Abstract
Introduction
Less-invasive circulatory support devices have been developed that require anastomosis to a peripheral artery. The Symphony Heart Assist System is a volume displacement pump sewn to the subclavian artery to provide partial circulatory support. The surgical configuration produces non-physiologic blood pressure and bidirectional flow in the subclavian artery. Our objective was to identify effects of altered hemodynamics on arterial structure and function.
Methods
In calves (n=23, 80-100kg), the Symphony pump was sewn end-to-side to the carotid artery. Acutely, carotid blood pressure and flow were recorded to evaluate hemodynamic changes. After medium-term support (1-4 weeks), carotid artery cross sections were studied. Histology and molecular assays evaluated architectural changes. Quantitative real-time PCR evaluated gene expression of matrix metalloproteinase (MMP)-2 and MMP-9 and connective tissue growth factor (CTGF). In vitro carotid arterial-ring studies evaluated physiological responses.
Results
During Symphony support, carotid arterial pressure was 200/15mmHg. Antegrade flow increased significantly (p<0.05) from 1.40±0.32 to 4.29±0.33L/min. Flow during native cardiac diastole reversed completely from 0.25±0.05 to -4.15±0.38L/min in carotid artery proximal to the anastomosis. After medium-term support, the carotid artery was significantly dilated with significantly thinner tunica media and thicker tunica adventitia versus controls. MMP-9 gene expression decreased significantly, CTGF gene expression increased significantly, and collagen, elastin, and total extracellular matrix increased significantly. Endothelial cells were significantly hypertrophied and produced significantly more von Willebrand factor. Endothelial apoptosis increased significantly. Platelet-endothelial interactions decreased significantly. Endothelial-independent contraction decreased significantly, whereas endothelial-dependant relaxation increased modestly.
Conclusions
Assisted circulation with a left ventricular assist device (LVAD) triggered arterial remodeling that allowed a peripheral artery to accommodate the altered hemodynamics of a novel partial-support pump. Further delineation of remodeling pathways may be of significance for the emerging field of partial circulatory support.
Keywords: LVAD, mechanical circulatory support, arterial remodeling, endothelial remodeling
Introduction
Mechanical circulatory support has evolved into a standard therapy for adult patients with advanced heart failure. With nearly 4,300 patients studied, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) reported a recent 2-year survival rate of 74% with current devices1. Yet concerns have been raised that abnormal hemodynamics in these patients may trigger unfavorable changes in myocardial structure2. In particular, prolonged unloading of the failing left ventricle may reduce myocardial mass and produce myocyte atrophy and ventricular stiffening.
Arterial remodeling during left ventricular assist device (LVAD) support has not received the same attention. It is well known that blood pressure and blood flow influence arterial architecture and function3, 4. However, when hemodynamics are maintained outside of the normal physiological range, the effect on arterial tissues is uncertain. Arteries exposed to supra-physiologic pressures, flows, and shear stress from an LVAD may undergo changes in size, architecture, and function.
A number of novel circulatory support devices are being developed that require an end-to-side anastomosis to a peripheral artery5, 6. This anatomical configuration forces a medium-sized artery to accommodate significant increases in blood flow and blood pressure, and with certain devices, bidirectional blood flow. The effects on arterial structure and function are unknown.
We studied a novel partial-support LVAD (Symphony Heart Assist System, Abiomed Inc., Danvers, MA) in a chronic bovine model. The Symphony is a 30 cc pump designed to deliver prolonged ambulatory partial circulatory support for months to years in patients with advanced heart failure. The pump is implanted posterior to the pectoralis major muscle, which precludes the need for a sternotomy or thoracotomy (Figure 1 B). A modified Gortex graft is sewn to the subclavian artery. The patient's continuous electrocardiogram (ECG) triggers real-time filling and emptying of the Symphony pump. The pump fills during native cardiac systole to reduce left ventricular afterload and ejects during native cardiac diastole to augment diastolic blood pressure. This results in improved coronary and systemic blood flow. By these mechanisms, the myocardial oxygen supply/demand relationship improves5, and end-organ perfusion increases. A clinical trial with the Symphony pump is currently underway in Canada and France7.
Figure 1.

A: The anatomical configuration of the Symphony Heart Assist System in cows is shown. B, C: The anatomical configuration of the Symphony Heart Assist System in humans is shown. In humans, the pump is connected to the subclavian artery. The pump fills during native cardiac systole to reduce afterload and ejects during native cardiac diastole to augment diastolic blood pressure and improve the myocardial oxygen supply/demand relationship. C, D: As a result, in the segment of artery between the anastomosis and the aorta, blood flows antegrade during pump filling and retrograde during pump ejection. D: At baseline, a normal profile of carotid arterial blood pressures and flow were present (left panels). During 1:1 support, in which each ventricular systole triggered pump filling and each ventricular diastole triggered pump ejection, non-physiologic pressures and flows were observed (middle panels). The aortic pressure tracings demonstrated effective diastolic augmentation during 1:1 support (top right panel) and 1:2 support (bottom right panel). E, F: Blood flows were quantified at baseline and during 1:1 support proximal and distal to the anastomosis. In the segment of vessel distal to the anastomosis, augmented flow was observed during native cardiac diastole when the pump ejected. In the segment of vessel proximal to the anastomosis, augmented flow was observed during native cardiac systole when the pump was filling. Complete flow reversal was observed in artery proximal to the anastomosis during pump ejection. Flow reversal did not occur distal to the anastomosis. Mean flow was unchanged in either segment of vessel.
The anatomical configuration of the Symphony produces a unique hemodynamic profile near the anastomosis. The segment of the subclavian artery between the outflow graft and the aorta experiences a non-physiologic pattern of alternating antegrade-retrograde blood flow during pump filling and emptying, respectively (Figure 1). The effects of altered pressure and bidirectional flow on arterial structure and function have not previously been studied. Consequently, we hypothesize that a non-physiological profile of blood flow triggers endothelial and arterial remodeling in vivo. We investigated structural and functional changes that occurred after weeks of support with the Symphony pump.
Methods
This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisville, Louisville, KY.
Study Overview
Pre-clinical testing of implantable devices requires a large animal model that closely mimics human cardiovascular anatomy and physiology8. Male calves (n=23, 80 to 100 kg) were used to evaluate the Symphony pump. Calves are the established industry standard to test safety, performance, reliability, and efficacy of LVADs9.
The study was performed according to Good Laboratory Practices (GLP) guidelines to determine preclinical safety of the pump. Animals were supported acutely (n=4), or for 1 week (n=10), 2 weeks (n=7), or 4 weeks (n=2) of uninterrupted support with the Symphony pump. Evaluation of the carotid artery near the anastomosis and contralateral carotid artery allowed each animal to be used as its own control.
The Symphony pump was implanted subcutaneously in the neck. An anastomosis was performed between the pump graft and the carotid artery as described below. This approach was chosen due to the similar size and structure of the bovine carotid artery and human subclavian artery (approximately 8 to 10 mm diameter) as well as the similar distance from the anastomosis to the aortic valve in humans and in calfs (approximately 8 to 10 cm distance)10.
After completing the planned duration of support, animals were euthanized. Carotid artery proximal to the anastomosis and contralateral carotid artery were harvested. Initially, histological and molecular analyses were performed on tissues from animals that underwent 1 week of support (n=8). Interesting histological changes in this group of animals led to more analyses in additional animals. We evaluated whether observed histological changes may have resulted from changes in matrix metalloproteinase (MMP) and connective tissue growth factor (CTGF). Quantitative real-time polymerase chain reaction (PCR) for MMP and CTGF gene expression was performed on carotid arteries from additional animals that underwent 1 week and 2 weeks of support (n=9). Later, to determine whether architectural changes were associated with abnormal vessel reactivity, isolated arterial ring studies were performed with carotid arteries from animals that underwent 2 weeks and 4 weeks of support (n=5). In these animals, live carotid artery sections were transferred to physiologic saline solution (PSS) for in vitro isolated ring preparations to determine endothelial-dependant and endothelial-independent vasoreactivity to pharmacological challenge as described below. In addition to histological and molecular analyses, carotid artery pressures and flows were measured acutely (n=4) to document the hemodynamic changes associated with partial support via an end-to-side anastomosis to a peripheral artery.
Surgical Preparation, Hemodynamic Measurements
One-day prior to surgery and continuing for the duration of the study, each animal received a daily dose of 75 mg oral clopidogrel. Six hours after surgery, animals were placed on heparin, which was titrated to maintain an activated clotting time >200 seconds. Coumadin was initiated on postoperative day three and titrated to maintain an INR of 2.5 to 3.5. Heparin was discontinued after the International Normalized Ratio (INR) was therapeutic.
Animals were anesthetized with 3 to 5% isoflurane and prepared for sterile surgery. Permanent fluid-filled catheters were placed in the right jugular vein for intravenous access and in the proximal right carotid artery for arterial blood pressure monitoring. A left 5th intercostal space mini-thoracotomy was performed to place screw-in epicardial electrocardiographic leads (Medtronic Inc., Minneapolis, MN).
The animal was repositioned, and a left neck incision was made. The Symphony pump (30 ml stroke volume) was implanted in a subcutaneous pocket (7cm × 7cm × 5cm) in the anterior neck. The percutaneous driveline was tunneled to the nape of the neck, externalized, and attached to a pneumatic driver (iPulse, Abiomed Inc., Danvers, MA). A modified 8 mm Gortex vascular graft was sewn end-to-side to the carotid artery and connected to the pump. Care was taken to completely de-air the pump. Timing of pump filling and emptying was triggered by the ECG with R-wave recognition software. Significant aortic diastolic augmentation and left ventricular afterload reduction were achieved (Figure 1 D, right panels).
In a subset of animals, carotid artery pressures and flows were measured acutely. Transit-time ultrasonic flow probes (Transonic, Ithaca, NY) were placed around the carotid artery proximal and distal to the anastomosis. High-fidelity single-tip micromanometer catheters (Millar Instruments Inc., Houston, TX) were placed directly in the carotid artery proximal and distal to the anastomosis. The Symphony was operated in an uninterrupted 1:1 support mode in which each ventricular systole resulted in pump filling and each ventricular diastole triggered pump ejection. Diastolic augmentation and afterload reduction were achieved. Hemodynamics were recorded for 30 seconds at baseline and during Symphony support.
After recovery, animals underwent uninterrupted support for 1 to 4 weeks. Upon completion of the planned duration of support, euthanasia was induced with an intravenous bolus of Beuthanasia-D Special (1 ml/5 kg).
Hemodynamic Data Reduction and Analysis
Hemodynamic transducers were pre- and post-calibrated against known physical standards to ensure measurement accuracy. Data were collected at 400 Hz, signal conditioned, and analog-to-digital converted for digital analysis using our GLP compliant data acquisition system11.
To determine hemodynamic values at baseline and during Symphony support, carotid artery flow and pressure waveforms were recorded to derive mean, peak antegrade, and peak retrograde carotid artery flow as well as systolic and diastolic arterial pressures proximal and distal to the anastomosis. Hemodynamic values were calculated on a beat-to-beat basis for each 30 second data epoch with the Hemodynamic Evaluation and Assessment Research Tool (HEART) program12 developed in Matlab (Mathworks Inc., Natick, MA). All analyzed beats in each data set (approximately 30 to 40 beats/30 second data set) were averaged to obtain a single representative mean value.
Isolated Carotid Arterial-Ring Preparation
After euthanasia, fresh carotid artery cross sections proximal to the anastomosis and from the control carotid artery were harvested, weighed, and immersed in 4°C PSS (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 12.5 mM NaHCO3, 11.1 mM glucose, pH 7.4). Carotid-arterial rings (∼2 mm thickness) were mounted in a tissue myobath containing PSS, maintained at 37°C, and bubbled with 95% O2 and 5% CO2. The carotid-arterial rings were mounted between two wires, stretched, and brought to a resting tension of 4 g. One of the mounted wires was connected to a force transducer. Carotid-arterial rings were allowed to equilibrate for approximately 1 hour prior to experimentation. Maximum contraction was induced with 100 mM K+ PSS. Endothelial-independent contraction was stimulated with phenylephrine (10-6 to 10-3 M). At the maximum contraction with phenylephrine, endothelial-independent relaxation was stimulated with sodium nitroprusside (10-6 to 10-3 M). Phenylephrine was again used to induce maximum contraction, and endothelium-dependant relaxation was stimulated with acetylcholine (10-6 to 10-3 M). Tissues were washed three times for 15 minutes with PSS between each pharmacological challenge and set of measurements. To minimize error due to variation of tissue weight, tension in grams was normalized to tissue weight in grams. Relaxation was calculated as a percentage of maximum contraction with phenylephrine.
Histology
Formalin-fixed carotid artery sections were embedded in paraffin, sectioned at 4 μm, deparaffinized, rehydrated, stained, and analyzed as previously described13, 14. Tunica media fibrosis was quantified with Masson's trichrome staining as the ratio of area occupied by collagen stain to the area of tunica media sampled. Tunica media elastin content was quantified with van Giesen's staining as the ratio of area occupied by elastin stain to the area of tunica media sampled. Total extracellular matrix was determined with fluorescein isothiocynate (FITC)-conjugated wheat-germ agglutinin staining (Molecular Probes, Invitrogen, Carlsbad, CA) as the ratio of area occupied by FITC stain to the area of tunica media sampled. Tunica media cell nuclear size was determined with 4′,6-diamidino-2-phenylindole (DAPI) co-nuclear staining as the ratio of area occupied by DAPI stain to the area of tunica media sampled divided by the number of counted nuclei in that field. Endothelial size was determined with FITC-conjugated isolectin-B4 staining by measuring the cross-sectional thickness of stained endothelium.
To determine the integrity of the endothelium, paraffin immunohistochemistry for cluster of differentiation (CD)41/61 was performed to establish whether platelets were adherent to intima denuded of endothelial cells. Standard antigen retrieval was performed. Sections were permeabilized (0.05% Saponin), blocked (2% bovine serum albumin, 2% Goat Serum, in phosphate-buffered saline), incubated with monoclonal mouse anti-bovine CD41/61 primary antibody (Abcam, 1/500) for 24 hours at 4°C and FITC-conjugated goat anti-mouse IgG (Invitrogen, 1/200) for 1 hr at room temperature. Similarly, paraffin immunohistochemistry for von Willebrand factor (vWF) was performed. After permealbilization and blocking, sections were incubated with rabbit anti-human vWF primary antibody (Dako, 1/500 dilution) overnight at 4°C and FITC-conjugated mouse anti-human IgG (Invitrogen, 1/200) for 1 hr at room temperature. Endothelial and tunica media cell apoptosis was determined with the DeadEnd fluorometric terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) System (Promega, Madison, WI), which catalytically incorporates fluorescein-12-dUTP at DNA strand breaks in cells actively undergoing programmed cell death.
Stains were viewed and photographed with light and epifluorescence microscopy (Nikon TE2000, Nikon Corp., Tokyo, Japan) and analyzed with Metamorph Imaging Software (Molecular Devices Inc., Sunnyvale, CA). For all histological stains, three random fields were analyzed per tissue section. Values were averaged to obtain a single representative mean value.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from snap-frozen carotid artery with TRIzol reagent (Invitrogen, Calsbad, CA), and complementary DNA (cDNA) was synthesized from 1 μg RNA with the iScript cDNA Synthesis kit (BioRad, Hercules, CA). Relative levels of messenger RNA (mRNA) for MMP-2, MMP-9, and CTGF were quantified by real-time PCR with the use of SYBR Green (Applied Biosystems, Carlsbad, CA). Data were normalized to 18s ribosomal RNA subunit expression by the ΔΔCT comparative method as previously described13. Primer pairs were:
MMP-2, 5′-CCTGGGCCCCGTCACT-3′, 5′-GAGATGCCGTCGAAGACGAT-3′
MMP-9, 5′-TTAGGAACCGCTTGCATTTCTT-3′, 5′-CCCCCTCCCTCAGAAAGTCT-3′
CTGF, 5′-TCCCACGGAGGGTCAAACT-3′, 5′-CATCACGGGACACCCATTC-3′
18s, 5′-CGAACGTCTGCCCTATCAACTT-3′, 5′-ACCCGTGGTCACCATGGTA-3′
Statistics
GraphPad, version 5.00 (Prism, GraphPad Software, Inc.) was used to perform statistical analyses and plot data. Paired student t-tests were used to compare hemodynamic measurements, histology, gene expression, immunohistochemistry, molecular measurements, and isolated ring measurements between the carotid artery adjacent to the location of the Symphony graft anastomosis and the contralateral (control) carotid artery. A p<0.05 (95% confidence) was considered statistically significant. All data were presented as mean±standard error.
Results
Hemodynamics
The Symphony pump altered carotid arterial pressure and blood flow (Figure 1). Carotid arterial blood pressure changed from approximately 90/50 mmHg at baseline to 200/15 mmHg during full support (Figure 1 D). Blood pressure values were similar proximal and distal to the anastomosis. During ejection of the pump during native cardiac diastole, approximately 33% of the blood volume flowed cephalad through the distal carotid artery. In contrast, 66% of the ejected blood volume flowed retrograde through the proximal carotid artery into the ascending aorta.
Proximal to the anastomosis, peak antegrade blood flow increased from a baseline flow of 1.40±0.32 L/min to 4.29±0.33 L/min during pump filling (p<0.001, Figure 1 D, F). The peak flow during pump ejection reversed completely from antegrade flow of 0.25±0.05 L/min to a retrograde flow of 4.15±0.38 L/min (P<0.001, Figure 1 D, F). Flow reversal in the proximal carotid artery during diastole resulted in antegrade flow augmentation into the aorta.
Distal to the anastomosis, the peak antegrade blood flow increased from a baseline value of 1.10±0.06 L/min to 2.17±0.21 L/min during pump ejection (p=0.01, Figure 1 D, E). Flow reversal did not occur in the distal carotid artery.
Gross Findings
Carotid artery near the anastomosis appeared dilated versus the control carotid artery. Faint ecchymosis was frequently observed within the arterial wall near the anastomosis (Figure 2 A). The control carotid artery appeared grossly normal.
Figure 2.

A: Gross carotid artery from the site of the anastomosis is shown. Faint ecchymosis was observed within the arterial wall. B-D: The diameter of the vessel lumen, tunica media, and tunica adventitia changed significantly versus the control carotid artery. E-H: Masson's trichrome staining of carotid artery cross sections demonstrated intimal hyperplasia and bands of extracellular matrix deposition within layers of smooth muscle in the tunica media near the anastomosis versus the controls. Carotid artery rings 2X, black bar=2,000 μm; insets 20X, black bar=200 μm. I: The total collagen content increased significantly in the tunica media near the anastomosis versus the controls. J-M: van Giesen's staining of carotid artery cross sections demonstrated increased elastin density (black stain) and elastin cross-linking in the tunica media and tunica adventitia near the anastomosis versus the controls. Vessel wall 10X, black bar =400 μm; insets 40X, black bar =100μm. N: The total elastin content increased significantly in the tunica media near the anastomosis versus the controls.
Histomorphometry
Carotid artery near the anastomosis was dilated by approximately 25% versus control carotid artery (p<0.01, Figure 2 B, E vs. G), which translated into an approximate 145% increase in luminal area. The tunica media was significantly thinner (p<0.01, Figure 2 C) and tunica adventitia was significantly thicker (p<0.01, Figure 2 D) than the controls. Intimal hyperplasia was clearly evident near the anastomosis (Figure 2 G, H).
Histology
Masson's trichrome staining indicated that the tunica media near the anastomosis contained significantly more collagen than control carotid arteries (p<0.05, Figure 2 E-I). Similarly, van Giesen's staining exhibited significantly more elastin near the anastomosis than in control carotid arteries (p<0.05, Figure 2 J-N). FITC-conjugated wheat-germ agglutinin staining demonstrated significantly elevated total extracellular matrix in the tunica media near the anastomosis versus controls (p<0.01, Figure 3 A-I). DAPI co-nuclear staining demonstrated that tunica media cell number and nuclear size were not statistically different.
Figure 3.
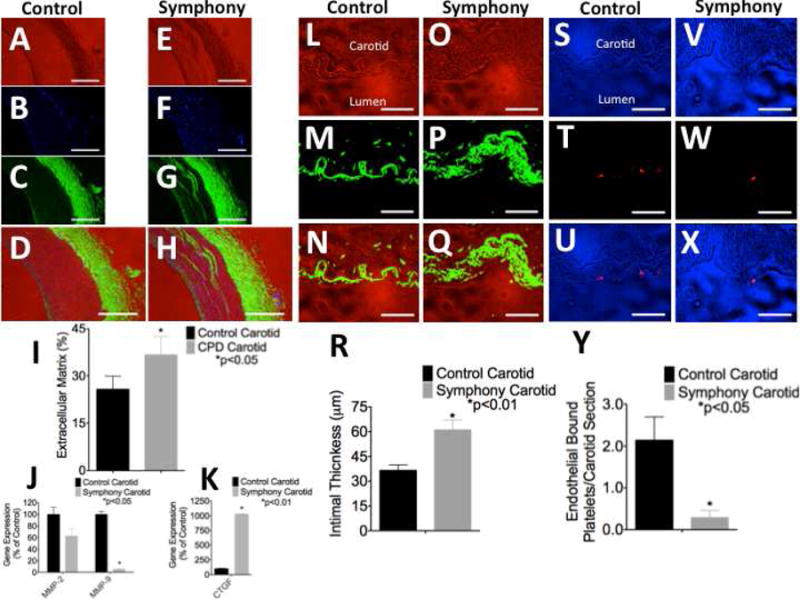
Wheat-germ agglutinin staining of carotid arterial cross sections demonstrated bands of infiltrating extracellular matrix within layers of the tunica media near the anastomosis versus the controls. A, E: Bright field images. B, F: DAPI co-nuclear stain. C, G: FITC-conjugated wheat-germ agglutinin stain. D, H: Triple overly. 4X, white bar=1000μm. I: The total extracellular matrix content increased significantly in the tunica media near the anastomosis versus the controls. J, K: Quantitative real-time PCR demonstrated (J) reduced matrix metalloproteinase (MMP) gene expression and (K) greatly enhanced connective tissue growth factor (CTGF) gene expression in carotid artery near the anastomosis versus controls. L-R: Isolectin-B4 staining of carotid arterial cross sections for endothelial cells demonstrated that the endothelium was circumferentially intact. Endothelial cells were significantly hypertrophied near the anastomosis versus the controls. L, O: Bright field images. M, P: FITC-conjugated isolectin-B4 stain. N, Q: Overlay. 40X, white bar=100μm. R: The thickness of the tunica intima increased significantly near the anastomosis versus the controls. S-Y: CD41/61 immunohistochemical staining for platelets demonstrated significantly reduced platelet-endothelial interactions near the anastomosis versus control carotid artery. Large platelet aggregates were not observed in any animal and further demonstrated that the endothelium was intact circumferentially within the carotid artery near the anastomosis. S, V: Bright field images. T, W: Texas red-conjugated CD41/61 stain. U, X: Overlay. 40X, white bar=100 μm. Y: Platelet-endothelial interactions were extremely rare but were significantly reduced near the anastomosis as compared to the controls.
MMP Gene Expression
Quantitative real-time PCR demonstrated significantly reduced gene expression of MMP-9 (p<0.05, Figure 3 J) in carotid artery near the anastomosis versus the controls. MMP-2 gene expression trended toward a decrease but did not reach statistical significance. Gene expression of CTGF increased significantly (p<0.01, Figure 3 K) by approximately 1,000% in carotid artery near the anastomosis versus the controls.
Molecular Studies
Isolectin-B4 staining demonstrated a significantly greater endothelial cell thickness near the anastomosis than in the control carotid arteries (p<0.01, Figure 3 L-R). vWF staining demonstrated significantly greater von Willebrand factor on the luminal surface of endothelial cells near the anastomosis than in the controls (p<0.01, Figure 4 A-J).
Figure 4.

von Willebrand factor immunohistochemical staining demonstrated that endothelial cells near the anastomosis were manufacturing a greater quantity of von Willebrand factor versus the controls. A, E: Bright field images. B, F: FITC-conjugated von Willebrand factor stain. C, G: DAPI co-nuclear stain. D, H: Overlay. 40X, white bar=100 μm. I, J: Total staining area and staining intensity for von Willebrand factor increased significantly in endothelium near the anastomosis as compared to controls. K-T: TUNEL staining for cells actively undergoing programmed cell death demonstrated a significantly higher rate of endothelial cell apoptosis near the anastomosis versus the controls. K, O: Bright field images. L, P: DAPI co-nuclear stain. M, Q: FITC-conjugated TUNEL+ stain. N, R: Overlay. 40X, white bar=100μm. S, T: The rate of endothelial cell apoptosis but not smooth muscle cell apoptosis increased significantly near the anastomosis versus controls.
Platelets adherent to endothelial cells were extremely rare near the anastomosis and in the controls and indicated that the endothelium was circumferentially intact in treated and control carotid arteries. CD41/61 staining for platelets demonstrated significantly fewer platelets adherent to endothelial cells near the anastomosis than in the controls (p<0.05, Figure 3 S-Y).
TUNEL staining demonstrated a significantly greater rate of endothelial cell apoptosis near the anastomosis than in the controls (p<0.01, Figure 4 K-T). The rate of tunica media cell apoptosis was not significantly different near the anastomosis than in the controls.
Carotid Endothelial-dependent and Endothelial-Independent Vasoreactivity
In carotid arterial rings proximal to the anastomosis, maximum contraction with high [K+] PSS was reduced compared to the controls (Figure 5 A). Similarly, endothelial-independent contraction with phenylephrine was significantly reduced (p<0.05, Figure 5 B). Endothelium-independent relaxation with sodium nitroprusside was not affected (Figure 5 C). Endothelial-dependant relaxation with acetylcholine trended toward an increase near the anastomosis but did not reach statistical significance (Figure 5 D).
Figure 5.

In vitro isolated carotid arterial-ring studies demonstrated (A) reduced total force generation from high K+ challenge, (B) significantly reduced force generation to endothelial-independent challenge with phenylephrine, (C) no change in relaxation with endothelial-independent challenge with NO, and (D) enhanced relaxation with endothelial-dependant challenge with acetylcholine.
Discussion
Less-Invasive Partial-Support LVADs
Recently, the National Heart, Lung, and Blood Institute issued a mission statement to support the development of long-term mechanical strategies with minimally invasive surgery to provide moderate hemodynamic support earlier in the progression of heart failure15. In parallel, investigators and industry have miniaturized LVADs for less-invasive support. Small devices implanted with less-invasive surgery are designed to provide “partial-support”. Partial unloading of the failing left ventricle may interrupt the progressive hemodynamic deterioration of heart failure and improve quality of life in patients with earlier stages of heart failure. Importantly, partial support may reduce native ventricular workload and augment myocardial blood flow to promote favorable myocardial remodeling before the onset of irreversible myocardial damage. Other potential benefits of less-invasive partial support may include reduced right ventricular failure, shorter hospital stays, and lower costs16. Initial clinical results with partial-support devices are encouraging6, 16-18.
Nearly a dozen new devices with novel support strategies are poised for clinical trial16. Certain devices are connected to a peripheral artery rather than the aorta. Anastomosis of the outflow graft to the subclavian artery was initially described by DeBakey when he implanted the first LVAD19 and has become a common site for less-invasive therapy. For example, the continuous-flow CircuLite Synergy Pocket Micro-Pump6 and the Symphony pump5 have adopted this configuration. This approach forces a medium-sized peripheral artery to accommodate non-physiologic hemodynamics and the volumetric flow of a larger artery. With some devices, complete flow reversal occurs. The effect of altered hemodynamics on arterial structure and function has not been well studied.
The Effect of Mechanical Loading on Arterial Remodeling
In this study, altered hemodynamics during circulatory support through a peripheral artery triggered endothelial and arterial remodeling. Specifically, abnormal blood pressure and flow activated multiple cellular programs that produced: 1) arterial dilatation, 2) a decreased ratio of tunica media to tunica adventitia, 3) increased deposition of collagen, elastin, and total extracellular matrix, 4) decreased gene expression of MMPs, 5) increased gene expression of CTGF, 6) decreased endothelial adhesion of platelets, 7) endothelial cell hypertrophy, 8) increased endothelial cell production of von Willebrand factor, 9) endothelial cell apoptosis, 10) enhanced endothelial-dependent relaxation, and 11) impaired endothelial-independent contraction. These changes within the endothelium, smooth muscle, and extracellular matrix allowed a peripheral artery to accommodate the altered hemodynamics of a novel partial-support LVAD.
Arterial remodeling is a natural consequence of pathological mechanical loading3, 4. Multiple clinical examples are well described. Malignant hypertension induces fibrinoid necrosis20. Chronic hypertension causes endothelial dysfunction, hyperplastic remodeling of the tunica intima and tunica media, and accelerated atherogenesis20. Heart failure produces endothelial dysfunction and arterial stiffening21. Furthermore, iatrogenic arterial remodeling similar to changes described in this report occurs in autogenous arteriovenous fistulas and during intraaortic balloon pump (IABP) counterpulsation. In an arteriovenous fistula for hemodialysis access, a medium-sized artery transitions from accommodating medium-volume laminar flow to high-volume turbulent flow with high shear stress. As a result, radial-cephalic fistulas undergo adaptive changes such as arterial dilatation22, intimal hyperplasia23, and smooth muscle cell apoptosis23, which ensure a functional fistula for chronic intravenous access. Likewise, an IABP produces supra-physiologic pulse pressures and systemic flow reversal24 that trigger arterial changes25. In these situations, abnormal mechanical forces influence the composition and function of the endothelium and the arterial wall.
Indeed, during normal pulsatile blood flow, three mechanical forces stimulate arterial cells: 1) cyclic circumferential stretching caused by a transmural radial force perpendicular to the vessel wall, 2) a constant uni-directional viscous drag-induced shear stress from erythrocytes and plasma flowing over the endothelium, and 3) cyclic axial stretching of the artery parallel to the direction of blood flow. These forces impart an atheroprotective effect that maintains normal arterial architecture26. The Symphony pump alters each of these forces. High pressure during systolic ejection increases radial expansion. Viscous drag changes from continuous and unidirectional to interrupted and bidirectional during each beat. Axial stretching changes from unidirectional to bidirectional during each beat.
Endothelial cell structure and function are heavily dependant on mechanical forces4. During Symphony support, endothelial cells near the anastomosis adapted to supra-physiologic pressures and turbulent, bidirectional flow. Despite arterial dilatation, the endothelium remained circumferentially intact. Endothelial hypertrophy, increased production of von Willebrand factor, enhanced endothelial-dependant relaxation, and increased endothelial apoptosis suggested increased metabolism within endothelial cells and a high cell turnover rate. Abnormal flow activated multiple cellular programs involved in endothelial cell size, metabolism, protein manufacturing, matrix deposition and remodeling, vasoreactivity, and life-cycle.
Tunica media structure and function are also dependant on mechanical forces3. During Symphony support, increased production of collagen and elastin were observed throughout the arterial wall. Decreased MMPs in the arterial wall may have resulted in the accumulation of extracellular matrix throughout the tunica media and tunica adventitia. Bands of matrix were deposited between layers of smooth muscle cells and interrupted the normal architecture of the tunica media. These structural changes likely prevented the artery from generating the same contractile force via alpha-adrenergic stimulation with phenylephrine during in vitro isolated ring studies.
We propose that non-physiologic arterial hemodynamic loading with the Symphony pump triggered arterial remodeling to accommodate a new flow profile within the vessel. Endothelial cells likely orchestrated remodeling of the media. In endothelial cells, mechanotransduction mechanisms modulate biophysical, biochemical, and gene regulatory responses to influence vascular remodeling and vascular reactivity4. Phasic hydrostatic pressures stimulate endothelial secretion of autocrine factors that modulate vascular smooth muscle cell orientation and organization of the extracellular matrix via MMP activity and growth factors3. Similarly, vasomotor tone is controlled largely by vasoactive substances released from the endothelium. As such, we speculate that altered pulsatility, flow, and shear stress within the carotid artery during Symphony support may have reset the balance of autocrine, growth, and vasoactive factors as well as matrix proteases. As a result, remodeling of the endothelium and arterial wall occurred.
Clinical Implications
Multiple devices that operate with different mechanisms of flow are clinically available or poised for clinical trials16. Each device produces a unique hemodynamic profile with varying pulsatility, turbulence, shear stress, and flow directionality. As such, each device produces hemodynamic derangements that may cause arterial remodeling.
We have shown that the Symphony pump, a pulsatile pump, generates excess pulsatility that triggers arterial remodeling. In contrast, during support with a continuous-flow LVAD, native pulsatility is nearly abolished27. Interestingly, there is significant evidence that prolonged non-pulsatile blood flow with a continuous-flow LVAD also produces arterial remodeling28-31 similar the changes described in this report with a pulsatile pump. These studies and our data demonstrate that altered pulsatility (increased or reduced) produces architectural and functional arterial changes. Therefore, we speculate that the altered flow profile with any LVAD will influence arterial tissues. The effect on long-term patient outcomes is uncertain. Further investigation of the remodeling pathways that we identified may be of significance for the field of mechanical circulatory support.
Cerebral perfusion is an important consideration with the Symphony pump. In this study, the end organs of all animals were examined carefully in cross-section for gross pathology and sings of thromboembolism. We did not observe infarcted brain tissue or the sequelae of a stroke in any animal in this study. Furthermore, during prior 30-day studies with the Symphony pump, in which support was delivered directly into the carotid artery, we did not observe neurological dysfunction in any animal10. In humans the Symphony pump will be anastomosed to the subclavian artery. As such, support will not occur directly into the carotid artery and cerebral vessels, which is even less likely to produce neurological dysfunction.
Limitations
The primary purpose of these implantations was to determine the safety of the Symphony pump in a pre-clinical large-animal model. In parallel, the opportunity to study vessel morphology and function near the anastomosis was available. As a result, tissues from specific durations of support were not always available for an equal sample size with each assay performed.
Animals were sacrificed after short-term support (weeks). Gross, histological, and molecular changes observed may be progressive upon longer exposure to non-physiological blood flow patterns. It is possible that prolonged support over years may result in subclavian aneurysm formation or arterial dissection. Similarly, prolonged Symphony support may influence arterial tissues distant from the anastomosis. Additional long-term studies in animals and in humans are necessary to characterize the effect of prolonged mechanical circulatory support on a peripheral artery as well as to develop management strategies for complications that may arise. Patients implanted with a Symphony pump will undergo serial ultrasound screening of the subclavian artery.
Conclusions
Altered hemodynamics with an LVAD induced arterial remodeling. Non-physiologic blood pressure and flow triggered a hypermetabolic state in endothelium, altered arterial gene expression, changes in the distribution and composition of the extracellular matrix, and changes in arterial vasoreactivity. These data suggest potential mechanistic pathways for future investigation that may be of significance for the emerging field of partial circulatory support. These data may also contribute to our understanding of arterial remodeling in hypertension, heart failure, arteriovenous fistulas, and prolonged IABP counterpulsation.
Acknowledgments
The authors acknowledge and thank the University of Louisville veterinary staff as well as Dr. Sumanth Prabhu, Dr. Guruprasad Giridharan, Dr. Leslie Sherwood, Kenneth Brittian, Sujith Dassanayaka, Mary Anne Huack, and Abiomed Inc. for their assistance.
Sources of Funding: Funding for this project was provided by National Institutes of Health Small Business Innovation Research grants 2R44HL083586-02A1, 2R44HL088760-02, and R43HL102981 as well as Kentucky Science and Technology Corporation grants KSTC-184-512-08-054 and KSTC-184-512-08-054.
Footnotes
Disclosures: Paul Spence, M.D. receives royalties for the Symphony pump. Thorsten Siess, Ph.D. is a paid employee of Abiomed Inc. Daniel Raess, M.D. is a paid employee of Abiomed Inc. Robert Dowling, M.D. is a paid consultant of Abiomed Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The fourth intermacs annual report: 4,000 implants and counting. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Drakos SG, Kfoury AG, Selzman CH, Verma DR, Nanas JN, Li DY, Stehlik J. Left ventricular assist device unloading effects on myocardial structure and function: Current status of the field and call for action. Current opinion in cardiology. 2011;26:245–255. doi: 10.1097/HCO.0b013e328345af13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. Journal of biomechanics. 2007;40:947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. Journal of biomechanics. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Bartoli CR, Wilson GC, Giridharan GA, Slaughter MS, Sherwood LC, Spence PA, Prabhu SD, Koenig SC. A novel subcutaneous counterpulsation device: Acute hemodynamic efficacy during pharmacologically induced hypertension, hypotension, and heart failure. Artificial Organs. 2010;34:537–545. doi: 10.1111/j.1525-1594.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyns BP, Simon A, Klotz S, Wittwer T, Schlensak C, Rega F, Burkhoff D. Clinical benefits of partial circulatory support in new york heart association class iiib and early class iv patients. Eur J Cardiothorac Surg. 2011;39:693–698. doi: 10.1016/j.ejcts.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 7.ClinicalTrials. Symphony: The implantable counter pulsation device (cpd) safety and feasability trial. 2012;2013 [Google Scholar]
- 8.Bertho E, Gagnon G. A comparative study in three dimension of the blood supply of the normal interventricular septum in human, canine, bovine, procine, ovine and equine heart. Diseases of the chest. 1964;46:251–262. doi: 10.1378/chest.46.3.251. [DOI] [PubMed] [Google Scholar]
- 9.Bartoli CR, Sherwood LC, Giridharan GA, Slaughter MS, Wead WB, Prabhu SD, Koenig SC. Bovine model of chronic ischemic cardiomyopathy: Implications for ventricular assist device research. Artificial organs. 2013 doi: 10.1111/aor.12129. [DOI] [PubMed] [Google Scholar]
- 10.Bartoli CR, Dowling RD, Wilson GC, Giridharan GA, Slaughter MS, Sherwood LC, Spence PA, Prabhu SD, Koenig SC. Response to letter to the editor: A novel subcutaneous counterpulsation device: Acute hemodynamic efficacy during pharmacologically induced hypertension, hypotension, and heart failure. Artif Organs. 2011;35:93–95. doi: 10.1111/j.1525-1594.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- 11.Koenig SC, Woolard C, Drew G, Unger L, Gillars K, Ewert D, Gray L, Pantalos G. Integrated data acquisition system for medical device testing and physiology research in compliance with good laboratory practices. Biomed Instrum Technol. 2004;38:229–240. doi: 10.2345/0899-8205(2004)38[229:IDASFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder MJ, Perreault B, Ewert DL, Koenig SC. Heart: An automated beat-to-beat cardiovascular analysis package using matlab. Comput Biol Med. 2004;34:371–388. doi: 10.1016/S0010-4825(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 13.Bartoli CR, Brittian KR, Giridharan GA, Koenig SC, Hamid T, Prabhu SD. Bovine model of doxorubicin-induced cardiomyopathy. J Biomed Biotechnol. 2011;2011:758736. doi: 10.1155/2011/758736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoli CR, Wead WB, Giridharan GA, Prabhu SD, Koenig SC, Dowling RD. Mechanism of myocardial ischemia with an anomalous left coronary artery from the right sinus of valsalva. The Journal of thoracic and cardiovascular surgery. 2012;144:402–408. doi: 10.1016/j.jtcvs.2011.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin JT, Mann DL. Nhlbi's program for vad therapy for moderately advanced heart failure: The revive-it pilot trial. J Card Fail. 2010;16:855–858. doi: 10.1016/j.cardfail.2010.06.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartoli CR, Dowling RD. The future of adult cardiac assist devices: Novel systems and mechanical circulatory support strategies. Cardiology clinics. 2011;29:559–582. doi: 10.1016/j.ccl.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayward CS, Peters WS, Merry AF, Ruygrok PN, Jansz P, O'Driscoll G, Larbalestier RI, Smith JA, Ho B, Legget ME, Milsom FP. Chronic extra-aortic balloon counterpulsation: First-in-human pilot study in end-stage heart failure. J Heart Lung Transplant. 2010;29:1427–1432. doi: 10.1016/j.healun.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Jeevanandam V, Jayakar D, Anderson AS, Martin S, Piccione W, Jr, Heroux AL, Wynne J, Stephenson LW, Hsu J, Freed PS, Kantrowitz A. Circulatory assistance with a permanent implantable iabp: Initial human experience. Circulation. 2002;106:I183–188. [PubMed] [Google Scholar]
- 19.DeBakey ME. Left ventricular bypass pump for cardiac assistance. Clinical experience. Am J Cardiol. 1971;27:3–11. doi: 10.1016/0002-9149(71)90076-2. [DOI] [PubMed] [Google Scholar]
- 20.Leitschuh M, Chobanian A. Vascular changes in hypertension. The Medical clinics of North America. 1987;71:827–841. doi: 10.1016/s0025-7125(16)30811-2. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M. Peripheral vascular remodeling in chronic heart failure: Clinical relevance and new conceptualization of its mechanisms. J Card Fail. 1999;5:127–138. doi: 10.1016/s1071-9164(99)90035-0. [DOI] [PubMed] [Google Scholar]
- 22.Ene-Iordache B, Mosconi L, Antiga L, Bruno S, Anghileri A, Remuzzi G, Remuzzi A. Radial artery remodeling in response to shear stress increase within arteriovenous fistula for hemodialysis access. Endothelium. 2003;10:95–102. doi: 10.1080/10623320303365. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa Y, Takemura G, Misao J, Kanoh M, Ohno M, Ohashi H, Takatsu H, Ito H, Fukuda K, Fujiwara T, Minatoguchi S, Fujiwara H. Apoptosis and overexpression of bax protein and bax mrna in smooth muscle cells within intimal hyperplasia of human radial arteries : Analysis with arteriovenous fistulas used for hemodialysis. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2066–2077. doi: 10.1161/01.atv.19.9.2066. [DOI] [PubMed] [Google Scholar]
- 24.Giridharan GA, Bartoli CR, Spence PA, Dowling RD, Koenig SC. Counterpulsation with symphony prevents retrograde carotid, aortic, and coronary flows observed with intra-aortic balloon pump support. Artificial organs. 2012;36:600–606. doi: 10.1111/j.1525-1594.2012.01456.x. [DOI] [PubMed] [Google Scholar]
- 25.Bia D, Zocalo Y, Armentano R, de Forteza E, Cabrera-Fischer E. Acute increase in reversal blood flow during counterpulsation is associated with vasoconstriction and changes in the aortic mechanics. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference; 2007. pp. 3986–3989. [DOI] [PubMed] [Google Scholar]
- 26.Cummins PM, von Offenberg Sweeney N, Killeen MT, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: A force to be reckoned with. American journal of physiology. Heart and circulatory physiology. 2007;292:H28–42. doi: 10.1152/ajpheart.00304.2006. [DOI] [PubMed] [Google Scholar]
- 27.Bartoli CR, Giridharan GA, Litwak KN, Sobieski M, Prabhu SD, Slaughter MS, Koenig SC. Hemodynamic responses to continuous versus pulsatile mechanical unloading of the failing left ventricle. Asaio Journal. 2010;56:410–416. doi: 10.1097/MAT.0b013e3181e7bf3c. [DOI] [PubMed] [Google Scholar]
- 28.Nishinaka T, Tatsumi E, Nishimura T, Shioya K, Ohnishi H, Taenaka Y, Takano H. Change in vasoconstrictive function during prolonged nonpulsatile left heart bypass. Artificial Organs. 2001;25:371–375. doi: 10.1046/j.1525-1594.2001.025005371.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi H, Itoh T, Nishinaka T, Tatsumi E, Fukuda T, Oshikawa M, Shioya K, Tsukiya T, Takewa Y, Homma A, Uesho K, Sato K, Takano H, Taenaka Y. Morphological changes of the arterial systems in the kidney under prolonged continuous flow left heart bypass. Artificial Organs. 2002;26:974–979. doi: 10.1046/j.1525-1594.2002.07135.x. [DOI] [PubMed] [Google Scholar]
- 30.Kihara S, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann Thorac Surg. 2003;75:178–183. doi: 10.1016/s0003-4975(02)04087-0. discussion 183. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura T, Tatsumi E, Taenaka Y, Nishinaka T, Nakatani T, Masuzawa T, Nakata M, Nakamura M, Endo S, Takano H. Effects of long-term nonpulsatile left heart bypass on the mechanical properties of the aortic wall. Asaio Journal. 1999;45:455–459. doi: 10.1097/00002480-199909000-00017. [DOI] [PubMed] [Google Scholar]


