Abstract
The IL-1 family members IL-36α (IL-1F6), IL-36β (IL-1F8) and IL-36γ (IL-1F9) and the receptor antagonist IL-36Ra (IL-1F5) constitute a novel signaling system that is poorly understood. We now show that these cytokines have profound effects on the skin immune system. Treatment of human keratinocytes with IL-36 cytokines significantly increased the expression of CXCL1, CXCL8, CCL3, CCL5, and CCL20, potent chemotactic agents for activated leukocytes, and IL-36α injected intradermally resulted in chemokine expression, leukocyte infiltration and acanthosis of mouse skin. Blood monocytes, myeloid dendritic cells (DC) and monocyte-derived DC (MO-DC) expressed IL-36R and responded to IL-36. In contrast, no direct effects of IL-36 on resting or activated human CD4+ or CD8+ T cells, or blood neutrophils, could be demonstrated. Monocytes expressed IL-1A, IL-1B and IL-6 mRNA and IL-1β and IL-6 protein and mDC upregulated surface expression of CD83, CD86 and HLADR and secretion of IL-1β and IL-6 after treatment with IL-36. Furthermore, IL-36α-treated MO-DC enhanced allogeneic CD4+ T cell proliferation, demonstrating that IL-36 can stimulate the maturation and function of DC and drive T cell proliferation. These data indicate that IL-36 cytokines actively propagate skin inflammation via the activation of keratinocytes, antigen presenting cells and, indirectly, T cells.
Keywords: skin, inflammation, dendritic cells, cytokines, IL-36
INTRODUCTION
Global definition of the human transcriptome has revealed many new members of the IL-1 family, including IL-36α (formerly known as IL-1F6)(1), IL-36β (IL-1F8)(2, 3), IL-36γ (IL-1F9)(2, 4) and IL-36Ra (IL-1F5), which, along with the IL-36 receptor (IL-1Rrp2), constitute an independent IL-1 signaling system analogous to IL-1α, -1β, -1Ra and IL-1RI. We (5) and others (1, 4) have recently demonstrated that IL-36Ra, IL-36α, IL-36β and IL-36γ mRNA and protein are elevated in skin plaques of the inflammatory disease psoriasis and keratinocytes (KC) were identified as the predominant source (4, 5). Emerging evidence from mouse models indicates a critical role for the IL-36 system in skin inflammation (1, 6, 7) and a crucial role for human IL-36Ra was recently highlighted, when missense mutations in IL36RN which affect the function of IL-36Ra were identified and associated with a form of generalized pustular psoriasis (8, 9). Despite these advances, the regulation and function of these new IL-1 family members is poorly understood. Given the over-expression of the IL-36 cytokines in psoriasis, we investigated the effects of IL-36 on KC, T cells and antigen presenting cells (APC) to better understand the role of IL-36 in inflammatory disease. We demonstrate that IL-36 cytokines induce expression of neutrophil, T cell and myeloid cell chemokines by KC and IL-36α induced immune cell infiltration in vivo in mice. Although human T cells or neutrophils did not express IL-36R, nor respond to exogenous IL-36 cytokines, monocytes, myeloid dendritic cells (mDC) and monocyte-derived DC (MO-DC) expressed both pre-requisite receptors for IL-36, IL-1RAcP and IL-36R, and responded to IL-36 by becoming activated and production of inflammatory cytokines. When cultured with allogeneic CD4+ T cells, IL-36α-treated MO-DC were also more potent drivers of allogeneic mixed lymphocyte reactions, demonstrating that IL-36 can stimulate the maturation and function of DC. These data are consistent with the notion that IL-36 contributes to skin inflammation by acting on KCs, DCs and indirectly upon T cells to drive tissue infiltration, cell proliferation and keratinocyte hyperproliferation, all hallmarks of lesional psoriatic skin.
MATERIALS AND METHODS
Keratinocyte culture
Normal human keratinocyte (NHK) cultures were established in serum-free medium optimized for high-density keratinocyte growth (Medium 154, Invitrogen/Cascade Biologics, Portland, OR) using sun-protected skin of 3 healthy adults as previously described (10). NHKs were used for experiments in the second or third passage. All cells were plated at 5000 cells/cm2 and maintained to 4 days’ post-confluence. Cultures were then starved of growth factors in unsupplemented medium M154 for 24 hours before use. Experiments were carried out under low calcium (0.1mM) conditions. Cultures were stimulated with truncated (11) recombinant human 1-2000ng/ml IL-36α, β or γ, IL-36Ra or IL-1β (R&D Systems, Minneapolis, MN). Informed consent was obtained from all subjects, under protocols approved by the Institutional Review Board of the University of Michigan. This study was conducted in compliance with good clinical practice and according to the Declaration of Helsinki Principles.
Mouse experiments
5μg rmIL-36α or BSA was injected intradermally into 2 separate dorsal regions of skin of 7 week old CD1 mice. Animals were treated every other day for 10 days. A circle was drawn around each site after every injection to ensure similar location between days. Prior to the first injection the animal was anesthetized with isoflourane and shaved. Four hours after the last injection, animals were euthanized and each injection site harvested. During tissue harvesting each injection site (~circular in nature) was dissected from the rest of the dorsal skin and then bisected. Half of each site was put into either 10% NBF or into TFM for histological and immunostaining analyses as previously described in detail (12). The other half was placed into tubes, snap frozen and total RNA extracted. QRT-PCR was performed and data normalized to the housekeeping gene 18S and expressed as fold change over BSA-treated controls (n=4). H&E staining and immunohistochemistry using Abs specific for CD4, CD8, CD11b, CD11c (BD Biosciences, San Jose, CA) and F4/80 (eBioscience, San Diego, CA), were also completed as described previously (12).
IL-36R expression
CD4+ and CD8+ T cells and CD14+ monocytes were prepared from PBMC by negative immunomagnetic selection (Miltenyi). Cells were typically >85% pure cultures as determined by flow cytometry using antibodies against CD3, CD4, CD8, CD14 as detailed below. Human mDC were magnetically isolated from PBMC using 2 rounds of positive selection for CD1c+ cells after negatively selecting CD19+ cells using the BDCA-1 microbead kit from Miltenyi. mDC were assessed to be 95% viable lineage (CD3, CD14, CD16, CD19, CD20, CD56) negative, HLA-DR+CD11c+CD123-phenotype by flow cytometry. Total RNA was isolated from CD4+ T cells, CD8+ T cells, mDC and monocytes and QRT-PCR for IL-1R1, IL-1RAcP and IL-36R was carried out as described below. Surface expression of IL-1R1, IL-1RAcP and IL-36R was detected by incubating cells with biotinylated polyclonal goat anti-human IL-1R1 (BAF269), IL-1RAcP (BAF676) and IL-36R (IL-1Rrp2, BAF872) antibodies (all 50μg/ml, R&D Systems) on ice for 30 min. After washing in FACS buffer, 25μl allophycocyanin-labeled streptavidin (BD), anti-CD3 (S4.1, Invitrogen), CD4 (S3.5, Invitrogen), CD8 (SK1, BD Biosciences), CD14 (M5E2, Biolegend, San Diego, CA) were added for 30 min at 4°C in the dark, and after 2 more washes cells were analyzed with an LSR2 flow cytometer (BD Bioscience).
T-Lymphocyte stimulation
CD3+ T cells were isolated from PBMC as above and incubated for 24h either unstimulated or with 1μg/ml CD3 and CD28 antibodies (BD Biosciences), together with 10ng/ml IL-1β or 100ng/ml truncated IL-36α, β or γ. Cultures were subsequently prepared for qRT-PCR or flow cytometry. Cells were washed in FACS buffer (PBS+0.5%BSA+0.1%NaN3) then stained with antibodies against CD3 (clone S4.1, Invitrogen), CD4 (OKT-4, eBioscience), CD8 (SK1, BD), CLA (HECA-452, Biolegend), CD103 (Ber-ACT8, Biolegend), CD25 (BC96, Biolegend), CD69 (FN50, Biolegend), CD54 (HCD54, Biolegend) and appropriate isotype-matched control antibodies for 30 min at 4°C in the dark. After 2 washes in FACS buffer, cells were analyzed using a BD LSR2 flow cytometer gating on lymphocytes expressing CD3 and CD4 or CD8.
Neutrophil isolation and stimulation
Neutrophils were isolated from heparinized peripheral blood as previously outlined (13). IL-1R1, IL-1RAcP and IL-36R expression were determined by FACS as described above gating on small, highly granular cells expressing CD11b. Neutrophil stimulations were performed with 10ng/ml IL-1β, 100ng/ml truncated IL-36α, β or γ, 100ng/ml LPS (Sigma) or 50ng/ml PMA (Sigma) for 4 hours. Conditioned media were assayed for CXCL-8 and TNF-α by ELISA (Duoset, R&D Systems).
Antigen-presenting cell isolation and culture
CD14+ monocytes and BDCA-1 mDC were prepared from PBMC as above and stimulated for 12h in round-bottomed 96-well culture plates in complete RPMI+10% FCS supplemented with 100ng/ml IL-36α, β or γ. Cells were analyzed by flow cytometry using antibodies against CD83 (HB15e, Biolegend), CD86 (FUN-1, BD Bioscience) and HLA-DR (LN3, eBioscience) and appropriate isotype controls. Alternatively, total RNA was prepared from cells and assessed for cytokine transcripts using QRT-PCR as detailed below. Conditioned media were analyzed for IL-1β and IL-6 using ELISA Duosets from R&D Systems.
Dendritic cell generation and culture
Human MO-DC were generated in vitro as previously described (14). Briefly, CD14+ monocytes were magnetically isolated from PBMC (Miltenyi) and cultured in RPMI containing 10% FCS supplemented with GM-CSF (100ng/ml) and IL-4 (20ng/ml) (R&D Systems). Cultures were fed on day 4. On day 8, DC were seeded into poly(2-hydroxyethyl-methacrylate) (Sigma) coated 12-well culture plates (Corning Costar) at a density of 1 million cells/well and stimulated for 2 days with a cocktail containing IL-6 (10ng/ml), PGE2 (0.1μM) along with IL-1β (10ng/ml), IL-36α, IL-36β or IL-36γ (100ng/ml) in 500μl complete RPMI. DC phenotype was analyzed by flow cytometry as described above using antibodies against CD86 (FUN-1, BD), HLA-DR (LN3, eBioscience), CD1a (HI149, eBioscience), CD11c (3.9, eBioscience), CD123 (6H6, Biolegend) and appropriate isotype control antibodies.
Allogeneic Mixed Lymphocyte Reaction
MO-DCs were matured as above with 10ng/ml IL-6, 0.1μM PGE2 and 10ng/ml IL-1β, 100ng/ml IL-36α and then on day 10, were incubated with 200,000 allogeneic CD4+ T cells at ratios of 1:20 and 1:100 for 5 days in round-bottomed 96-well culture plates (NUNC). In some cases T cells were pre-labeled with CFSE (Invitrogen) before culture as directed by manufacturer. Cells were stained with anti-CD3 (HIT3a, Biolegend) for 20 min at 4°C then treated with 200μl of 1μg/ml DAPI (Invitrogen) in PIPES buffer for 10 min at room temperature. Cells were analyzed by flow cytometry gating on lymphocytes with the DAPI detection channel set for linear detection.
Real Time Quantitative Reverse Transcription PCR (QRT-PCR)
Total RNA was isolated (RNeasy Mini kit, Qiagen), reverse transcribed (High Capacity cDNA Transcription kit, Applied Biosystems Inc., Foster City, CA) and transcripts quantified using a 7900HT Fast Real-time PCR system (Applied Biosystems) normalizing to the expression of the housekeeping gene ribosomal protein, large, P0 (RPLP0). Taqman primer sets were purchased from Applied Biosystems. IL1A Hs00174092_m1, IL1B Hs00174097_m, IL-6 Hs00174131_m1, CXCL1 Hs00236937_m1, CXCL9 Hs00171065_m1, CXCL10 Hs00171042_m1, CXCL11 Hs00171138_m1, CCL1 Hs00171072_m1, CCL2 Hs00234140_m1, CCL3 Hs00234142_m1, CCL4 Hs99999148_m1, CCL5 Hs00174575_m1, CCL7 Hs00171147_m1, CCL20 Hs00355476_m1, IL-1R1 Hs00168392_m1, IL1RAP Hs00370506_m1, IL1rL2 (IL36R) Hs00909276_m1 and RPLP0 Hs99999902_m1 were used in this study.
Statistical Analysis
Data were tested for normality and statistical significance calculated using 2-way Student’s t-tests, Mann Whitney test or 1-way ANOVA with Dunnet’s post-test as appropriate using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
IL-36 induces chemokine expression by human keratinocytes
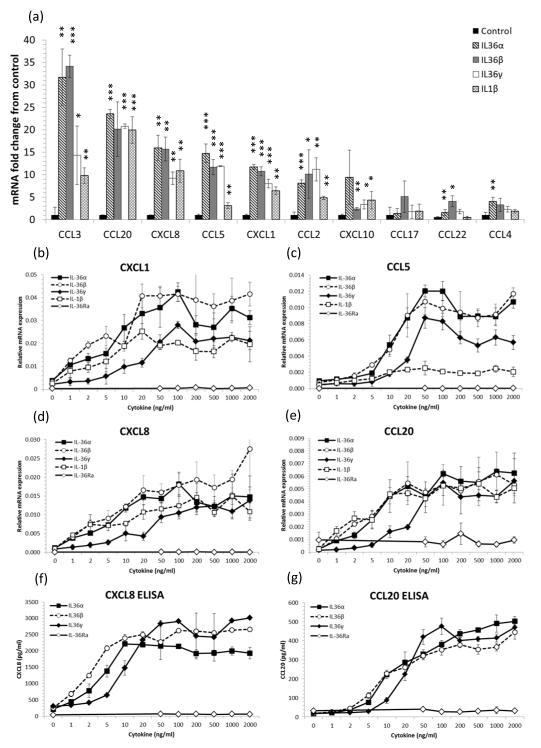
We have previously shown that human keratinocytes are an important source of antimicrobial peptides when exposed to IL-36 family cytokines (5). We now demonstrate that IL-36 cytokines induce the robust expression of chemokines that drive immune cell chemotaxis. Treatment of NHK with IL-36α, IL-36β or IL-36γ lead to significant increases in the macrophage (CCL3, CCL4, CCL5, CCL2, CCL17, CCL22) and T cell chemoattractants (CCL20, CCL5, CCL2, CCL17, CCL22) and the neutrophil chemokines (CXCL8, CCL20 and CXCL1) (Figure 1a). Moreover, all IL-36R agonists but not IL-36Ra, dose-dependently induced CXCL1, CCL5, CXCL8 and CCL20 mRNA expression and CXCL8 and CCL20 protein secretion by NHK (Figure 1b-e), demonstrating that following IL-36 exposure, KC are potent sources of macrophage, T cell and neutrophil chemokines.
Figure 1. IL-36 cytokines induce chemokine expression by keratinocytes.
4-day post-confluent normal human keratinocytes (NHK) were stimulated for 24h with recombinant truncated IL-36R ligands or IL-1β. Total RNA was extracted and mRNA transcripts quantified by qRT-PCR relative to the housekeeping gene RPL-P0 and conditioned medium assayed by ELISA. 100ng/ml IL-36α, IL-36β and IL-36γ significantly induced T cell chemokine mRNA expression compared with untreated cells, mean ± S.D. (n=3) (a). IL-36α, IL-36β, IL-36γ and IL-1β but not IL-36Ra dose-dependently induced CXCL1, CCL5, CXCL8 and CCL20 mRNA expression (b-e) and CXCL8 and CCL20 protein secretion (f and g) by keratinocytes. Mean ± S.D. (n=3). Statistical significance indicated by * p<0.05, t** p<0.01 or *** p<0.001, Student’s t-test.
IL-36 induces myeloid cell infiltration of skin concomitant with chemokine and growth factor induction
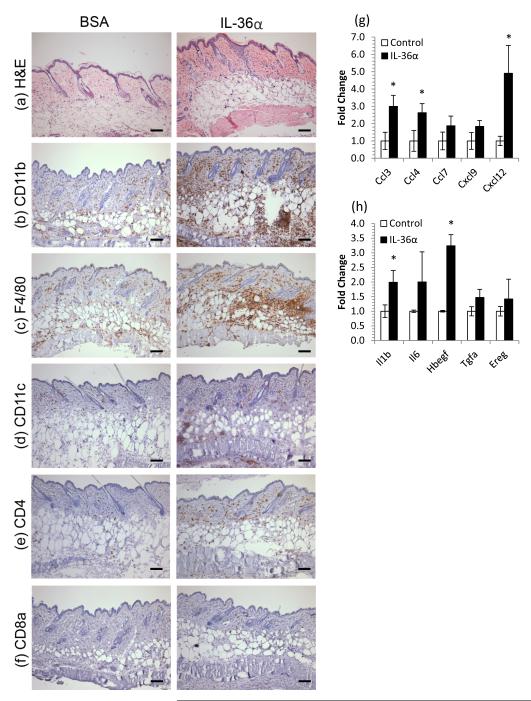
Given that IL-36 induced chemokine expression by human KC in vitro, we sought to test whether this drove cell chemotaxis in vivo by injecting 5μg rmIL-36α or BSA intradermally into CD1 mice every other day for 10 days. By day 10, the mouse skin did not appear erythemic or thickened, but histologically, a mild acanthosis and an increase in eosinophilic dermal collagen was evident along with a very pronounced leukocytic infiltrate (Figure 2a). The infiltrate was striking in its largely granulocytic character (Figure 2b-d) with few T cells (Figures 2e and f). QRT-PCR revealed that IL-36 treatment induced significant fold-changes in a number of leukocyte chemokines including CCL3, CCL4 and CXCL12 (Figure 2g) as well as IL-1β and HB-EGF (Figure 2h, all p<0.05, fold change vs. BSA control, n=4), further supporting a role for IL-36 in facilitating immunocyte recruitment to inflamed skin.
Figure 2. IL-36 induces myeloid cell infiltration of skin concomitant with chemokine and growth factor induction.
5μg rmIL-36α or BSA was injected intradermally into CD1 mice every other day for 10 days. Back skin was harvested, snap frozen, processed for RNA and histochemistry. IL-36α treatment lead to acanthosis and an increase in eosinophilic dermal collagen (a) and striking infiltration of granulocytes (CD11b, b), macrophages (F4/80, c), dendritic cells (CD11c, d) CD4+ cells (e) but not CD8+ cells (f). These changes were accompanied by increases in chemokines (g) and cytokines/growth factors (h). Mean ± SEM (n=4 mice). Statistical significance indicated by * p<0.05 (2-tailed t-test). Scale bar = 100μm.
Antigen-presenting cells but not T cells express IL-36R
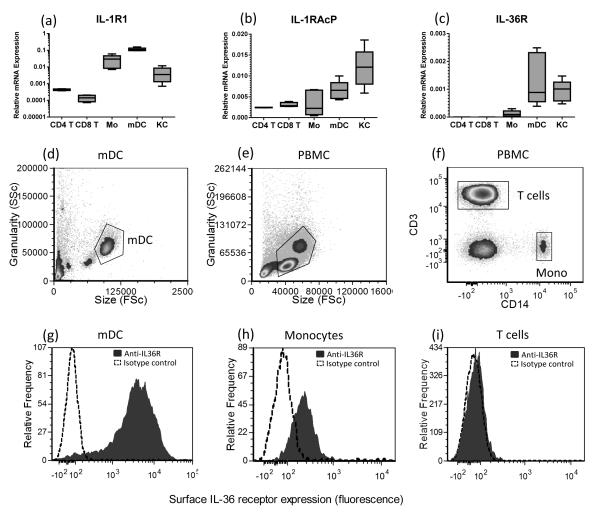
Given that KC expressed chemokines in response to IL-36 treatment and IL-36 injected intradermally lead to leukocyte infiltration, we questioned whether T cells, neutrophils and APC would also respond to IL-36 cytokines. First we examined whether isolated blood CD4+ T cells, CD8+ T cells, monocytes or mDC expressed IL-36R. After magnetic separation, giving >85% pure CD4+ T cells, CD8+ T cells, monocytes and >95% mDC from PBMC, we isolated RNA and performed QRT-PCR. Along with monocytes, mDC and primary keratinocytes (NHK), both subsets of T cells expressed the IL-1R1 (Figure 3a) and IL-1RAcP (Figure 3b) receptors. IL-36R transcripts were not detectable from CD4+ or CD8+ T cells (Figure 3c); however, monocytes, mDC and NHK expressed IL-36R mRNA (Figure 3c). To demonstrate cell surface IL-36 receptor expression we stained T cells, monocytes and mDC with antibodies against IL-36R and used flow cytometry to show that IL-36R was most strongly expressed on the surface of mDC (Figure 3d), which was approximately 10-fold more than on monocytes (Figure 3e), and absent from the surface of T cells (Figure 3f). In contrast to myeloid cells, blood neutrophils showed no expression of IL-36R and failed to respond to IL-36 treatment (Supplementary Figure 1). Likewise, neither resting nor CD3/CD28 activated CD4+ nor CD8+ T cells responded to IL-36 treatment (Supplementary Figure 2).
Figure 3. Human APC but not T cells express the IL-36 receptor.
Keratinocytes (KC), monocytes and myeloid DC (mDC) express IL-1R1, IL-1RAcP and IL-36R mRNA transcripts, however, CD4+ and CD8+ T cells were found not to express IL-36R as determined by qRT-PCR (a-c, n=4 donors). Flow cytometric analysis reveals that in contrast to mDC (d and g) and monocytes (e, f and h), T cells (e, f and i) did not express surface IL-36R. Filled histogram: anti-IL36R, dotted histogram, isotype control antibody. Flow cytometry gating shown in panels 3d-f; Flow cytometry data are representative of 6 donors.
IL-36 activates and induces antigen-presenting cells to secrete IL-1 and IL-6
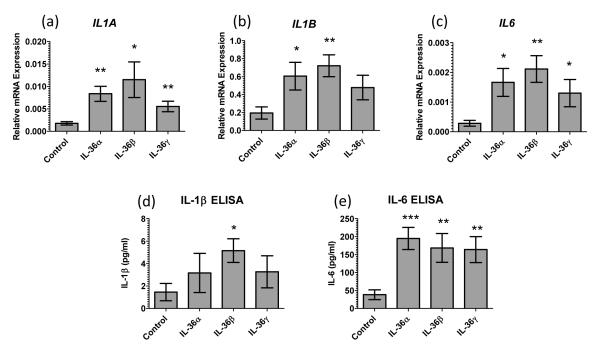
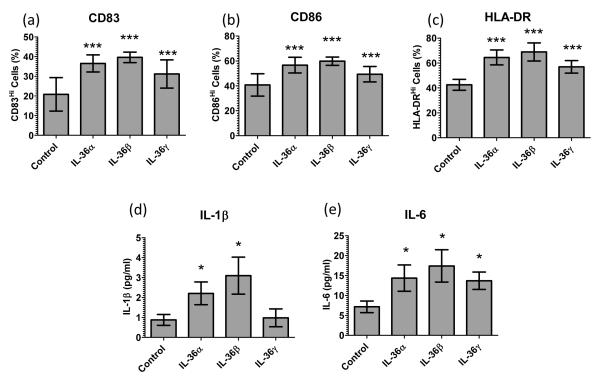
Given that monocytes and mDC expressed the pre-requisite receptors for IL-36, we stimulated monocyte and mDC cultures with 100ng/ml IL-36α, β or γ and, in contrast to T cells (Supplemental Figure 2), monocytes were activated and significantly upregulated expression of IL-1A, IL-1B and IL-6 mRNA (Figure 4a-c) after 12 hours of IL-36 treatment, which is supported by significantly increased IL-1β and IL-6 protein secretion into the conditioned medium (Figure 4d and e). Likewise, mDC treated with IL-36 cytokines for 12 hours significantly increased the proportion of cells with strong CD83, CD86 and HLA-DR expression as determined flow cytometrically (Figure 5a-c). Moreover, when conditioned media were assayed by ELISA both IL-1β and IL-6 were significantly elevated in IL-36α and β treated mDC cultures (Figure 5d and e). Of note is that the responses of mDC to IL-36 were not mediated by the secreted IL-1β or IL-6 (Supplemental Figure 3).
Figure 4. IL-36 cytokines induce monocyte expression of inflammatory cytokines.
Monocytes treated with 100ng/ml IL-36 cytokines for 12h upregulate IL-1A, IL-1B and IL-6, cytokine transcripts (n=9 donors, a-c). Significantly elevated levels of IL-1β and IL-6 were detected in the conditioned culture media after 12h (n=6 donors, e and f). Bars, mean ± SEM. Statistical significance indicated by * p<0.05, ** p<0.01, *** p<0.001 (2-tailed t-test).
Figure 5. IL-36 cytokines facilitate myeloid DC activation and cytokine secretion.
Ex vivo blood myeloid DC treated with 100ng/ml IL-36 cytokines for 12h up-regulate CD83 (a), CD86 (b) and HLA-DR (c) expression (FACS, n=6 donors) and secretion of IL-1B (d) and IL-6 (e) (ELISA, n=9). Bars, mean ± SD. Statistical significance indicated by * p<0.05, ** p<0.01, *** p<0.001 (2-tailed t-test).
Dendritic cells matured in the presence of IL-36 have increased activity
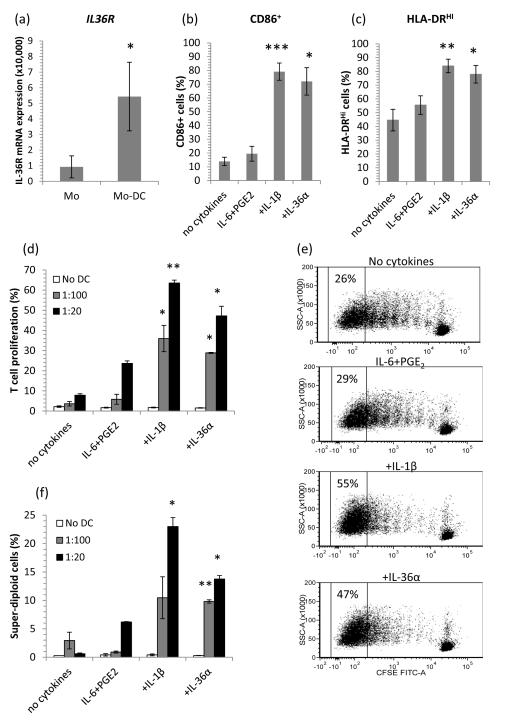
Given that primary blood mDC typically compose only 2% of the PBMC population isolated from blood, we sought to use in vitro monocyte-derived DC (MO-DC) as a surrogate APC. We cultured MO-DC and found IL-36R was expressed 6-fold more abundantly by CD1a+CD14−MODC than precursor CD1a−CD14+ monocytes (Figure 6a). We next examined whether IL-36 could alter MO-DC phenotype during maturation. After 6 days in culture, immature MO-DC were stimulated for 48 hours with a cytokine cocktail which promoted DC maturation in an IL-1 dependent manner; A combination of IL-6 and PGE2 with IL-1β, IL-36α, IL-36β or IL-36γ, significantly activated DC as illustrated by 3-fold increases in CD86 with IL-36α (p=0.014, Figure 6b) and significantly increased proportions of DC strongly expressing HLA-DR compared with IL-6 and PGE2 alone (p=0.03, Figure 6c).
Figure 6. Monocyte-derived dendritic cells (MO-DC) have enhanced IL-36R expression and IL-36 cytokines facilitate DC maturation, driving T cell proliferation.
MO-DC expressed 6-fold more IL-36R mRNA than their monocyte precursors (qRT-PCR, n=6, a). When cultured with 10ng/ml IL-6+0.1μM PGE2 and either 10ng/ml IL-1β or 100ng/ml IL-36α, MODC significantly increased their surface expression of CD86 (b) and HLA-DR (c), indicative of DC maturation (48hr, bars, mean ± SEM, n=8 donors). MO-DC matured with 10ng/ml IL-6 + 0.1μM PGE2 and 10ng/ml TNF-α, 10ng/ml IL-1β or 100ng/ml IL-36α drove allogeneic T cell proliferation as determined by CFSE dye dilution (d and e) or DAPI labeling of DNA (f). No DC (T cells only), 1:100 DC:T cell ratio, 1:20 DC:T cell ratio showing mean ± SEM (n=4 donors). Statistical significance indicated by * p<0.05, ** p<0.01, *** p<0.001 compared with IL-6+PGE2 (2-tailed t-test).
Having established that IL-36 could alter DC phenotype we began to assess whether this translated into altered DC function. Thus we co-cultured IL-36-matured DCs with allogeneic T cells at DC:T cell ratios of 1:20 (Figure 6d and e) and 1:100 (Figure 6d) for 5 days and monitored T cell proliferation using CFSE and DAPI labeling of T cells. Compared with basal stimulation with IL-6 and PGE2 only, DC matured with IL-1β or IL-36α induced significant increases in T cell proliferation in the allogeneic mixed lymphocyte reaction in terms of both CFSE dilution (Figure 6d and e) and increased numbers of super-diploid cells (Figure 6f).
DISCUSSION
Skin inflammation such as that seen in psoriasis results from the multipartite interactions of, at least, KC, T cells, antigen presenting cells (APC), fibroblasts and endothelial cells (15, 16). In this scenario, KC are not passive and may initiate inflammatory cascades following physical stress, UV irradiation or infection (17, 18). KC are a major source of IL-36 cytokines, particularly during inflammation (1, 4, 5, 19, 20). We now demonstrate that KC, when treated with IL-36 cytokines are potent sources of chemokines active upon T cells and APC (Figure 1) and this activity was not mediated via IL-1β or IL-6 (Supplemental Figure 3). In the present study we focused on the use of the recently characterized, highly active truncated IL-36 cytokines, which have been shown to bind IL-36R and activated cells in the ng/ml range (11). The truncated IL-36α and β were at least 40-fold more potent than their full-length counterparts ((11) and Supplemental Figure 4). Interestingly truncated IL-36γ appears to be equipotent to IL-36α and β, yet the full length version showed little to no agonist activity on KC or APCs.
Many of the inflammatory mediators induced by IL-36 have been demonstrated to be over-expressed in psoriatic skin lesions including CCL3, CCL5, CXCL1, CXCL8, and CCL20 (5, 21, 22) suggesting that IL-36 may contribute to the chemokine environment of inflamed skin. Indeed, intradermal injection of recombinant murine IL-36α to mouse skin resulted in chemokine expression, leukocyte infiltration and inflammation (Figure 2). These data are in accord with an earlier model of murine IL-36 expression, where murine IL-36α was overexpressed in basal mouse epidermis under the control of a K14 promoter (1) resulting in a similar pattern of chemokine induction and cell infiltration. Moreover, IL-36 cytokines were shown to be essential mediators in the KC-APC crosstalk required to drive imiquimod-induced psoriasiform dermatitis in mice (7). In this case, stimulation of APC via TLR7 leads to KC activation and IL36R-dependent release of chemokines such as CXCL1 and CCL20 and recruitment of macrophages and neutrophils to the skin (7).
KC expressing the above chemokines recruit T cells, APC, and neutrophils into the skin, thus we next examined whether IL-36 was also active on these immune cells. It was recently shown that mouse CD4+ but not CD8+ T cells respond to murine IL-36 in an IL-36R-dependent manner (6) and that IL-36 synergizes with IL-12 to drive a potent murine Th1 response (26). Unlike mouse cells, however, human CD4+ and CD8+ T cells do not express IL-36R nor respond to IL-36 neither when resting nor under CD3/CD28 stimulation (Supplemental Figure 2). We show however that APCs such as CD14+ monocytes, CD1a+CD14− MO-DC and CD1c+ mDC do express IL-36R (Figures 2 and 6). Recently, Mutamba et al., demonstrated expression of IL-36R (IL-1RL2) by MO-DC and plasmacytoid DC, but not undifferentiated monocytes, myeloid DC or bulk PBMC (27) and these cells responded to IL-36R ligation. We show that MO-DC express approximately six times more IL-36R transcript than their precursor monocytes (Figure 6a) and this is expression is further doubled in blood myeloid DC (Figure 2c).
DC activated with a cocktail of IL-6, PGE2 and either IL-1β or IL-36α upregulated CD86 and HLA-DR surface expression (Figure 6b and c). This is in accord with the ability of murine IL-36 cytokines to activate mouse bone-marrow derived DC (6) and IL-36β to drive maturation of MO-DC(27). This is a key observation as HLA-DR presents peptide antigens to CD4+ T cells and CD86 binds to CD28 on the T cell providing the costimulatory second signal essential for priming naïve T cells. We next investigated the functional consequences of IL-36 activity on DCs and incubated IL-36-matured DCs with allogeneic CD4+ T cells. DCs treated with IL-36 promoted increased T cell proliferation (Figure 6d-f), which strongly suggests that IL-36 may influence T cell function in skin via its effects on APCs. Moreover, we demonstrate that IL-36 cytokines induce APC expression of IL-1β and IL-6 (Figures 4 and 5), which potentially contributes to a pro-Th17 environment (28). The epidermal hyperproliferation in psoriasis is now thought to be driven, at least in part, by IL-17 and IL-22 secreting T cells infiltrating the skin (29). In humans, naïve CD4+ T cells differentiate into mature Th17 cells in vitro in response to IL-1β, IL-6 and/or IL-23 (29-31) and we have recently demonstrated that IL-1β and/or IL-23 can promote the survival and expansion of Th17 cells from the memory T cell pool (32). This is a particularly important observation, as memory T cells are likely to be the most important T cell subset for the maintenance of psoriasis (33, 34).
A form of generalized pustular psoriasis (GPP) has recently been associated with missense mutations in IL36RN (8, 9) that affect the structure and function of IL-36Ra protein, leading to unrestrained IL-36 agonist activity. Although GPP has a strong neutrophil component we could not demonstrate IL-36R expression by neutrophils or direct activity of IL-36 on neutrophils (Supplemental Figure 1). GPP is also is associated with increased activation of Th17 and Th22 cells during the disease flare (35) and interestingly, Th17 T cells, immature DC, γδ T cells and neutrophils all express CCR6 (21, 36, 37), and we show that IL-36 is a strong inducer of the CCR6 ligand CCL20 expression by KC (Figure 1) consistent with a role for the IL-36-CCL20-CCR6 axis in driving psoriatic inflammation (7).
IL-1 family members have also been shown to act synergistically with other cytokines and growth factors (38) and in this respect IL-36α, β, γ are no exception, with synergism reported with IL-17A, TNF-α (39) and IL-1β (40) extending the potential of IL-36 in the skin, particularly on psoriatic keratinocytes (41). This may be particularly relevant given that psoriasis is now considered a mixed Th1/Th17 disease and that IL-36 upregulates the expression of a number of IFN-γ-induced chemokines.
Our data presented here, together with our previous findings (5), data from others using mice (1, 7), and studies of the IL36RN mutations associated with pustular psoriasis (8, 9) all suggest an important role for the IL-36 system in skin inflammation: IL-36 induces KC expression of antimicrobial peptides, matrix metalloproteinases (5) and chemokines (Figure 1) which recruit T cells and APCs. IL-36 activates APCs and biases their cytokine profile (Figures 4-6 and (27)) which further drives the inflammatory response. Taken together, these data are consistent with the notion that IL-36 cytokines, which we have shown to be overexpressed in psoriasis (5), can influence the phenotype and function of DC with subsequent changes in T cell activity.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Ann Pero and Sarah Laponsa for subject recruitment and Dr. Xianying Xing, Marybeth Riblett and Candace Matheny for excellent technical assistance.
AJ is supported by The American Skin Association, The Dermatology Foundation, a Babcock Foundation Endowment and NIH K01 AR064765. JEG is supported by NIH K08 AR060802, the National Psoriasis Foundation, Taubman Medical Research Institute (as the Kenneth and Frances Eisenberg Emerging Scholar) and a Babcock Foundation Endowment. NLW is supported by grants from the National Psoriasis Foundation and NIH P30AR39750, P50AR05508, R01AR063437, and R01AR062546.
Abbreviations
- NHK
normal human keratinocyte
- KC
keratinocyte
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- mDC
myeloid dendritic cell
- MO-DC
monocyte-derived dendritic cell
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- 1.Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK, Sims JE, Peschon JJ. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- 3.Magne D, Palmer G, Barton JL, Mezin F, Talabot-Ayer D, Bas S, Duffy T, Noger M, Guerne PA, Nicklin MJ, Gabay C. The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblasts and articular chondrocytes. Arthritis Res Ther. 2006;8:R80. doi: 10.1186/ar1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, Wagner J, Edwards G, Clifford T, Menon S, Bazan JF, Kastelein RA. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 5.Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, Wohn C, Prens EP, Wang F, Maier LE, Kang S, Voorhees JJ, Elder JT, Gudjonsson JE. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, Gabay C. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 7.Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, Towne JE, Douangpanya J, Pons C, Mansour S, Serre V, Makni H, Mahfoudh N, Fakhfakh F, Bodemer C, Feingold J, Hadj-Rabia S, Favre M, Genin E, Sahbatou M, Munnich A, Casanova JL, Sims JE, Turki H, Bachelez H, Smahi A. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 9.Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, Knight J, Spain SL, Nestle FO, Burden AD, Capon F, Trembath RC, Barker JN. Mutations in IL36RN/IL1F5 Are Associated with the Severe Episodic Inflammatory Skin Disease Known as Generalized Pustular Psoriasis. Am J Hum Genet. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder JT, Fisher GJ, Zhang QY, Eisen D, Krust A, Kastner P, Chambon P, Voorhees JJ. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991;96:425–433. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- 11.Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, Sims JE. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36{alpha}, IL-36{beta} and IL-36{gamma}) or antagonist (IL-36Ra) activity. J Biol Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, Askew D, Gilliam AC, McCormick TS, Ward NL. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark RA, Nauseef WM. Isolation and functional analysis of neutrophils. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0723s19. Chapter 7:Unit 7 23. [DOI] [PubMed] [Google Scholar]
- 14.Tedder TF, Jansen PJ. Isolation and generation of human dendritic cells. Current protocols in Immunology. 2001 doi: 10.1002/0471142735.im0732s23. Chapter 7:Unit 7.32. [DOI] [PubMed] [Google Scholar]
- 15.Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR, Nair RP. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 16.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clinical and experimental immunology. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickoloff BJ. Keratinocytes regain momentum as instigators of cutaneous inflammation. Trends Mol Med. 2006;12:102–106. doi: 10.1016/j.molmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang LM, Suarez-Farinas M, Krueger JG. Human Keratinocytes’ Response to Injury Upregulates CCL20 and Other Genes Linking Innate and Adaptive Immunity. J Invest Dermatol. 2011;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Krueger JG, Kao MC, Lee E, Du F, Menter A, Wong WH, Bowcock AM. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiological genomics. 2003;13:69–78. doi: 10.1152/physiolgenomics.00157.2002. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, Catron D, Buchanan ME, Muller A, deWaal Malefyt R, Deng G, Orozco R, Ruzicka T, Lehmann P, Lebecque S, Caux C, Zlotnik A. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 22.Johnston A, Guzman AM, Swindell WR, Wang F, Kang S, Gudjonsson JE. Early tissue responses in psoriasis to the anti-TNF-alpha biologic etanercept suggest reduced IL-17R expression and signalling. Br J Dermatol. 2014 doi: 10.1111/bjd.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Varona R, Cadenas V, Gomez L, Martinez AC, Marquez G. CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- 25.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, Sallusto F, Sims JE, Gabay C. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 27.Mutamba S, Allison A, Mahida Y, Barrow P, Foster N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur J Immunol. 2012;42:607–617. doi: 10.1002/eji.201142035. [DOI] [PubMed] [Google Scholar]
- 28.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 30.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I, Bruce A, Gudjonsson J, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling T, Elder J, Zou W. Induction of IL-17+ T cell trafficking and development by IFN-gamma: Mechanism and pathological relevance in psoriasis. Journal of Immunology. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8 T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teraki Y, Tanaka S, Hitomi K, Izaki S. A case of generalized psoriasiform and pustular eruption induced by infliximab: evidence for skin-homing Th17 in the pathogenesis. Br J Dermatol. 2010;163:1347–1351. doi: 10.1111/j.1365-2133.2010.10002.x. [DOI] [PubMed] [Google Scholar]
- 36.Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, Zlotnik A, Schall TJ. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–3963. [PubMed] [Google Scholar]
- 38.Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011;131:329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O’Toole M, Young DA, Fouser LA, Nickerson-Nutter C, Collins M, Dunussi-Joannopoulos K, Medley QG. Inter-Regulation of Th17 Cytokines and the IL-36 Cytokines In Vitro and In Vivo: Implications in Psoriasis Pathogenesis. J Invest Dermatol. 2011;131:2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen TT, Niyonsaba F, Ushio H, Akiyama T, Kiatsurayanon C, Smithrithee R, Ikeda S, Okumura K, Ogawa H. Interleukin-36 cytokines enhance the production of host defense peptides psoriasin and LL-37 by human keratinocytes through activation of MAPKs and NF-kappaB. J Dermatol Sci. 2012;68:63–66. doi: 10.1016/j.jdermsci.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Muhr P, Zeitvogel J, Heitland I, Werfel T, Wittmann M. Expression of interleukin (IL)-1 family members upon stimulation with IL-17 differs in keratinocytes derived from patients with psoriasis and healthy donors. Br J Dermatol. 2011;165:189–193. doi: 10.1111/j.1365-2133.2011.10302.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.