Abstract
Background
Somatic complaints and altered interoceptive awareness are common features in the clinical presentation of major depressive disorder (MDD). Recently, neurobiological evidence has accumulated demonstrating that the insula is one of the primary cortical structures underlying interoceptive awareness. Abnormal interoceptive representation within the insula may thus contribute to the pathophysiology and symptomatology of MDD.
Methods
We compared fMRI blood oxygenation level-dependent (BOLD) responses between twenty unmedicated adults with MDD and twenty healthy control participants during a task requiring attention to visceral interoceptive sensations and also assessed the relationship of this BOLD response to depression severity, as rated using the Hamilton Depression Rating Scale (HDRS). Additionally, we examined between-group differences in insula resting-state functional connectivity, and its relationship to HDRS ratings of depression severity.
Results
Relative to the healthy controls, unmedicated MDD subjects exhibited decreased activity bilaterally in the dorsal mid-insula cortex (dmIC) during interoception, as well as within a network of brain regions implicated previously in emotion and visceral control. Activity within the insula during the interoceptive attention task was negatively correlated with both depression severity and somatic symptom severity in depressed subjects. MDD also was associated with greater resting-state functional connectivity between the dmIC and limbic brain regions implicated previously in MDD, including the amygdala, subgenual prefrontal cortex, and orbitofrontal cortex. Moreover, functional connectivity between these regions and the dmIC was positively correlated with depression severity.
Conclusions
MDD and the somatic symptoms of depression are associated with abnormal interoceptive representation within the insula.
Keywords: Interoception, major depressive disorder, insula, fMRI, functional connectivity, depression severity
INTRODUCTION
Some of the most pervasive symptoms of major depressive disorder (MDD) involve somatic disturbances and an altered sense of body awareness (1; 2). In particular, multiple behavioral and psychophysiological studies have reported decreased heartbeat perception in individuals with MDD (3–8). Despite these findings, the role of interoception in mood disorders remains poorly understood. Influential theoretical accounts and accompanying empirical evidence suggest that emotion and decision making are grounded in the perception of interoceptive signals (9–11), which accords well with MDD patients’ clinical reports of emotional dissociation (12). With the advent of theories stating that mood and anxiety disorders are fundamentally disorders of interoception (13) there is clearly a need to understand the neural bases of interoception, and how the function of interoception-related brain regions may be related to depressive symptoms.
A limited number of previous interoception studies in MDD have employed behavioral measures of heartbeat perception accuracy as the primary metric of interoceptive awareness, with varied results (4; 5). Some studies reported decreased accuracy in depression, but these findings were either based on data from sub-clinical populations (6), or from populations with varied medication status (5; 7; 8) confounding the interpretation of their results in regards to an effect of major depression. In contrast, both clinical and sub-clinical levels of anxiety were associated with increased interoceptive accuracy (3; 14), a finding that appears challenging to reconcile with the reports of decreased accuracy in depression, since depressive and anxiety symptoms occur concomitantly in most patients with MDD. Findings based on heartbeat perception accuracy are further complicated by the fact that healthy subjects often do not show reliable heartbeat perception accuracy (3). Consequently, the interpretation of interoceptive accuracy metrics is challenging. Importantly, the recent finding that heartbeat evoked potential is reduced in depressed subjects (7) suggests that, apart from differences in interoceptive accuracy, the neural basis underlying the interoceptive signal itself may be disturbed in depression.
In healthy humans recent neuropsychological and functional neuroimaging studies have established a role for the insula in interoceptive awareness (15–18). The insula receives afferent projections from the vagus nerve via the nucleus of the solitary tract (NST) and the parvocellular portion of the ventroposteromedial nucleus of the thalamus (VPMpc) that convey visceral information important for homeostatic regulation (19–21). Meta-analyses of human neuroimaging studies which parcellated the insula among various functional domains, have associated interoception with the activity of mid-insular cortex (22; 23), a region that appears homeostatically sensitive (24). Likewise, hemodynamic activity increases in mid-insular cortex in human subjects performing tasks involving visceral interoceptive attention (15; 25; 26) or direct visceral stimulation (27; 28). Notably, the mid-insula regions underlying interoception appear at least partially dissociable from dorsal and ventral anterior insula regions involved in cognitive and emotional processing, respectively (15), suggesting the hypothesis that multiple insula regions play distinct roles in the symptoms expressed in MDD, with somatic abnormalities observed in MDD conceivably resulting either from mood and anxiety-related pathophysiology within the insula (29–32), or from abnormal visceral afferent input into this region (13).
The extensive structural and functional connectivity between the insula and other brain structures also provides indirect evidence for its role in MDD. The insula has strong functional connectivity to the medial prefrontal network of regions such as the subgenual prefrontal cortex (sgPFC) and other ventromedial prefrontal cortex (vmPFC) regions that play major roles in visceromotor regulation and exhibit increased metabolism and resting-state functional connectivity in the depressed versus the non-depressed phases of MDD (15; 33–35). The insula also shares substantial anatomical connections with the amygdala and orbitofrontal cortex (OFC)(36–38), regions which display molecular, histological, and functional abnormalities in MDD (33; 39–41).
Given the reported interoceptive deficits in MDD and the insula’s role in interoception, it is surprising that to date, no published study has directly investigated insula function during interoception in depressed subjects. If the neural circuitry involving viscerosensory regions of the insula underlies altered interoception in MDD, then we should observe the following:
During an interoceptive attention task, subjects with MDD will exhibit decreased hemodynamic response within the mid-insula.
The magnitude of the hemodynamic response in the insula during interoceptive attention will correlate with behavioral measures of depression severity and the severity of depressed subjects’ self-reported somatic symptoms.
Given this region’s extensive limbic connectivity (35; 42), in MDD patients the mid-insula will exhibit increased functional connectivity to other limbic or paralimbic structures, particularly to the ventromedial PFC and other regions implicated in depression, such as the amygdala and OFC.
The magnitude of functional connectivity to these regions will also relate to depression severity.
METHODS AND MATERIALS
Participants
Forty right-handed, native English-speaking volunteers between the ages of 21 and 50 years participated in the study: twenty subjects with MDD (13 female; mean[SD] age=36[9] years; range=21–50 years) and twenty healthy control subjects (12 female; mean[SD] age=33[7] years; range=21–45 years). All subjects underwent clinical screening assessments including a Structured Clinical Interview for Diagnosis (SCID) performed by Master’s-level clinicians with experience in psychiatric diagnosis. In addition, for every subject, the SCID results were compared to those of psychiatric interviews performed by a research psychiatrist, with any discrepancies between the two assessments resolved prior to inclusion in the study. All depressed subjects met DSM-IV criteria for MDD in a current major depressive episode. Depression severity was assessed using the 25-item Hamilton Depression Rating Scale (HDRS)(43). Anxiety severity was assessed using the Hamilton Anxiety Rating Scale (HARS)(44).
Volunteers were excluded from participation if they had been exposed to psychotropic medications or other drugs likely to affect cerebral function or blood flow within three weeks (six weeks for fluoxetine), or had manifested a major neurological or medical disorder, substance abuse, a past history of traumatic brain injury, or current pregnancy. Additionally, healthy control subjects were excluded for having met criteria for any Axis I psychiatric disorder on the SCID.
All subjects received compensation for their participation and provided written informed consent as approved by the University of Oklahoma Institutional Review Board.
Experimental Design
A high-resolution anatomical MRI scan was obtained for each subject, followed by a 450-second resting state BOLD-fMRI scan, during which the subject viewed a black fixation mark against a white background. During this time, they were asked to keep their eyes open, focus on the fixation mark, clear their mind, and not think of anything in particular.
After the resting-state scan, each subject completed three additional fMRI scans while undergoing the Focused Awareness task. Within each 9-minute, 10-second scan they alternated between two experimental conditions, the Interoceptive Attention condition and the Exteroceptive Attention condition. During the interoception condition, the word “HEART”, “STOMACH”, or “BLADDER” was presented for 10 seconds in black font against a white background. During this time, subjects were instructed to focus attention on the intensity of the sensations experienced from that organ, such as heartbeat or stomach and bladder distension. Previous research has demonstrated that focal attention on a perceptual modality amplifies activity in brain regions underlying that modality (45–47). The interoceptive attention task used here capitalizes on this attentional spotlight effect by instructing participants to focus on their naturally occurring interoceptive sensations. We have previously demonstrated in healthy adults that this task is effective at mapping interoceptive regions in the insula (15). As an exteroceptive attention control condition, subjects fixated on the word ‘TARGET’ which randomly switched to the lowercase ‘target’ for 500 ms durations during the 10-second exteroceptive task trial. Subjects were instructed to attend to the exteroceptive target and to count the number of times they saw the lowercase word during each 10-second trial. Following one-half of the trials of each condition, the subjects were shown for 5 seconds a number line with values from 1 to 7, and asked to indicate via a magnetic resonance-compatible scroll wheel either the intensity of the sensations (with “1” indicating no sensation, and “7” indicating an extremely strong sensation), or the number of targets perceived in the preceding trial. These ratings were included to help ensure that subjects remained attentive to the task. After receiving verbal instructions about how to perform the tasks, all subjects practiced the interoception and exteroceptive tasks prior to performing the tasks in the scanner, were observed to make stimulus intensity responses, and finally were asked to indicate whether they had any remaining questions about the task demands.
For additional imaging task details, see Supplemental Methods.
Data Acquisition
Functional and structural MR images were collected using a General Electric Discovery MR750 whole-body 3-Tesla MRI scanner, using a scalable 32-channel digital MRI receiver capable of performing massively-parallel fMRI. A brain-dedicated receive-only 32-element coil array (Nova Medical Inc), optimized for parallel imaging, was used for MRI signal reception. A single-shot gradient-recalled echo-planar imaging (EPI) sequence with Sensitivity Encoding (SENSE) depicting blood oxygenation level-dependent (BOLD) contrast was used for functional scans (see Supplemental Methods for detailed scan parameters). Simultaneous physiological pulse oximetry and respiration waveform recordings were collected for each fMRI run. The pulse oximetry readings were used to calculate heart rate during functional scans (see Supplemental Methods).
Data Preprocessing and Subject-level Statistical Analyses
Functional image preprocessing was performed using AFNI (http://afni.nimh.nih.gov/afni), as detailed in the Supplemental Methods. Each subject’s data from the Focused Awareness task was analyzed using a multiple linear regression model.
Group Analyses
A whole-brain voxel-wise analysis was conducted to examine group differences in heartbeat interoceptive attention. The beta values derived from the contrast of heartbeat interoception versus the exteroceptive control condition, which indicate the mean percent signal change during interoceptive attention relative to exteroception, were extracted for each subject. These values were then included in a two-sample random effects t-test. Additionally, the HDRS scores of the depressed subjects were used to conduct a whole-brain voxel-wise correlation analysis examining the relationship between heartbeat interoceptive attention and depression severity. Both analyses were performed using the AFNI program 3dttest++, and subsequently corrected for multiple comparisons at p<.05 (see Supplemental Methods for details).
Region-of-Interest (ROI) Analyses
Using the dorsal mid-insula (dmIC) clusters identified in the voxel-wise analysis above (Figure 1), the average beta coefficients for stomach and bladder interoception versus exteroception were extracted within these ROIs, in order to examine group differences in these modalities. The relationship between insular activation during interoceptive attention and behavioral measures of depression and anxiety was also examined by calculating the correlation between the beta values for heartbeat attention versus exteroception within the dmIC clusters and the depressed subjects’ HDRS and HARS scores, respectively. Following this, post hoc analyses were conducted to further specify this relationship using the HDRS subscales as defined by Cleary and Guy (1977) (48). As we were primarily interested in the relationship of these variables and activity within these regions, these analyses were performed in an external statistical analysis suite.
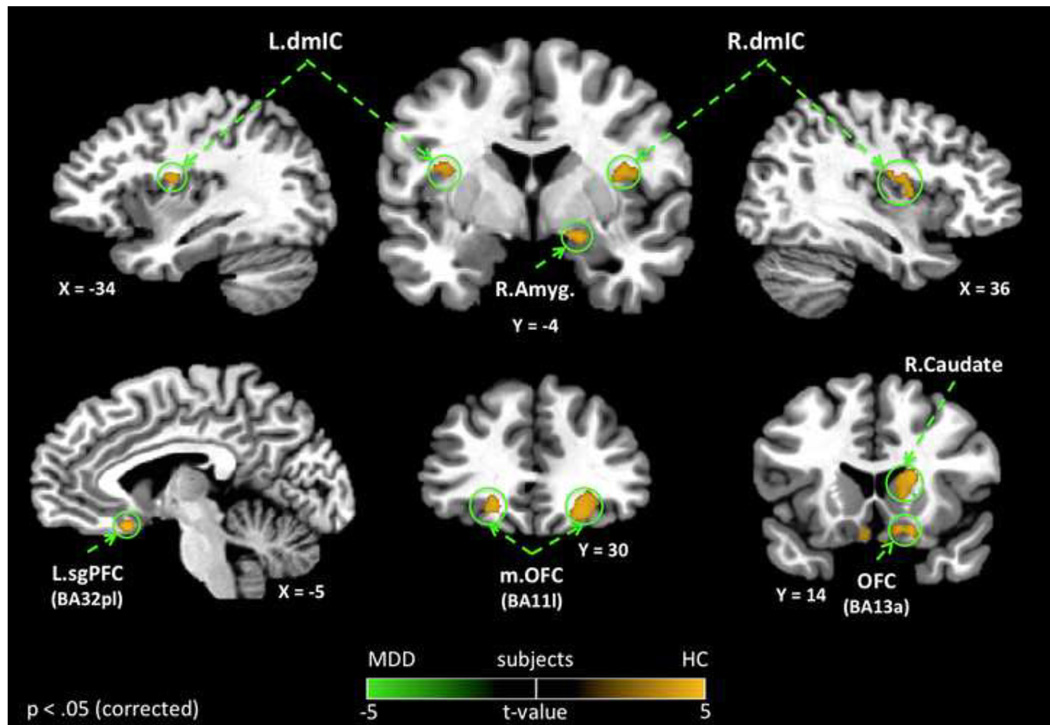
Figure 1. Group differences in heartbeat interoception.
Depressed subjects (MDD) exhibited decreased hemodynamic activity compared to healthy subjects (HC) within multiple brain regions during attention to heartbeat sensations. Group differences in heartbeat interoception were observed in bilateral dmIC, bilateral OFC, as well as right amygdala. Importantly, the group differences in heartbeat interoception within the insula were confined to regions of the dmIC that have been implicated in primary viscerosensory representation within the insula (15). All results shown were corrected for multiple comparisons at pcorrected < .05. dmIC – Dorsal Mid-Insula, Amyg. - Amygdala; OFC - Orbitofrontal Cortex, sgPFC - Subgenual Prefrontal Cortex
Functional connectivity analyses
Because our primary interests were group differences in interoception-related brain activity, and earlier studies demonstrated homeostatic sensitivity and selectivity for interoceptive attention in the dmIC (15; 24), this region was used as the seed for functional connectivity analyses of the resting-state BOLD image data. The seed time-series from both dmIC ROIs (Figure 1) was used to identify brain regions that showed group differences in functional connectivity to the dmIC, as well as regions where dmIC resting-state functional connectivity was associated with behavioral measures of depression or anxiety. These analyses were performed using the AFNI program 3dttest++, and the resulting statistical maps were corrected for multiple comparisons at p<.05 (see Supplemental Materials for details).
RESULTS
The depression severity for the MDD group ranged from mild to severe (11 to 34), with a mean HDRS score of 23.1 (SD=7.5; the demographic and clinical characteristics appear in Table 1 and Supplemental Results.). All of the MDD subjects were currently unmedicated, and none were currently undergoing psychotherapy. Eight of the MDD subjects were drug naive, and among those who previously had taken medications, the mean time free of psychotropic medications was 8.3 years (SD=7.3 years). Nine MDD subjects had secondary, comorbid anxiety disorders (social phobia n=4, PTSD n=3, simple phobia n=1, panic disorder n=1); the performance and behavioral measures of these subjects did not differ from those without secondary comorbid anxiety diagnoses (see Supplemental Materials).
Table 1.
Demographic and clinical characteristics of the study samples
| Demographics | ||||
|---|---|---|---|---|
| HC | MDD | t38 | p | |
| Sample size (n) | 20 | 20 | ||
| Age – yrs.(sd) | 33(7) | 36(9) | −1.3 | 0.20 |
| Gender | 12F | 13F | ||
| Body Mass Index (kg/m2) | 27.4 | 27.8 | −.5 | .79 |
| Resting Heart Rate (bpm) | 66(10) | 65(8) | .5 | .63 |
| Task Heart Rate (bpm)c | 68(8) | 66(8) | .8 | .43 |
| HDRS - mean(sd) | 1.2(1.6) | 23.1(7.5) | −12.0 | <.001 |
| HARS - mean(sd) | 1.2(1.9) | 17.0(4.9) | −13.6 | <.001 |
| Age of Onset - yrs.(sd) | NA | 19(10) | ||
| Illness Durationa - mos.(sd) | NA | 61(75) | ||
| Drug-Naïve (n/20) | NA | 8/20 | ||
| Currently Unmedicated | NA | 20/20 | ||
| Mean Duration Drug-freeb - mos.(sd) | NA | 99(87) | ||
| Comorbid Anxiety Disorder (n) | 0 | 9 | ||
of current MDE
of non-drug-naïve subjects
Heart Rate calculated during interoception tasks did not differ from average heart rate during the Focused Awareness task.
Imaging Results
Group differences in activity during interoceptive attention
Voxel-wise analysis revealed that depressed subjects exhibited decreased hemodynamic response during interoceptive attention to heartbeat sensations, specifically within the bilateral dmIC (Figure 1, Table 2). No other regions of the insula exhibited group differences in the hemodynamic response to heartbeat attention. Outside of the insula, depressed subjects exhibited significantly lower BOLD activity during heartbeat attention in multiple brain regions implicated in emotional, sensory, and reward processing including: the right amygdala, sgPFC (located in the putative prelimbic region corresponding to Brodmann Area 32pl)(38; 49), lateral OFC, posterior OFC (BA13a; located near the caudal part of the olfactory sulcus) and right caudate nucleus (Figure 1, Table 2).
Table 2.
Brain regions exhibiting differences in the hemodynamic response to heartbeat interoception versus exteroceptive attention between healthy and depressed subjects
| Side / Locationa | Peak Coordinatesb | t18 | Volume | ||
|---|---|---|---|---|---|
| X | Y | Z | (mm3) | ||
| R Lateral OFC (BA11l) | +24 | +29 | −8 | 4.01 | 1474 |
| R Caudate | +18 | +17 | +14 | 4.46 | 1104 |
| L Lateral OFC (BA11l) | −18 | +31 | −5 | 3.78 | 568 |
| L Superior Parietal Lobule | −27 | −60 | +48 | 4.39 | 568 |
| R dmIC | +39 | −6 | +16 | 3.62 | 563 |
| R Posterior OFC (BA13a) | +18 | +15 | −12 | 3.87 | 520 |
| L dmIC | −34 | −6 | +16 | 3.30 | 279 |
| R Amygdala | +18 | −3 | −10 | 3.90 | 252 |
| Left sgPFC (BA32pl) | −4 | +17 | −14 | 3.59 | 161 |
In all cases, activity was greater in healthy subjects compared to the MDD group.
All coordinates reported according to Talairach stereotaxic atlas (73). This format uses three numbers (X,Y,Z) to describe the distance from the anterior commissure. The X,Y,Z dimensions refer to right(+)-to-left(−), anterior(+)-to-posterior(−), and dorsal(+)-to-ventral(−) respectively.
Abbreviations: BA – Brodmann Area; dmIC – Dorsal Mid-Insula; OFC - Orbitofrontal Cortex; sgPFC - Subgenual Prefrontal Cortex
A subsequent ROI analysis within the dmIC clusters revealed that depressed subjects also exhibited decreased BOLD activity for stomach and bladder attention bilaterally in the dmIC (Figure S1, Table S2; also see Figure S5 and Table S12 for results from voxelwise analyses of group differences during stomach and bladder interoception).
The relationship between heartbeat interoceptive attention and behavioral symptom severity
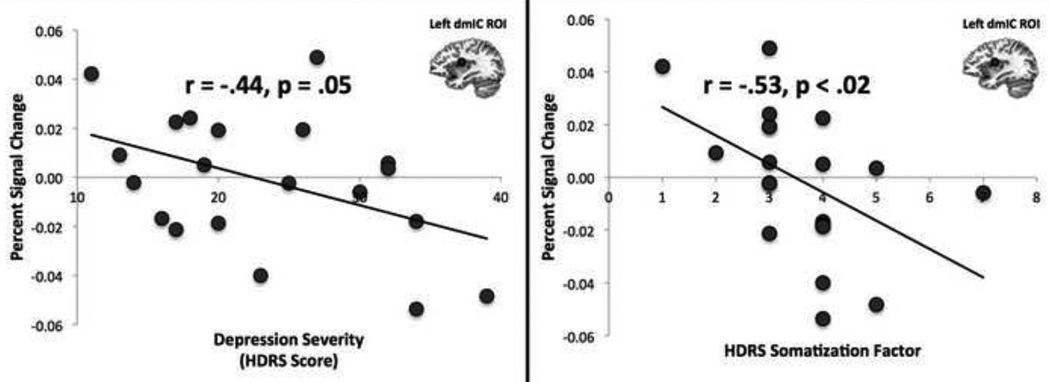
Using ROI analyses within the left dmIC cluster identified in the heartbeat attention contrast (Figure 1), a negative correlation between BOLD activity during heartbeat interoceptive attention and HDRS measures of depression severity was observed within the MDD group (Left Insula: r=−.44; p=.05; Figure 2, Table S3). Importantly, this appears to be largely attributable to the subjects’ somatic-depressive complaints, as only the HDRS somatization subscale (48) exhibited a significant relationship with dmIC activity (r=−.53, p<.02; Figure 2, Table S4). None of the other HDRS subscales significantly related to dmIC activity (p>.5).
Figure 2. Dorsal mid-insula activation during heartbeat interoception is correlated with depression severity and the severity of somatic symptoms.
Within the left dmIC – which was identified in Figure 1– a significant negative correlation was observed between depressed subjects’ hemodynamic response during heartbeat interoceptive attention and scores on the Hamilton Depression Rating Scale (HDRS). A significant negative correlation was also observed between hemodynamic response and the HDRS somatization sub-scale (48) (See Supplemental Methods). Values on the X-axis indicate scores on the HDRS, which was administered prior to the fMRI scan. Values on the Y-axis are beta coefficients representing percent signal change during heartbeat interoception within the left dmIC cluster from Figure 1. Circular ROIs in Left dmIC are for illustrative purposes only.
Additionally, voxelwise analyses outside the dMIC ROI revealed that depressed subjects exhibited a significant negative correlation between BOLD activity during heartbeat attention and HDRS measures of depression severity within left ventral anterior insular cortex and left ventral and dorsal mid-insula (Figure S2, Table S5). Other regions exhibiting a negative correlation between depression severity and heartbeat interoception activity included the bilateral amygdala and left posterior OFC.
Functional Connectivity Results
Group differences in functional connectivity to the dmIC
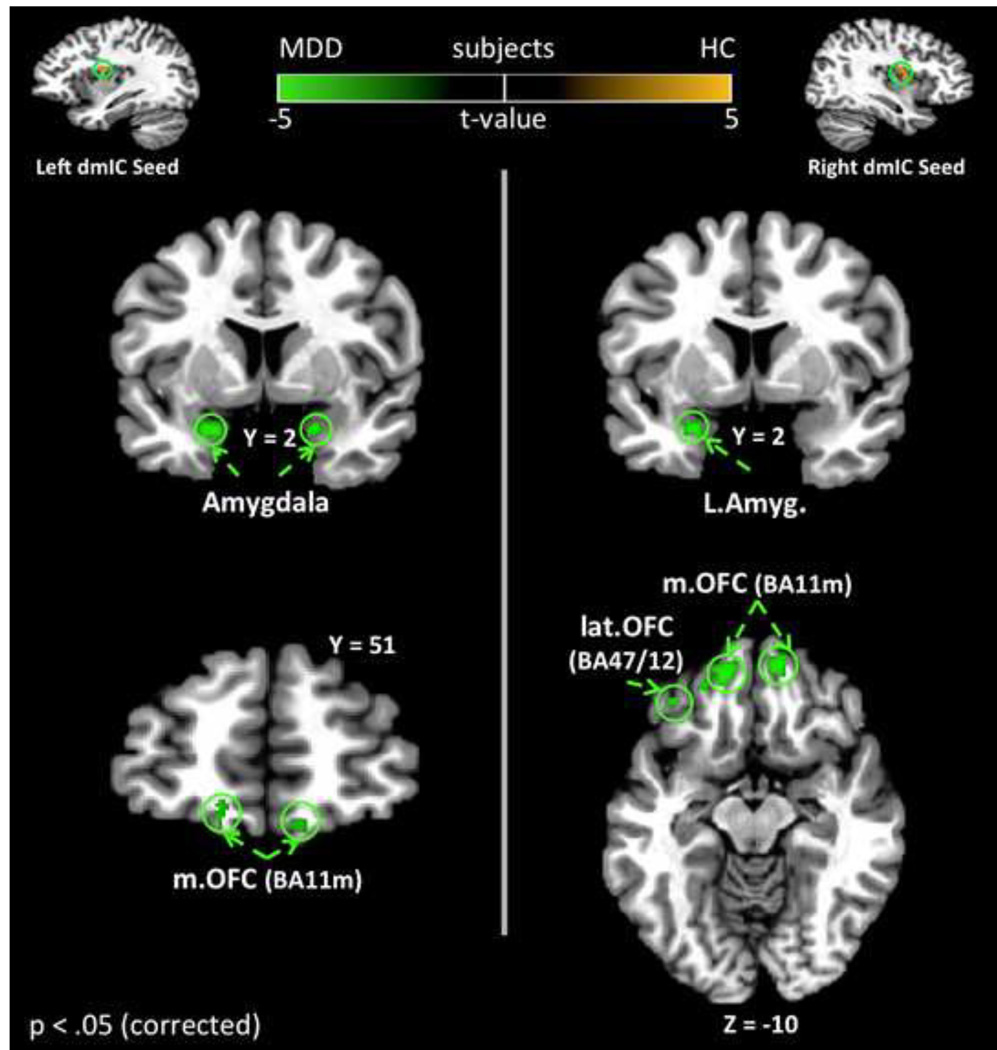
Depressed subjects exhibited significantly greater resting-state functional connectivity between the dmIC and multiple brain regions involved in affective and sensory processing (Figure 3, Table 3). Notably, bilateral dmIC exhibited significantly greater functional connectivity to the amygdala and medial OFC in depressed versus healthy subjects.
Figure 3. Group Differences in BOLD resting-state functional connectivity to the dorsal mid insular cortex.
The left and right dmIC regions – identified in Figure 1 – were used as seeds for a comparison of resting state functional connectivity between healthy and depressed subjects. Many of the circled regions, including the amygdala and orbitofrontal cortex, have previously been implicated in the pathophysiology of MDD. In the present study, depressed participants exhibited significantly stronger resting state functional connectivity between these regions and the dmIC. All results corrected for multiple comparisons at pcorrected < .05. dmIC – Dorsal Mid-Insula, Amyg. - Amygdala; OFC - Orbitofrontal Cortex
Table 3.
Brain regions exhibiting greater dmIC resting-state functional connectivity in the MDD subjects compared to healthy participants.
| Side / Location | Peak Coordinatesa | t38 | Volume | ||
|---|---|---|---|---|---|
| X | Y | Z | (mm3) | ||
| Left dmIC Seed | |||||
| Depressed > Healthy | |||||
| R Middle Temporal Gyrus | +45 | −68 | +7 | −4.66 | 477 |
| L Amygdala | −20 | +3 | −19 | −4.04 | 311 |
| R Middle Occipital Gyrus | +27 | −66 | +23 | −4.11 | 268 |
| L Medial OFC (BA11m) | −13 | +45 | −10 | −3.37 | 177 |
| R Medial OFC (BA11m) | +10 | +52 | −12 | −4.17 | 166 |
| R Amygdala | +22 | +3 | −19 | −3.43 | 129 |
| Healthy > Depressed | |||||
| R Cerebellum | +18 | −75 | −22 | 4.87 | 423 |
| Right dmIC Seed | |||||
| Depressed > Healthy | |||||
| L Medial OFC (BA11m) | −11 | +48 | −8 | −4.55 | 745 |
| R Medial OFC (BA11m) | +10 | +53 | −14 | −4.61 | 562 |
| R Middle Temporal Gyrus | +52 | −68 | +13 | −4.29 | 466 |
| R Middle Occipital Gyrus | +25 | −64 | +20 | −3.97 | 316 |
| L Precentral Gyrus | −29 | −8 | +48 | −4.83 | 263 |
| L Amygdala | −24 | +3 | −19 | −3.99 | 198 |
| L Lateral OFC (BA47/12) | −36 | +36 | −7 | −3.09 | 118 |
All coordinates reported according to Talairach stereotaxic atlas (73). This format uses three numbers (X,Y,Z) to describe the distance from the anterior commissure. The X,Y,Z dimensions refer to right(+)-to-left(−), anterior(+)-to-posterior(−), and dorsal(+)-to-ventral(−) respectively.
Abbreviations defined in legend for table 2
Functional connectivity to the dmIC is associated with depression severity
Within the MDD group depression severity, as measured by the HDRS, was positively associated with functional connectivity between the dmIC and both the left amygdala and the medial OFC (Figure S2, Table S5), regions that also exhibited increased dmIC functional connectivity in MDD subjects versus control subjects (Figure 3, Table 3). Functional connectivity between the dmIC and both posterior OFC and sgPFC, regions exhibiting decreased activity during heartbeat attention, was also positively correlated to depression severity. Additionally, dmIC connectivity with both anterior and posterior insula was greater with increasing depression severity.
COMMENT
Prior findings have demonstrated that MDD is associated with interoceptive deficits assessed behaviorally (4–7), and that the insula contributes to interoception in healthy humans (15–17). These findings warranted the prediction that MDD patients would exhibit abnormal insula hemodynamic activity during interoceptive attention, and that this activity would be related to depression severity. Both hypotheses were confirmed in the present study. Within the dorsal mid-insula, as well as a network of brain regions involved in emotion and visceral control, unmedicated and currently depressed adults exhibited decreased activation during interoceptive attention, relative to healthy controls. This reduced dmIC activity in depression was observed during attention to interoceptive signals broadly (i.e., attention to heartbeat, stomach, and bladder sensations), potentially consistent with evidence that behavioral measures of interoceptive sensitivity are correlated across interoceptive modalities (50). The function of the dorsal mid-insula region identified here has been previously identified as being homeostatically-sensitive (24) and selective for interoception (15). Crucially, this region appears to constitute the human homologue of a location identified in macaque monkeys as the terminus of a major vagal afferent pathway, originating from the entrance of the vagus nerve into the solitary nuclear complex in the medulla, through the VPMpc nucleus of the thalamus, and ending in the dorsal insula/frontal operculum (19–21).
Although relatively more attention has been paid to the role of the anterior insula in mood disorders, there is a growing body of evidence that the dmIC is also critically affected. For example, recent studies have highlighted mood and anxiety-related abnormalities in dmIC grey matter volume (29), GABA-benzodiazepine site binding potential (30), and regional cerebral blood flow (31). Until the present study, however, the functional significance of these findings had not been explored. Our data suggest that structural and functional abnormalities in the dmIC present in depressed subjects may lead to altered information processing of interoceptive signals, as indexed by the diminished hemodynamic response of the insula during interoceptive attention.
Hemodynamic response during heartbeat interoception was significantly negatively correlated with HDRS symptom severity in the dmIC, as was the relationship between hemodynamic response and severity of somatic symptoms. Additionally, a relationship between depression severity and BOLD activity during interoception was observed in the amygdala, as well as in the ventral anterior insula and ventral mid-insula. The ventral anterior region of the insula implicated here is rostrally contiguous with the caudal orbitofrontal cortex and is the insula region most strongly associated with emotion (22; 49). Additionally, ventral anterior insula metabolism may also serve as a predictive measure of response to distinct treatment methods for depression (32).
Recently, Paulus and Stein have theorized that amplification of interoceptive signals within the insula contributes to the pathogenesis of depression (13). By this account, the emotional dysregulation and negative affect associated with MDD (2) result from amplified interoceptive background noise that interferes with an individual’s ability to generate accurate predictions about how external stimuli will affect one’s homeostatic state and general well-being. We envision at least two scenarios by which increased basal noise within the dmIC might contribute to the pathology of MDD. First, the finding in the present study that resting-state functional connectivity between the amygdala and the dmIC is not only increased in MDD, but is predictive of depression severity, suggests that interoceptive deficits and amygdala pathophysiology in MDD may be functionally related. Pathologically increased amygdala activity in MDD subjects (33; 51) might propagate to the insula, thereby leading to maladaptive information processing in this region. The resulting reduced interoceptive signal-to-noise ratio could thus interfere with depressed subjects’ ability to reliably discriminate afferent homeostatic signals, perhaps also causing a form of emotional allodynia (52; 53) (i.e., where psychic pain or visceral discomfort is caused by a non-painful stimulus), which conceivably may account for the idiopathic pain states that commonly manifest in MDD (1). This is supported by recent evidence implicating the dmIC in the interaction between chronic pain and depression (52; 54; 55), as well as the present study’s finding of increased dmIC connectivity in depressed subjects to anterior and posterior insula regions previously demonstrated to be involved in emotional and pain processing (56–58). Alternately, peripheral pathology, perhaps due to heightened pain responses (1) or chronic inflammation (59; 60), which directly affects dmIC function (61), could be propagated to the amygdala, heightening its activity and resulting in the exaggerated emotional responses and negative emotional processing bias observed in MDD (33; 51; 62). The precise origin of this increased basal noise in the dmIC, whether peripheral or limbic, cannot be inferred from the present data, due to the multiple reciprocal neural connections along the entire length of the insula (36; 37). (See Supplemental Discussion for more discussion on interoception in anxiety and depression). Ultimately, due to the difficulties inherent in interpreting group differences in hemodynamic response, our data cannot elucidate the specific pathophysiology underlying the abnormal BOLD activity observed during interoception in MDD. Future studies using other imaging modalities that can provide more direct assays of neural activity (e.g., electrophysiological or glucose metabolic activity) within the dmIC during interoception may be helpful in resolving this question.
Consistent with previous findings in resting-state studies of MDD, depressed subjects exhibited increased functional connectivity between default mode network regions such as the sgPFC (35; 42; 63), as well as brain regions previously implicated in the pathophysiology of depression, most notably the amygdala and OFC (33; 38). MDD is associated with neuropathological and neuroimaging abnormalities within the sgPFC (33; 40; 41) and CBF and glucose metabolism in the sgPFC also correlate with depression severity (33; 64). Based upon cytoarchitecture and connectivity, Öngür, Ferry, and Price (2003) (49) suggest that BA32pl is the human homologue of rodent prelimbic cortex, a region associated with the enhancement of fear responses mediated via the amygdala (49; 65) (this region is adjacent to but distinct from BA25, a region of sgPFC considered to be the human homologue of infralimbic cortex (49), which also has been implicated in depression (66; 67)). Given our observation that both the amygdala and the sgPFC (BA32pl) exhibited functional connectivity to the dmIC that was positively correlated with depression severity, the present study offers neurophysiological findings that appear compatible with preclinical evidence that activity within this region increases emotional expression mediated via the central nucleus of the amygdala (65).
Similarly, volumetric abnormalities as well as increased rCBF and metabolism in the OFC have been reported in neuroimaging and post-mortem neuropathological studies of MDD (33; 39; 68), and lesions of the OFC increase the risk for developing MDD (69). The posterior orbitofrontal cortex (BA13a) constitutes one of the three areas identified by Öngür & Price as forming critical junctions between the medial (visceromotor) and orbital (sensory) prefrontal cortical networks (38; 49; 70). The other two junction areas between these networks, BA45a and BA12o/47s, previously were shown to exhibit increased CBF and metabolism in MDD (38; 71). The involvement of BA13a in decreased interoceptive activity observed in depressed subjects in the present study, as well as the significant correlation between functional connectivity to the dmIC and depression severity, implicates this region in the pathophysiology of MDD as well. The involvement of this area of convergence between networks is noteworthy because the other PFC areas where abnormalities were observed in depressed patients under interoception and under resting connectivity with dmIC implicate both the medial prefrontal (sgPFC) and the orbital (OFC regions 11l and 11m) networks (tables 2,3; figures 2,3)(38; 49; 70).
Conclusion
Major depressive disorder is associated with a reduced hemodynamic response during interoception within the dmIC, a primary viscerosensory region of the insula, as well as in a network of regions involved in emotional and visceral control, many of which have been implicated previously in depression. Additionally, ventral anterior and ventral and dorsal mid-insula hemodynamic activity during interoception correlates inversely with depression severity. This reduction in task-related activity is accompanied by greater functional connectivity between the dmIC and this network of regions under the resting condition, to an extent that is positively correlated with depression severity. Consistent with the findings of this study, the vagal nerve stimulation-induced BOLD response, specifically within right dmIC, is positively correlated with HDRS scores in treatment-resistant depression (72). Combined with its role in vagal afferent signaling and extensive limbic connectivity, these findings suggest that changes in dmIC activity play a mechanistic role in the efficacy of vagal nerve stimulation treatment for depression. Future studies are needed to determine if the abnormal interoceptive activity we observed reflects a state or trait effect, by assessing the hemodynamic correlates of interoception in individuals studied in the remitted condition of MDD, and individuals who are at high familial risk for developing MDD. It will also be important to explore the effect of antidepressant medications on group differences in interoception, and the mechanisms by which pharmacological interventions may exert their influence in this region of the cortex. The findings of the present study demonstrate that abnormal activity and connectivity within primary viscerosensory regions of the insula play an important role in the depressive symptoms experienced by individuals with MDD, and may offer a promising therapeutic target for depression.
Supplementary Material
Acknowledgments
The authors thank Kara Kerr for assistance with data management, Rayus Kuplicki for assistance with resting-state data pre-processing, as well as Joan Collins, Jessica Santiago, Lisa Kinyon, and Michele Drevets for their help with subject assessment and recruitment. This research was supported by NIMH (K01MH096175-01) grant to WKS, a grant from the Oklahoma Center for the Advancement of Science and Technology (OCAST HR10-141) to WKS, a NARSAD Young Investigator Award to WKS, a grant to WKS from the Oklahoma Tobacco Research Center, the Laureate Institute for Brain Research, and The William K. Warren Foundation. JAA also supported by the Wilfred A. Woobank Fellowship from the University of Tulsa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
WCD has consulted for Johnson & Johnson Pharmaceuticals, Inc. and RBM/ Myriad, Inc., and currently is an employee of Johnson & Johnson, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Lépine J-P, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S3–S7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 2.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 3.Willem Van der Does AJ, Antony MM, Ehlers A, Barsky AJ. Heartbeat perception in panic disorder: a reanalysis. Behav Res Ther. 2000;38:47–62. doi: 10.1016/s0005-7967(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 4.Mussgay L, Klinkenberg N, Rüddel H. Heart Beat Perception in Patients with Depressive, Somatoform, and Personality Disorders. J Psychophysiol. 1999;13:27–36. [Google Scholar]
- 5.Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behav Res Ther. 2007;45:1921–1930. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Pollatos O, Traut-Mattausch E, Schandry R. Differential effects of anxiety and depression on interoceptive accuracy. Depress Anxiety. 2009;26:167–173. doi: 10.1002/da.20504. [DOI] [PubMed] [Google Scholar]
- 7.Terhaar J, Viola FC, Bär K-J, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123:1950–1957. doi: 10.1016/j.clinph.2012.02.086. [DOI] [PubMed] [Google Scholar]
- 8.Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in Major Depressive Disorder. J Affect Disord. 2013;151:780–785. doi: 10.1016/j.jad.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond, B, Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 10.Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum brain mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert BM, Pollatos O, Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. Int J Psychophysiol. 2007;65:214–227. doi: 10.1016/j.ijpsycho.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Honkalampi K, Hintikka J, Laukkanen E, Lehtonen J, Viinamäki H. Alexithymia and depression: a prospective study of patients with major depressive disorder. Psychosomatics. 2001;42:229–234. doi: 10.1176/appi.psy.42.3.229. [DOI] [PubMed] [Google Scholar]
- 13.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clin Psychology Rev. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum brain mapp. 2013;34:2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 17.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 19.Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 22.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage. 2012;61:1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, et al. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. 2013 doi: 10.1038/nn.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Farb NAS, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex. 2013;23:114–126. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G-J, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Stephan E, Pardo JV, Faris PL, Hartman BK, Kim SW, Ivanov EH, et al. Functional neuroimaging of gastric distention. J Gastrointest Surg. 2003;7:740–749. doi: 10.1016/s1091-255x(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 29.Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, Frey KA. Reduced gamma-aminobutyric acid(A)-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Arch Gen Psychiatry. 2007;64:793–800. doi: 10.1001/archpsyc.64.7.793. [DOI] [PubMed] [Google Scholar]
- 31.Jabbi M, Kippenhan JS, Kohn P, Marenco S, Mervis CB, Morris CA, et al. The Williams syndrome chromosome 7q11.23 hemideletion confers hypersocial, anxious personality coupled with altered insula structure and function. Proc Natl Acad Sci USA. 2012;109:E860–E866. doi: 10.1073/pnas.1114774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 35.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 2003;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 37.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 38.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacol. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 41.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kühn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23 doi: 10.1136/jnnp.23.1.56. BMJ Group56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 45.Jäncke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- 46.Johansen-Berg H, Christensen V, Woolrich M, Matthews PM. Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport. 2000;11:1237–1241. doi: 10.1097/00001756-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 47.Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cleary P, Guy W. Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res. 1977;1:115–120. [Google Scholar]
- 49.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 50.Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE. 2012;7:e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 52.Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- 53.Strigo IA, Simmons AN, Matthews SC, Craig ADB, Paulus MP. Increased affective bias revealed using experimental graded heat stimuli in young depressed adults: evidence of "emotional allodynia". Psychosom Med. 2008;70:338–344. doi: 10.1097/PSY.0b013e3181656a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 55.Seifert CL, Valet M, Pfaffenrath V, Boecker H, Rüther KV, Tölle TR, Sprenger T. Neurometabolic correlates of depression and disability in episodic cluster headache. J Neurol. 2011;258:123–131. doi: 10.1007/s00415-010-5704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 57.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 58.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24:1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 63.Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng L-L, Hu D. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 65.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 69.MacFall JR, Payne ME, Provenzale JE, Krishnan KR. Medial orbital frontal lesions in late-onset depression. Biol Psychiatry. 2001;49:803–806. doi: 10.1016/s0006-3223(00)01113-6. [DOI] [PubMed] [Google Scholar]
- 70.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 71.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nahas Z, Teneback C, Chae J-H, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacol. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 73.Talairach J, Tournoux JTP. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.