Abstract
A crucial event in the metastatic cascade is the extravasation of circulating cancer cells from blood capillaries to the surrounding tissues. The past 5 years have been characterized by a significant evolution in the development of in vitro extravasation models, which moved from traditional transmigration chambers to more sophisticated microfluidic devices, enabling the study of complex cell–cell and cell–matrix interactions in multicellular, controlled environments. These advanced assays could be applied to screen easily and rapidly a broad spectrum of molecules inhibiting cancer cell endothelial adhesion and extravasation, thus contributing to the design of more focused in vivo tests.
Keywords: extravasation, cancer, in vitro, microfluidics, drug
The past four decades were characterized by promising successes in cancer treatment and detection, through the development of devices reducing surgical invasiveness or enabling early diagnosis, and the discovery of drugs blocking primary tumor progression, thus reducing cancer mortality and improving life quality for patients with terminal disease [1]. As discussed in a recent scientific report by the American Cancer Society, the relative 5-year survival rate for all cancers diagnosed between 2002 and 2008 in the USA was 68%, significantly higher compared with the 49% reported for 1975–1977 [2]. However, despite great advances in basic cancer molecular and cell biology with the discovery of oncogenes [3], tumor suppressor mechanisms [4] and cytokines involved in cancer progression [5], the spread of primary tumors toward distant organs and the subsequent metastatic colonization is still responsible for 90% of cancer-associated mortality [6].
In vitro assays can be beneficial to study cancer cell invasion and migration, and for the development of new anticancer drugs [7]. In particular, human 3D models can closely mimic the pathophysiological microenvironment [8], combining multiple cell types and molecular factors in a controlled system, thus bridging the gap between simplified 2D assays, which lack the structural architecture of body tissues and force cells to adapt to an artificial flat and stiff surface [9], and complex, expensive in vivo studies, often performed using animal models that might fail to reproduce features of human tumors [10]. Significant advances have been made since the development of soft lithography techniques, which enable microfabrication of structures and channels with poly-dimethyl-siloxane (PDMS) for microfluidic applications, thus replacing traditional plastic surface devices, and patterning of cells and biomolecules [11]. Microfluidic devices with embedded 3D cultures are currently used to study cancer cell behavior within in vivo-like microenvironments [12] and new promising applications, including paper-based multilayer constructs, have been developed to control oxygen and nutrient gradients [13].
Modeling the multiple steps of the metastatic cascade represents a challenge that could pave the way for the discovery of new antimetastatic drugs [14]. In particular, the extravasation process represents a crucial point that leads to the invasion of specific secondary sites, with the subsequent growth of metastatic tumors; thus, detailed studies are necessary to clarify the interaction between specific primary tumors and secondary target organs [15].
Following an introductory section on cancer metastases, in this review, we focus on in vitro models to study cancer cell invasion, migration and, particularly, extravasation. We also discuss microfluidic applications to investigate extravasation processes and other metastasis-related phenomena. Finally, we present in vitro and in vivo models that can be used to study the effects of therapeutics on cancer cell extravasation, underscoring how highly specific microfluidic models could provide a significant breakthrough in the screening process of antimetastasis drugs.
Cancer cell odyssey in the metastatic cascade
Tumors arising from epithelial tissues represent approximately 80% of life-threatening cancers because of their ability to metastasize in different secondary organs [16]. The complex metastatic process can be conceptually divided into two main phases, namely the physical translocation of cancer cells from the primary tumor to distant sites, and their subsequent colonization (Figure 1). More specifically, several sequential and interrelated steps can be recognized in the former phase, including loss of cellular adhesion, acquisition of increased invasiveness and motility owing to genetic and epigenetic alterations, and induction of tumor angiogenesis leading to entry into the circulatory or lymphatic systems, a process known as intravasation [6,17]. After intravasation, those cells that survive in the circulation might undergo extravasation, which includes several steps, such as cells becoming trapped in a remote vessel or adhered to its endothelium and transmigrating into tissues, to initiate the development of secondary tumors [18–22].
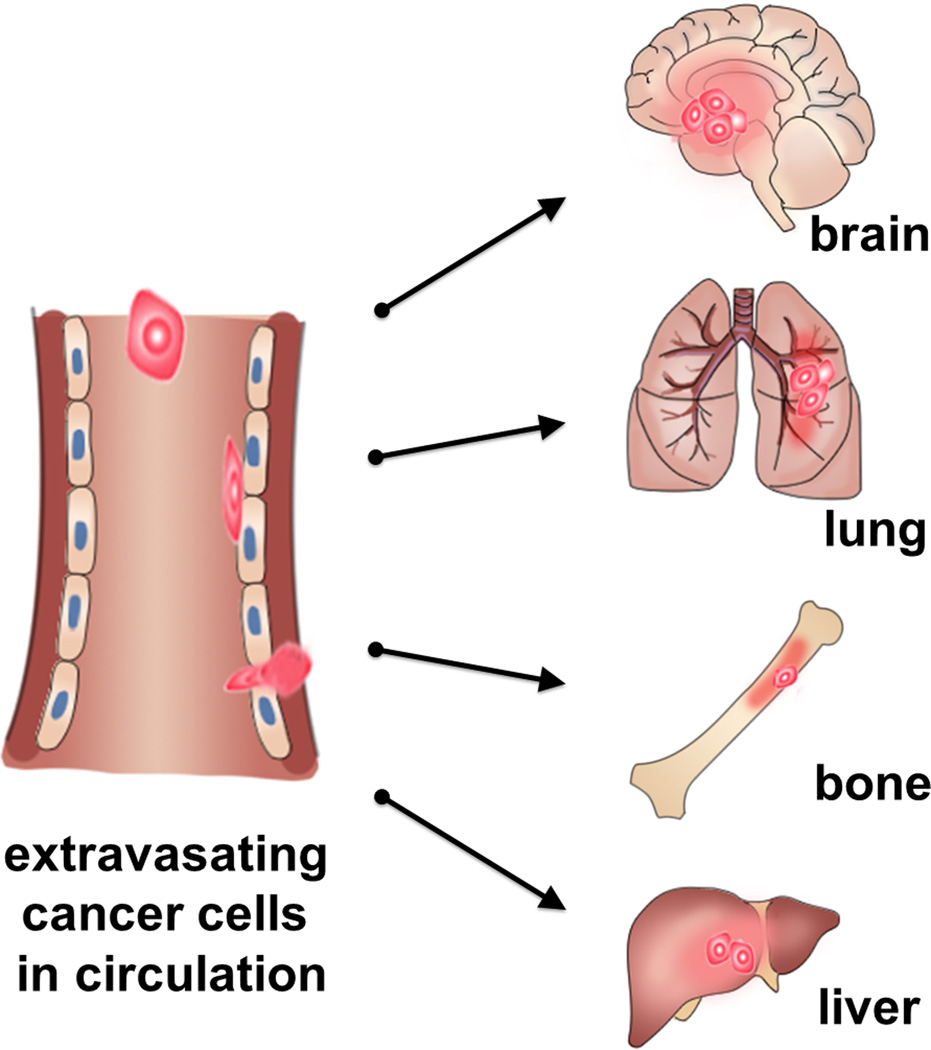
Figure 1.
Schematic of extravasation cascade. Primary cancer cells travel in the circulatory system and transmigrate across the endothelium to extravasate into secondary sites and colonize at organs such as lung, bone, liver and brain. Predominant primary cancer sites where initial dissemination occurs include breast, pancreas, prostate gland, colon and lung.
In one scenario of extravasation, circulating tumor cells (CTCs) showing a leukocyte-like rolling behavior on the vascular walls [23] establish transient, metastable contacts with the endothelium mediated by endothelial cell surface molecules, such as E-selectin and P-selectin, and cancer cell counter-receptors, such as sialyl Lewis-a/x [24,25]. Subsequently, a firmer adhesion is mediated by adhesive molecules on the endothelium, such as vascular cell adhesion molecules (VCAMs), whose expression can be triggered by cancer cells themselves [26], and cancer cell integrins, while chemo-attractant molecules promote trans-endothelial migration toward the surrounding tissues [8]. An alternative view is that CTCs, being relatively large, are physically trapped in the small vessels of the microcirculation, become activated, and transmigrate [27].
Steven Paget’s ‘seed and soil’ hypothesis represents a milestone in the study of mechanisms governing metastases, based on the assumption that the interplay between specific cancer cell types and a properly receptive microenvironment guides the metastatic spread of primary tumors to distant organs [28]. However, Paget’s theory was challenged by James Ewing, who proposed that the main factor leading to metastases is represented by the anatomy of blood and lymphatic vessels and by circulatory patterns between primary tumors and specific secondary sites [29]. It is now accepted that these theories are not mutually exclusive: scientists have shown how CTCs migrating from the primary tumors target a well-defined subset of organs, specific for each tumor type. This tissue tropism is partially because of the anatomy of the circulatory system, not only leading to physical trapping as described above, but also influenced by the interaction between ‘seed cells’ and ‘receptive soils’ [15–17,30].
Endothelial cells in the vasculature of different organs express different surface receptors and specific chemokines are secreted by host cells of individual tissues [31,32]. Moreover, the ‘pre-metastatic niche model’ states that growth factors secreted by the primary tumor can prime specific tissues for cancer engraftment, determining the attraction of tumor-associated cells, which contribute to the development of a receptive environment [29,33–35] and promoting specific cancer cell homing. Particularly, breast cancer cells often metastasize to the bone and autopsy studies have demonstrated that 70% of patients with breast cancer have skeletal metastases, which represent the major cause of lethality and induce pain, spinal cord compression and fractures, severely compromising quality of life [36,37].
Unraveling the multiple steps of extravasation could enable the identification of new anticancer drugs to inhibit the adhesion and/or transendothelial migration of metastatic cells. In vitro testing platforms represent a useful tool, but the lack of organ-specific models, reproducing the human in vivo microenvironment and tissue tropism shown by specific cancer cells, constitutes a significant limitation among current systems.
In vivo and in vitro cancer models for invasion, migration, extravasation and colonization
Although no in vivo or in vitro model fully replicates the complex milieu of factors that influence metastasis in humans, there have been numerous studies devoted to understanding cancer cell invasion, migration and interactions with the endothelium, which comprise different stages of cancer metastasis. Conventional studies of metastasis have been mostly limited to in vivo mouse models because there is a lack of tumor models and methods to study the associated processes in vitro. Mouse models provide a platform to screen for genes involved in metastasis for specific organs or proteins that mediate cancer invasion [38–40]. Roles of chemical factors and different signaling mechanisms that trigger each step of metastasis have also been studied [41–43]. In particular, in the case of cancer cell extravasation, in vivo video microscopy of tail-vein injected cancer cells to mouse has been the primary means of investigation [21,44]. Moreover, advanced in vivo models were developed to study metastasis through direct injection of breast cancer cells either intravenously or directly to specific organs [45,46], and intravital video microscopy was used to visualize the interactions of cancer cells in the circulatory system and the metastatic site in a more physiologically relevant manner. However, the main disadvantages of in vivo models are that they make it difficult to perform tightly regulated, parametric studies and quantification is limited [47].
Earlier in vitro models relating to cancer metastasis investigated cancer cell invasion and migration across matrices of various types under different mechanical and/or chemical cues [48]. There were also studies that focused on interactions of two cell types by modeling cancer cell adhesion to the endothelium, with an emphasis on the changes imposed in cell morphology and monolayer biomechanical properties [49,50]. Furthermore, use of the Boyden chamber and/or transwell assays for simulating cell migration and cancer cell invasion across the endothelium has been widely accepted. These models have been a popular choice because they overcome some of the limitations of in vivo experiments (e.g. parametric studies, quantification, non-human cells, etc.) by providing more regulated environments with tunable parameters and using human cell types. However, limitations still exist in that the Boyden chamber enables limited control over the local environment and complex multicellular interactions cannot be accurately analyzed because of limited imaging capabilities.
In recognition of the need for a new generation of in vitro platforms, optically accessible and better mimicking physiological conditions through controlled microenvironments, recent research has led to the creation of a new class of in vitro testing methodologies using the emergent technologies of microfluidics. Although acknowledging that in vitro systems cannot fully reproduce the complexity of in vivo situation, microfluidic devices provide the opportunity to create organ-specific microenvironments and explore the development of metastasis of different cancer types, including migration through gels as well as real-time imaging of invasion and extravasation.
Microfluidic tools for in vitro cancer models
Microfluidics has revolutionized the field of cell biology, enabling researchers to develop advanced 3D assays in highly controlled microenvironments [51], characterized by spatiotemporal tunable chemical gradients, interstitial flows and shear stresses, complex interactions among multiple cell types and small reagent volumes compared with traditional assays [12,52,53]. As a result, microfluidics is one of the most promising technologies to develop and optimize complex in vitro cancer models, mimicking multiple steps of the metastatic cascade from primary tumor local invasion to extravasation in secondary loci.
In recent work by Haessler and co-authors [54], the migratory behavior and migrational speed of metastatic breast cancer cells MDA-MB-231 were investigated under a controlled interstitial flow within a 3D microfluidic chamber. The results demonstrated how the interstitial flow increased the percentage of migrating cancer cells and induced a superior persistence (the ratio between the cell net displacement in a specific direction and the cell total path length) for specific cell subpopulations, either in the positive or negative interstitial flow direction. These data promote the idea that small, aggressive and resistant subpopulations of cells have a crucial role in cancer, being characterized by phenotypes leading to drug resistance and metastatic dissemination. The morphology and invasiveness of breast cancer cells were also investigated by Liu and colleagues [55], who showed that MCF-7 breast cancer cells generated protrusions and migrated up an epidermal growth factor (EGF) gradient within a 3D basement membrane extract gel with a matrix metalloproteinase (MMP)-dependent proteolytic activity. On the same topic, the Beebe group developed a simple and effective microfluidic device to study the transition from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC) in a co-culture system with human mammary fibroblasts, demonstrating that the presence of both soluble factors and cell–cell contacts accelerates the transition and that increasing the distance between cell populations leads to incomplete transition, with carcinoma cells retaining their rounded morphology [56]. An advanced microfluidic model was recently proposed by Zervantonakis et al. to investigate the mechanism underlying cancer cell intravasation, showing how tumor necrosis factor alpha (TNF-α) secreted by macrophages interacting with cancer cells can promote endothelial barrier impairment and subsequent cancer cell transmigration [57].
The above mentioned models represent just a few of the studies recently developed to investigate the initial events of the metastatic cascade, whereas other specific assays were designed to analyze the final steps of cancer cell journey within the circulatory system. In particular, a few interesting models have been developed over the past 5 years to study the adhesion and extravasation of cancer cells (Figure 2). The Takayama group designed a microfluidic device to recreate the adhesion of cancer cells to an endothelial monolayer under physiological flow conditions. They demonstrated both that breast cancer cell receptors chemokine (C-X-C motif) receptor 4 (CXCR4) and CXCR7 are involved in the adhesion process and that a CXCL12-conditioned environment can act on endothelial cells, enhancing breast cancer cell adhesion in a CXCR4- or CXCR7-independent manner [58]. Similarly, Shin and co-authors developed a complex platform to analyze the metastatic process from intravasation to the downstream endothelial adhesion in a single chip. In particular, they showed how colon cancer cell adhesion is dependent both on E-selectin expression by endothelial cells and shear stress levels, finding an optimal value at 3 dyne/cm2. Moreover, they found a significant decrease in cell adhesion when cancer cells were treated with the extravasation inhibitor CA19-9 antibody [59]. Extravasation events were monitored by Zhang et al., who analyzed the transmigration of salivary gland adenoic cystic carcinoma (ACC) cell aggregates in a gel matrix, demonstrating how cell clusters can adhere to, but not extravasate through, the endothelium without CXCL12 stimulation. Interestingly, a CXCL12 concentration-dependent transmigration behavior was highlighted, and the addition of the CXCR4 antagonist AMD3100 inhibited cell aggregate extravasation, although it failed to cause detachment of aggregates from the endothelial monolayer. Finally, cell–cell junctions in the endothelial monolayer appeared completely destroyed at the site of transmigration [60]. A similar model was designed by the Kamm group to investigate single breast cancer cell extravasation through an endothelial monolayer within a collagen gel matrix. Transmigration can be closely monitored in this microfluidic device with a high resolution imaging system, showing that extravasation events within the matrix occur in the first 24 h following cancer cell introduction, and are associated with a significant increase in endothelial monolayer permeability owing to disruption of vascular endothelial (VE)–cadherin junctions [61]. A more physiological model was recently developed by the same group to analyze the extravasation ability of different cancer cell types within a microvascular network, demonstrating the effect of inflammatory cytokines and accurately describing the transmigration event, which is characterized by initial thin cancer cell protrusions followed by extrusion of the nucleus and cell body [62]. It is well known that cancer cells undergo deformation processes before adhesion and extravasation through the endothelial lining. Chaw and colleagues developed a multistep microfluidic device to study the effect of cell deformation on viability and proliferation, revealing that different cancer cell lines under mechanical stresses were characterized by reduced viability and increased doubling times, thus suggesting a significant change in their biological activity. Moreover, they quantified the migration rate and the percentage of cells capable of migrating through 30-Mm-wide microgaps coated with Matrigel, with or without an endothelial cell lining. They demonstrated the different roles of basement membrane coating and the endothelial monolayer, the former slowing down cancer cell migration and the latter reducing the total number of transmigrating cells [63].
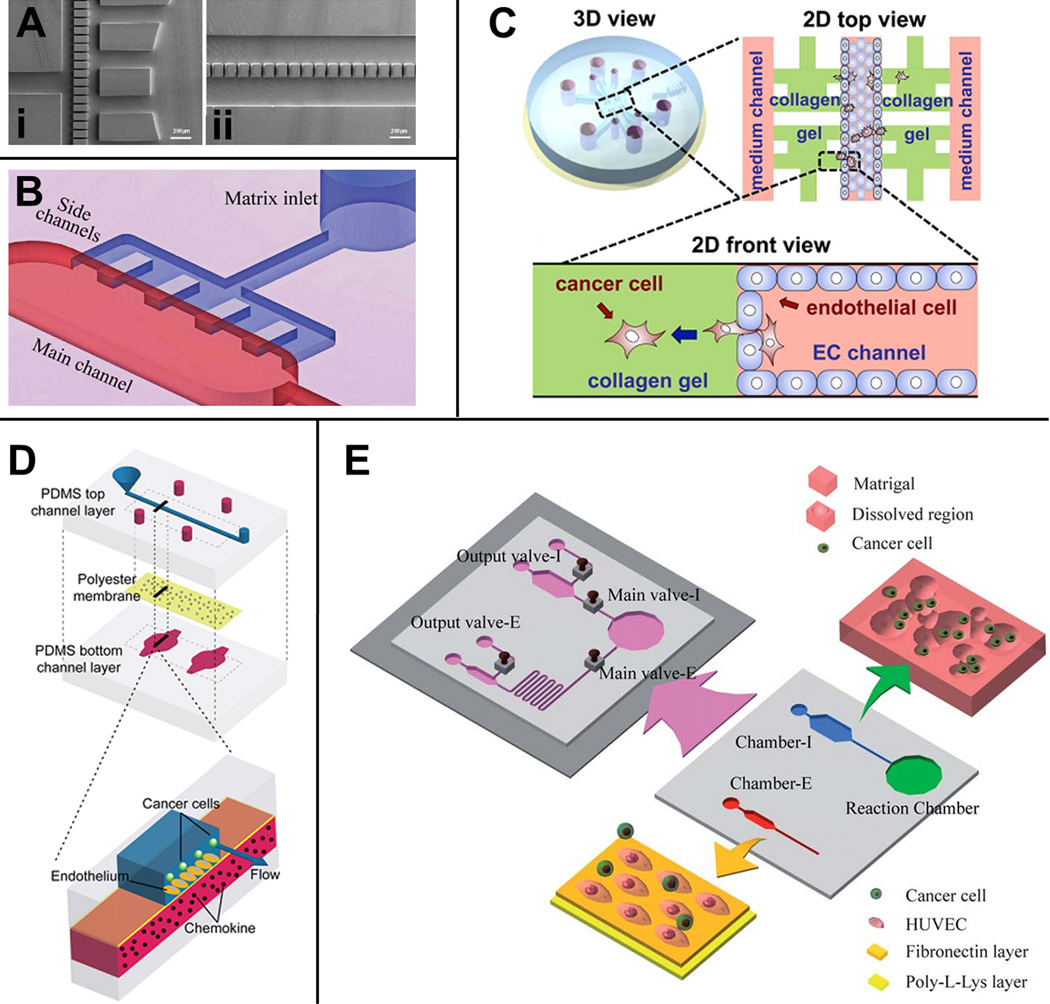
Figure 2.
Microfluidic models for extravasation. (a) Multi-step microfluidic device developed by Chaw and colleagues. Deformation chamber with 10-Mm wide gaps through which cells are forced (i) and transmigration chamber with a row of 30-Mm wide gaps where cell migration and invasion occur (ii). (b) Microfluidic device for the study of transendothelial migration of cancer aggregates [60]. Schematic representation of a single vessel unit characterized by a main fluidic channel and five lateral regions, where an extracellular matrix-mimicking gel can be easily reproduced to study salivary gland adenoic cystic carcinoma cell aggregate transmigration. (c) In vitro model developed by Jeon et al. to investigate breast cancer cell extravasation in a collagen gel matrix [61]. 3D view of the microfluidic platform characterized by three fluidic channels and two gel channels. 2D top view and front view showing the endothelialized central fluidic channel where cancer cell extravasation occurs through eight regions of interest. (d) Microdevice designed by the Takayama group to analyze the intravascular adhesion of breast cancer cells. Schematic of the multilayered device characterized by an endothelial cell-coated polyester membrane separating a top channel where cancer cells flow and a chemokine-containing bottom channel. (e) Microfluidic platform for the study of intraand extravasation events [59]. Chamber I represents the intravasation chamber for cell migration and invasion, whereas chamber E constitutes the extravasation chamber where cancer cell adhesion events on an endothelial monolayer can be detected. A reaction chamber is designed to condition cells upstream from the extravasation chamber. Screw valves enable the researcher to control independently the fluid flowing through each chamber. Reproduced, with permission, from [62] (a), [60] (b), [61] (c), [58] (d) and [59] (e). Abbreviations: EC, endothelial cells; HUVEC, human umbilical vein endothelial cell; PDMS, poly-dimethyl-siloxane.
Leukocyte extravasation, which shares many similarities with cancer cell extravasation, has been more extensively studied. Schaff and co-authors [64] designed a microfluidic device to test neutrophil capture, rolling and deceleration on an endothelial monolayer under controlled shear stress conditions, with the capability of including chemokine gradients; moreover, they coupled a computational model to predict shear stresses and leukocyte trajectories. This platform could be used to analyze cancer cell adhesion and extravasation if 3D hydrogels mimicking an extracellular matrix were to be included. Chau and colleagues [65] focused on the development of a microdevice to study the effect of multiple shear stress conditions on endothelial cell morphology, nuclear size, perimeter and secretory activity. Although they did not investigate the adhesion of circulating cells, this platform could be easily adapted to perform detailed studies on cancer cell or leukocyte adhesion. An exhaustive discussion of in vitro assays for leukocyte adhesion with useful insights on computational models can be found in recent reviews by Bianchi [66] and Hanzlik [67]. Finally, we highlight the versatility of a previously described platform [7], which was used to analyze leukocyte transendothelial migration under the influence of inflammatory stimuli [68] as well as cancer cell extravasation [61].
The extravasation assays discussed in this section (Table 1) allowed investigation for the first time of adhesion and transmigration processes of single or aggregated cancer cells, eventually coupled with chemokine gradients or inhibitory molecules. Despite the significant step forward provided by these models, they lack the organ specificity that different cancer cell types exhibit in vivo. With the increasing interest in organ-specific chemokines and endothelium adhesion molecules guiding extravasation of specific CTCs, microfluidic devices mimicking different organ microenvironments could prove useful in identifying which surface receptor–ligand interactions are most crucial and in developing targeted therapeutics.
Table 1.
| In vivo extravasation model | |||||
|---|---|---|---|---|---|
| Animal model | Primary cancer cells | Metastatic site | Key molecule and/or gene investigated |
Blocking molecule and/or drug | Refs |
| Mouse (intravenous injection) | Mouse melanoma cells | Lung | E-selectins and/or integrins | Synthetic sialyl Lewis X and/or fibronectin-derived RGDS peptide analog Ar(DRGDS)3 | [69] |
| Breast cancer cells | Lung | HIF/Ang-like 4/L1-CAM | Digoxin | [43] | |
| Mouse (mesenteric vein injection) | Mouse melanoma cells | Liver | VLA-6 (α6β1) integrin | VLA-6 monoclonalantibody MA6 | [70] |
| Mouse (intracardiac injection) | Breast cancer cells | Bone and other tissues | Lysyloxidase | β-Aminopropionitrile | [71] |
| Mouse (subcutaneous tissue of back) | Osteosarcoma cells | Lung | VEGF | Pazopanib | [74] |
| Mouse (lateral tail vein injection) | Tumorigenic Chinese hamster fibroblasts and colon carcinoma cells | Lung | NF-kB and/or E-selectin | Lovastatin and/or glycyrrhizic acid and/or Rac1 inhibitors | [73] |
| Mouse (subcutaneous or lateral tail vein injection) | Mouse melanoma cells and mouse Lewis lung carcinoma cells | Lung | P2Y2 and/or Munc13-4 | − | [76] |
| Transparent zebrafish embryos (intracardiac injection) | Breast cancer cells | Tail intersegmental vessels | Twist and/or ITGB1 and/or VEGFA | − | [75] |
| In vitro microfluidic extravasation model | |||||||
|---|---|---|---|---|---|---|---|
| Vascular cells | Cancer cells | Process modeled |
Key molecule investigated |
Matrix | Flow | Permeability | Refs |
| Microvascular endothelial cells | Hepatocellular carcinoma; cervical carcinoma; breast carcinoma | Extravasation (no matrix) | - | Matrigel (micro-gapcoating): no matrix | − | − | [63] |
| Dermal microvascular cells | Breast cancer cells | Adhesion | CXCL12-TNFα-AMD3100-11G8 | Polyester membrane with 400-nm pores | + | − | [58] |
| Umbilical vein endothelial cells | Colon cancer cells | Adhesion | MMP2 inh- MMP 9 inh- GM6001- CA19-9 |
Matrigel 4 mg/ml (intravasation chamber)-0.1 mg/ml poly-L-lysine coating 1 50 Mg/ml fibronectin coating (extravasation chamber) | + | − | [59] |
| Salivary gland adenoic cystic adenocarcinoma cells | Extravasation | CXCL12-AMD3100 | Basement membrane extract | − | − | [60] | |
| Breast cancer cells; fibrosarcoma cell line | Extravasation | TNF-α | Fibrin gel (2.5 mg/ml) | − | + | [62] | |
| Microvascular endothelial cells | Breast cancer cells | Extravasation | - | Collagen type I (2 mg/ml) | − | + | [61] |
Targeting cancer cell extravasation: in vitro and in vivo drug-screening models to block the metastatic cascade
Inhibiting cancer cell extravasation represents a promising strategy to break the metastatic cascade, coupled with other therapies to stop tumor growth, inhibit tumor angiogenesis and prevent epithelial to mesenchymal transition (EMT) and intravasation events. These, in combination with early diagnosis techniques, could significantly improve the efficacy of cancer therapies.
One of the first studies performed on cancer cell extravasation was conducted by Saiki and co-authors, who investigated the effects of synthetic sialyl Lewis X and fibronectin-derived RGDS peptide analog on lung metastases generated by in vivo intravenous injection of melanoma cells. The former molecule was found to inhibit the interaction with the endothelium, whereas the latter limited cancer cell invasion into the basement membrane, thus affecting a later step of the extravasation process [69]. A second pioneering study was conducted by Hangan and colleagues, who analyzed VLA-6, an integrin receptor mediating cancer cell adhesion to the endothelium. By performing in vivo studies on mice and simple in vitro models with chemotaxis chambers, researchers discovered that, although the VLA-6 monoclonal antibody MA6 did not affect adhesion, it inhibited melanoma cell movement on laminin substrates. Thus, their findings suggested an active role for the VLA-6 receptor in providing both cell movement and adhesion and, more specifically, demonstrated an absence of alterations in the focal adhesion kinase phosphorylation, which is involved in motility [70]. Furthermore, in vivo experiments demonstrated a reduced ability of MA6-treated mouse melanoma cells to extravasate the liver vasculature.
Recent work has concentrated on a wide spectrum of biomolecules involved in cancer cell extravasation. The Jirik group found that the extracellular matrix remodeling enzyme lysyl oxidase (LOX) inhibitor β-aminopropionitrile (BAPN) was able to reduce the number of breast cancer metastases in treated mice, without affecting established loci. LOX seemed to be involved in the initial steps of extravasation and tissue colonization and could represent a potential candidate for advanced drug screening tests [71]. Based on findings reporting that radiotherapy can increase the metastatic potential of surviving cells [72], Hamalukic and colleagues performed a promising study in which the adhesion of ionizing radiation-stimulated cancer cells and/or endothelial cells was limited by treatment with HMG-CoA reductase inhibitor lovastatin, sialyl Lewis X mimetic drug and Rac1 inhibitor (Figure 3). In vivo studies confirmed the potential role of the lipid-lowering drug lovastatin in mice, counteracting the increased extravasation effect induced by radiation therapy [73]. Tanaka and co-authors used the osteosarcoma cell line OS LM8 within standard transendothelial migration assays to show the ability of these malignant cells to compromise an endothelial barrier and demonstrate that inhibiting the vascular endothelial growth factor (VEGF) signaling through the tyrosine kinase inhibitor pazopanib limited extravasation. In vivo studies seemed to confirm that anti-VEGF therapies could limit lung metastases from osteosarcoma [74]. Furthermore, the reduced O2 availability within the tumor environment can promote the expression of hypoxia inducible factors (HIFs), which increase the level of proteins involved in cancer progression. HIFs can induce angiopoietin-like 4 and L1 cell adhesion molecule production by cancer cells, which in turn promote extravasation into lungs [43]. A recent elegant in vivo model on transparent zebrafish developed by the Klemke group demonstrated that MDA breast cancer cells engineered to overexpress the metastatic gene Twist were characterized by increased extravasation ability compared with wild-type cells. Moreover, they reported the process switched to a β1-integrin-independent mechanism, with interesting implications on the use of small interfering RNAs [75]. Finally, we highlight a recent study on tumor cell-activated platelets that were shown to release adenine nucleotides that interacted with the P2Y2 endothelial cell receptor, thus promoting openings in the endothelial barrier and subsequent cancer cell extravasation [76].
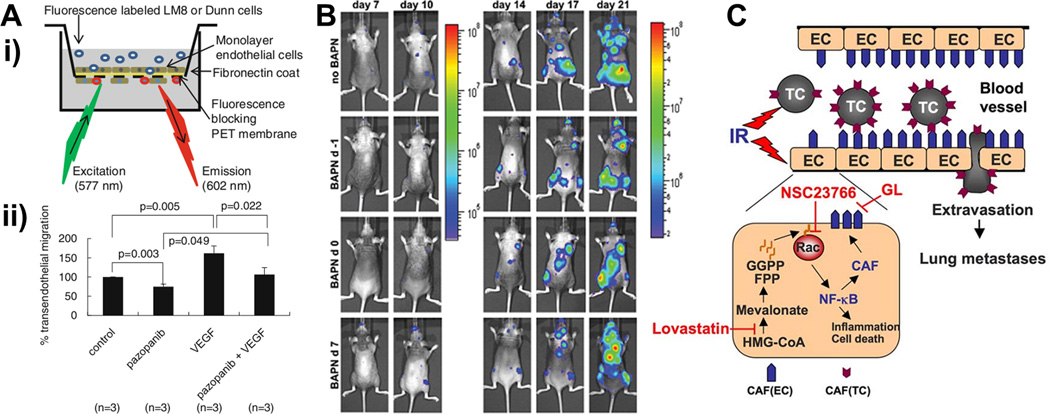
Figure 3.
In vivo and in vitro models developed for screening drugs acting on extravasation. (a) Transendothelial migration of the highly metastatic LM8 osteosarcoma (OS) cell and Dunn OS cell lines [73]. Cancer cells were applied on a monolayer of endothelial cell (EC)-coated fluorescence-blocking membrane and transmigration investigated after 12 h (i). Comparison between control LM8 cells, pazopanib [a vascular endothelial growth factor (VEGF)- inhibitor], VEGF or a combination of VEGF and pazopanib (ii). Tanaka and co-authors analyzed the effect of this anti-VEGF therapy through subsequent in vivo mouse studies, with promising results to limit lung metastases from osteosarcoma. (b) Bioluminescence imaging showing the metastatic progression of breast cancer cells between day 7 and day 21 after cancer cell intracardiac injection. Bondareva and colleagues investigated the role of the lysyl oxidase (LOX) inhibitor β-aminopropionitrile (BAPN) on metastatic growth [70]. Comparison among nontreated (no BAPN) and treated mice with drug administration daily performed starting 1 day before (BAPN d-1), the same day (BAPN d0) and 7 days after (BAPN d7) cancer cell injection. (c) Ionizing radiation (IR) can increase tumor cell (TC)-EC adhesion and subsequent TC extravasation and generation of lung metastases. Hamalukic and colleagues investigated the effects of lovastatin and Rac1 inhibitors (NSC23766) on the expression of endothelial cell adhesion molecules and the role of glycyrrhizic acid (GL) as E-selectin antagonist [72]. Reproduced, with permission, from [73] (a), [70] (b) and [72] (c).. Abbreviations: CAF, cell adhesion factor; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; NF-κB, nuclear factor kappa B,
The identified molecules (Table 1) could represent promising targets for future antimetastatic therapies, but additional studies are required to clarify their mechanisms of action and possible interactions with different cell types. In this framework, microfluidic cancer models, as reported in the previous section, pave the way to a new class of assays lying between traditional 2D models and in vivo studies. These advanced in vitro models enable strict control of multiple spatiotemporal parameters, while maintaining drug gradients and co-cultures of multiple human cell types in physiological 3D matrices. Their optimization could lead to the development of more focused in vivo screenings, which although being essential, are becoming increasingly expensive and surrounded by ethical problems.
Conclusions and future potential
Significant steps forward have been made over the past few years in the treatment of cancer, but the development of effective antimetastatic therapies still remains an issue. Traditional in vitro models do not enable parametric studies on cell–cell and cell–matrix interactions in complex, spatiotemporal tunable environments. Microfluidics can contribute to the establishment of advanced in vitro cancer models, overcoming limitations of traditional methods in cancer modeling and anticancer drug screening. Organ-specific microfluidic models are the leading edge of current in vitro research, with the possibility of multi organ-specific microfluidic models. Our hope is that they will contribute to improve knowledge on cancer biology and provide useful data to be subsequently tested through animal models and preclinical trials.
Highlights.
Extravasation of circulating cancer cells is one of the critical events in metastasis
Microfluidic platform allows multicellular studies in controlled environment
Adhesion and transmigration of cancer cells are studied in extravasation assays
In vitro systems can contribute to the design of more focused in vivo tests
Acknowledgments
Support from the National Cancer Institute (R33 CA174550-01 and R21 CA140096) and the Italian Ministry of Health, fellowship support to S.B. provided by the Fondazione Fratelli Agostino and Enrico Rocca through the Progetto Rocca Doctoral Fellowship and support to J.S. Jeon provided by Repligen Fellowship in Cancer Research and Draper Fellowship are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: How can we unravel the mechanisms underlying cancer cell extravasation and the organ-specificity of primary tumor dissemination? This review focuses on in vitro extravasation models, highlighting recent advances provided by microfluidics platforms.
References
- 1.Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2013. American Cancer Society; 2013. [Google Scholar]
- 3.Leung CT, Brugge JS. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature. 2012;482:410–413. doi: 10.1038/nature10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplon J, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 5.Owens P, et al. Bone morphogenetic proteins stimulate mammary fibroblasts to promote mammary carcinoma cell invasion. PLoS ONE. 2013;8:e67533. doi: 10.1371/journal.pone.0067533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 7.Shin Y, et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussos ET, et al. Chemotaxis in cancer. Nat. Rev. Cancer. 2011;11:573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, et al. Three-dimensional cell culture matrices: state of the art. Tissue Eng. B. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 10.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Whitesides GM, et al. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 12.Chung S, et al. Microfluidic platforms for studies of angiogenesis, cell migration, and cell-cell interactions. Sixth International Bio-Fluid Mechanics Symposium and Workshop March 28–30 2008 Pasadena, California. Ann. Biomed. Eng. 2010;38:1164–1177. doi: 10.1007/s10439-010-9899-3. [DOI] [PubMed] [Google Scholar]
- 13.Derda R, et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. SciUSA. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thuma F, et al. The metastasis-associated molecule C4.4A promotes tissue invasion and anchorage independence by associating with the alpha6beta4 integrin. Mol. Oncol. 2013 doi: 10.1016/j.molonc.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers AF, et al. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 16.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 18.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 22.Kebers F, et al. Induction of endothelial cell apoptosis by solid tumor cells. Exp. Cell. Res. 1998;240:197–205. doi: 10.1006/excr.1998.3935. [DOI] [PubMed] [Google Scholar]
- 23.Miles FL, et al. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin. Exp. Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda M, et al. C-type lectins and sialyl Lewis X oligosaccharides. Versatile roles in cell-cell interaction. J. Cell. Biol. 1999;147:467–470. doi: 10.1083/jcb.147.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zipin A, et al. Tumor-microenvironment interactions: the fucose-generating FX enzyme controls adhesive properties of colorectal cancer cells. Cancer Res. 2004;64:6571–6578. doi: 10.1158/0008-5472.CAN-03-4038. [DOI] [PubMed] [Google Scholar]
- 26.Khatib AM, et al. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am. J. Pathol. 2005;167:749–759. doi: 10.1016/S0002-9440(10)62048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crissman JD, et al. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 1988;48:4065–4072. [PubMed] [Google Scholar]
- 28.Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 29.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen DX, et al. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 31.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 32.Uehara H, et al. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J. Natl. Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 33.Hiratsuka S, et al. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 34.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussard KM, et al. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 37.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl. 8):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 41.Hsu YL, et al. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/Snail signaling pathway. Oncogene. 2012 doi: 10.1038/onc.2012.444. [DOI] [PubMed] [Google Scholar]
- 42.Claffey KP, et al. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 43.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. SciUSA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonald IC, et al. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 2002;24:885–893. doi: 10.1002/bies.10156. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein RH, et al. Of mice and (wo)men: mouse models of breast cancer metastasis to bone. J. Bone Miner. Res. 2010;25:431–436. doi: 10.1002/jbmr.68. [DOI] [PubMed] [Google Scholar]
- 46.Kuperwasser C, et al. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- 47.Hendrix MJ, et al. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987;38:137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- 48.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion: lessons from the alpha6beta 4 integrin. Semin. Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 49.Mierke CT. Cancer cells regulate biomechanical properties of human microvascular endothelial cells. J. Biol. Chem. 2011;286:40025–40037. doi: 10.1074/jbc.M111.256172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer RH, Nicolson GL. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc. Natl. Acad. SciUSA. 1979;76:5704–5708. doi: 10.1073/pnas.76.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424:870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 52.Lii J, et al. Real-time microfluidic system for studying mammalian cells in 3D microenvironments. Anal. Chem. 2008;80:3640–3647. doi: 10.1021/ac8000034. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, et al. Microfluidics-based devices: new tools for studying cancer and cancer stem cell migration. Biomicrofluidics. 2011;5:13412. doi: 10.1063/1.3555195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haessler U, et al. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr. Biol. 2012;4:401–409. doi: 10.1039/c1ib00128k. [DOI] [PubMed] [Google Scholar]
- 55.Liu T, et al. A microfluidic device for characterizing the invasion of cancer cells in 3-D matrix. Electrophoresis. 2009;30:4285–4291. doi: 10.1002/elps.200900289. [DOI] [PubMed] [Google Scholar]
- 56.Sung KE, et al. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr. Biol. 2011;3:439–450. doi: 10.1039/c0ib00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zervantonakis IK, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. SciUSA. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song JW, et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS ONE. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin MK, et al. Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip. 2011;11:3880–3887. doi: 10.1039/c1lc20671k. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, et al. A microfluidic-based device for study of transendothelial invasion of tumor aggregates in realtime. Lab Chip. 2012;12:2837–2842. doi: 10.1039/c2lc00030j. [DOI] [PubMed] [Google Scholar]
- 61.Jeon JS, et al. In vitro model of tumor cell extravasation. PLoS ONE. 2013;8:e56910. doi: 10.1371/journal.pone.0056910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen MB, et al. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 2013;5:1262–1271. doi: 10.1039/c3ib40149a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaw KC, et al. Multi-step microfluidic device for studying cancer metastasis. Lab Chip. 2007;7:1041–1047. doi: 10.1039/b707399m. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Chau L, et al. A novel multishear microdevice for studying cell mechanics. Lab Chip. 2009;9:1897–1902. doi: 10.1039/b823180j. [DOI] [PubMed] [Google Scholar]
- 66.Bianchi E, et al. Microfluidics for in vitro biomimetic shear stress-dependent leukocyte adhesion assays. J. Biomech. 2013;46:276–283. doi: 10.1016/j.jbiomech.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Hanzlik J, et al. Biomimetic leukocyte adhesion: a review of microfluidic and computational approaches and applications. J. Bionic Eng. 2008;5:317–327. [Google Scholar]
- 68.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saiki I, et al. Functional role of sialyl Lewis X and fibronectin-derived RGDS peptide analogue on tumor-cell arrest in lungs followed by extravasation. Int. J. Cancer. 1996;65:833–839. doi: 10.1002/(SICI)1097-0215(19960315)65:6<833::AID-IJC21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 70.Hangan D, et al. An epitope on VLA-6 (alpha6beta1) integrin involved in migration but not adhesion is required for extravasation of murine melanoma B16F1 cells in liver. Cancer Res. 1997;57:3812–3817. [PubMed] [Google Scholar]
- 71.Bondareva A, et al. The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS ONE. 2009;4:e5620. doi: 10.1371/journal.pone.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camphausen K, et al. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61:2207–2211. [PubMed] [Google Scholar]
- 73.Hamalukic M, et al. Rac1-regulated endothelial radiation response stimulates extravasation and metastasis that can be blocked by HMG-CoA reductase inhibitors. PLoS ONE. 2011;6:e26413. doi: 10.1371/journal.pone.0026413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanaka T, et al. Dynamic analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a candidate for anti-metastasis therapy. Clin. Exp. Metastasis. 2013;30:369–379. doi: 10.1007/s10585-012-9543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stoletov K, et al. Visualizing extravasation dynamics of metastatic tumor cells. J. Cell Sci. 2010;123:2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schumacher D, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]