis after IL-2/αCD40 resulted in their accumulation such that the majority of Tregs in these mice were GFP− transferred MRL-Fas(lpr) Tregs (Fig. 8A). Tregs from MRL-Fas(lpr) mice had higher Foxp3 expression (data not shown) and greater suppressor function on a per-cell basis, as compared with WT Tregs (Fig. 8B). The absolute numbers of adoptively transferred WT Tregs were significantly reduced after IL-2/αCD40 treatment, whereas the numbers of MRL-Fas(lpr)Tregs remained unchanged (Fig. 8C), consistent with the premise that transferred MRL-Fas(lpr) Tregs are not eliminated over the course of IL-2/αCD40 treatment. The primary tumor sizes in WT mice receiving MRL-Fas(lpr) Tregs were indistinguishable between VC and IL-2/αCD40 treatment groups, suggesting that the antitumor efficacy of IL-2/αCD40 was abrogated in these mice (Fig. 8D). In contrast, primary tumors in mice receiving WT Tregs were significantly reduced in IL-2/αCD40–treated mice, as compared with controls. Therefore, the antitumor efficacy of IL-2/αCD40 is lost when Tregs are deficient in functional Fas expression, because these cells are unable to be removed.
FIGURE 8.
Fas-deficient Tregs are not removed from the tumor microenvironment and abrogate the antitumor efficacy of IL-2/αCD40 therapy. On various days after tumor inoculation (day 0), endogenous (eGFP+) and adoptively transferred (eGFP−) leukocytes were analyzed in the blood, spleens, and tumors of recipient mice (A). Cells were gated by forward and side scatter and CD45 expression. Samples were obtained from an IL-2/αCD40–treated mouse, representative of five mice. (B) The suppressive effects of WT and MRL-Fas(lpr) Tregs were compared. Teffs from WT and MRL-Fas(lpr) mice were cocultured with Tregs at the indicated ratios (the 10:0 ratio indicates control Teffs plated alone). (C) Tregs were enumerated from tumors by multiplying the percentage of CD4+/CD25+ cells by the number of CD45+ leukocytes (n = 5; **p < 0.01 compared with VC). (D) Primary tumors were weighed on day 21 (n = 10; *p < 0.03 compared with VC).
The Fas-mediated loss of MDSCs in the 4T1 tumor microenvironment is required for the antitumor efficacy of IL-2/αCD40 therapy
Having established the importance of Fas-mediated Treg removal toward successful treatment of Renca tumors, we also explored the 4T1 tumor model because accumulation of MDSCs in this model is well established. IL-2/αCD40 treatment significantly reduced 4T1 tumor area (Fig. 9A). Although αCD40 controlled tumors out to day 14, the tumors eventually grew to be indistinguishable from controls. In MRL-Fas(lpr) mice, the antitumor efficacy of IL-2/αCD40 was abolished (Fig. 9B). We confirmed the requirement for the Fas-mediated removal of MDSC toward the efficacy of IL-2/αCD40 treatment by performing a similar adoptive transfer experiment to that described earlier for Renca/Tregs. When MDSC-depleted mice were reconstituted with MDSCs from MRL-Fas(lpr) mice, the antitumor efficacy of IL-2/αCD40 was abrogated (Fig. 9C). In contrast, antitumor efficacy was retained in mice reconstituted with MDSCs from WT mice, demonstrating the importance of Fas-mediated MDSC removal to the successful immunotherapy of 4T1 tumors.
FIGURE 9.
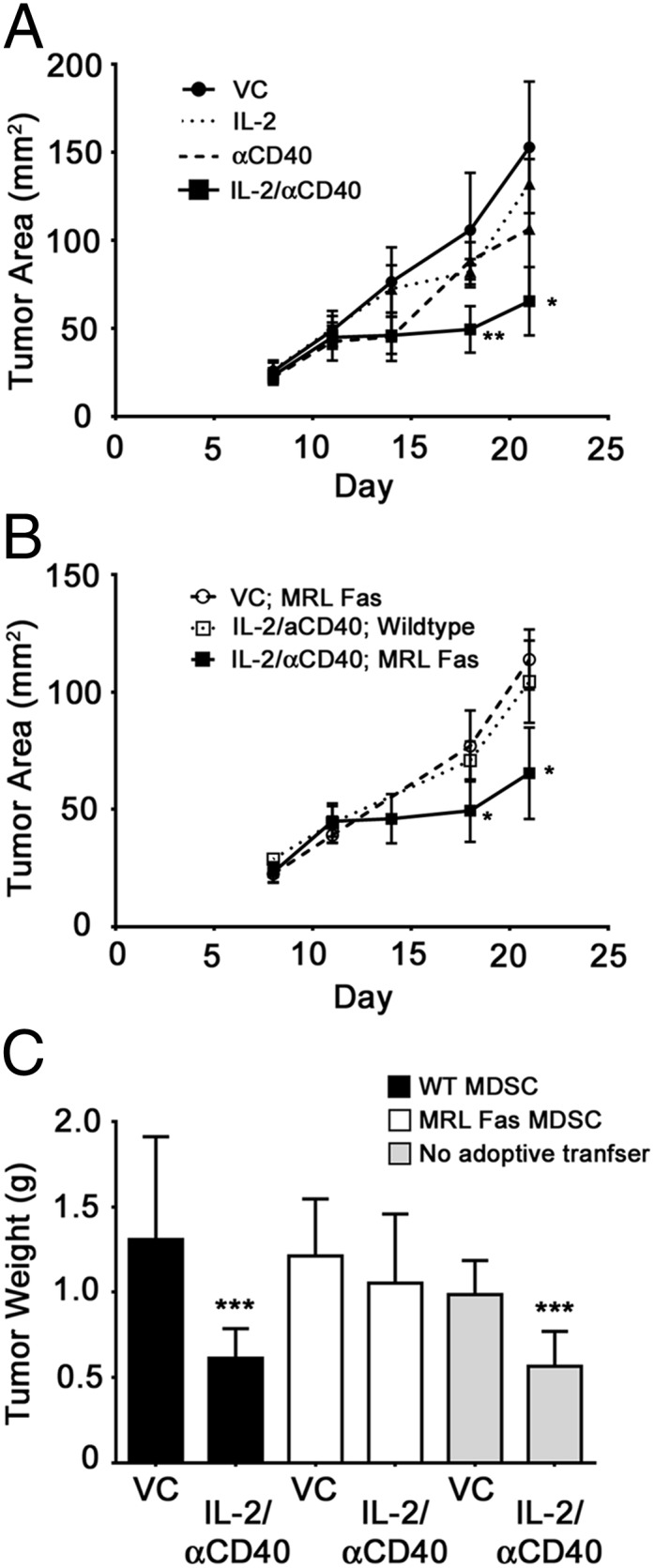
The antitumor efficacy of IL-2/αCD40 in the 4T1 tumor model is dependent on Fas, and Fas-deficient MDSCs abrogate the antitumor efficacy of this therapy. 4T1 tumor-bearing mice were treated with VC or IL-2/αCD40 beginning on day 11 after tumor inoculation. (A) Primary tumor area was measured biweekly beginning on day 7 (*p < 0.03, **p < 0.01 as compared with all other treatment groups on days 18 and 21; 10 mice/group). (B) Primary tumors in MRL Fas mice were measured; the WT VC group is shown for comparison. (C) GR-1–depleted mice were reconstituted with MDSCs from either WT (black bars) or MRL Fas (white bars) mice. Gray bars denote control mice that did not receive any depleting Ab or adoptive transfer. Primary tumors were weighed on day 21 (n = 10; ***p < 0.005 as compared with the corresponding VC).
Discussion
Several studies have highlighted the susceptibility of Tregs and MDSCs to Fas-dependent apoptosis, because of heightened activation under certain inflammatory conditions (9, 11, 12). Deng and colleagues (28) recently showed the combination of irradiation and anti–PD-L1 treatment synergistically activated cytotoxic T cells and promoted MDSC apoptosis, although the mechanism in their study was mediated by TNF rather than IFN-γ and a possible role for Fas was not shown. A role for Fas in the elimination of Tregs was also shown by intratumoral administration of FasL (14). Treg removal enhances tumor-specific immune responses and represents a critical component of cancer vaccine strategies (1, 2). In our study, we demonstrated that IL-2/αCD40 induces Fas-mediated elimination of Tregs and MDSCs from the tumor microenvironment. We also demonstrated the importance of suppressor cell removal for durable antitumor responses after immunotherapy, although it needs to be recognized that the well-established immune dysregulation in MRL Fas(lpr) mice may complicate the interpretations of adoptive transfer studies. Although Fas ligand expression in RCC is associated with tumor stage, its coordinated upregulation in tumors after IL-2/αCD40 treatment transforms the tumor microenvironment into a setting where Fas-mediated preferential depletion of Tregs and MDSC occurs.
Lymphocyte sensitivity to apoptosis is regulated by the rate of cell proliferation. Tregs have faster turnover rates and higher sensitivity to apoptosis as compared with Teffs (11, 12). Immunotherapies such as IL-2/αCD40 further increase proliferation-driven expansion of splenic Tregs (27). One of the most important cytokines to the suppressor function of Tregs is IL-2 (29, 30). Tregs are avid consumers of IL-2, and IL-2 decreases their susceptibility to Fas-mediated apoptosis in vitro, even though they may express Fas (9, 11). It may appear paradoxical that IL-2–based immunotherapy renders Tregs more susceptible to apoptosis. However, the sensitivity of Tregs to Fas-induced apoptosis under in vitro culturing conditions was primarily modulated by direct cell–cell interactions between CD25+CD4+ and CD25−CD4+ T cells (9) and competition between CD25+ Tregs and CD25− Teffs for IL-2 binding. It is therefore conceivable that the homeostatic effects of IL-2 in vivo during the course of immunotherapy are complex and capable of sensitizing Tregs to apoptosis through enhanced proliferation and the likely collaboration with other proinflammatory cytokines.
A critical component of IL-2/αCD40 immunotherapy is the infiltration of primary tumors by T and NK cells (23). Furthermore, the antitumor efficacy of IL-2/αCD40 depends on CD8+ T cells, IFN-γ, and Fas signaling (22). We causally link these phenomena with the active elimination of suppressor cell populations from within primary tumors. The infiltration of tumors by CD8+ T cells is noteworthy because they express Fas ligand and were essential for the apoptosis of suppressor cell populations. Fas ligand has also been described on Tregs, where it plays an important role in depleting effector Th1 cells (10, 31, 32). We also noted Fas ligand expression on Gr1Lo myeloid cells and Gr1Hi granulocytes, which can also induce apoptosis (33). Tumor-specific loss of Fas+ Tregs and MDSCs might be attributed to upregulated Fas ligand expression throughout tumor tissue after IL-2/αCD40 immunotherapy.
Fas was expressed by Tregs and MDSCs, as well as the tumor (34). Tregs (10, 31) and MDSCs (15, 16) express Fas in vivo, and MDSCs were shown to apoptose in response to T cell–derived Fas ligand expression in vitro (16). Our findings provide mechanistic insight to these results, in that we demonstrate the in vivo potential for immunotherapies to render these cells amenable to Fas-mediated death. Whereas inflammation resulting from IL-2/αCD40 treatment reduced MDSC numbers in our study, another study using IL-1β overexpressed by 4T1 breast cancer cells showed reduced caspase activity in MDSCs and protection from apoptosis (15). Several distinctions between that study and ours are noteworthy. First, the cellular (tumor versus hematopoietic cells) sources of inflammatory mediators may dictate MDSC susceptibility to apoptosis, particularly if levels of cytokines produced differ (endogenous versus ectopic overexpression). Second, the profile of which cytokines predominate may contribute to the susceptibility of MDSCs to apoptosis. IL-1β may result in protection of MDSCs from apoptosis, whereas other Th1 cytokines (e.g., IFN-γ, IL-12) principally associated with IL-2/αCD40 therapy and TNF after irradiation/anti–PD-L1 combination therapy (28) may favor their susceptibility to cell death.
The dramatic contrast between Treg and MDSC loss in the primary tumor and their peripheral expansion might be attributed to tissue-specific factors, namely, the preferential expression of IFN-γ by activated CD8+ cells and engagement of the Fas/Fas ligand signaling pathway in the tumor microenvironment. Although tumor-associated Fas/Fas ligand expression has been associated with killing of activated T cells (18, 20) and poor survival in RCC (19), our results highlight the potential for immunotherapeutic strategies capable of harnessing this pathway to elicit the removal of Fas-sensitive immunoregulatory cells specifically within the tumor microenvironment.
Acknowledgments
We thank Drs. Marston Linehan and Youfeng Yang of the Urologic Oncology Branch, National Cancer Institute, for analysis of human RCC. We thank Drs. John Ortaldo and Giorgio Trinchieri for critically reviewing this manuscript. We thank Donna Butcher of the Pathology/Histotechnology Laboratory (Frederick National Laboratory for Cancer Research) for immunohistochemical staining.
The online version of this article contains supplemental material.
- αCD40
- agonistic CD40 Ab
- eGFP
- enhanced GFP
- FasL
- Fas ligand
- MDSC
- myeloid-derived suppressor cell
- qPCR
- quantitative PCR
- RCC
- renal cell carcinoma
- Renca
- renal adenocarcinoma
- Teff
- effector T cell
- Treg
- regulatory T cell
- VC
- vehicle control
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Le D. T., Jaffee E. M. 2012. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 72: 3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poehlein C. H., Haley D. P., Walker E. B., Fox B. A. 2009. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur. J. Immunol. 39: 3121–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ko, J. S., A. H. Zea, B. I. Rini, J. L. Ireland, P. Elson, P. Cohen, A. Golshayan, P. A. Rayman, L. Wood, J. Garcia, et al. 2009. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15: 2148–2157. [DOI] [PubMed]
- 4.Chen J. J., Sun Y., Nabel G. J. 1998. Regulation of the proinflammatory effects of Fas ligand (CD95L). Science 282: 1714–1717 [DOI] [PubMed] [Google Scholar]
- 5.Alderson M. R., Tough T. W., Davis-Smith T., Braddy S., Falk B., Schooley K. A., Goodwin R. G., Smith C. A., Ramsdell F., Lynch D. H. 1995. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer P. H. 2000. CD95’s deadly mission in the immune system. Nature 407: 789–795 [DOI] [PubMed] [Google Scholar]
- 7.Muzio M., Chinnaiyan A. M., Kischkel F. C., O’Rourke K., Shevchenko A., Ni J., Scaffidi C., Bretz J. D., Zhang M., Gentz R., et al. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death—inducing signaling complex. Cell 85: 817–827 [DOI] [PubMed] [Google Scholar]
- 8.Kischkel F. C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P. H., Peter M. E. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14: 5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banz A., Pontoux C., Papiernik M. 2002. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J. Immunol. 169: 750–757 [DOI] [PubMed] [Google Scholar]
- 10.Yolcu E. S., Ash S., Kaminitz A., Sagiv Y., Askenasy N., Yarkoni S. 2008. Apoptosis as a mechanism of T-regulatory cell homeostasis and suppression. Immunol. Cell Biol. 86: 650–658 [DOI] [PubMed] [Google Scholar]
- 11.Fisson S., Darrasse-Jèze G., Litvinova E., Septier F., Klatzmann D., Liblau R., Salomon B. L. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198: 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritzsching B., Oberle N., Eberhardt N., Quick S., Haas J., Wildemann B., Krammer P. H., Suri-Payer E. 2005. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J. Immunol. 175: 32–36 [DOI] [PubMed] [Google Scholar]
- 13.Fritzsching B., Oberle N., Pauly E., Geffers R., Buer J., Poschl J., Krammer P., Linderkamp O., Suri-Payer E. 2006. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood 108: 3371–3378 [DOI] [PubMed] [Google Scholar]
- 14.Chen A., Liu S., Park D., Kang Y., Zheng G. 2007. Depleting intratumoral CD4+CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Res. 67: 1291–1298 [DOI] [PubMed] [Google Scholar]
- 15. Chornoguz, O., L. Grmai, P. Sinha, K.A. Artemenko, R.A. Zubarev, and S. Ostrand-Rosenberg. 2011. Proteomic pathway analysis reveals inflammation increases myeloid-derived suppressor cell resistance to apoptosis. Mol Cell Proteomics. 10: M110.002980. [DOI] [PMC free article] [PubMed]
- 16.Sinha P., Chornoguz O., Clements V. K., Artemenko K. A., Zubarev R. A., Ostrand-Rosenberg S. 2011. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood 117: 5381–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsässer-Beile U., Gierschner D., Welchner T., Wetterauer U. 2003. Different expression of Fas and Fas ligand in tumor infiltrating and peripheral lymphocytes of patients with renal cell carcinomas. Anticancer Res. 23(1A): 433–437 [PubMed] [Google Scholar]
- 18.Das T., Sa G., Paszkiewicz-Kozik E., Hilston C., Molto L., Rayman P., Kudo D., Biswas K., Bukowski R. M., Finke J. H., Tannenbaum C. S. 2008. Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathways. J. Immunol. 180: 4687–4696 [DOI] [PubMed] [Google Scholar]
- 19.Sejima T., Morizane S., Hinata N., Yao A., Isoyama T., Saito M., Takenaka A. 2012. Fas expression in renal cell carcinoma accurately predicts patient survival after radical nephrectomy. Urol. Int. 88: 263–270 [DOI] [PubMed] [Google Scholar]
- 20.O’Connell J., Bennett M. W., O’Sullivan G. C., Collins J. K., Shanahan F. 1999. Fas counter-attack—the best form of tumor defense? Nat. Med. 5: 267–268 [DOI] [PubMed] [Google Scholar]
- 21.Williams P. D., Pontes E. J., Murphy G. P. 1981. Studies of the growth of a murine renal cell carcinoma and its metastatic patterns. Res. Commun. Chem. Pathol. Pharmacol. 34: 345–349 [PubMed] [Google Scholar]
- 22.Murphy W. J., Welniak L., Back T., Hixon J., Subleski J., Seki N., Wigginton J. M., Wilson S. E., Blazar B. R., Malyguine A. M., et al. 2003. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J. Immunol. 170: 2727–2733 [DOI] [PubMed] [Google Scholar]
- 23.Weiss J. M., Back T. C., Scarzello A. J., Subleski J. J., Hall V. L., Stauffer J. K., Chen X., Micic D., Alderson K., Murphy W. J., Wiltrout R. H. 2009. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc. Natl. Acad. Sci. USA 106: 19455–19460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N., Yamaguchi N., Nagao F., Matsuo S., Maeda H., Okumura K., Yagita H. 1997. Polymorphism of murine Fas ligand that affects the biological activity. Proc. Natl. Acad. Sci. USA 94: 3914–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 26.Tang Q., Adams J. Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E., Piccirillo C. A., Salomon B. L., Bluestone J. A. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28: 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alderson K. L., Zhou Q., Berner V., Wilkins D. E., Weiss J. M., Blazar B. R., Welniak L. A., Wiltrout R. H., Redelman D., Murphy W. J. 2008. Regulatory and conventional CD4+ T cells show differential effects correlating with PD-1 and B7-H1 expression after immunotherapy. J. Immunol. 180: 2981–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R. R., Fu Y. X. 2014. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setoguchi R., Hori S., Takahashi T., Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot J. D., Rasmussen J. P., Gavin M. A., Rudensky A. Y. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6: 1142–1151 [DOI] [PubMed] [Google Scholar]
- 31.Strauss L., Bergmann C., Whiteside T. L. 2009. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J. Immunol. 182: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber S. E., Harbertson J., Godebu E., Mros G. A., Padrick R. C., Carson B. D., Ziegler S. F., Bradley L. M. 2006. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J. Immunol. 176: 4730–4739 [DOI] [PubMed] [Google Scholar]
- 33.Serrao K. L., Fortenberry J. D., Owens M. L., Harris F. L., Brown L. A. 2001. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am. J. Physiol. Lung Cell. Mol. Physiol. 280: L298–L305 [DOI] [PubMed] [Google Scholar]
- 34.Lee J. K., Seki N., Sayers T. J., Subleski J., Gruys E. M., Murphy W. J., Wiltrout R. H. 2005. Constitutive expression of functional CD40 on mouse renal cancer cells: induction of Fas and Fas-mediated killing by CD40L. Cell. Immunol. 235: 145–152 [DOI] [PubMed] [Google Scholar]