Abstract
Pulsed high-intensity focused ultrasound (pHIFU) has been demonstrated to enhance vascular permeability, disrupt tumor barriers and enhance drug penetration into tumor tissue through acoustic cavitation. Monitoring of cavitation activity during pHIFU treatments and knowing the ultrasound pressure levels sufficient to reliably induce cavitation in a given tissue are therefore very important. Here, three metrics of cavitation activity induced by pHIFU and evaluated by confocal passive cavitation detection were introduced: cavitation probability, cavitation persistence and the level of the broadband acoustic emissions. These metrics were used to characterize cavitation activity in several ex vivo tissue types (bovine tongue and liver and porcine adipose tissue and kidney) and gel phantoms (polyacrylamide and agarose) at varying peak-rarefactional focal pressures (1–12 MPa) during the following pHIFU protocol: frequency 1.1 MHz, pulse duration 1 ms, pulse repetition frequency 1 Hz. To evaluate the relevance of the measurements in ex vivo tissue, cavitation metrics were also investigated and compared in the ex vivo and in vivo murine pancreatic tumors that develop spontaneously in transgenic KPC mice and closely recapitulate human disease in their morphology. The cavitation threshold, defined at 50 % cavitation probability, was found to vary broadly among the investigated tissues (within 2.5–10 MPa), depending mostly on the water-lipid ratio that characterizes the tissue composition. Cavitation persistence and the intensity of broadband emissions depended both on tissue structure and lipid concentration. Both the cavitation threshold and broadband noise emission level were similar between ex vivo and in vivo pancreatic tumor tissue. The largest difference between in vivo and ex vivo settings was found in the pattern of cavitation occurrence throughout pHIFU exposure: it was sporadic in vivo, but ex vivo it decreased rapidly and stopped over the first few pulses. Cavitation activity depended on the interplay between the destruction and circulation of cavitation nuclei, which are not only used up by HIFU treatment but also replenished or carried away by circulation in vivo. These findings are important for treatment planning and optimization in pHIFU-induced drug delivery, in particular for pancreatic tumors.
Keywords: pulsed high-intensity focused ultrasound, pHIFU, cavitation, drug delivery, pancreatic cancer, passive cavitation detection
INTRODUCTION
The use of pulsed high-intensity focused ultrasound (pHIFU) to enhance the penetration of chemotherapeutic agents into solid tumors is a promising new approach in cancer therapy (Frenkel 2008; Pua and Zhong 2009; Mo et al. 2012). Cavitation is commonly considered to be the key mechanism in pHIFU-mediated drug delivery, and the treatment protocols are designed to promote cavitation, but suppress tissue heating by using short HIFU pulses, and delivering ultrasound at low pulse repetition frequencies. Ultrasound contrast agents (UCAs) are often administered to enhance cavitation activity and reduce the cavitation threshold (Ferrara 2007). It has also been demonstrated that pHIFU can be used without the aid of UCAs to induce cavitation in the brain and open the blood brain barrier (Vykhodtseva 1995) or, in combination with tissue plasminogen activator, to improve the rate of thrombolysis in vivo (Stone et al. 2007). However, reports of quantitative cavitation activity measurements during in vivo studies without UCAs are scarce. The major goal of this study was to characterize the cavitation activity induced by 1.1 MHz pHIFU exposures for in vivo murine pancreatic tumors, with a long term perspective of applying this treatment for chemotherapeutic drug delivery to treat pancreatic cancer.
In ultrasound therapy studies, it is often useful to perform pilot experiments in the controlled environment of ex vivo tissues and tissue-mimicking gel phantoms, before pursuing the treatments in vivo (Coussios et al. 2007). However, the quantitative difference in the cavitation nucleation threshold and cavitation activity metrics between in vivo and ex vivo conditions in the same tissue type has been a subject of long-term debate. Commonly, the cavitation threshold is believed to be lower for ex vivo tissues, primarily due to the absence of circulation ex vivo, and the presence of higher dissolved gas concentrations resulting from tissue decomposition, outgassing and potential exposure to air during tissue sample preparation (Church 2002; Holland and Apfel 1990). Cavitation activity and the threshold for cavitation nucleation also depend significantly on tissue composition (Maxwell et al. 2013); therefore, the results obtained for a single tissue type may not be uniformly applicable to other tissues. Thus, the second goal of this work was to compare the different metrics of cavitation activity in ex vivo and in vivo pancreatic tumor tissue, and to compare these metrics among different types of ex vivo tissues.
Multiple techniques are available and have been shown useful for characterizing cavitation activity during HIFU exposures (McLaughlan et al. 2010a), with passive cavitation detection (PCD) of broadband noise, produced by collapsing bubbles during inertial cavitation, being by far the most widely used technique (Madanshetty 1991; Hwang et al. 2006). Several different ways to interpret the signals acquired by PCD, i.e. cavitation activity metrics, are currently in use. Inertial cavitation dose - the root-mean-square (RMS) amplitude, in the frequency domain, of the broadband noise within a frequency window located between the harmonic peaks - was introduced and correlated to endothelial cell damage and hemolysis in vivo in several studies (Hwang et al. 2006; Chen et al. 2003), it was also validated to monitor in vitro drug release from liposomes (Somaglino et al. 2011). Probability of cavitation occurrence is another metric, which indicates the likelihood of observing a cavitation event at a given HIFU peak-rarefactional pressure level and pulse duration and was used to study cavitation thresholds in different ex vivo tissues and gel phantoms (Maxwell et al. 2013; Kyriakou et al. 2011; Khokhlova et al. 2009). A third metric – cavitation persistence - should be introduced to describe how long cavitation lasts when pHIFU is consistently delivered at a single treatment location. This indicator is useful for optimization of the treatment duration. Cavitation noise level relates to the intensity of bubble collapse, cavitation probablity relates to the presence of cavitation nuclei while the cavitation persistence relates to the replenishement of cavitation nuclei. If one considers these three metrics with respect to the design of exposures for cavitation-mediated drug delivery, all of them appear equally important and complementary for the following reasons. Inertial cavitation noise level is an measure of the extent of the cavitation activity, and does not allow differentiation between multiple subtle emissions and infrequent powerful emissions, which can occur throughout the pulsed HIFU exposure. Neither probability, nor persistence of cavitation occurrence are sufficient for proper treatment planning: probability of cavitation nucleation may be high, but the collapses not violent enough to perturb the tissue and facilitate drug transport. In in vivo conditions where multiple pHIFU pulses are delivered, neither probabiliy nor noise level reflects how long cavitation persist because cavitation nuclei could be both replenished or carried away by in vivo circulation and used up by pHIFU treatment. A cavitation occurrence with the same probability and noise level but high persistence could result in a higher cavitation dose than one with low persistence. In this study, all three metrics were employed to obtain a better understanding of cavitation activity patterns in different media - gel phantoms, ex vivo tissues and in vivo murine pancreatic tumors.
METHODS
Experimental setup
A customized pre-clinical focused ultrasound system (VIFU 2000, Alpinion US Inc, Bothell, WA), was used for pHIFU exposures and cavitation monitoring. The system included a 1.1-MHz HIFU transducer (64 mm aperture and radius of curvature) with a circular central opening of 38 mm in diameter, into which a focused ring-shaped PCD transducer and a B-mode imaging probe (C4-12 phased array, working frequency: 4 MHz-12 MHz, center frequency: 7 MHz, Alpinion, Korea) were built (Fig. 1a,b). The signals received by the PCD were amplified (Panametrics PR5072, Waltham, MA, USA) and recorded by a digital oscilloscope (Picoscope 4424, Pico Technology, St. Netos, Cambridgeshire, UK) at the sampling frequency of 50 MHz. The PCD (outer diameter 38 mm, inner diameter 33 mm, radius of curvature 64 mm) was made of 70 µm PVDF film and had a frequency band of 2.3 – 8.8 MHz at the −6 dB level. The geometric foci of the PCD and the HIFU transducers were aligned in the axial direction, so that the overlap of the focal areas was maximized. The dimensions of the focal areas for both transducers were simulated numerically using Field II (Jensen 1992), and confirmed using hydrophone measurements to be 19 mm × 0.5 mm and 16 mm × 1.5 mm at the −6 dB level for the PCD and HIFU transducers, respectively. The samples undergoing HIFU exposures (gel phantoms, ex vivo tissue or tumor-bearing mice in vivo) were positioned at the HIFU focus using a computer-controlled 3D positioning system. The HIFU focus location was preregistered with the imaging system (E-CUBE series, Alpinion, Korea) and was confirmed by a fiber optic probe hydrophone (FOPH 2000; RP Acoustics, Leutenbach, Germany) measurement through acoustic field mapping. Therefore, the HIFU focus was positioned within the sample using B-mode image guidance. All of the exposures were performed in a water tank, which was connected to a degassing system and a heating unit.
FIG. 1.
(a) Schematic illustration of the HIFU treatment system. The 1.1 MHz HIFU transducer, ring-shaped PCD transducer and an ultrasound imaging probe (P4–12) were aligned confocally and coaxially and built into the side of the acrylic water tank. The focal areas of the PCD and HIFU at −6 dB level are shown in gray and black, respectively. (b) Photograph of the HIFU transducer, PCD transducer, and the ultrasound imaging probe arrangement. (c) Focal HIFU waveforms measured in water using fiber-optic probe hydrophone (FOPH) at different electric power levels (15, 150 and 800 Watts). The corresponding spatial peak, pulse averaged (ISPPA) intensities were 100 W/cm2, 2180 W/cm2 and 18850 W/cm2. The arrows indicate the shock front that forms at the focal region at high output powers.
The HIFU exposures used in this study had the same pulsing protocol (1 ms pulse duration, 1 Hz pulse repetition frequency, 60 s exposure duration), and differed only in the focal pressure levels. Millisecond-long pulses are commonly used in drug delivery treatments (Hynynen 2008); however, in most studies the pulse duration is somewhat longer (10–100 ms). In the present investigation, a shorter, 1 ms, pulse duration was chosen to avoid substantial thermal effects, especially at the larger focal pressure levels. Low duty factor (0.001) also prevented heat accumulation. The use of longer pulses is likely to alter cavitation activity, partially through heating of the surrounding tissue, and is an important parameter to address in the future studies.
The focal waveforms produced by the HIFU transducer in water at different power levels were measured by the fiber optic probe hydrophone and are presented in Fig.1c. The in situ peak- rarefactional acoustic pressure at focus ranged from 1.6–12.4 MPa, and the peak-compressional acoustic pressure at focus ranged from 1.9–77 MPa, which corresponded to 15–800 W of pulse average electrical output power. The maximum deviation of the measured pressure level is within 10 % according to the manufacturer’s specification. Note, that at the medium output levels, the focal waveform is significantly nonlinearly distorted, and at the higher output levels (over 500 W or 10 MPa peak-rarefactional pressure) contains a shock front.
In order to estimate the in situ focal pressures from the measurements in water, we used a modified derating procedure developed in previous work (Khokhlova et al. 2011), that accounts for not only tissue attenuation, but also for the higher coefficient of nonlinearity in tissue compared to water. Briefly, in that method the focal HIFU waveforms are first measured in water at each output electric power setting, Wel. The in situ focal pressure waveform at a depth l in tissue, at the power level Wel. is then calculated as follows: 1) Take the focal waveform measured in water at lower source excitation power, W’el, defined as:
| (1) |
2) Multiply the corresponding waveform by the ratio β/βwater.
Here βwater=3.5, the values for attenuation for different tissues and gel phantoms were taken from the literature (Lafon et al. 2005; Duck 1990; Kyriakou et al. 2011; Normand et al. 2000) as follows: for bovine liver α = 0.05 Np/cm, for porcine kidney α = 0.11Np/cm, for bovine tongue α = 0.15 Np/cm, for porcine adipose tissue α = 0.25 Np/cm, for agarose gel α = 0.011 Np/cm, for polyacrylamide gel α = 0.016 Np/cm. The nonlinear parameter was considered as β = 4 for all the investigated tissues, which is equivalent to our previous measurement in bovine cardiac tissue and liver tissue (Khokhlova et al. 2011). The nonlinear parameter in gel phantoms was considered equivalent to that of water (β = 3.5).
Although tissue temperature elevation in tissues was assumed to be small, at the highest output levels used in this study the waveforms contain a shock front, which leads to an efficient heat deposition. Therefore, even a 1-ms pulse could cause a noticeable temperature elevation within the super-focused area where the shocks are present. Tissue temperature elevation ΔT, caused by the absorption of shocks within a short pulse with duration Δt can be estimated using weak shock theory (Hamilton and Blackstock 1998):
| (2) |
where As is the shock amplitude, f0 is the fundamental HIFU frequency, ρ is the tissue density, c is the sound velocity in tissue, and cp is the specific heat capacity of tissue. In the case of the highest output setting used in this study, the peak temperature elevation in tissues resulting from a single HIFU pulse was 17 °C. We followed the cylindrical Gaussian beam approximation introduced by Parker (1983) to account for heat diffusion between HIFU pulses and evaluate the focal temperature elevation throughout the 60-second pulsed exposure. The necessary tissue properties (density, sound speed, heat capacity, thermal diffusivity) were taken from literature (Duck 1990). The resulting mean steady-state temperature increase was 6.5 °C. However, this calculation is only valid for shocked waveforms. At the lower transducer output levels, when the waveform is nonlinearly distorted, but not shocked, the peak temperature is dominated by the absorption of the main harmonic. The highest calculated temperature elevation in that case in adipose tissue (which has the highest absorption coefficient of all the tissues tested) per pulse and averaged over the exposure are 1 °C and 2 °C correspondingly.
PCD signal processing and analysis
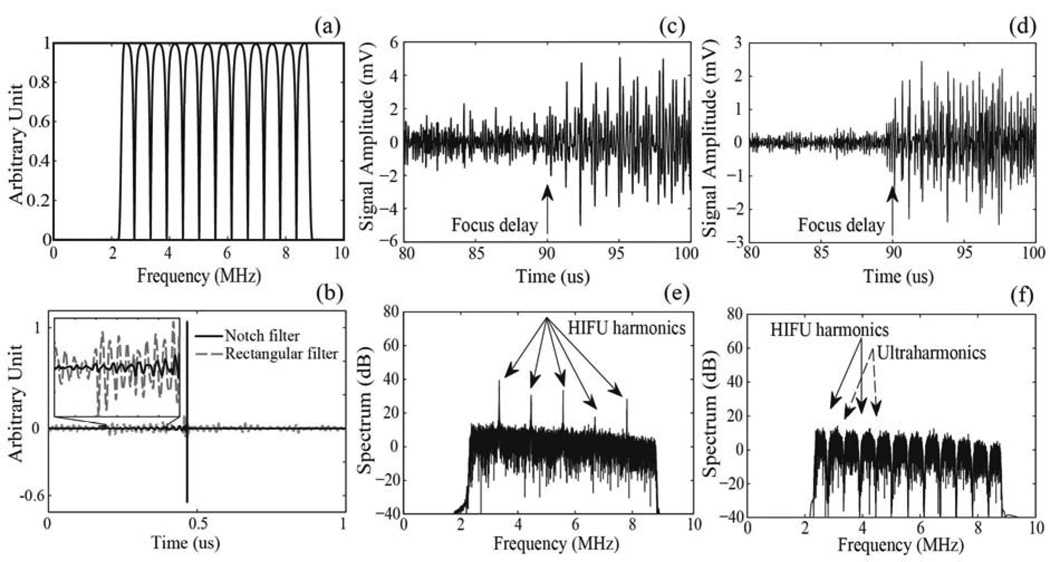
During the HIFU exposures, a series of 1 ms duration broadband signals were acquired by the PCD transducer and processed using a custom-made program using MATLAB (MATLAB 2010b, The MathWorks, Natick, MA, USA) as follows. First, each signal was band- pass filtered in the frequency domain (MATLAB function fir1), with the filter band of 2.3–8.8 MHz, which corresponds to the sensitive band of the PCD, in order to eliminate the main HIFU harmonic backscattered from the sample as well as high-frequency noise. Since the majority of the focal HIFU waveforms used in this study were significantly nonlinearly distorted, the backscattered harmonics of the HIFU wave dominated the band-pass filtered PCD signal (Fig.2c, e). To eliminate the harmonic, ultraharmonic and superharmonic content in the PCD signal, a notch-shaped comb filter (MATLAB function iirnotch, notch bandwidth 100 kHz) was applied (Fig.2a). The bandwidth of 100 kHz is the half-maximum width of the spectral peak at each harmonic, so the comb filter removes the contribution from each harmonic and ultraharmonic. The resulting frequency spectrum was only associated with inertial cavitation of bubbles (Fig.2f). The filtered PCD signal in the time domain is shown in Fig. 2d. The notch filter was used here because it introduces minimal artifacts into the signal in the time domain as compared to a rectangular shaped comb filter that has been widely used for extracting broadband noise (McLaughlan 2010b). Figure 2b demonstrates the difference in the level of artifacts introduced by the notch and the rectangular filters when filtering a short broadband unipolar pulse).
FIG. 2.
(a) A combination of a band-pass filter (2.3 – 8.8MHz) and a notch shaped comb filter with a notch bandwidth of 100 kHz applied to the PCD signals in the frequency domain to suppress the harmonics of HIFU backscattered by tissue and the ultraharmonics generated by stably oscillating bubbles. (b) A short unipolar signal, modeling the acoustic signal produced by an inertial bubble collapse, after filtering by a band-pass and notch filter combination used in the current study (solid line) and a rectangular band-pass and comb filter combination (dashed line). As seen, the filtering procedure used in this study introduces fewer artifacts to the signal in the time domain compared to the rectangular comb filter. An example of a band-pass filtered signal detected by the PCD transducer before (c) and after (d) notch filtering. The corresponding frequency spectra are shown in (e) and (f). The signal was recorded during HIFU exposure of a polyacrylamide gel phantom at peak-rarefactional focal pressure of 5 MPa.
The filtered PCD signal was further analyzed to obtain two metrics: a binary evaluation of whether a cavitation event took place within the 1 ms interval and, if a cavitation event was observed, a measure of the cavitation activity in the form of the broadband noise amplitude. The binary measure relied on the assumption that the signals from collapsing bubbles within a sample were expected to arrive with a certain time delay corresponding to the double distance to the sample surface. The signals arriving before that timepoint were considered as background noise. The cavitation event was considered observed if its arrival time exceeded the aforementioned time delay, and if the signal amplitude exceeded the maximum amplitude of the background noise by – the Rose criterion. This criterion ensures that the signal is distinguishable from a simple statistical variation of the background noise with a 98% confidence level (Rose 1974). The measure of cavitation activity was obtained by integration over the broadband noise components in the frequency domain. This metric is similar to that used by Hwang et al. (2006).
It is known that the surface of tissue samples, and the animal skin in the case of in vivo exposures, often harbors many more small air bubbles and cavitation nuclei than the bulk tissue. Cavitation events at a given peak-rarefactional pressure level are therefore more likely to occur at the sample surface rather than within the sample. In all the ex vivo tissue and gel phantom exposures, HIFU focus was intentionally placed no less than 1 cm deep within the tissue to avoid cavitation at the surface. However, since the axial size of the focal regions of HIFU transducers are rather large, cavitation at the sample surface could still occur at the highest power outputs. Furthermore, in the case of in vivo experiments, the depth of tumor location in some cases was less than 1 cm, which made cavitation at the skin surface more probable. To address that problem, if the cavitation events occurred at the sample surface (according to the arrival time of the signal to the PCD transducer) and did not occur at the focus, the corresponding PCD signal was excluded from the study. Therefore, surface cavitation was monitored and excluded from the analysis.
Gel phantoms and ex vivo tissue samples
Two types of tissue mimicking phantoms were used in this study – polyacrylamide (7% w/v) and agarose gels (1.5% w/v). We chose these two types of gels because they were acoustically similar but very different in the polymertization process, and hence gas content and the distribution of gas cavitation nuclei were also likely to differ. Polyacrylamide (PA) gel is optically transparent, which makes it very convenient for optical observation of the cavitation bubbles, and multiple studies on bubble dynamics were performed in PA phantoms (Khokhlova et al. 2006; Lafon et al. 2005; Khokhlova et al. 2011). The additional advantage of PA gel phantoms is that polymerization takes place at room temperature, therefore, proteins like egg white or bovine serum albumin (BSA) can be added to the gel to serve as indicators for thermal denaturation induced by HIFU. Note, however, that in the present study no protein constituents were added to the gel to avoid the introduction of additional cavitation nuclei. To prepare the samples, the liquid mixture of PA gel constituents was degassed for 1 h in a desiccant chamber by a vacuum pump (VTE8, Thomas, Sheboygan, WI) (Lafon et al. 2005). The degassed mixture was poured into a custom mold (5 cm wide by 5 cm tall by 8 cm deep). The polymerization was then initiated by the addition of a 10 % (w/v) ammonium persulfate solution (APS, Sigma) and N,N,N’,N’-tetra-methylethylene/diamine (TEMED, Sigma).
Agarose gel is another popular tissue-mimicking material, which is non-toxic and can therefore be used in cell seeding experiments (Liu and Zhong 2006; Maxwell et al. 2010). Relevant to the current study, the agarose gel is likely to be different from PA gel in terms of gas content, because of the difference in the polymerization and degassing procedures. Agarose powder (Type VII; Sigma-Aldrich Co., St. Louis, Missouri, USA) was added to distilled water (1.5 % w/v agarose/water), and the solution was placed into an autoclave and allowed to boil for 20 minutes to displace any dissolved gas. The solution was then poured into a custom mold (5 cm wide by 5 cm tall by 8 cm deep) and rapidly cooled down for fast polymerization. The elastic properties of both polyacrylamide gel (7 % w/v) and agarose gel (1.5 % w/v) are very similar, 18.1 KPa (Ahearne et al. 2005) and 17 KPa (Gautreau et al. 2006) respectively. Both of the PA and agarose gel phantoms were imaged using B-mode ultrasound both before and after the exposures, and no residual hyperechoic regions were observed.
The bovine and porcine ex vivo tissue - porcine adipose tissue, bovine tongue, porcine kidney and bovine liver - was obtained from an abattoir on the same day as experiments and stored in phosphate buffered saline and on ice until experiments were performed. The four types of tissues were selected due to their difference in structure and composition. The tissue was cut into samples to fit in a custom-designed tissue holder (8 cm wide by 8 cm tall by 2.7 cm deep) and was brought to room temperature and degassed for 1 h in a desiccant chamber immediately prior to experiments.
In the in vivo experiments, a transgenic mouse model of pancreatic ductal adenocarcinoma was used. The KrasLSL.G12D/+; p53R172H/+; PdxCretg/+ (or KPC) model closely recapitulates the genetic mutations, clinical symptoms and histopathology found in human pancreatic cancer, unlike xenograft or orthotopic models (Olive et al. 2009). The tumors generally have a moderately differentiated ductal morphology with extensive dense stromal matrix that, together with poorly developed vasculature presents a major obstacle to chemotherapeutic drug penetration. In KPC mice, pancreatic cancer cells are surrounded by dense stromal matrix, and are therefore highly fibrotic, which impairs drug delivery. The KPC mouse model is thus the most relevant model in the studies on ultrasound-induced chemotherapeutic drug delivery in pancreatic cancer. All of the animal experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. KPC mice were used for the study when their tumor size reached 1 cm according to diagnostic ultrasound examination. The animal was anesthetized by inhalation of isoflurane, and the abdomen was depilated and washed. The animal was then placed into a specially designed holder and submerged into the heated water tank (at 36 °C) for the HIFU exposure.
Experimental procedures and measurements
In the experiments with gel phantoms and ex vivo tissue, the samples were positioned so that the HIFU focus was 10 mm below the sample surface. Pulsed HIFU exposures were delivered, with peak-rarefactional in situ pressures varying within the 1.5–12.5 MPa range in increments of 0.5–1 MPa. After each exposure, the HIFU focus was moved by 3 mm to another spot within the sample to account for the sample heterogeneity. At each peak-rarefactional pressure level, 5–10 spots were sonicated. In the in vivo experiments, the total number of exposure spots was limited by the tumor size and the number of animals. Therefore, only five different HIFU output levels were used with the in situ peak-rarefactional pressures range from 5–11 MPa, 4–12 treatment spots per level, and the distance between the treatment spots was reduced to 2 mm. The HIFU focus was placed within the tumor, 8–12 mm below the animal skin depending on the depth of the tumor location. In a separate set of experiments, the pancreatic tumor was removed after the animal was sacrificed and used for the cavitation activity measurements similar to those described for ex vivo tissue. Four treatment spots per pressure level were used in that experiment.
A PCD signal was acquired during the delivery of each HIFU pulse using a digital oscilloscope and was stored at the host PC. The PCD signals were then filtered as described in the section “PCD signal processing and analysis”, and batch-processed to extract the following metrics of cavitation activity in a certain tissue type or phantom: probability of cavitation occurrence, cavitation persistence and broadband noise amplitude.
Probability of cavitation occurrence at a certain HIFU output level is defined here as the ratio (expressed in percent) of the number of HIFU focus locations, at which at least one cavitation event was observed throughout the exposure, to the total number of spots treated at this HIFU output level. The expected probability values have a step size of 10 % to 20 % depending on the number of treated spots (5–10 spots were used). As defined previously, the cavitation event was considered observed if its arrival time exceeded the time delay corresponding to the HIFU focus, and if the signal amplitude exceeded the maximum amplitude of the background noise by per the Rose criterion.
Cavitation persistence is defined as the percentage of the HIFU pulses that induced a cavitation event among the pulses delivered at a single treatment spot. The expected cavitation persistence has a step size of 1.67 % as 60 HIFU pulses were delivered at each spot. Cavitation persistence at each HIFU output level was averaged among the different HIFU focus locations.
Broadband noise amplitude is the root-mean-square (RMS) value of the filtered PCD signal, representing the broadband noise amplitude emitted by collapsing bubbles, in the time domain. The RMS calculation was performed starting from the time delay corresponding to the HIFU focus, and over the HIFU pulse duration. Note that this metric was only applied to the signals in which a cavitation event was observed. The broadband noise amplitude at each pulse was averaged over all 60 HIFU pulses delivered to the different HIFU focus locations.
The mean and standard deviation values of cavitation persistence and broadband noise amplitude are calculated in a certain tissue type, at a certain HIFU output level. Note, that all of the peak-rarefactional pressure values reported in the Results section are in situ values, derated from measurements in water.
RESULTS
Cavitation activity in water and gel phantoms
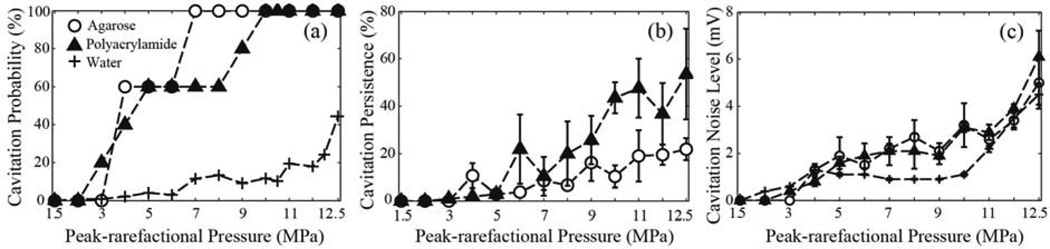
Figure 3 shows the three metrics – cavitation probability, cavitation persistence and cavitation noise level - at different HIFU in situ peak-rarefactional focal pressures, measured in agarose and polyacrylamide gels and degassed, deionized water, with a gas saturation level of 26 %. For all the following results presented in this study, the curves were compared using a two-sample t-test at a 95 % confidence level. The probability of cavitation occurrence is similar in the gel phantoms at lower pressure levels: the 50 % probability is reached at p− = 4–5 MPa. The probability of 100 %, however, is reached at a lower pressure level in the agarose gel (7 MPa) than in the PA gel (10 MPa). This may be due to the higher inhomogeneity of the cross-linking structure, observed optically in PA compared to agarose and, therefore, higher inhomogeneity in nuclei distribution. The probability of cavitation in degassed, deionized water was lower than in both gels, and did not reach 50 % within the peak-rarefactional pressure limits of the study. This result is consistent with findings by others, that pure water has a very high cavitation threshold (Maxwell et al. 2013; Gateau et al. 2011). Since HIFU causes streaming in water, each HIFU pulse was assumed to be incident at a different volume of water. Therefore, the cavitation persistence (that, according to the definition, is calculated for a single HIFU focal spot location) was not calculated for water. In order to measure cavitation persistence in water, one would need to utilize a small, sealed and acoustically transparent chamber to contain the fluid (Maxwell et al. 2013).
FIG. 3.
Dependence of the metrics of cavitation activity on the in situ HIFU peak-rarefactional focal pressure in deionized water and in water-based tissue phantoms – polyacrylamide and agarose gels: (a) the probability of cavitation occurrence, calculated over 5–10 HIFU focus locations in the phantom for each peak-rarefactional pressure level, (b) cavitation persistence, calculated over 60 HIFU pulses delivered to a single HIFU focus location, and averaged over 5–10 focus locations, and (c) broadband cavitation noise level, averaged over all recorded PCD signals, that contained a cavitation event, at each HIFU pressure level. In the case of water, the probability is calculated over 100 HIFU pulses delivered to the water volume of the tank at each pressure level. As seen, the probability of cavitation occurrence is similar in both gel phantoms, and is significantly lower in water. The persistence of cavitaiton in polyacrylamide is, however, considerably larger, which is most probably because it has more pre-existing nuclei compared to agarose gel or water. Cavitation noise level for all three media is within the experimental error, and increases steadily with peak pressure, which indicates that bubble collapses become more violent and/or a larger number of cavitation bubbles form in the focal region.
In both gel phantoms, cavitation persistence (Fig.3b) grows steadily with the increase of peak-rarefactional focal pressure. An important observation, also reported previously by our group (Khokhlova et al. 2011), is that cavitation activity, if any, always occurs within the first several pulses of HIFU, and then stops, and the following HIFU pulses, delivered at the same spot, do not induce cavitation. According to the high-speed camera observations from our previous studies, this is likely due to the fact that the available cavitation nuclei present within the focal volume are used within the first pulses, and the remnants of the cavitating bubbles are pushed away from the focus into the post-focal region by acoustic radiation force (Khokhlova et al. 2011). Another possible mechanism is the fragmentation of the cavitation bubbles into small-sized voids that are more likely to dissolve quickly or less likely to cavitate, before the arrival of the next HIFU pulse (Leighton 1994). The cavitation persistence is significantly higher in PA gel than in the agarose gel (p-value < 0.05) at higher pressure levels (9–12.5 MPa), suggesting that PA overall has a higher concentration of the cavitation nuclei compared to agarose. We speculate that this is due to a lower number of cavitation nuclei within the HIFU focal area, which results from different dissolution and polymerization processes in between agarose gel and PA gel. The degassing procedures used for the two gel phantoms were also different. PA gel was degassed using a dessicant chamber, whereas the agarose gel was degassed by boiling, which seems to be a more efficient procedure for removing the cavitation nuclei. Another potential explanation is higher levels of streaming within the focal area that result in displacing the bubbles towards the postfocal region.
The average amplitude of the broadband noise (Fig. 3c) emitted by collapsing bubbles is very similar for both of the gel phantoms and water at most peak-rarefactional pressure levels. Although cavitation is expected to be more violent in water than in gel phantoms due to the lack of restricting polymer matrix, this effect may be offset due to greater effects of streaming in water compared to gels. Therefore, the intensity of bubble collapses appears similar in all three media, but cavitation is much less likely to occur in water than in gel phantoms.
Cavitation activity in ex vivo tissue
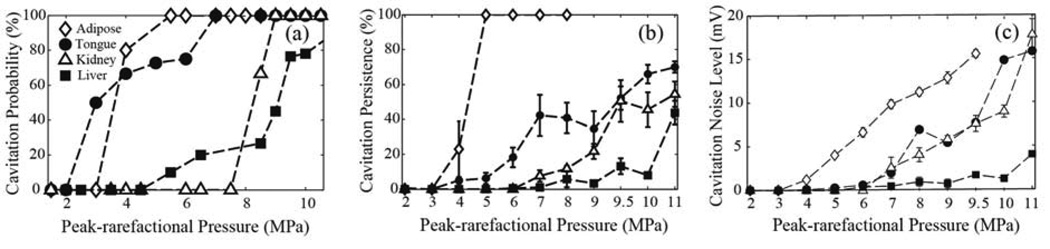
Comparison of the metrics of cavitation activity in the four ex vivo tissue types – porcine adipose tissue, bovine tongue, porcine kidney and bovine liver – are presented in Figure 4. The probability of cavitation occurrence (Fig. 4a) is highest in porcine adipose tissue, which contains 65 % fat (Monziols 2007), and is somewhat lower in bovine tongue, which contains 15–24 % fat (O’Brien 1977). Porcine kidney and bovine liver have a much lower fat content, which are 12 % and 2 % respectively. In adipose tissue, fat is distributed rather uniformly, whereas in the tongue tissue, areas of fat are located in between muscle bundles. On the other hand, water content increased across the four tissue types, 11.4–30.5 % adipose, 60–72 % tongue, 72.3–80.5 % kidney, 72.8–75.6 % liver (Duck 1990). Although the multiple interfaces between water-based and fatty tissue in the tongue may be considered as efficient cavitation nuclei, the data suggest that cavitation is easier to induce in the more homogenous fatty tissue. The probability of cavitation in kidney is bifurcated, changing abruptly from 0 to 100 % between peak-rarefactional pressures of 7 and 9 MPa. This is likely an indication of high homogeneity in the distribution of cavitation nuclei throughout the tissue. In liver tissue, the increase of probability with pressure is slower, and at higher pressure levels (over 7 MPa) the probability of cavitation is lower than in all other tissues. The elastic properties of porcine adipose tissue, bovine tongue, porcine kidney and bovine liver, with bulk modulus of 2.01 GPa, 2.4 GPa (Lakes 1998), 2.54 GPa and 2.70 GPa (Duck 1990) suggests that cavitation is less likely to be induced when tissue is stiffer.
FIG. 4.
Dependence of the metrics of cavitation activity on the in situ HIFU peak-rarefactional focal pressure in ex vivo tissues: bovine tongue and liver and porcine subcutaneous adipose tissue and kidney: (a) the probability of cavitation occurrence, calculated over 5–10 HIFU focus locations in tissue for each peak-rarefactional pressure level (b) cavitation persistence, caluclated over 60 HIFU pulses delivered at a single focus location, and averaged over 5–10 focus locations, and (c) cavitation noise level, averaged over each HIFU pulse delivered at a certain pressure level. As seen, all three metrics are highest in adipose tissue at most HIFU pressure levels. In tongue, which contains 15–24% lipid and 60–74% water, the probability and persistence of cavitation are considerably higher than in water-based tissues (liver and kidney), but the cavitation noise level is comparable to that in kidney. All three metrics are lowest in liver.
The pattern of cavitation occurrence in all tissues throughout the 60-pulse exposure is similar to that in the gel phantoms: at lower pressure levels cavitation activity is only observed following the first few HIFU pulses. The cavitation persistence (Fig. 4b) in the adipose tissue is significantly higher than in the tongue, kidney and liver (p-value < 0.05) respectively at each pressure level. Since adipose tissue is very cohesive, consisting of loose connective tissue holding clusters of adipocytes and is also very viscous, both effects that may increase cavitation persistence - streaming and dissolution of the bubble remnants - are expected to be less pronounced than in water-based tissues.
Similar to other metrics, the cavitation noise level (Fig. 4c) is highest in adipose tissue. Cavitation noise in tongue is lower, and very similar to that in kidney, despite the differences in tissue structure and composition. In liver, cavitation noise level is the lowest.
Cavitation activity in mouse pancreatic tumors in vivo and ex vivo
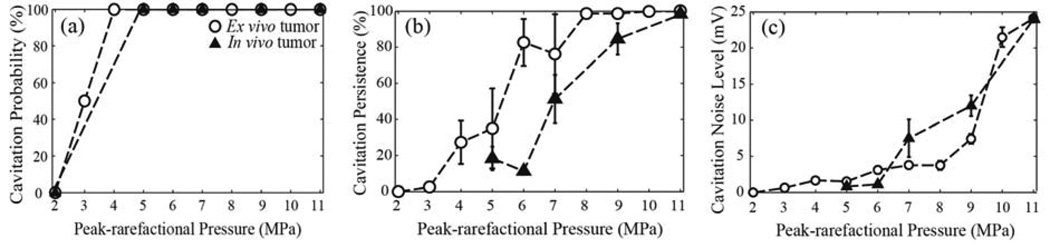
Cavitation activity metrics obtained from the pancreatic tumors of KPC mice in vivo and ex vivo are presented in Figure 5. Surprisingly, the cavitation probability (Fig. 5a) was 100 % for the in vivo tissue, at the entire range of peak-rarefactional pressures used (5–11 MPa), and reached 100 % in the ex vivo tissue at the same peak-rarefactional pressure level as that of fat (p− = 4 MPa). An important difference in the pattern of cavitation occurrence throughout a single 60-pulse exposure is shown in Fig.6. In the ex vivo tissue, the cavitation occurs following the first few pulses of HIFU, similarly to other ex vivo tissues and gel phantoms, whereas in vivo, cavitation events are distributed sporadically throughout the exposure (Fig. 6a). The cavitation noise level (Fig. 6b) declines in both cases over the course of the exposure, but the decline is much more rapid in the ex vivo case. In the in vivo case, the cavitation noise level also fluctuates in a random manner. We speculate that this is likely due to the replenishment of the nuclei within the HIFU focal area by the circulation. The cavitation persistence was significantly lower in the in vivo case compared to ex vivo within the 60s exposure (p-value < 0.05), and only reached 100 % at the highest peak-rarefactional pressure level tested (11 MPa). The cavitation noise level, indicating the intensity of cavitation bubble collapses, was mostly similar (within the standard deviation) in the in vivo and ex vivo setting.
FIG. 5.
Dependence of the metrics of cavitation activity on the in situ HIFU peak-rarefactional focal pressure in the pancreatic tumor of a KPC mouse in vivo (open symbols) and ex vivo (solid symbols).(a) The probability of cavitation occurrence, calculated over 4–12 HIFU focus locations in the in vivo tumor tissue and 4 focus locations in the ex vivo tumor tissue for each peak-rarefactional pressure level. (b) cavitation persistence, caluclated over 60 HIFU pulses delivered at a single focus location, and averaged over the different focus locations, and (c) cavitation noise level, averaged over each HIFU pulse delivered at a certain pressure level. Both in vivo and ex vivo, the pressure sufficient to achieve 100% probability and persistence lies around 11 MPa. Cavitation noise level gradually increases when peak-rarefactional pressure increases, in both ex vivo and in vivo case.
FIG. 6.
A representative illustration of the behavior of cavitation activity metrics throughout pulsed HIFU exposure of an ex vivo (solid symbols) and in vivo (open symbols) pancreatic tumor of KPC mice. The in situ HIFU peak-rarefactional focal pressure was 5 MPa in both cases. (a) Binary metric of cavitation occurrence within each HIFU pulse: “1” indicates that a cavitation event was observed, “0” indicates no cavitation events. In the ex vivo tumor tissue, the cavitation events only occur within the first few HIFU pulses, whereas in the in vivo case they occur sporadically throughout the exposure. (b) Cavitation noise level recorded during each HIFU pulse. In the ex vivo tumor tissue, the cavitation noise level gradually declines over the first few HIFU pulses, which is typical for other ex vivo tissues and gel phantoms. In the in vivo tumor, the cavitation noise level fluctuates randomly and slightly declines throughout the exposure.
DISCUSSION
In this work, we compared the metrics of cavitation activity induced by pulsed 1.1 MHz HIFU exposures in different ex vivo tissues, in vivo pancreatic tumor tissue and different tissue-mimicking phantoms, within the range of peak-rarefactional focal pressures of 1.5 – 12 MPa. Three different metrics of cavitation activity were used: cavitation probability, persistence and broadband noise level, and their dependence on the HIFU focal peak-rarefactional pressure was investigated.
The probability of cavitation as a function of peak-rarefactional pressure generally followed a sigmoid curve for all tested media, with the probability equal to zero at the low peak-rarefactional pressures (below 2 MPa) and approaching or reaching 100 % at the higher pressures. The width of the transition region of the sigmoid curve (where the probability has any value other than 0 or 100 %) may be associated with the degree of tissue heterogeneity at the macroscopic level, i.e. the heterogeneity of the spatial distribution of the cavitation nuclei. Our data supports this assumption: tissues with a lower degree of heterogeneity – adipose tissue, kidney cortex and pancreatic tumors in KPC mice – had a much narrower transition region (2 MPa) compared to more heterogenous tissues – liver and skeletal muscle (over 5 MPa). Liver is composed of mm-scale lobules, separated by connective tissue, and organized around biliary structures; tongue muscle is composed of muscle fiber bundles with adipose tissue inclusions. The adipose tissue (pork belly) is highly homogenous, and so is kidney cortex, which is composed of micron-scale channels. Pancreatic tumors, which form spontaneously in KPC mice, have a very homogenous structure and are very rarely necrotic (Olive et al. 2009). The blood vessels present in the tumor are often nearly collapsed due to high pressure within the tumor.
Following this line of thought, one would expect a high degree of homogeneity and hence a narrow transition region in the gel phantoms. However, a broad transition region was observed in the case of agarose gel (4 MPa), and even broader – for the PA gel. This may be explained by the difference in crosslinking polymer of the gels, mechanical structure and, therefore, the number of gas nuclei distributed within the two types of gel.
The peak-rarefactional pressure, which corresponds to 50 % or 100 % cavitation probability level, is often referred to as the cavitation threshold (Maxwell et al. 2013; Kyriakou et al. 2011). Of all the media, tested in this work, the lowest cavitation threshold, defined at the level of 50 % probability, was observed in porcine adipose tissue, bovine tongue and murine pancreatic tumor tissue (2.5–3 MPa). In both of the gel phantoms a somewhat higher cavitation threshold (3.5–4.5 MPa) was found, and it was the highest in bovine liver and porcine kidney (9 MPa and 8 MPa, respectively). Probability of cavitation in deionized water did not reach 50 % within the peak-rarefactional pressure range that was tested here. Comparison of these results to the threshold values reported by others at the same HIFU frequency is difficult and not always relevant due to the differences in the sonication regime and the associated tissue temperature elevation. For example, the cavitation threshold reported by McLaughlan et al. (2010a) for ex vivo bovine liver tissue sonicated continuously at 1.1 MHz for 4 s was much lower (~2 MPa) than that observed here. In that study, the thermal effects were readily visible, and tissue denaturation was observed. Tissue heating and denaturation may considerably alter the cavitation bubble behavior. In other studies, the cavitation threshold was measured in sheep brain in vivo (Gateau et al. 2011) and in different ex vivo tissues (Maxwell et al. 2013) in the opposite extreme case of very short, microsecond-duration pulses. The reported threshold values were much larger than the peak-rarefactional pressure range used in this study: 30 MPa in kidney, 27 MPa in deionized, degassed water, 15 MPa in adipose tissue (at 1 MHz) and 18 MPa in sheep brain in vivo at 0.66 MHz. This discrepancy may be due to pulse duration, a 50 % threshold for 1-cycle and 1000-cycle pulses are different, or the likely lower sensitivity of the bubble detection method employed in these studies: the authors used HIFU backscattered signals from the bubbles as the indication of cavitation, and the bubble has to grow to a relatively large size to be detectable in such a way (Maxwell et al. 2013). However, the results are similar to our data in that the threshold in adipose tissue or tissue with high fat content is substantially lower than in water based tissue, such as kidney. In a study by Kyriakou et al. (2011) cavitation detection method was similar to that used here, and the cavitation threshold for adipose tissue was reported as 0.82 MPa at 0.5 MHz and over 2.1 MPa for 1.1 MHz, which is in agreement with our results.
Commonly, the cavitation threshold is assumed to be larger in vivo than ex vivo in the same tissue type. Ex vivo tissue is likely to have higher gas content after it is removed from the living organism, mostly due to tissue outgassing, exposure of the tissue to air and tissue decomposition over time. We have found the thresholds and the cavitation noise levels to be remarkably similar for murine pancreatic tumor tissue ex vivo and in vivo, and close to these for porcine adipose tissue and bovine tongue. This may be, in part, due to the fact that KPC tumors are enclosed in a dense stromal matrix that prevents the penetration of air into the tissue after the tumor is removed. The measurements on the ex vivo tumor were performed within two hours after the tumor was excised, so that tissue decomposition processes were unlikely to occur. The other differences – the presence of circulation in vivo and the difference in tissue temperature (20 °C ex vivo and 36 °C in vivo) did not appear to have influenced the cavitation threshold and the cavitation noise level.
The largest difference between the in vivo and the ex vivo pancreatic tumor tissue was in the pattern of cavitation occurrence throughout the pulsed HIFU exposure. In the cases of all of the tested ex vivo tissues (including the pancreatic tumor) and phantoms, cavitation only occurred during the first few HIFU pulses of the exposure, and the cavitation noise level quickly declined with the number of HIFU pulses delivered, as illustrated in Figure 6. In the in vivo case, cavitation events occurred sporadically throughout the exposure, and the cavitation noise level fluctuated in a random manner from one HIFU pulse to the next. We speculate that this is due to the presence of circulation in the in vivo case. The blood flow speed in murine pancreatic tissue in vivo can be estimated as 0.5 mm/s (Nyman et al. 2010), therefore, the blood volume within the 1.5 mm-wide HIFU focal area is at least partially renewed between the subsequent HIFU pulses (one HIFU pulse per second). Note, that this renewal of the cavitation nuclei by the circulation was significant even in the present case of very poorly vascularized pancreatic tumors (Olive et al. 2009). The interplay between the destruction of cavitation bubbles by HIFU pulses and replenishment of cavitation nuclei by blood perfusion is likely to influence the cavitation persistence in in vivo conditions.
The cavitation persistence, as measured over 60 HIFU pulses, was higher ex vivo than in vivo. In the in vivo case, cavitation persistence reached 100 % only at the highest peak-rarefactional pressure level (11 MPa). This, again, may be attributable to the presence of circulation in vivo, which not only supplies the nuclei, but also carries them and the cavitation bubble remnants away from the HIFU focal area. In the ex vivo case, cavitation nuclei are contained in the tumor tissue and are not diminished unless they result in cavitation bubbles and are pushed away from the focal region by radiation force or broken into daughter bubbles and dissolved.
The long-term goal of this work was to identify the optimal pressure levels for pHIFU exposures aimed at drug delivery to pancreatic tumors. Clearly, all three metrics of cavitation introduced here are important for this optimization, and we have observed a difference in the behavior of cavitation probability/persistence and the cavitation noise level with an increase of pressure level. The probability of cavitation reached 100 % at 5 MPa, and the persistence exceeded 50 % at 7 MPa in both ex vivo and in vivo pancreatic tumors. However, the broadband noise level continued to increase steadily as the peak-rarefactional pressure was further increased (6–11 MPa). It is not clear yet whether reaching high levels of probability and persistence alone are enough for efficient drug delivery or whether the intensity of bubble collapses (characterized by broadband emissions) has to reach a certain threshold. This question will be addressed in our future studies on pHIFU-aided drug delivery to pancreatic tumors.
CONCLUSIONS
In the present manuscript, the metrics of cavitation activity (probability, persistence and broadband noise level) induced by pulsed HIFU exposures were studied in tissue-mimicking gel phantoms, different ex vivo tissues and murine pancreatic tumors in vivo, using passive cavitation detection. Cavitation thresholds in these different media were identified at the HIFU frequency of 1.1 MHz and varied within 2.5 – 10 MPa, depending mostly on the relative concentration of water and lipid in tissue. The results demonstrated an important difference in the pattern of cavitation occurrence in vivo and ex vivo: cavitation activity ceased after only a few HIFU pulses in ex vivo tissue, but occurred sporadically throughout the HIFU exposure in vivo. Pulsed HIFU exposures used in this study were designed to introduce minimal tissue heating (short pulses, low duty cycle), but to enhance mechanical tissue damage introduced by cavitating bubbles and/or acoustic radiation force. Such exposures are most commonly needed in HIFU-aided drug and gene delivery, and the conclusions drawn in this study would be relevant in these applications, but likely not in the ablative applications, where tissue temperature is considerably elevated.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Adam Maxwell and Dr. Constantin Coussios for productive discussions and advice. This work was supported by NIH grants 1R01CA154451 and 1K01EB015745.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tong Li, Email: tongli@u.washington.edu, Tel.: 206-616-6787, Fax: 206-543-6785, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
Hong Chen, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
Tatiana Khokhlova, Division of Gastroenterology, Department of Medicine, University of Washington, 1959 NE Pacific Street, Seattle, WA, 98195 and Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
Yak-Nam Wang, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
Wayne Kreider, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
Xuemei He, Department of Ultrasound Imaging, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China, 400016.
Joo Ha Hwang, Division of Gastroenterology, Department of Medicine, University of Washington, 1959 NE Pacific Street, Seattle, WA, 98195 and Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of Washington, 1013 NE 40th Street, Seattle WA 98105.
REFERENCES
- Ahearne M, Yang Y, El Haj AJ, Then KY, Liu KK. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. Journal of the Royal Society Interface. 2005;2:455–463. doi: 10.1098/rsif.2005.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-S, Matula TJ, Brayman AA, Crum LA. A comparison of the fragmentation thresholds and inertial cavitation doses of different ultrasound contrast agents. The Journal of the Acoustical Society of America. 2003;113:643–651. doi: 10.1121/1.1529667. [DOI] [PubMed] [Google Scholar]
- Chow CK. Fatty acids in foods and their health implications. Third Edition. CRC Press; 2007. p. 390. [Google Scholar]
- Church CC. Spontaneous homogeneous nucleation, intertial cavitation and the safety of diagnostic ultrasound. Untrasound in medine & biology. 2002;28:1349–1364. doi: 10.1016/s0301-5629(02)00579-3. [DOI] [PubMed] [Google Scholar]
- Coussios CC, Farny CH, ter Haar G, Roy RA. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU) International journal of hyperthermia. 2007;23:105–120. doi: 10.1080/02656730701194131. [DOI] [PubMed] [Google Scholar]
- Duck F. Physical properties of tissue: a comprehensive reference book. London: Academic; 1990. pp. 9–137. [Google Scholar]
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annual review of biomedical engineering. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced drug delivery reviews. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateau J, Aubry J-F, Chauvet D, Boch A-L, Fink M, Tanter M. In vivo bubble nucleation probability in sheep brain tissue. Physics in medicine and biology. 2011;56:7001–7015. doi: 10.1088/0031-9155/56/22/001. [DOI] [PubMed] [Google Scholar]
- Gautreau Z, Griffin J, Peterson T, Thongpradit P. Characterizing viscoelastic properties of polyacrylamide gels(Bachelor thesis) Worcester Polytechnic Institute; 2006. pp. 55–57. [Google Scholar]
- Hamilton M, Blackstock D. Nonlinear Acoustics. London: Academic Press; 1998. pp. 105–106. [Google Scholar]
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound environment. The Jounal of the Acoustical Society of America. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound in medicine & biology. 2006;32:1611–1619. doi: 10.1016/j.ultrasmedbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Hynynen K. Ultrasound for drug and gene delivery to the brain. Advanced drug delivery reviews. 2008;60:1209–1217. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. Calculation of Pressure Fields from Arbitrarily Shaped, Apodized, and Excited Ultrasound Transducers. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 1992;39:262–267. doi: 10.1109/58.139123. [DOI] [PubMed] [Google Scholar]
- Khokhlova TD, Canney MS, Khokhlova VA, Sapozhnikov OA, Crum LA, Bailey MR. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. The Journal of the Acoustical Society of America. 2011;130:3498–3510. doi: 10.1121/1.3626152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova TD, Canney MS, Lee D, Kenneth IM, Crum LA, Khokhlova VA, Bailey MR. Magnetic resonance imaging of boiling induced by high intensity focused ultrasound. The Journal of the Acoustical Society of America. 2009;125:2420–2431. doi: 10.1121/1.3081393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum LA. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. The Journal of the Acoustical Society of America. 2006;119:1834–1848. doi: 10.1121/1.2161440. [DOI] [PubMed] [Google Scholar]
- Kyriakou Z, Corral-Baques MI, Amat A, Coussios C-C. HIFU-induced cavitation and heating in ex vivo porcine subcutaneous fat. Ultrasound in Medicine & Biology. 2011;37:568–579. doi: 10.1016/j.ultrasmedbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Lafon C, Zderic V, Noble ML, Yuen JC, Kaczkowski PJ, Sapozhnikov OA, Chavrier F, Crum LA, Vaezy S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound in medicine & biology. 2005;31:1383–1389. doi: 10.1016/j.ultrasmedbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lakes RS. Viscoelastic solids. First Edition. CRC Press; 1998. p. 271. [Google Scholar]
- Leighton TG. The acoustic bubble. Academic Press Inc; 1994. p. 335. [Google Scholar]
- Liu Y, Zhong P. P2G-6 high Intensity focused ultrasound induced transgene activation in a cell-embedded tissue mimicking phantom. IEEE Ultrasonics Symposium. 2006:1746–1749. [Google Scholar]
- Madanshetty SI, Apfel RE. Acoustic microcavitation: enhancement and applications. The Journal of the Acoustical Society of America. 1991;90:1508–1514. doi: 10.1121/1.401890. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Cain Ca, Hall TL, Fowlkes JB, Xu Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound in medicine & biology. 2013;39:449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Wang T-Y, Yuan L, Duryea AP, Xu Z, Cain CA. A tissue phantom for visualization and measurement of ultrasound-induced cavitation damage. Ultrasound in medicine & biology. 2010;36:2132–2143. doi: 10.1016/j.ultrasmedbio.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlan J, Rivens I, Leighton T, ter Haar G. A study of bubble activity generated in ex vivo tissue by high intensity focused ultrasound. Ultrasound in medicine & biology. 2010a;36:1327–1344. doi: 10.1016/j.ultrasmedbio.2010.05.011. [DOI] [PubMed] [Google Scholar]
- McLaughlan J, Rivens I, Leighton T, ter Haar G. Commentary on the detection of bubble activity generated in ex-vivo tissue by high intensity focused ultrasound (HIFU) with respect to the generation of therapeutic lesions in tissue for the treatment of cancer. Ultrasound in medicine & biology. 2010b;330 doi: 10.1016/j.ultrasmedbio.2010.05.011. ISVR Technical Report. [DOI] [PubMed] [Google Scholar]
- Mo S, Coussios C, Seymour L, Carlisle R. Ultrasound-enhanced drug delivery for cancer. Expert opinion on drug delivery. 2012;9:1525–1538. doi: 10.1517/17425247.2012.739603. [DOI] [PubMed] [Google Scholar]
- Monziols M, Bonneau M, Davenel A, Kouba M. Comparison of the lipid content and fatty acid composition of intermuscular and subcutaneous adipose tissues in pig carcasses. Meat science. 2007;76:54–60. doi: 10.1016/j.meatsci.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Normand V, Lootens DL, Amici E, Plucknett KP, Aymard P. New insight into agarose gel mechanical properties. Biomacromolecules. 2000;1:730–738. doi: 10.1021/bm005583j. [DOI] [PubMed] [Google Scholar]
- Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. American journal of physiology Endocrinology and metabolism. 2010;298:E807–E814. doi: 10.1152/ajpendo.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien W. The role of collagen in determining ultrasonic propagation properties in tissue. New York: Plenum Publishing Corporation; 1977. pp. 37–50. [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, Mcintyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ. The thermal pulse decay technique for measuring ultrasonic absorption coefficients. The Jounal of the Acoustical Society of America. 1983;74:1356–1361. [Google Scholar]
- Pua EC, Zhong P. Ultrasound-mediated drug delivery. IEEE engineering in medicine and biology magazine. 2009;28:64–75. doi: 10.1109/MEMB.2008.931017. [DOI] [PubMed] [Google Scholar]
- Rose A. Human and electronic vison. New York: Plenum Press; 1974. [Google Scholar]
- Roy RA, Atchley AA, Crum LA, Fowlkes JB, Reidy JJ. A precise technique for the measurement of acoustic cavitation thresholds and some preliminary results. The Jounal of the Acoustical Society of America. 1985;78:1799–1805. doi: 10.1121/1.392767. [DOI] [PubMed] [Google Scholar]
- Somaglino L, Bouchoux G, Mestas J-L, Lafon C. Validation of an acoustic cavitation dose with hydroxyl radical production generated by inertial cavitation in pulsed mode: application to in vitro drug release from liposomes. Ultrasonics sonochemistry. 2011;18:577–588. doi: 10.1016/j.ultsonch.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Stone MJ, Frenkel V, Dromi S, Thomas P, Lewis RP, Li KCP, Horne M, Wood BJ. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thrombosis research. 2007;121:193–202. doi: 10.1016/j.thromres.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vykhodtseva NI. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brian in vivo. Ultrasound in Medine & Biology. 1995;21:969–979. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]