Abstract
TNF-α is a pleotropic cytokine, which has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-α is first expressed as a transmembrane (mem) protein that is proteolytically processed to release a soluble (sol) form. memTNF-α and solTNF-α have been shown to exert distinct tissue-protective or -pathologic effects in several disease models. However, the relative contributions of memTNF-α or solTNF-α in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing non-cleavable memTNF-α or lacking TNF-α entirely and examined the outcomes. We found that solTNF-α, but not memTNF-α, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-α, there was a significant increase in the CD8+ T-cell response, including virus-specific CD8+ T-cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-α mediates these immunoregulatory effects primarily through TNF receptor 1 (TNFR1), since mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-α. We also found that solTNF-α expression was required early during infection to regulate the magnitude of the CD8+ T-cell response indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-α to release solTNF-α is a critical event regulating the immune response during influenza infection.
Keywords: TNF-α, TNF-α processing, influenza, immunopathology, CD8+ T cells
Introduction
Influenza A virus is a respiratory pathogen that is capable of causing significant pulmonary pathology in humans (1). Infection of alveolar macrophages and respiratory epithelial cells with virus results in production of cytokines and chemokines such as TNF-α, IL-6, CCL2, CXCL10, and many others (2, 3). This results in progressive recruitment of macrophages, neutrophils, and CD8+ T cells into the lungs and airways and virus is cleared 7 to 10 days post-infection (4, 5). CD8+ T cells, operating both through direct cytolysis of infected cells and production of cytokines (such as TNF-α and IFN-γ), have a vital role in the clearance of influenza virus from the lung as mice deficient in T cells succumb very late to infection with delayed and muted pulmonary pathology and very high systemic viral titers (6, 7). There are many important variables that can determine the severity of illness and lung injury following influenza infection and it is believed that a dysregulated host response can contribute to a significant portion of this pathology (8, 9).
Traditionally, TNF-α has been considered to be a pro-inflammatory cytokine as it plays an important role mediating many disease processes including sepsis, sarcoidosis, and rheumatoid arthritis, among others (10–12). In this regard, TNF-α has been shown to exacerbate inflammation and enhance morbidity during influenza infection. Neutralization of TNF-α during influenza infection can reduce pulmonary infiltration and lung injury and prolong survival without impairing viral clearance (13, 14). Furthermore, we have shown that in a transgenic mouse model of severe influenza infection, effector CD8+ T-cell production of TNF-α is required to induce pulmonary infiltration and diffuse alveolar damage (15, 16). TNF-α mediates its effects by signaling through two distinct receptors, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2) (17). Consistent with a pro-inflammatory role for TNF-α during influenza infection, mice deficient in TNFR1 exhibit prolonged survival, reduced morbidity, and pulmonary infiltration (18, 19). Furthermore, we have demonstrated that both TNFR1 and TNFR2 signaling contribute to immunopathology during effector CD8+ T-cell clearance of influenza virus (15, 20).
Despite strong evidence supporting a pro-inflammatory role for TNF-α, it is becoming increasingly clear that TNF-α is also capable of exerting immunoregulatory effects during infection and disease. The clinical use of anti-TNF-α biological agents such as etanercept, infliximab, adalimumab has been associated with serious adverse events such as lupus and demyelinating disease and an increased risk for infection and certain malignancies (21). In mice, TNF-α deficiency has been shown to exacerbate experimental autoimmune encephalitis and lupus indicating that TNF-α can suppress inflammatory responses (22, 23). It was also recently revealed that TNF-α has an immunosuppressive role during influenza infection (24). In the complete absence of TNF-α, infected mice exhibited increased morbidity and an increased and prolonged CD8+ T-cell response, with enhanced inflammation and injury (24). Studies involving mice deficient in IL-15 have supported the hypothesis that the magnitude of the CD8+ T-cell response is associated with the extent of lung injury during influenza infection (25). However, the cellular mechanisms by which TNF-α limits the magnitude and duration of the CD8+ T-cell response, and mitigation of immunopathology during influenza infection are unclear.
Previous studies investigating the immunoregulatory roles of TNF-α have utilized genetic deletions or antibody neutralization of TNF-α. However, these methods block the effects of both memTNF-α and solTNF-α signaling. TNF-α is first expressed as a transmembrane protein that is proteolytically processed by ADAM17 (a disintegrin and metalloprotease or TNF-α converting enzyme, TACE) to release a solTNF-α (26). Evidence to date indicates that memTNF-α and solTNF-α have both distinct as well as overlapping biological functions. In mice exclusively expressing non-cleavable memTNF-α, it was demonstrated that memTNF-α was sufficient to provide protection against M. tuberculosis infection (27). In addition, these mice were also protected against septic shock and pulmonary fibrosis suggesting that memTNF-α did not mediate some of the deleterious effects of solTNF-α while still preserving some protective effects (28, 29). However, the respective roles of memTNF-α and solTNF-α in regulating immune responses and immunopathology during influenza infection remain unclear.
In this study, we investigated the differential impact of memTNF-α and solTNF-α in regulating immune responses to a sub-lethal influenza virus infection using mice that exclusively express a non-cleavable memTNF-α or which were entirely deficient in TNF-α. We found that solTNF-α but not memTNF-α was required early during infection to limit the magnitude of the immune response and the extent of lung immunopathology. In the absence of solTNF-α, there was a significant increase in CD8+ T-cell accumulation late in infection, including virus-specific CD8+ effector T-cells. The enhanced CD8+ T-cell response in the absence of solTNF-α appeared to drive the increased lung injury as depletion of CD8+ T cells attenuated the extent of lung injury. Overall, the findings of this study suggest that proteolytic processing of memTNF-α to solTNF-α is a critical immunoregulatory event during influenza infection. Moreover, our observations are important for understanding how early events during infection can shape the contraction of the effector phase and the extent of pathologic injury.
Methods
Mice
Seven-week-old C57BL/6, Thy1.1, and CD45.1 mice or mice deficient in either TNFR1 (TNFR1−/−) or TNFR2 (TNFR2−/−) were purchased from Jackson Laboratories (Bar Harbor, ME). TNF-α knockout (TNF−/−) breeding pair on a C57BL/6 background was purchased from Taconic (Germantown, NY). Breeding pairs of mice that only express membrane-bound TNF-α created by knocking-in a non-cleavable Δ1–9, K11E TNF-α allele (memTNFΔ1–9, K11E KI) were generously provided by Dr. William Rigby (Dartmouth College) (28). Mice were bred and maintained in a pathogen-free environment and all experiments used seven- to twelve-week-old female mice. All animal studies were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at Geisel School of Medicine at Dartmouth.
Reagents
The following monoclonal antibodies were purchased from Biolegend (San Diego, CA) as conjugated to FITC, PE, PE-Cy7, PerCP-Cy5.5, Alexa-647, or APC-Cy7: CD4 (GK1.5), CD8 (53-6.7), CD45.1 (A20), CD90.1 (OX-7), CD107a (1D4B), annexin V, and Bcl-2 (BCL/10C4). PE-conjugated tetramer PA224–233 and APC-conjugated tetramer NP366–374 were prepared by the NIH Tetramer Core Facility (Atlanta, GA). Anti-mouse CD16/32 was purchased from DartLab (Lebanon, NH). For antibody neutralization experiments, anti-TNF-α (XT3.11), anti-CD8a (2.43), and rat IgG1 isotype controls (HRPN, LTF-2) were purchased from BioXCell (West Lebanon, NH). Recombinant mouse solTNF-α was purchased from BioLegend.
Viral infection and treatment
Mice were anesthetized with an intraperitoneal injection of ketamine/xylazine and inoculated intranasally with one-tenth the median lethal dose of mouse-adapted influenza A/PR/8/34 (H1N1) virus. Morbidity as measured by weight loss was monitored daily after infection. Peripheral oxygen saturation (SpO2) of conscious mice was measured before and after infection using a MouseOx system (Starr Life Sciences Corp., Allison Park, PA). For TNF-α neutralization, mice received 500µg of anti-TNF-α or isotype control antibodies by intraperitoneal injection on the days indicated. For CD8 depletion, mice received 300µg of anti-CD8a or isotype control antibodies by intraperitoneal injection on days 1 and 4 post-infection. For solTNF-α treatment, 2µg of recombinant mouse solTNF-α was intranasally administered at the time of infection.
Viral titers
At 3, 8 and 14 days post-infection, whole lungs were homogenized in PBS, snap frozen, and stored at −80°C. Tenfold serial dilutions of lung samples were applied in triplicate to Madin-Darby canine kidney (MDCK) cells in a 96-well plate and incubated at 37°C for 5 days. Microwells containing influenza virus were identified by chicken red blood cell hemagglutination and the 50% tissue culture infective dose (TCID50) was calculated (30).
Bronchoalveolar lavage (BAL) and tissue preparation
Airway cells and cytokines were collected by lavaging the lungs four times with a single 1 mL volume of PBS via an incision in the trachea. BAL fluid (BALF) was centrifuged and the fluid was stored at −80°C for cytokine and chemokine analysis. ELISA and Millipore Mouse 32-plex Luminex assay was used to determine the expression of cytokines and chemokines. ELISA was used to determine the level of albumin, a marker of vascular leakage, in BALF (Bethyl Laboratories). Lung and mediastinal lymph node (MLN) single-cell suspensions were prepared by passing these tissues through 70µm and 40µm nylon cell strainers, respectively. Red blood cells were lysed using Gey's solution. Total viable cell counts were obtained by counting the cells on a hemocytometer with trypan blue exclusion. Identification of specific cell populations was determined by analyzing the samples on a flow cytometer as previously described (30). For lung histology, lungs were inflated with 0.5% low melting point agarose in PBS, fixed in formalin, sectioned, and stained with hematoxylin and eosin as previously described (16).
Activation-induced cell death assays
CD8+ T cells recovered from BAL on day 8 post-infection were resuspended in complete media with IL-2 and stimulated with 10 µg/mL plate-bound anti-CD3 in a 96-well plate at 37°C, 5% CO2. After 72 hours, cells were stained with annexin V and the frequency of apoptotic cells was determined by flow cytometry. Bcl-2 expression was analyzed on fixed permeabilized cells by flow cytometry. Alternatively, splenocytes were harvested from day 10 post-infected CD45.1 and TNFR1−/− mice and equal numbers of NP366–374-specific CD8+ T cells from CD45.1 (CD45.1+/Thy1.1−) and TNFR1−/− (CD45.1−/Thy1.1−) mice were adoptively transferred into naive Thy1.1 (CD45.1−/Thy1.1+) mice one day prior to infection. CD8+ T cells were recovered from the BAL on day 7 post-infection and the frequency of transferred NP366–374-specific CD8+ T cells from CD45.1 and TNFR1−/− mice was determined by flow cytometry.
Flow cytometric analysis
Cells were blocked with anti-mouse CD16/32 and then stained with specific antibodies or isotype controls as described previously (31). For tetramer staining, cells were blocked with anti-mouse CD16/32, incubated with tetramer for 1 hour at room temperature, and stained for additional surface markers. Flow cytometry was performed on a FACS Calibur, FACS Canto cytometer (BD Biosciences, San Jose, CA), or MacsQuant Analyzer (Miltenyi Biotec, San Diego, CA) and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Statistical analyses were performed with GraphPad Prism (GraphPad Software Inc., La Jolla, CA) using one-way ANOVA with Tukey post-hoc tests and 95% confidence interval or two-tailed unpaired t-test with 95% confidence interval. Data are presented as the mean ± standard deviation.
Results
solTNF-α serves to mitigate lung injury during influenza infection
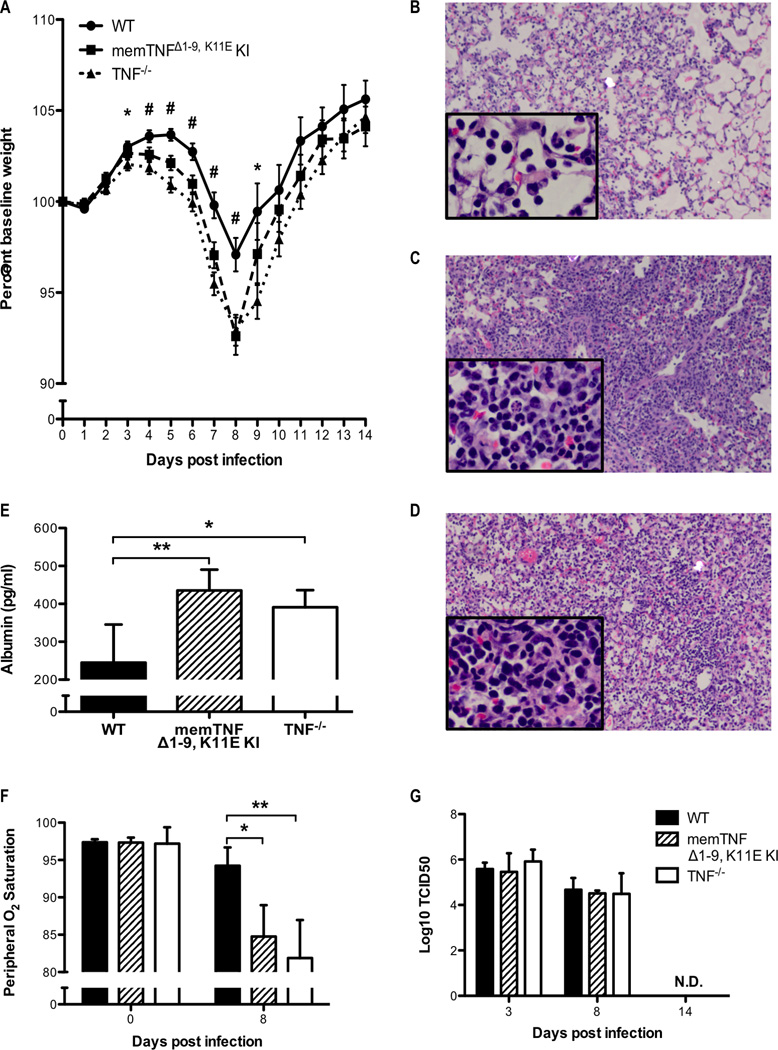
It was recently demonstrated that in the complete absence of TNF-α there is an increase in immunopathology during influenza virus infection, suggesting a dominant anti-inflammatory effect (24). Since memTNF-α and solTNF-α have been shown to have both distinct and overlapping effects, we sought to characterize the roles of memTNF-α and solTNF-α in regulating immunopathology during influenza infection. Therefore, we infected TNF−/−, memTNFΔ1–9, K11E KI, and control wild-type C57BL/6 (WT) mice with a sub-lethal dose of influenza A virus. We found that both memTNFΔ1–9, K11E KI and TNF−/− mice experienced greater weight loss morbidity, compared to WT mice (Figure 1A). Histologically, memTNFΔ1–9, K11E KI and TNF−/− mice exhibited similarly increased alveolar airspace and interstitial inflammatory cell infiltration with increased alveolar damage compared to WT mice (Figures 1B, 1C, 1D). This histological observation of enhanced lung injury prompted our measurement of albumin leakage into the alveoli and we found that both infected memTNFΔ1–9, K11E KI and TNF−/− mice had increased levels of albumin in the airways compared to WT, indicating enhanced disruption of the distal epithelial barrier function (Figure 1E). The physiologic impact of the pulmonary pathology observed in both memTNFΔ1–9, K11E KI and TNF−/− mice was confirmed by a greater reduction in peripheral oxygen saturation after viral infection compared to WT mice (Figure 1F). Taken together, these results indicate that expression of memTNF-α alone is insufficient to limit the extent of lung injury during clearance of influenza infection and suggest that the anti-inflammatory effects of TNF-α are mediated by solTNF-α.
Figure 1. Enhanced morbidity and lung injury in memTNFΔ1–9, K11E KI and TNF−/− mice following influenza infection.
WT, memTNFΔ1–9, K11E KI, and TNF−/− mice were infected with one-tenth the median lethal dose of mouse-adapted influenza A/PR/8/34 virus. (A) Weight loss was monitored daily after infection and the percent change in baseline weight was calculated. Data represents mean ± standard error from multiple independent experiments (initial n value is 88 for WT, 75 for memTNFΔ1–9, K11E KI, and 79 for TNF−/−). #P<0.05 memTNFΔ1–9, K11E KI or TNF−/− compared to WT and *P<0.05 TNF−/− compared to WT. Representative H&E stained lung sections from (B) WT, (C) memTNFΔ1–9, K11E KI, and (D) TNF−/− mice harvested 8 days post-infection are shown at 10× magnification with 40× inset. (E) BALF was assessed for albumin by ELISA on day 8 post-infection. (F) Peripheral oxygen saturation levels were measured by the MouseOx System. (G) Viral titers were determined from whole-lung homogenates using the TCID50 method. Data represent mean ± standard deviation. Each group consists of 3–5 mice. Data are representative of at least three independent experiments. *P<0.05, **P<0.01.
Both solTNF-α and memTNF-α are dispensable for influenza virus clearance
Next, we measured influenza viral titers in whole lung homogenates to investigate the role of memTNF-α and solTNF-α in viral clearance. All mice were capable of complete virus clearance, as no virus was detected in the lungs 14 days post infection (Figure 1G). Importantly, no significant differences in viral titers were observed on days 3 or 8 post-infection (Figure 1G). These data strongly suggest that the enhanced morbidity and pathology we observed in memTNFΔ1–9, K11E KI and TNF−/− mice was not due to inability of the host to control or clear the virus.
Dysregulated cytokine and chemokine responses in the absence of solTNF-α
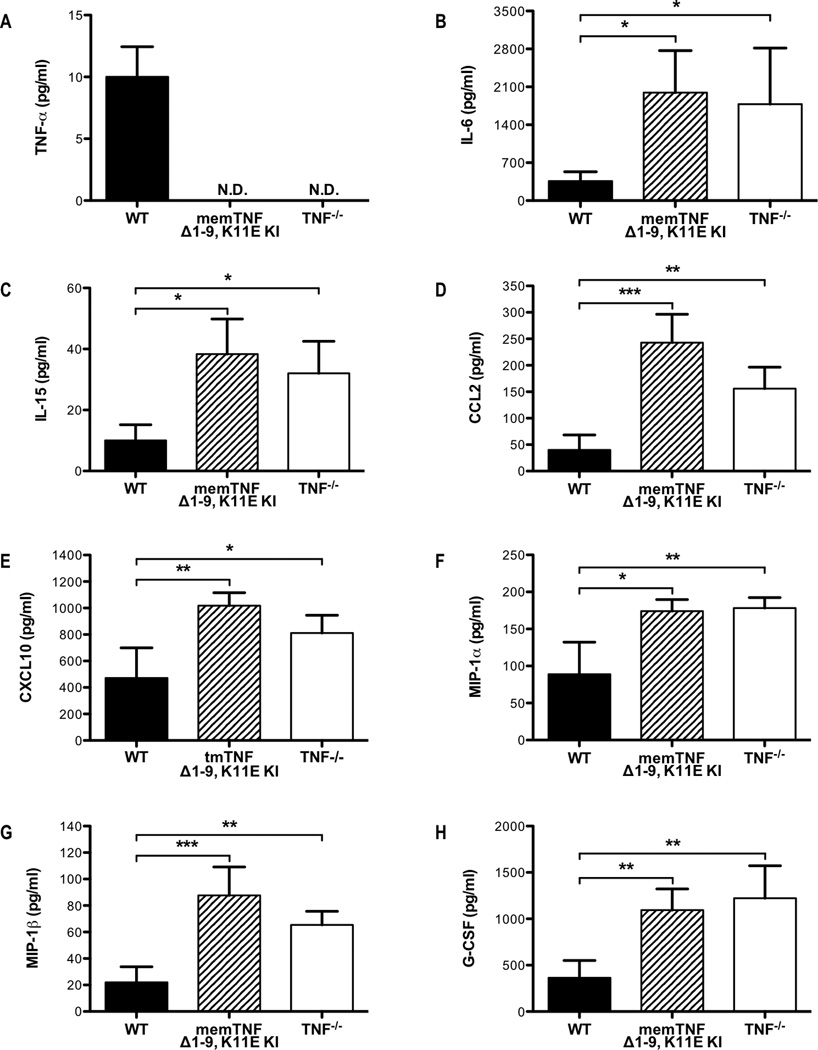
To investigate the molecular mechanisms by which solTNF-α mitigates lung injury during influenza infection, we examined the expression of cytokines and chemokines in the airways 8 days post-infection. As expected solTNF-α was present in lavage samples from WT mice but not detected in samples recovered from memTNFΔ1–9, K11E KI and TNF−/− mice (Figure 2A). However, total TNF-α production does not appear to be affected in memTNFΔ1–9, K11E KI mice, as TNF-α expression in stimulated NP366–374-specific CD8+ T cells derived from memTNFΔ1–9, K11E KI mice was comparable to that of WT CD8+ T cells (Supplemental Figure 1). We observed increases in the BALF levels of IL-6 and IL-15 in the airways of memTNFΔ1–9, K11E KI and TNF−/− mice compared to WT mice (Figures 2B, 2C). Both of these cytokines have important roles in regulating the magnitude of the T-cell response to influenza infection (25, 32). Moreover, there were increases in the levels of CCL2 and CXCL10 in the airways of memTNFΔ1–9, K11E KI and TNF−/− mice compared to WT mice (Figures 2D, 2E). This is similar to the enhanced levels of CCL2 and CXCL10 observed by Damjanovic et al. in the airways of TNF−/− mice during influenza infection (24). We also observed enhanced levels of MIP-1α, MIP-1β, and G-CSF in the airways of memTNFΔ1–9, K11E KI and TNF−/− mice compared to WT mice (Figures 2F, 2G, 2H). Following infection with highly pathogenic influenza viruses, higher levels of CCL2, MIP-1α, MIP-1β, IL-6, and G-CSF in the lungs and higher levels of CCL2, CXCL10, and IL-6 in the serum have been observed in mice and humans, respectively (9, 33–35). These data suggest that solTNF-α expression functions in part to limit cytokine and chemokine expression following influenza infection.
Figure 2. Dysregulated cytokine and chemokine responses in memTNFΔ1–9, K11E KI and TNF−/− mice following influenza infection.
WT, memTNFΔ1–9, K11E KI, and TNF−/− mice were infected with influenza virus. BALF was recovered on day 8 post-infection and the levels of (A) TNF-α, (B) IL-6, (C) IL-15, (D) CCL2, (E) CXCL10, (F) MIP-1α, (G) MIP-1β, and (H) G-CSF were determined by Millipore Mouse 32-plex Luminex assay. Data represent mean ± standard deviation. Each group consists of 4–6 mice from one or two independent experiments. *P<0.05, **P<0.01, ***P<0.005.
solTNF-α limits the magnitude of the CD8+ T-cell response during influenza infection
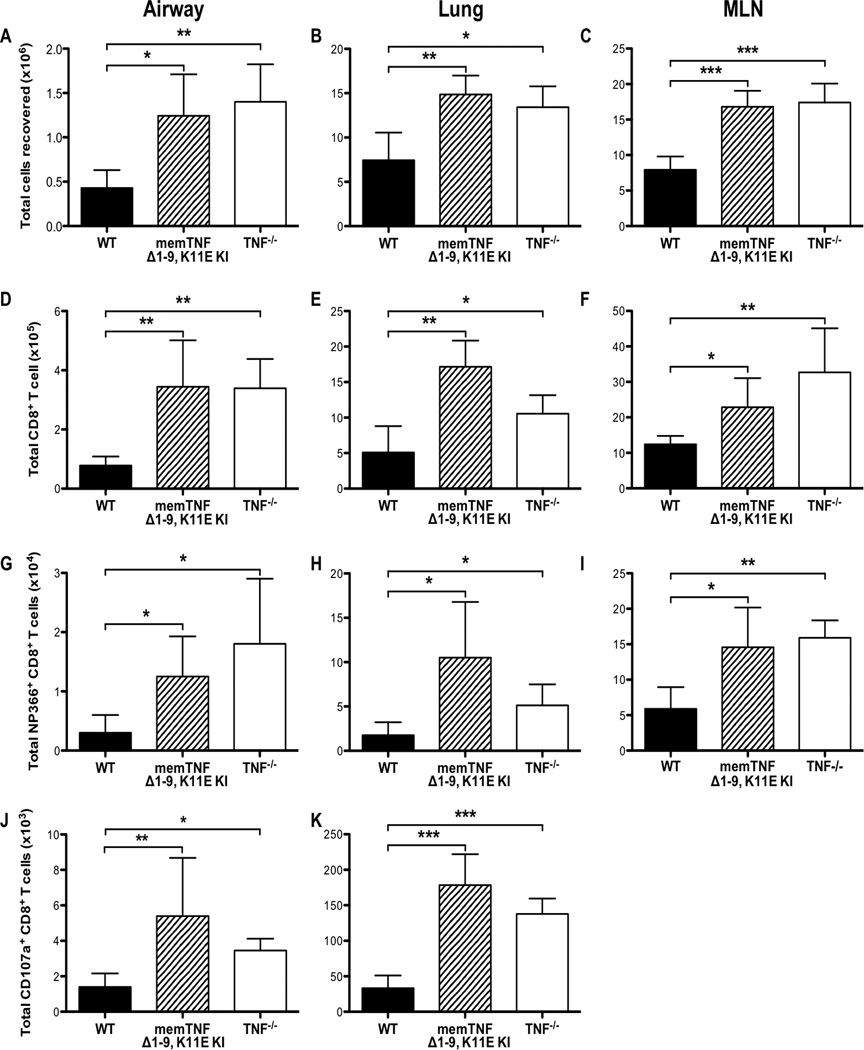
Since we observed a dysregulation of cytokine and chemokine expression, we next investigated whether there were differences in the immune response that could contribute to the increased lung injury we observed after infection in memTNFΔ1–9, K11E KI and TNF−/− mice. We recovered cells from the airways, whole lung, and MLN harvested from animals 8 days after infection and found an increase in the total number of cells present in the airways, lung, and MLN of both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice (Figures 3A, 3B, 3C). Using flow cytometry to identify specific cell populations, we observed a significant enhancement in the CD8+ T-cell response in all tissues examined of both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice (Figures 3D, 3E, 3F). Total numbers of CD4+ T cells were also increased in both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice (Supplemental Figures 2A, 2B, 2C). To characterize further the CD8+ T-cell response, we examined the virus-specific response to the H-2kb-restricted influenza virus CD8+ T-cell epitopes, NP366–374 and PA224–233. We found a greater number of both NP366–374-specific and PA224–233-specific CD8+ T cells in both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice (Figures 3G, 3H, 3I; Supplemental Figures 2D, 2E, 2F). The enhanced influenza-specific CD8+ T-cell response that was observed in the absence of TNF-α was due in part to the elevated levels of IL-15 observed in the airways of these mice, as blockade of IL-15 signaling attenuated both the total and NP366–374-specific CD8+ T-cell responses in TNF−/− mice (Supplemental Figures 3A, 3B). These data indicate that TNF-α serves to limit the magnitude of the influenza-specific T-cell response. We also found a significant increase in the total number of monocyte-derived macrophages and neutrophils in the airways of both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice on day 8 post-infection (data not shown), indicating that solTNF-α expression was also required to limit the infiltration by other inflammatory cell types following influenza viral challenge.
Figure 3. Enhanced CD8+ T-cell responses in memTNFΔ1–9, K11E KI and TNF−/− mice following influenza infection.
WT, memTNFΔ1–9, K11E KI, and TNF−/− mice were infected with a sub-lethal dose of influenza virus and cells were harvested from the airways, lung, and MLN on day 8 post-infection. Total number of viable cells recovered from (A) BAL, (B) lung, and (C) MLN. Total number of CD8+ T cells from (D) BAL, (E) lung, and (F) MLN. Total number of NP366–374-specific CD8+ T cells from (G) BAL, (H) lung, and (I) MLN. Total number of CD107a+CD8+ T cells from (J) BAL and (K) lung. Data represent mean ± standard deviation. Each group consists of 4–6 mice. Data are representative of at least three independent experiments. *P<0.05, **P<0.01, ***P<0.005.
Enhanced CD8+ T-cell responses promote lung injury in the absence of solTNF-α
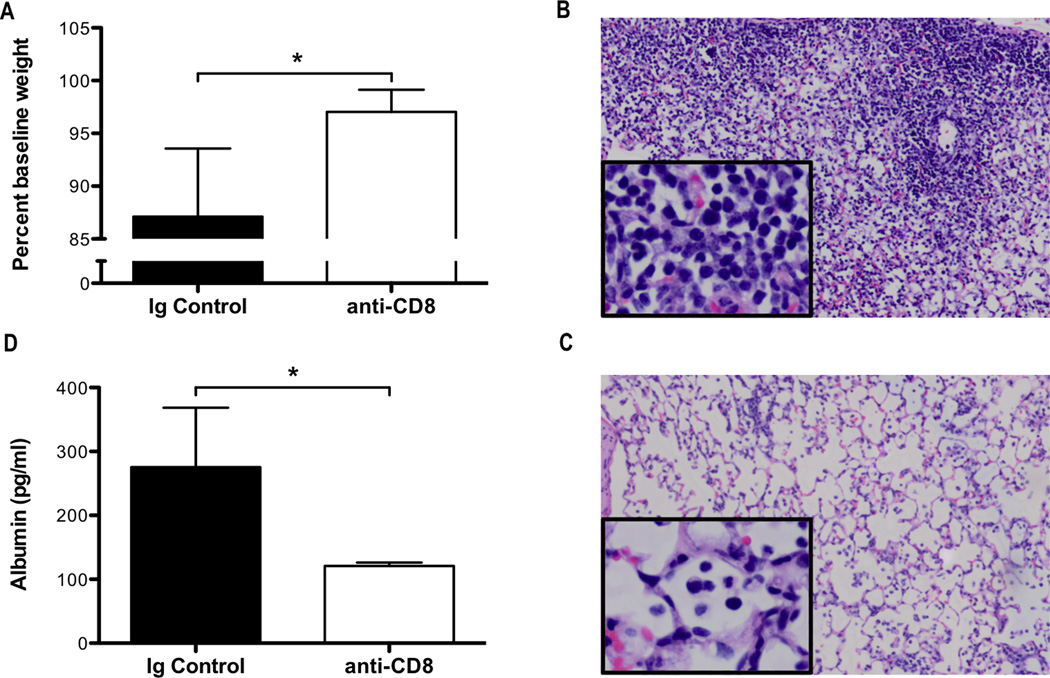
Since we observed an enhanced CD8+ T-cell response in both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice (Figures 3B, 3C, 3D), at a time that corresponded with the peak in morbidity (weight loss; Figure 1A), we hypothesized that the increased magnitude of the CD8+ T-cell response and effector function promoted the enhanced injury observed in these mice. We first examined the total number of cytotoxic CD8+ T cells expressing CD107a, a marker of recent degranulation, in the airways and lungs following influenza infection. We observed an increase in the total number of cytotoxic CD8+ T cells expressing CD107a in the airways and lungs of both memTNFΔ1–9, K11E KI and TNF−/− mice, compared to WT mice on day 8 post-infection suggesting that increased CD8+ T-cell cytotoxicity could lead to enhanced lung injury (Figure 3J, 3K). To test whether the increased number of cytotoxic CD8+ T cells in the absence of TNF-α contributed to the enhanced lung injury, we depleted CD8+ T-cells in TNF−/− mice by administering anti-CD8a antibody on days 1 and 4 post-infection. Following CD8+ T-cell depletion, we observed a reduction in weight loss morbidity on day 8 post-infection (Figure 4A). In contrast to TNF−/− mice that received control antibody (Figure 4B), antibody depletion of CD8+ T cells reduced infiltration of the airspaces and limited the extent of alveolar damage (Figure 4C). The reduction in immunopathology, which was evident on histologic analysis, corresponded to a reduction in BALF albumin in the airways of CD8+ T-cell depleted mice after infection (Figure 4D). Taken together, these data indicate that the enhanced CD8+ T-cell response that occurs in the absence of TNF-α drives enhanced lung injury during influenza infection and we infer, based upon the data presented above, that solTNF-α regulates the extent of the CD8+ T-cell response.
Figure 4. Depletion of CD8+ T cells in TNF−/− attenuates lung injury following influenza infection.
Influenza infected TNF−/− mice were intraperitoneally administered 300µg of anti-CD8 or rat IgG1 on days 1 and 4 post-infection. (A) Weight loss was monitored daily and the percent baseline weight change for day 8 post-infection was calculated. Representative H&E stained lung sections from mice receiving (B) rat IgG1 or (C) anti-CD8 harvested 8 days post-infection are shown at 10× magnification with 40× inset. (D) ELISA was used to determine the levels of albumin in the BALF 8 days post-infection. Data represent mean ± standard deviation. Each group consists of 3–4 mice. Data are representative of two independent experiments. *P<0.05.
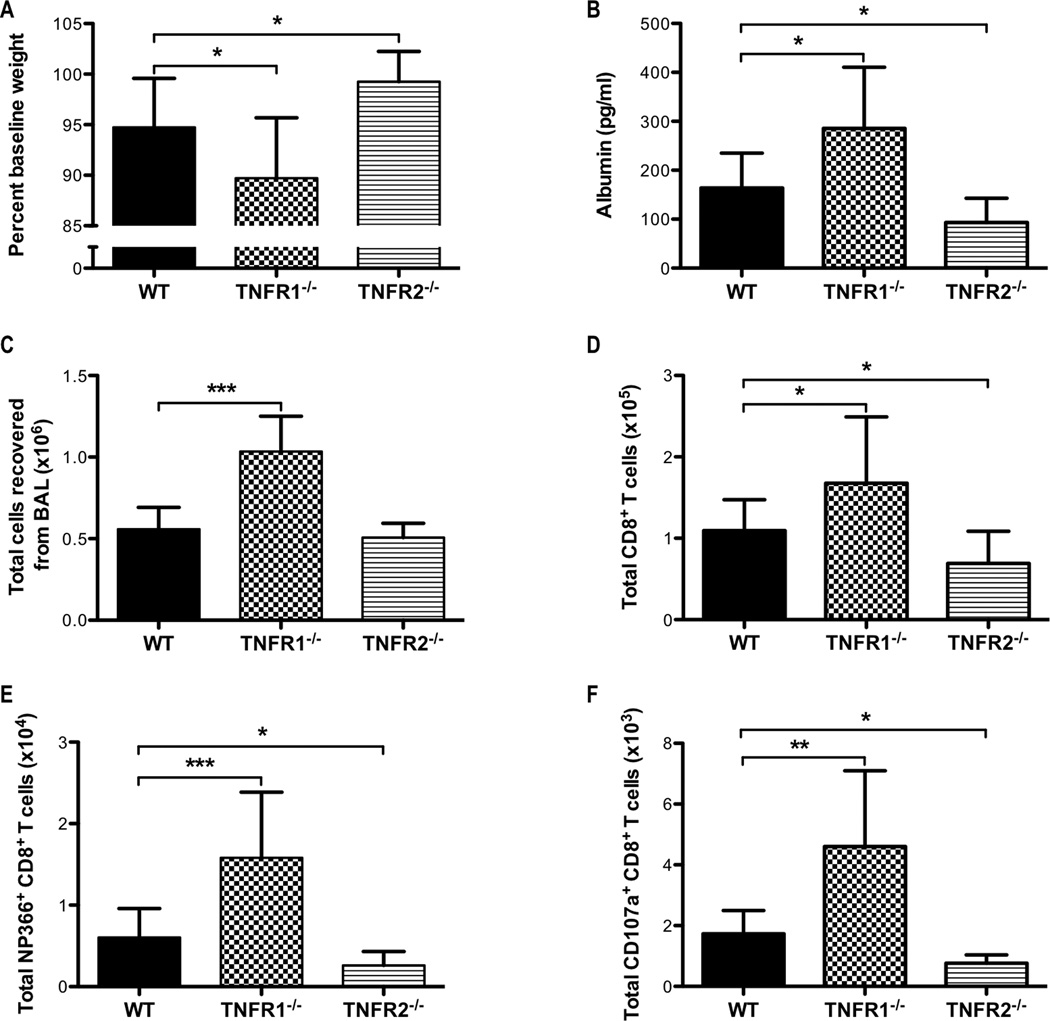
TNFR1 signaling is required to limit the magnitude of the CD8+ T-cell response and the subsequent lung injury
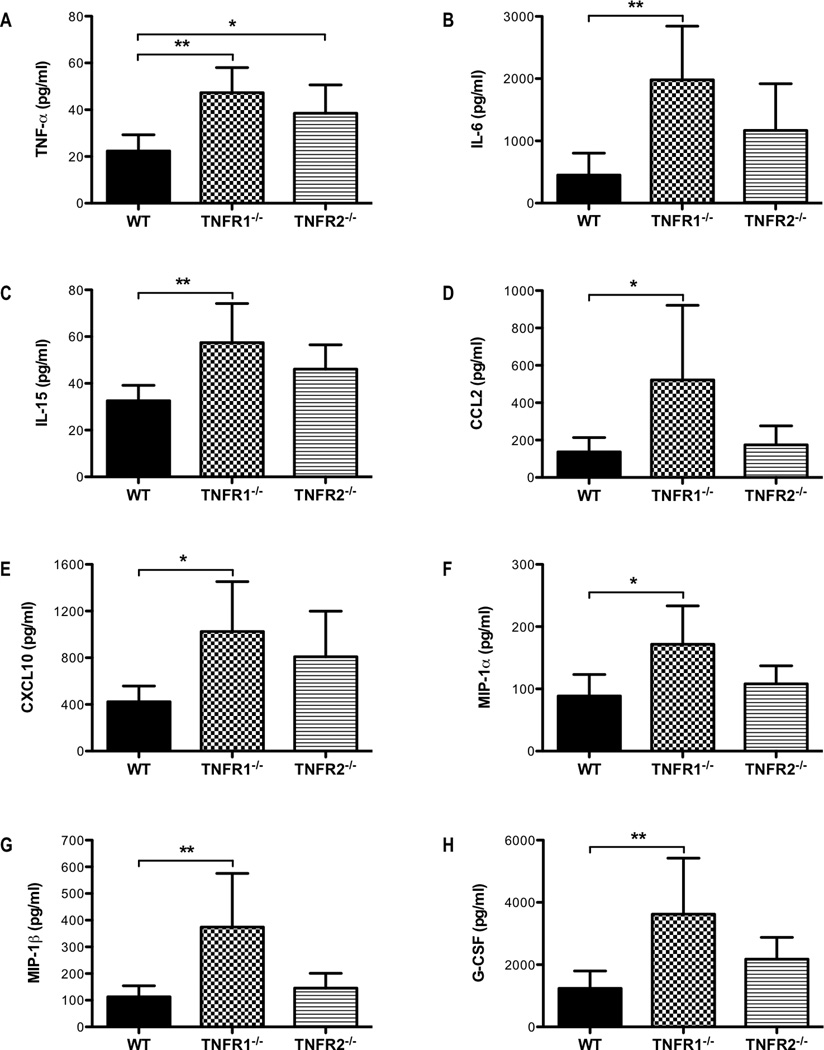
To examine the mechanisms by which solTNF-α constrains the T-cell response during influenza infection, we challenged TNFR1−/− or TNFR2−/− mice with influenza virus and examined the inflammatory responses. We found that TNFR1−/−, but not TNFR2−/−, mice recapitulated many of the effects we observed in both memTNFΔ1–9, K11E KI and TNF−/− mice. TNFR1−/− mice exhibited enhanced morbidity, as indicated by a greater loss in percent baseline weight compared to WT mice on day 8 post-infection (Figure 5A). TNFR1−/− mice also exhibited more severe lung injury as measured by elevated albumin levels in the airway compared to WT mice (Figure 5B). Interestingly, TNFR2−/− mice experienced less weight loss and lung injury when compared to WT mice (Figures 5A, 5B). We also observed dysregulated cytokine and chemokine responses in TNFR1−/− mice compared to WT mice (Figure 6). Similar to memTNFΔ1–9, K11E KI and TNF−/− mice, there were elevated levels of IL-6, IL-15, CCL2, CXCL10, MIP-1α, MIP-1β, and G-CSF in the airways of TNFR1−/− mice when compared to WT mice (Figure 6). Interestingly, solTNF-α was elevated in the airways of both TNFR1−/− and TNFR2−/− compared to WT mice suggesting that these receptors may play an important feedback role in regulating the levels of solTNF-α in the lung (Figure 6A). Consistent with the exacerbated injury and inflammation, there was an increase in the total number of inflammatory cells recovered from the BAL of TNFR1−/− mice compared to WT mice (Figure 5C). We also observed an increase in the total number of monocyte-derived macrophages and neutrophils in the BAL of TNFR1−/− mice compared to WT mice (data not shown). In contrast, no differences in the total number of cells and total number of monocyte-derived macrophages or neutrophils were observed between WT and TNFR2−/− mice (Figure 5C, data not shown). Moreover, the total number, NP366–374-specific, and cytotoxic CD107a expressing CD8+ T cells were increased in the airways of TNFR1−/− mice compared to WT mice 8 days after influenza infection (Figures 5D, 5E, 5F). Despite having intact solTNF-α and memTNF-α, TNFR1−/− mice exhibited enhanced CD8+ T-cell responses and exacerbated pulmonary pathology following influenza infection. This is consistent with the general belief that solTNF-α primarily mediates its effects through TNFR1 (36, 37) and reinforces our hypothesis that solTNF-α signaling primarily through TNFR1 is required to limit the magnitude of the effector CD8+ T-cell response and the extent of lung immunopathology during influenza infection. The attenuated lung injury and reduced cytotoxic CD8+ T-cell response in TNFR2−/− mice compared to WT mice (Figures 5D, 5E, 5F) further suggests that the magnitude of the CD8+ T-cell response likely impacts the extent of immunopathology following influenza infection.
Figure 5. Enhanced lung injury and CD8+ T-cell responses in TNFR1−/− mice following influenza infection.
WT, TNFR1−/−, and TNFR2−/− mice were infected with a sub-lethal dose of influenza virus. (A) Weight loss was monitored daily and the percent baseline weight change for day 8 post-infection was calculated. (B) ELISA was used to determine the levels of albumin in the BALF 8 days post-infection. (C) The total number of viable cells recovered from BAL day 8 post-infection. Flow cytometry was used to analyze the total number of (D) CD8+, (E) NP366–374-specific CD8+, and (F) CD107a+CD8+ T cells. Data represent mean ± standard deviation. Each group consists of 4–6 mice. Data are representative of at least two independent experiments. *P<0.05, **P<0.01, ***P<0.005.
Figure 6. Dysregulated cytokine and chemokine responses in TNFR1−/− mice following influenza infection.
WT, TNFR1−/−, and TNFR2−/− mice were infected with influenza virus. BALF was recovered on day 8 post-infection and the levels of (A) TNF-α, (B) IL-6, (C) IL-15, (D) CCL2, (E) CXCL10, (F) MIP-1α, (G) MIP-1β, and (H) G-CSF were determined by Millipore Mouse 32-plex Luminex assay. Data represent mean ± standard deviation. Each group consists of 4–6 mice from one or two independent experiments. *P<0.05, **P<0.01, ***P<0.005.
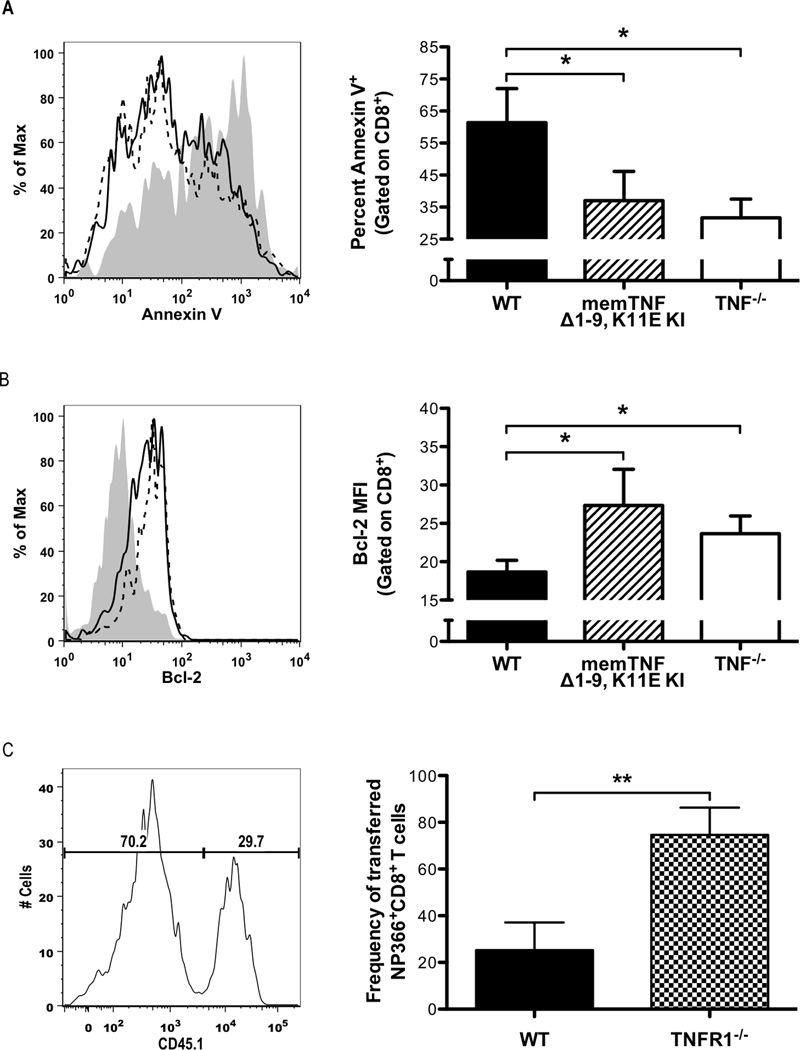
CD8+ T cells primed in the absence of solTNF-α are more resistant to activation-induced cell death
Since we observed an increased CD8+ T-cell response in the absence of solTNF-α, we hypothesized that the enhanced magnitude of the CD8+ T-cell response could be due to either reduced cell death or enhanced proliferation. We assessed these possibilities and we did not observe any differences in the ability of CD8+ T cells recovered from WT, memTNFΔ1–9, K11E KI, or TNF−/− mice to proliferate ex vivo in response to anti-CD3 stimulation (data not shown). However, as shown in Figure 7A, we found that CD8+ T cells recovered from both memTNFΔ1–9, K11E KI and TNF−/− mice were more resistant to activation-induced cell death than CD8+ T cells from WT mice, as indicated by a lower frequency of annexin V+ in both memTNFΔ1–9, K11E KI and TNF−/− CD8+ T cells, compared to WT CD8+ T cells after 72 hours of restimulation with anti-CD3. The increased resistance to activation-induced cell death in both memTNFΔ1–9, K11E KI and TNF−/− CD8+ T cells correlated with enhanced expression of the anti-apoptotic protein, Bcl-2, compared to WT CD8+ T cells (Figure 7B). To further test this hypothesis, we performed a dual-transfer experiment where we transferred equal numbers of NP366–374-specific CD8+ T cells from WT and TNFR1−/− mice into a congenic recipient that was subsequently infected with influenza virus. When we harvested cells from the airways 7 days post-infection, we recovered a significantly greater frequency of TNFR1−/− NP366–374-specific CD8+ T cells compared to transferred WT cells (Figure 7C). Taken together, these data suggest that solTNF-α affects the susceptibility of CD8+ T cells to activation-induced cell death and that the increased resistance of CD8+ T cells to cell death observed in the absence of solTNF-α may contribute to the enhanced CD8+ T-cell responses observed following influenza infection.
Figure 7. CD8+ T cells primed in the absence of solTNF-α are less susceptible to activation-induced cell death.
CD8+ T cells were recovered from the airways of WT, memTNFΔ1–9, K11E KI, and TNF−/− mice 8 days post-influenza infection. Cells were restimulated in vitro for 72 hours with plate-bound anti-CD3. (A) Frequency of annexin V+CD8+ T cells with representative histogram (left) and quantitative graph (right). Gray represents WT, solid line represents memTNFΔ1–9, K11E KI, and dashed line represents TNF−/−. (B) Bcl-2 expression in CD8+ T cells with representative histogram (left) and quantified MFI graph (right). Splenocytes containing equal numbers of NP366–374-specific CD8+ T cells from day 10 post-infection WT (CD45.1+/Thy1.1−) and TNFR1−/− (CD45.1−/Thy1.1−) mice were adoptively transferred into naive Thy1.1 (CD45.1−/Thy1.1+) mice one day before infection. (C) On day 7 post-infection, cells were recovered from the lung and the frequency of transferred NP366–374-specific CD8+ T cells from WT (CD8+/NP366–374+/CD45.1+/Thy1.1−) and TNFR1−/− (CD8+/NP366–374+/CD45.1−/Thy1.1−) mice were determined with representative histogram (left) and quantified MFI graph (right). Data represent mean ± standard deviation. Data are representative of at least two independent experiments with 3 mice per group. *P<0.05, **P<0.01.
solTNF-α has a predominant effect early during infection to limit the magnitude of the T-cell response
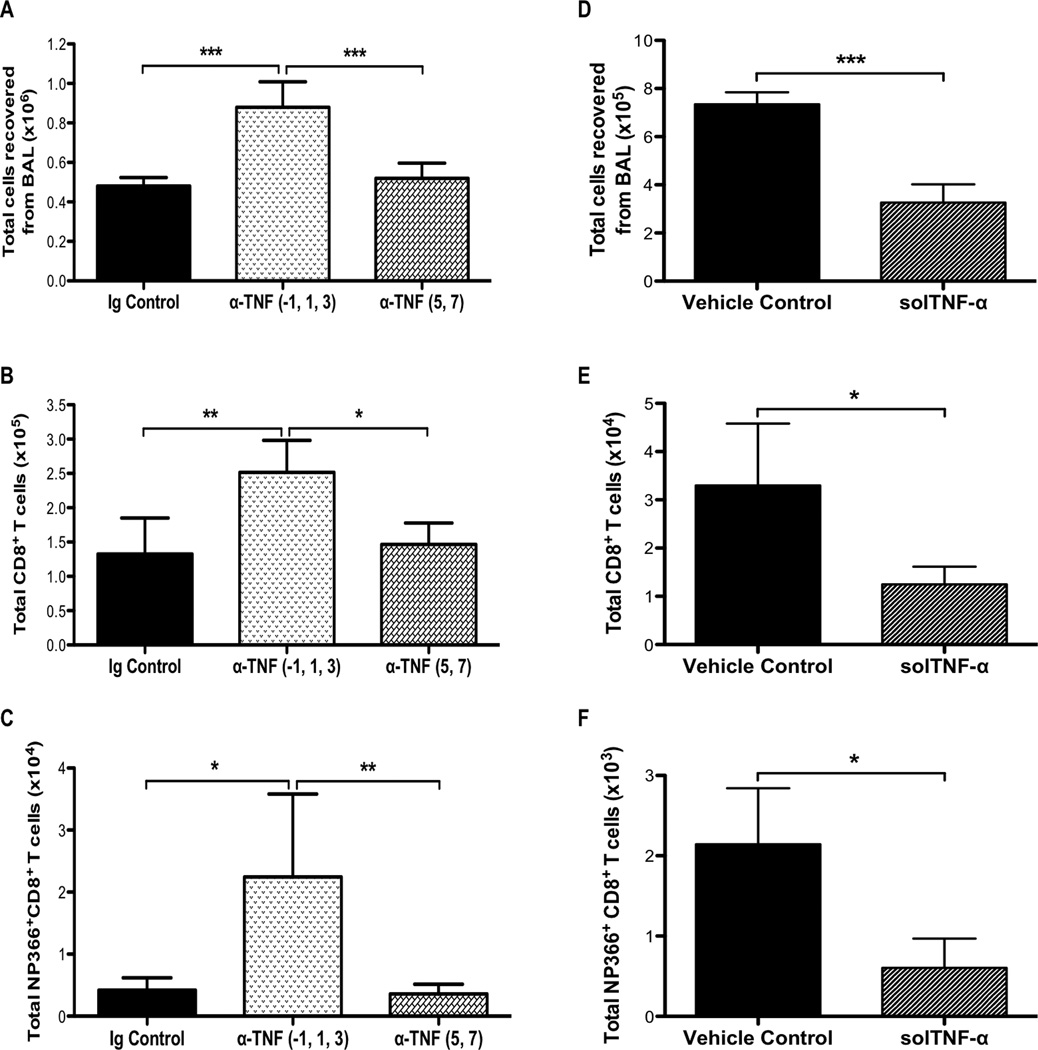
solTNF-α has been shown to sensitize T cells to activation-induced cell death during T-cell priming but not restimulation in vitro (38). Since we observed resistance to activation-induced cell death in effector CD8+ T cells derived from memTNFΔ1–9, K11E KI and TNF−/− mice, we examined whether solTNF-α was required during T-cell priming in vivo to limit the magnitude of the T-cell response during influenza infection. To test this, we used a TNF-α neutralizing antibody to deplete TNF-α at different time points during influenza infection in WT mice. We injected anti-TNF-α (or IgG1 control antibody) during T-cell priming (one day prior to infection and days 1 and 3 post-infection) or during the effector phase (days 5 and 7 post-infection). We found that TNF-α depletion early during T-cell priming, but not later during the effector phase, resulted in an increase in the total number of cells recovered from the airways 8 days post-infection compared to IgG1 control treated mice (Figure 8A). Moreover, there was an increase in the total number of CD8+ T cells in the airway following early TNF-α depletion compared to later TNF-α depletion or IgG1 control (Figure 8B). The enhancement in the CD8+ T-cell response included the virus-specific response, as there were greater numbers of NP366–374-specific CD8+ T cells following early TNF-α depletion (Figure 8C). To further test whether TNF-α has a predominant effect early during infection, we intranasally administered mouse recombinant solTNF-α to memTNFΔ1–9, K11E KI mice at the time of infection. We found that solTNF-α delivery reduced the total number of cells recovered from the airways on day 8 post-infection, which included a reduction in the total number and NP366–374-specific CD8+ T-cell responses (Figures 8D, 8E, 8F). These data indicate that early inflammatory events can shape the effector response. Moreover, it suggests that TNF-α expression (and by inference solTNF-α), as well as TNFR1, early after infection is critical for regulating the magnitude of the T-cell response during the effector phase of immune-mediated clearance of influenza infection.
Figure 8. TNF-α expression is required early during infection to limit the size of the T-cell response.
WT mice received anti-TNF-α or IgG1 control by intraperitoneal injection on days −1, 1, and 3 or days 5 and 7 post-infection. (A) Total number of viable cells recovered from the airways 8 days post-infection. Flow cytometry was used to analyze the total number of (B) CD8+ and (C) NP366–374-specific CD8+ T cells. (D) Alternatively, memTNFΔ1–9, K11E KI mice were intranasally administered 2µg mouse recombinant solTNF-α at the time of infection and the total number of viable cells from the airways was enumerated on day 8 post-infection. Flow cytometry was used to analyze the total number of (E) CD8+ and (F) NP366–374-specific CD8+ T cells. Data represents mean ± standard deviation. Each group consists of 3–5 mice per group. Data are representative of two independent experiments. *P<0.05, **P<0.01, ***P<0.005.
Discussion
In this study, we demonstrated that expression of solTNF-α was critical in regulating immunopathology during influenza infection. Neither memTNF-α nor solTNF-α were required for clearance of influenza virus from the lungs. However, solTNF-α expression was required to limit pulmonary inflammation and infiltration. Increased cytokine and chemokine expression as well as increased cellular infiltration, including an increase in the virus-specific CD8+ T-cell response, was observed in memTNFΔ1–9, K11E KI and TNF−/− mice following a sub-lethal influenza infection. This enhanced CD8+ T-cell response in the absence of solTNF-α appeared to drive exacerbated lung injury as depletion of CD8+ T cells in TNF−/− mice attenuated the extent of pulmonary pathology. The immunoregulatory effects of solTNF-α appear to be mediated by TNFR1, since TNFR1−/−, but not TNFR2−/−, mice recapitulated the enhanced immune responses and exacerbated injury observed in infected memTNFΔ1–9, K11E KI and TNF−/− mice. Furthermore, we found that solTNF-α expression in the first few days after influenza infection was required to limit the effector CD8+ T-cell response later during infection, suggesting that proteolytic processing of TNF-α during the priming phase in influenza infection is required to limit the immune response and mitigate lung immunopathology.
The observation that TNF-α was required to limit the size and duration of the immune response and pulmonary injury in the clearance of influenza infection is consistent with recent observations made by Damjanovic et al., who also identified an immunoregulatory role for TNF-α during influenza infection (24). Despite the use of male mice and a different mouse-adapted strain of H1N1 influenza virus, the results were quite similar. Both their study and ours report increased morbidity and pulmonary inflammation (with cellular infiltration) in the absence of TNF-α. Our study identifies solTNF-α as the form of TNF-α required to regulate the immune response and limit lung injury during influenza infection since memTNFΔ1–9, K11E KI mice exhibited a phenotype most similar to TNF−/− mice. We also identified TNFR1 as the TNFR required to mediate the immunoregulatory effects of solTNF-α since TNFR1−/− mice experienced increased morbidity and an enhanced T-cell response. Similarly, both TNF-α and TNFR1 have been implicated in limiting the magnitude and duration of the CD8+ T-cell response during lymphocytic choriomeningitis virus infection (39, 40). Interestingly, we found a significant attenuation of injury despite no differences in the total number of cells present in the airways of TNFR2−/− mice when compared to WT mice. However, there were fewer cytotoxic CD8+ T cells, including fewer virus-specific CD8+ T cells, recovered from the airways of TNFR2−/− mice suggesting that the attenuated injury observed in these mice was due in part to failure in sustaining an antiviral T-cell response. This is consistent with previous studies that reported deficiencies in cytotoxic CD8+ T-cell survival and function in mice lacking TNFR2 (41, 42). These observations suggest that solTNF-α signaling through TNFR1 is required to limit immune responses during influenza infection and TNFR2 signaling may be required for survival and cytotoxic function of CD8+ T cells.
Our observations suggest that the enhanced morbidity that occurred in the absence of solTNF-α or TNFR1, the peak of which occurred 8 days post-infection during a time when the T-cell response was also peaking, was largely driven by virus-specific CD8+ T-cell cytotoxicity. We observed a significantly greater number of CD8+ T cells expressing CD107a, a marker for cells that have recently degranulated, in the airways of memTNFΔ1–9, K11E KI, TNF−/−, and TNFR1−/− mice. Moreover, depletion of CD8+ T cells in TNF−/− mice attenuated lung injury after influenza infection. It is not unprecedented for injury to be associated with acquisition of cytotoxic function by CD8+ T cells. For example, the onset of hepatocellular injury in a model of hepatitis B virus (HBV) infection, measured by serum alanine aminotransferase levels, was concurrent with the acquisition of cytotoxic activity by HBV-specific CD8+ T cells (43). Furthermore, IL-15 has been shown to partly contribute to influenza immunopathology by regulating the virus-specific CD8+ T cell response (25). We observed elevated IL-15 levels in the airways of both memTNFΔ1–9, K11E KI and TNF−/− mice, and blockade of IL-15 signaling in TNF−/− mice attenuated CD8+ T-cell responses. This suggests that the enhanced IL-15 levels in the absence of solTNF-α may contribute to the increased magnitude of the CD8+ T-cell response and the subsequent exacerbation in lung injury. The caveat here is that while increased cytotoxicity may contribute to enhanced injury, it may also be required for clearance of influenza virus from the lungs by CD8+ T cells (44). Thus, the CD8+ T-cell response needs to be tightly regulated to ensure specific elimination of infected target cells and successful resolution of infection while minimizing both the killing of uninfected bystander cells and release of proinflammatory mediators.
Strikingly, we found that TNF-α expression was required during T-cell priming to limit the size of the effector response. It has been suggested that inflammation early during infection is important in mediating CD8+ T-cell contraction (45, 46). Neutralization of TNF-α early during infection but not later during the effector phase resulted in an increase in the number of CD8+ T cells, including virus-specific CD8+ T cells. Furthermore, we found that CD8+ T cells that were primed in memTNFΔ1–9, K11E KI and TNF−/− mice were more resistance to activation-induced cell death. This may have been due in part to increased expression of anti-apoptotic proteins such as Bcl-2. Consistent with these observations, it was previously reported that solTNF-α was required during T-cell priming in vitro to sensitize these cells to activation-induced cell death (38). This suggests that effector CD8+ T cells generated when TNF-α is neutralized during priming or in a TNF-deficient environment may be intrinsically different from effector CD8+ T cells generated in a TNF-sufficient environment. However, we still observed significant contraction of the T-cell response in WT mice and both memTNFΔ1–9, K11E KI and TNF−/− mice. Despite T-cell contraction, there remained a significantly higher number of virus-specific CD8+ T cells present in the airways of these mice and raises the possibility that enhanced T-cell numbers may persist to memory and provide better protection against a secondary challenge.
These observations also raise the critical question of whether TNF-α acts directly on T cells during priming to limit the size of the effector phase or if TNF-α acts indirectly to regulate the T-cell response. It was recently shown in an adoptive transfer model that TNFR2-deficient adoptively transferred virus-specific CD8+ T cells showed significantly impaired contraction, indicating that TNF-α can act directly on the T-cell to regulate its response (47). It has also been demonstrated that TNF-α may have an important role in the activation and survival of several immunosuppressive cell types. TNF-α signaling has been shown to be important for the accumulation of myeloid-derived suppressor cells at the site of inflammation and for their suppressive activity (48, 49). Moreover, TNFR1-dependent TNF-α signaling has been implicated in the development of macrophages capable of suppressing T-cell proliferation in vitro (50). TNF-α may also be also required for the expansion and suppressive function of CD4+CD25+ regulatory T cells (51). Therefore, TNF-α deficiency may impair the development of regulatory cells, which may contribute to limiting the size of the immune response and minimize injury during influenza infection. Further studies are required to determine whether mice lacking solTNF-α have any changes in the absolute number or function of suppressor cells.
In conclusion, proteolytic processing of TNF-α is a critical event regulating the magnitude and duration of the immune response to influenza infection. Furthermore, solTNF-α expression is required early during infection to limit the magnitude of the effector response. This may have important implications for adverse events following the use of anti-TNF-α biological agents. Whereas neutralization of TNF-α later during infection may reduce inflammation and limit injury, TNF-α neutralization early during infection and disease may actually enhance the inflammatory response and exacerbate immunopathology. The critical sources of TNF-α early during infection that limit the immune responses remain to be determined and future studies are required to elucidate the cellular mechanisms by which TNF-α expression during T-cell priming limits the magnitude of the effector response.
Supplementary Material
Acknowledgments
We thank Dr. Mark Schneider for critical reading of this manuscript. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of PE-conjugated PA224–233 and APC-conjugated NP366–374 tetramers. We also acknowledge DartLab: Immunoassay and Flow Cytometry Shared Resource at the Geisel School of Medicine at Dartmouth.
This work was supported by PHS grants T32 A107363 (MPD) and U19AI 083024 (RIE). The authors gratefully acknowledge the generous support of the Brody Idiopathic Pulmonary Fibrosis Research Fund, and the Ira Jerome Brody '44 Memorial Fund.
Abbreviations used in this article
- BAL
bronchalveolar lavage
- BALF
bronchoalveolar lavage fluid
- MLN
mediastinal lymph node
- memTNF-α
transmembrane TNF-α
- solTNF-α
soluble TNF-α
References
- 1.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, Deshpande C, Mollura DJ, Morens DM, Bray M, Travis WD, Taubenberger JK. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Archives of pathology & laboratory medicine. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, Mason RJ, Chan MC. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85:6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, Rincon M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal immunology. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao H, Hollenbaugh JA, Zand MS, Holden-Wiltse J, Mosmann TR, Perelson AS, Wu H, Topham DJ. Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza A virus. J Virol. 2010;84:6687–6698. doi: 10.1128/JVI.00266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimomura E, Suzuki F, Ishida N. Characterization of cells infiltrating the lungs of x-irradiated and nude mice after influenza virus infection. Microbiology and immunology. 1982;26:129–138. doi: 10.1111/j.1348-0421.1982.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 7.Wells MA, Albrecht P, Ennis FA. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126:1036–1041. [PubMed] [Google Scholar]
- 8.Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, Alves VA, Malheiros DM, Auler JO, Jr, Ferreira AF, Borsato MR, Bezerra SM, Gutierrez PS, Caldini ET, Pasqualucci CA, Dolhnikoff M, Saldiva PH. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2009;181:72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 9.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, Kuo GC, Beutler B, Cotran RS, Cerami, and A, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. The Journal of experimental medicine. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 12.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. The EMBO journal. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peper RL, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microbial pathogenesis. 1995;19:175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- 14.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. European journal of immunology. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- 16.DeBerge MP, Ely KH, Cheng GS, Enelow RI. ADAM17-mediated processing of TNF-alpha expressed by antiviral effector CD8+ T cells is required for severe T-cell-mediated lung injury. PloS one. 2013;8:e79340. doi: 10.1371/journal.pone.0079340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends in cell biology. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 18.Belisle SE, Tisoncik JR, Korth MJ, Carter VS, Proll SC, Swayne DE, Pantin-Jackwood M, Tumpey TM, Katze MG. Genomic profiling of tumor necrosis factor alpha (TNF-alpha) receptor and interleukin-1 receptor knockout mice reveals a link between TNF-alpha signaling and increased severity of 1918 pandemic influenza virus infection. J Virol. 2010;84:12576–12588. doi: 10.1128/JVI.01310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. Journal of virology. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Zhao MQ, Xu L, Ramana CV, Declercq W, Vandenabeele P, Enelow RI. Requirement for tumor necrosis factor-receptor 2 in alveolar chemokine expression depends upon the form of the ligand. American journal of respiratory cell and molecular biology. 2005;33:463–469. doi: 10.1165/rcmb.2005-0204OC. [DOI] [PubMed] [Google Scholar]
- 21.Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. The Journal of dermatological treatment. 2004;15:280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- 22.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. The Journal of experimental medicine. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontoyiannis D, Kollias G. Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol. 2000;30:2038–2047. doi: 10.1002/1521-4141(200007)30:7<2038::AID-IMMU2038>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Damjanovic D, Divangahi M, Kugathasan K, Small CL, Zganiacz A, Brown EG, Hogaboam CM, Gauldie J, Xing Z. Negative regulation of lung inflammation and immunopathology by TNF-alpha during acute influenza infection. The American journal of pathology. 2011;179:2963–2976. doi: 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura R, Maeda N, Shibata K, Yamada H, Kase T, Yoshikai Y. Interleukin-15 is critical in the pathogenesis of influenza a virus-induced acute lung injury. J Virol. 2010;84:5574–5582. doi: 10.1128/JVI.02030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 27.Olleros ML, Guler R, Corazza N, Vesin D, Eugster HP, Marchal G, Chavarot P, Mueller C, Garcia I. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guerin infection in the absence of secreted TNF and lymphotoxin-alpha. J Immunol. 2002;168:3394–3401. doi: 10.4049/jimmunol.168.7.3394. [DOI] [PubMed] [Google Scholar]
- 28.Mueller C, Corazza N, Trachsel-Loseth S, Eugster HP, Buhler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. The Journal of biological chemistry. 1999;274:38112–38118. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- 29.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, Kollias G, Aidinis V. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PloS one. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environmental health perspectives. 2009;117:1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, Gallimore AM. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS pathogens. 2008;4:e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, Usui T, Murata T, Maeda Y, Watanabe S, Suresh M, Suzuki T, Suzuki Y, Feldmann H, Kawaoka Y. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 34.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. Journal of virology. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 38.Muller S, Rihs S, Schneider JM, Paredes BE, Seibold I, Brunner T, Mueller C. Soluble TNF-alpha but not transmembrane TNF-alpha sensitizes T cells for enhanced activation-induced cell death. Eur J Immunol. 2009;39:3171–3180. doi: 10.1002/eji.200939554. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Suresh M. A role for TNF in limiting the duration of CTL effector phase and magnitude of CD8 T cell memory. J Leukoc Biol. 2007;82:1201–1211. doi: 10.1189/jlb.0407240. [DOI] [PubMed] [Google Scholar]
- 40.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kafrouni MI, Brown GR, Thiele DL. The role of TNF-TNFR2 interactions in generation of CTL responses and clearance of hepatic adenovirus infection. J Leukoc Biol. 2003;74:564–571. doi: 10.1189/jlb.0103035. [DOI] [PubMed] [Google Scholar]
- 42.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176:1026–1035. doi: 10.4049/jimmunol.176.2.1026. [DOI] [PubMed] [Google Scholar]
- 43.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. The Journal of experimental medicine. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 46.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 47.Wortzman ME, Lin GH, Watts TH. Intrinsic TNF/TNFR2 Interactions Fine-Tune the CD8 T Cell Response to Respiratory Influenza Virus Infection in Mice. PloS one. 2013;8:e68911. doi: 10.1371/journal.pone.0068911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor Necrosis Factor-alpha Blocks Differentiation and Enhances Suppressive Activity of Immature Myeloid Cells during Chronic Inflammation. Immunity. 2013 doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. The Journal of clinical investigation. 2012;122:4094–4104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raveney BJ, Copland DA, Calder CJ, Dick AD, Nicholson LB. TNFR1 signalling is a critical checkpoint for developing macrophages that control of T-cell proliferation. Immunology. 2010;131:340–349. doi: 10.1111/j.1365-2567.2010.03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.