Abstract
Eosinophilic esophagitis (EoE) is a clinicopathologic disease of increasing prevalence in children and adults. The triggering antigen in EoE is often a food that initiates a cascade of Th2 associated interleukins such as IL-5, -13, and chemokines such as eotaxin-3 as well as esophageal eosinophilia and mastocytosis. Amino acid based formulas have high efficacy rates in EoE and constituted the first evidence for food triggered esophageal eosinophilia. Animal models have demonstrated the sufficiency of food antigens in triggering both the inflammatory and remodeling complications of EoE. Food elimination diets followed by single food introduction with repeat biopsy have proven the efficacy of empiric and allergy testing based elimination diets in children and adults. Although the ideal allergy test for identifying food antigens in EoE remains to be elucidated, the utility of food skin prick combined with atopy patch testing has been shown in large pediatric cohorts. By comparison, smaller, non-U.S. adult cohorts have not had similar results. Currently, a positive test on food allergy evaluation suggests a food trigger for EoE but does not substitute for biopsy based tissue evaluation following food removal and re-introduction. The higher rates of food anaphylaxis in children with EoE, potential loss of tolerance to IgE positive foods that can occur with food avoidance, and the high rates of other atopic diatheses in EoE subjects all support the evaluation of EoE subject by an allergist, consideration for allergy testing, and an integrated approach by allergists, gastroenterologists, and pathologists in EoE management.
Food antigens and EoE pathogenesis
Eosinophilic esophagitis (EoE) is a clinicopathologic entity of increasing worldwide prevalence that affects both children and adults (1, 2). EoE is a chronic, immune, antigen mediated disorder with a pathogenesis akin to other allergic diseases such as asthma and eczema in which an antigen induces a cascade of Th2 interleukins and chemokines in addition to inflammatory cell infiltration (1, 3, 4). The diagnosis of EoE relies on the presence of a robust esophageal eosinophilia of ≥15 eosinophils per high power field which persists after a PPI trial (1). The process is frequently pan-esophageal and accompanied by histologic remodeling inclusive of submucosal fibrosis and angiogenesis which translates clinically into esophageal rigidity and dysmotility and symptoms of dysphagia (5–10). Important molecular factors for eosinophilia and remodeling include IL-5, -13, eotaxin-3, and TGFβ1 (3– 5, 11–14).
Food antigens clearly function as antigenic triggers for EoE induction and exacerbation in pediatric and adult populations (15–19). In 1995, Kelley and Sampson postulated that acid resistant esophageal eosinophilia could be due to food antigen exposure in children. Based on this hypothesis, these investigators treated children with gastrointestinal symptoms and esophageal eosinophilia with amino acid formula. Following a minimum of 6 weeks of treatment, all of the children experienced resolution or improvement of symptoms with significant reductions in esophageal eosinophilia (17). Since then, these data have been validated at multiple centers. Indeed, amino acid formulas are one of the most effective EoE therapies with resolution rates often higher than 96% in children (16, 18, 20, 21). The removal of all food antigens from the adult diet is also effective in resolving EoE with improvements in endoscopic and histologic features in 72% of subjects following 4 weeks of treatment (22).
A second line of evidence in support of food antigens in the pathogenesis of EoE is the clinicopathologic response to specific food elimination in the form of empiric elimination diets (15, 16, 23, 24). Empiric elimination of specific food groups (milk, egg, soy, wheat, peanuts/tree nuts, fish/shellfish) is highly effective in controlling EoE associated symptoms, endoscopic abnormalities, and eosinophilia. In children and adults, the empiric elimination diet resolves EoE in over 60% of subjects (15, 16, 18, 25). Food antigen elimination can also resolve fibrosis, at least in children (26). As such this food elimination diet not only provides a therapeutic remedy in EoE, but also provides proof of concept that food antigens are EoE instigators. Further evidence for the sufficiency of food antigens in initiating EoE comes from experimental models in which both peanut and egg exposure can cause the accumulation of eosinophils in the murine esophagus (6, 27). In these animal model systems, food antigen exposure induces many features of EoE inclusive of basal cell proliferation, esophageal eosinophilia and mastocytosis, and lamina propria remodeling with fibrosis (6, 27).
Allergy Testing
Allergy testing is generally aimed at elucidating 2 distinct mechanisms of hypersensitivity. Immediate reactions are gauged by the presence of IgE in the context of clinical history and the presence of food specific IgE. Clinically meaningful immediate food hypersensitivity reactions are defined as the presence of food specific IgE and a reproducible clinical reaction occurring within minutes to a few hours (up to about 4 hours) following ingestion of the instigating food. Food allergy in general, however, is defined as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food” (28). Food specific IgE can be detected either by skin prick testing (SPT) or serum IgE testing. Skin prick testing assesses both the presence and the function of mast cell bound IgE while serum IgE documents the presence and quantity of food specific IgE. SPT can be done using commercially available food extracts or fresh foods. The more common testing reagents in food allergy are commercially prepared food extracts. IgE food allergy testing is standardized and validated in the context of immediate reactions such as hives, eczema flares, angioedema, and symptoms of anaphylaxis. However, food specific IgE is detectable in some individuals even in the absence of clinical reactions to the particular food. The clinical state of having IgE to a food is referred to as “sensitization” (28). When this is coupled with a predictable and repeatable clinical reaction upon food ingestion it is termed an “allergy”. Subjects who eat a food and have no detectable clinical reaction are “tolerant”. It can be difficult to accurately predict if a sensitized person who has never consumed the food to which he/she is sensitized will have a reaction upon consumption of that food. However, predicting the likelihood of a response is usually based on the level of serum specific IgE and/or on the size of the wheal on SPT. In addition, the loss of food tolerance can occur when a food sensitized subject begins to avoid the food to which they carry IgE. This loss of tolerance can occur relatively rapidly, in weeks to months, and is manifested by the onset of clinical immediate hypersensitivity reactions such as hives, angioedema, and/or respiratory distress upon ingestion of the previously tolerated food. Current data suggest that continued consumption of a food once desensitization has occurred is likely an essential component for the maintenance of tolerance (29).
Serum IgE is done by a number of laboratories. However, the literature for predictive tests for serum IgE testing in pediatric subjects with immediate food hypersensitivity is based on the Phadia ImmunoCAP system. A study assessing this system in comparison to other serum IgE food tests (Turbo-MP and Immunlite) found that values were significantly different for milk, egg, and peanut (30). As such, the results obtained from different tests are not comparable. Testing, previously done by radioactivity and known as “RAST” (radioimmunoassay) has been entirely replaced by non-radioactive ELISA testing. Unlike IgG testing, which is entirely unwarranted in food allergy with the exception of IgG4 for research purposes, IgE testing requires the detection of nanogram quantities of antibody. This is due to the fact that the majority of IgE is bound to tissue cells such as mast cells and basophils. The pre-bound nature of IgE to its receptor is pivotal for the rapid time course of immediate hypersensitivity since the cell is essentially “primed” for its allergic response. In addition, the predictive values for reactions based on serum food IgE are specific to the food and the age of the subject such that younger children can have immediate reactions at lower food specific serum IgE levels than older children. Given the complexity of food allergy testing and evaluation, referral to an allergist is warranted when there are concerns for immediate hypersensitivity (see below).
The second type of food allergy testing is atopy patch testing (APT) which is used to assess the presence of non-IgE, cell mediated food reactions. The histologic recrudescence of EoE following food re-introduction is relatively rapid, within 3–7 days (15, 22). This time course supports a delayed type hypersensitivity/cell mediated mechanism. In contrast, there is no clinical evidence for reactions that are as rapid as that seen in subjects with food-induced anaphylaxis, that is within minutes of food consumption, or that food impactions are due to sudden esophageal spasm due to allergen exposure. The most studied delayed allergy testing is atopy patch testing (APT). The most rigorously investigated APT has been in the context of chemical and environmental contact dermatitis. In this context, both the specific chemical components and the best vehicle for solubilizing the chemical have been standardized. Results of food APT has been most closely evaluated in eczema (31–34). APT is done by placing fresh or rehydrated foods in metal Finn chambers, applying the chambers to the back for 48 hours, and reading the results at 72 hours using European guidelines for the analysis of food patch testing (35). For food APT in EoE, there have been no studies that have incorporated skin biopsies in order to verify the presence of an immunologic infiltrate at the cutaneous site of a positive patch test.
Food Allergy Testing in EoE
Both cell mediated and IgE mediated mechanisms may be at work in the EoE esophagus. Immunologic class switch machinery is present at elevated levels in the EoE esophagus as are B cells, IgE/IgE receptor positive cells, and interleukins such as IL-13 that promote class switch to IgE (3, 36, 37). As such, there is a precedent for in situ esophageal specific IgE production. Animal models of allergen induced experimental EoE show a dependence on T cells, recombination, and basophils but not on B cells or mast cells for disease induction (38, 39). In addition, animal models deficient in IgE have EoE induction suggesting that if IgE plays a role in sustaining or exacerbating but not in inciting EoE (38, 39). Interestingly, there have been reports of EoE onset during oral desensitization trials for egg and milk (40, 41). This clearly suggests that the mechanism of food allergy in EoE is not through IgE but, instead, through a cellular process.
The current data for allergy testing to elucidate food triggers in EoE support a number of conclusions. First, allergy testing for foods may be more useful in the pediatric than the adult population. In this context, it is important to keep the natural history of the atopic march in mind. That is, young children tend to have food sensitization and allergic responses while older children and adults have aeroallergen allergies. Second, the negative predictive values for foods generally tend to be superior to the positive predictive values. That is to say, if SPT is negative for a food allergen, there a greater than 90% chance that the patient will not have an IgE mediated reaction. An exception to the negative predictive value of food testing in EoE is milk (21). Third, the presence of food specific IgE can be due to cross reactivity with environmental allergens. For example, a patient may have wheat specific IgE due to a grass allergy. Fourth, the current data does not support a role for serum IgE based dietary elimination and also does not support the use of serum food allergy panels. Lastly, although positive SPT and/or APT to foods may reveal a food EoE trigger, the testing does not provide an alternative to endoscopic and biopsy evaluation following food elimination and reintroduction.
Generally, EoE subjects are highly atopic and tend to have poly-sensitization to both food and aeroallergens with children having more food sensitization and adults having more aeroallergen sensitization, consistent with the natural history of allergy. Both SPT and APT in EoE have been more rigorously studied in the pediatric population. Currently IgE food testing has been most rigorously studied via SPT. The exact extent that sensitization reflects clinically relevant EoE food triggers continues to be investigated; the current data are summarized below in this review. However, based on the current observations and our understanding of EoE disease mechanisms, isolated IgE testing via SPT or serum is very unlikely to provide meaningful data for generating a foundation for elimination diets.
Overall, 77% percent of children have at least 1 positive SPT to foods and up to 50% of adults have positive testing to foods (21, 42). In adult patients with EoE, peanut, egg and soy are most commonly positive on IgE testing (43). A recent study of adult EoE patients in Spain showed that 45% (total n=22) have no positive foods by SPT using food extracts while 27% had no food positives by fresh food SPT, suggesting that fresh foods may be more sensitive for detecting mast cell bound food specific IgE (44). Overall, legumes were the most common positive foods identified by either commercial or fresh food extract (44). It is common that serum specific IgE to food is detected in EoE patients but there is a paucity of data on the utility of serum IgE for guiding the design of elimination diets (1). One pediatric study demonstrated that serum IgE was more sensitive than SPT for finding food specific IgE but did not utilize serum data for dietary intervention (45). It is important to note that among pediatric EoE subjects there are higher rates of immediate food hypersensitivity reactions (urticaria, anaphylaxis) than in the general population (15–24% in EoE as compared with rates of 3.9% in the general population) with peanuts, eggs, and milk being the most common triggers of anaphylaxis (1, 21, 30, 46). As such the presence/level of food IgE may predict a potential for anaphylaxis if the food is not being consumed regularly.
Current data, largely from the Children’s Hospital of Philadelphia, has built predictive values for SPT, APT and combined SPT and APT in EoE (21). Similar to what is seen in IgE mediated food allergy, the negative predictive value of food allergy SPT exceeds the positive predictive value. While positive predictive values varied from 26%–96% depending on the food (average of 47%), the negative predictive value was >90% for all food with the exception of egg, wheat, and soy (which ranged from 79–90%) and milk (30%) (21). APT predictive values followed a similar trend with negative predictive values averaging 90% except milk (31%) and positive predictive values averaging 44%. Combining SPT and APT for building an elimination diet increased the negative predictive value so that all foods had negative predictive values equal to or in excess of 93% except for milk (44%), wheat (88%). However, the positive predictive values were not greatly increased (average of 44%) (21). In a study from a second center, the negative predictive values for milk, egg, and wheat were each slightly lower (milk 40%, egg 56%, wheat 67%) (18). Sensitivity and specificity were also increased by using the combination of prick and patch tests with sensitivity of rates of 65–95% (with the exception of milk and pork which were in the 50% range) and specificity rates of 78–90% (inclusive for all foods) (21).
Given these data, the issue remains whether food allergy testing provides a useful tool in EoE. The pediatric data from two separate centers show that this may be the case. One study demonstrated that either empiric food elimination of milk, egg, soy, wheat, peanuts/tree nuts, fish/shellfish or an APT+SPT directed elimination diet had equivalent response rate of 53% (21). Due to the poor predictive values of milk testing, the empiric elimination of milk along with a targeted, testing based diet resulted in a 77% histologic response rate in children with EoE, a rate higher than that seen with empiric or targeted elimination alone (21). Children who used a targeted diet based on testing had to avoid fewer foods (average of 3 based on SPT and 2.7 on APT) than those on empiric elimination (8 food groups with multiple foods in the tree nut, shellfish, and fish groups) (21). In a second study, testing based diets resulted in a 65% response rate in children (18).
Both of the pediatric studies included large cohorts of 319 and 98 subjects who were systematically tested for foods with repeat endoscopy and biopsy in order to assess histologic response following single food reintroduction. This type of large cohort data does not currently exist for the adult population but smaller studies have not demonstrated success rates that mirror the pediatric data. In a group of 15 adult subjects treated prospectively with SPT and APT based elimination diets, 4 (26%) and 1 (6.7%) achieved complete and partial histologic resolution, respectively (44). In a study of 30 adult subjects treated with the empiric 6-food elimination diet, SPT alone would have predicted only 13% of the triggering food allergens (15). APT was not performed in this study. In addition, a recent retrospective case review showed that in 61 Canadian children, only 14% had positive APT (47). The reason for the differences between the results at these centers is not clear but could include differences in age, genetics, and geography all of which could affect EoE phenotype.
Future Diagnostic Tests for Food Allergies
Currently, the best approach to testing for food allergens that underlie the clinicopathologic features of EoE is not certain. While IgE testing is standardized and validated in immediate hypersensitivity reactions, the role of IgE in largely cell mediated disorders like EoE is questionable. It is certainly possible that local IgE production is not reflected in the peripheral serum or in the cutaneous mast cell population. In such a scenario, SPT and serum IgE would not accurately reflect EoE food triggers.
There are a number of future diagnostic tools for food allergies that have been primarily developed to assess for immediate hypersensitivity. To date none have been systematically studied to identify food allergens in EoE. Peptide microarrays gauge the repertoire of IgE in patient serum and are limited to linear as opposed to conformational epitopes. Component-resolved diagnostic testing assesses which particular epitopes within a food antigen are recognized by patient serum. These can be used in the context of food-pollen syndrome in which a local immune mucosal response occurs to a food due to its botanical cross-reactivity with pollen and can help to predict the severity of an IgE mediated response to food (48, 49). Lastly, basophil release assays and analysis of activated basophils in the periphery using basophil activation markers such as CD63 and CD203c may be of utility (50, 51). One study to date has found an increased number of IL-33 receptor positive basophils in the periphery of pediatric EoE subjects (52). Lastly, the numbers of peripheral eosinophils can correlate with the numbers of tissue eosinophils (53). As such, serum eosinophil markers such as eosinophil peroxidase may function as markers of cellular allergy in EoE (54).
One of the most intriguing findings in eosinophilic gastroenteritis patients is that food specific, CD4 positive, IL-5 producing T cells can be found in the peripheral blood. By contrast, IL-4 producing food specific CD4+ T cells are found in subjects with immediate hypersensitivity (55). IL-5 producing T cells require longer culture times to develop in vitro and the production of IL-5 is dependent on chromatin remodeling (56). Currently it is not clear whether these IL-5 positive, CD4+ T cells exist in the esophagus as well as the periphery but is will be intriguing to learn if assays for peripheral food specific T cells can function as markers for EoE food triggers.
Clinical Recommendations and Conclusions
In conclusion, the role of food allergy testing in EoE subjects continues to require more investigation. Current data in children show that it is reasonable to utilize a SPT and APT based strategy in order to build an elimination diet. However, there is a paucity of data to support the use of such a strategy in adult subjects. Generally, targeted testing based diets will have almost equivalent success rates as empiric food elimination (6 food elimination diet) but may require elimination of fewer foods. It is possible that the foods eliminated using testing based diets will leave food in the diet that the child prefers, an issue that plays in to adequate weight gain in pediatric patients. There is a paucity of data to support the use of serum food specific IgE for building elimination diets in children or adults. Serum IgE food specific IgE panels should not be utilized for EoE. In addition, other immunoglobulin testing to foods, such as IgG, is not indicated since the presence of IgG to a food only reflects that this food has been ingested in the person’s lifetime and has been recognized by the immune system not that the food functions as an antigenic trigger. Indeed, certain types of IgG, specifically IgG4, are associated with tolerance rather than allergy. Importantly, positive food SPT or APT in EoE suggest the specific food trigger but do not substitute for biopsy evaluation following food elimination and re-introduction in order to verify the inciting food.
Food testing should be geared towards those foods that trigger EoE more commonly and to those foods that are consumed regularly in the diet. Testing to foods, especially IgE testing, leads to recognition of food sensitizations that may not be not clinically relevant and that, upon elimination, could result in the loss of tolerance to the foods. Although not published for EoE subjects, personal experience and expert opinion support the possibility for loss of tolerance following a period of food avoidance in a sensitized subject. Loss of tolerance to peanuts during avoidance has been clearly documented in the context of immediate food hypersensitivity (29). As such, food elimination must be applied judiciously and with clear explanation of potential risks and benefits to the patient. If a food with a large SPT or high serum level is removed from the diet, an allergist will often consider prescribing injectable epinephrine in case of accidental ingestion in the context of loss of tolerance. If foods are eliminated either empirically or using a testing based strategy, repeat food testing for immediate hypersensitivity reactions via SPT or serum IgE is warranted prior to food reintroduction. This will facilitate decisions of whether the food should be re-introduced in a controlled setting such as with a food challenge in the allergist’s office. In the case of a large SPT reaction (e.g. a wheal larger than 8mm which can be >95% predictive of a clinical immediate hypersensitivity reaction) or a high level of serum specific IgE (the predictive level will depend on the food and the age of the subject), it would be clinically justified to re-introduce the food in an allergist’s office. An allergist’s office is recommended since anaphylactic reactions are expected during standard clinical care due to the use of interventions such as allergen immunotherapy. As such, the staff is appropriately trained to recognize and treat anaphylaxis. In addition, the necessary medications (epinephrine, antihistamines, prednisone, oxygen, i.v. and intubation equipment) are readily available. Reintroduction can be done as an open food challenge where escalating doses of the food antigen are introduced in a controlled setting using defined oral challenge guidelines (57).
As a new disease, the natural history of EoE is unclear. Current data suggest that the disease is chronic and that >90% of pediatric subjects have disease recrudescence with food reintroduction (58). In addition, only 8% of children will become tolerant to all food which cause their EoE (58). This is in stark contrast to the natural history of pediatric immediate hypersensitivity to most foods in which case 80% of children will eventually outgrow an allergy to milk, egg, wheat, and/or soy (28). It seems, then, that EoE is a food driven immunologic process that remains intact for many years in most people. Whether the food or aeroallergen sensitization patterns associate with EoE natural history is unclear. However, studies in other allergic diseases such as asthma suggest that this is a possibility. For example, subjects with indoor allergen sensitization tend to have persistent or recurrent asthma (59).
It is likely that there is interplay between the various atopic diatheses in a single individual. As such, as a part of EoE management, it is important to assess and manage the multiple allergic disorders in a single individual. A classic asthma study demonstrated that when subjects with both house dust mite allergy and steroid dependent asthma were placed in a hospital setting with strict dust mite avoidance, there was decreased medication use and increased asthma control thereby illustrating the powerful impact of antigen avoidance on asthma control (60). It is also common that atopic diatheses flare simultaneously. For example, a viral infection can lead to simultaneous asthma and eczema exacerbations. Whether this occurs in EoE remains to be assessed. There is ample evidence from animal models that aeroallergens (dust mite, cockroach, Aspergillus species) can induce EoE. Pollens can cause both esophageal eosinophil accumulation as well as EoE (61–63). More recent evidence supports that aeroallergen immunotherapy may be a management strategy in EoE (64). Since both the human and animal model evidence that aeroallergens can trigger EoE, the season of an EoE exacerbation should be taken into consideration (62, 65). Given these scenarios, it is important to assess and manage all atopic diatheses in addition to food allergies in a subject with EoE.
In conclusion, there is clearly a role for an immunologic food reaction in EoE adults and children. Although the perfect test to determine food antigen triggers in EoE is still pending, given its allergic nature, the increased rates of immediate hypersensitivity, potential complications such as loss of tolerance during food avoidance, and the multiple concurrent allergic diatheses that occur in EoE subjects, this is a disease best served by an integrated clinical approach that involves gastroenterologists, allergists, and pathologists.
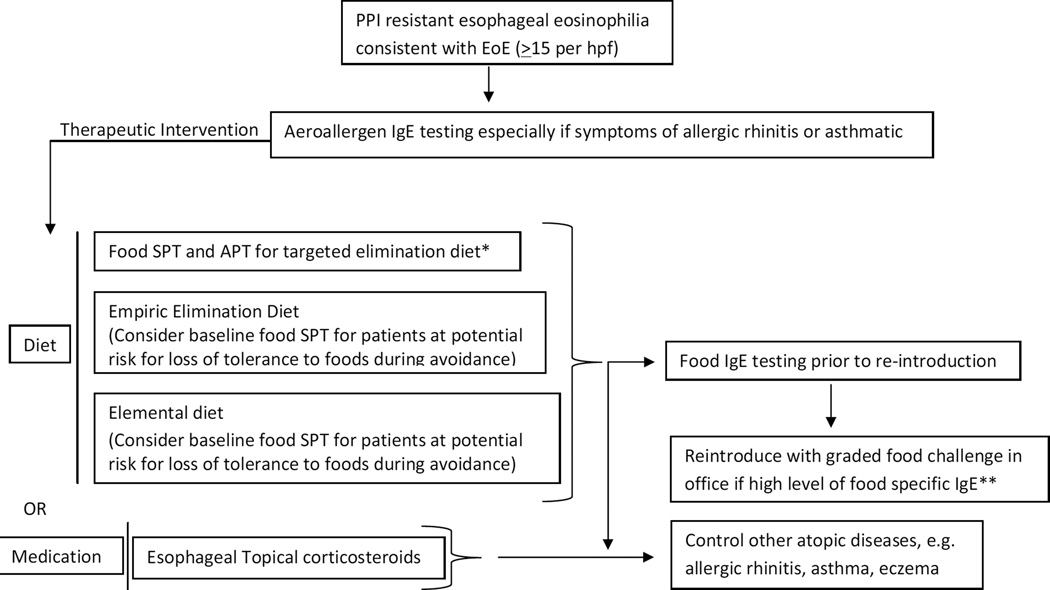
Figure 1.
Testing algorithm
*Data supportive in pediatrics
**Must have appropriate staff, medications, and equipment in place to deal with anaphylactic reaction, allergist’s office is recommended
Table I.
| Type of Test | Mechanism | Utility in EoE | References |
|---|---|---|---|
| Skin Prick Test (SPT) | Presence and function of specific IgE | Can be helpful to assess triggering foods in pediatric EoE when used in combination with APT Of importance when assessing potential for immediate hypersensitivity following food elimination Not of use as an isolated test for food triggers in EoE May not be of significant utility in adult EoE |
Spergel et al, JACI 2013 Henderson et al, JACI 2013 Boyce et al, JACI 2011 Liacouras et al, JACI 2011 Spergel et al, JACI 2013 Gonsalves et al, Gastro 2012 |
| Food Specific Serum IgE | Presence and level of specific IgE | Often detectable but not currently recommended for identifying food triggers in pediatric or adult EoE Of importance when assessing potential for immediate hypersensitivity following food elimination |
Multiple showing detectable levels, summarized in Liacouras et al, JACI 2011 Liacouras et al, JACI 2011 |
| Atopy Patch Test | Delayed hypersensitivity* | Can be helpful to assess triggering foods in pediatric EoE when used in combination with SPT Unclear utility in adult EoE |
Spergel et al, JACI 2012 Henderson et al, JACI 2012 Molina-Infante et al, JACI 2012 |
No current data demonstrating that there is a cutaneous immunologic infiltrate at the site of the patch test in EoE subjects.
Acknowledgments
Support: SA is supported by NIH/NIAID A01 AI092135 and DOD FA1000044
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. e26; quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:1066–1078. doi: 10.1016/j.cgh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, Rothenberg ME. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein E, Cho JY, Rosenthal P, Chao J, Miller M, Pham A, Aceves SS, Varki A, Broide DH. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–416. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:99–118. doi: 10.1016/j.giec.2007.09.009. ix. [DOI] [PubMed] [Google Scholar]
- 9.Persad R, Huynh HQ, Hao L, Ha JR, Sergi C, Srivastava R, Persad S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. J Pediatr Gastroenterol Nutr. 2012;55:251–260. doi: 10.1097/MPG.0b013e31824b6391. [DOI] [PubMed] [Google Scholar]
- 10.Nurko S, Rosen R, Furuta GT. Esophageal dysmotility in children with eosinophilic esophagitis: a study using prolonged esophageal manometry. Am J Gastroenterol. 2009;104:3050–3057. doi: 10.1038/ajg.2009.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 13.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–1537. doi: 10.1053/j.gastro.2010.07.048. 1537 e1521. [DOI] [PubMed] [Google Scholar]
- 14.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204. doi: 10.1016/j.jaci.2010.08.050. e1194. [DOI] [PubMed] [Google Scholar]
- 15.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–1459. doi: 10.1053/j.gastro.2012.03.001. e1451. [DOI] [PubMed] [Google Scholar]
- 16.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, Melin-Aldana H, Li BU. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–1512. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, Rothenberg ME. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570–1578. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Cervera J, Angueira T, Rodriguez-Dominguez B, Arias A, Yague-Compadre JL, Lucendo AJ. Successful Food Elimination Therapy in Adult Eosinophilic Esophagitis: Not All Patients are the Same. J Clin Gastroenterol. 2012 doi: 10.1097/MCG.0b013e3182432259. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 21.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, Liacouras CA. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–467. doi: 10.1016/j.jaci.2012.05.021. e465. [DOI] [PubMed] [Google Scholar]
- 22.Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, Gleich GJ, Adler DG, Clayton F. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108:759–766. doi: 10.1038/ajg.2012.468. [DOI] [PubMed] [Google Scholar]
- 23.Kagalwalla AF, Amsden K, Shah A, Ritz S, Manuel-Rubio M, Dunne K, Nelson SP, Wershil BK, Melin-Aldana H. Cow's Milk Elimination: A Novel Dietary Approach to Treat Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2012 doi: 10.1097/MPG.0b013e318268da40. [DOI] [PubMed] [Google Scholar]
- 24.Kagalwalla AF, Shah A, Li BU, Sentongo TA, Ritz S, Manuel-Rubio M, Jacques K, Wang D, Melin-Aldana H, Nelson SP. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–149. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Castillo S, Arias A, Lucendo AJ. Treatment of eosinophilic esophagitis: how should we manage the disease? J Clin Gastroenterol. 2010;44:663–671. doi: 10.1097/MCG.0b013e3181f189af. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012;67:1299–1307. doi: 10.1111/j.1398-9995.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 27.Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G645–G654. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. Peanut allergy: recurrence and its management. J Allergy Clin Immunol. 2004;114:1195–1201. doi: 10.1016/j.jaci.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Boyce JA, Assa'a A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011;27:253–267. doi: 10.1016/j.nut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Niggemann B, Reibel S, Wahn U. The atopy patch test (APT)--a useful tool for the diagnosis of food allergy in children with atopic dermatitis. Allergy. 2000;55:281–285. doi: 10.1034/j.1398-9995.2000.00464.x. [DOI] [PubMed] [Google Scholar]
- 32.Mehl A, Rolinck-Werninghaus C, Staden U, Verstege A, Wahn U, Beyer K, Niggemann B. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923–929. doi: 10.1016/j.jaci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Devillers AC, de Waard-van der Spek FB, Mulder PG, Oranje AP. Delayed-and immediate-type reactions in the atopy patch test with food allergens in young children with atopic dermatitis. Pediatr Allergy Immunol. 2009;20:53–58. doi: 10.1111/j.1399-3038.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 34.Giusti F, Seidenari S. Patch testing with egg represents a useful integration to diagnosis of egg allergy in children with atopic dermatitis. Pediatr Dermatol. 2005;22:109–111. doi: 10.1111/j.1525-1470.2005.22202.x. [DOI] [PubMed] [Google Scholar]
- 35.Heine RG, Verstege A, Mehl A, Staden U, Rolinck-Werninghaus C, Niggemann B. Proposal for a standardized interpretation of the atopy patch test in children with atopic dermatitis and suspected food allergy. Pediatr Allergy Immunol. 2006;17:213–217. doi: 10.1111/j.1399-3038.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 36.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, Putnam PE, Abonia JP, Santos J, Rothenberg ME. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen EH, Hornick JL, Dehlink E, Dokter M, Baker A, Fiebiger E, Nurko S. Comparative analysis of FcepsilonRI expression patterns in patients with eosinophilic and reflux esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:584–592. doi: 10.1097/MPG.0b013e3181de7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 39.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, Benitez AJ, Ruymann KR, Muir AB, Hill DA, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridolo E, De Angelis GL, Dall'aglio P. Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol. 2011;106:73–74. doi: 10.1016/j.anai.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol. 2012;129:1155–1157. doi: 10.1016/j.jaci.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 42.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44:22–27. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 43.Castellano Mdel R, Cimbollek S, Quiralte J. Defining the role of food allergy in a population of adult patients with eosinophilic esophagitis. Inflamm Allergy Drug Targets. 2010;9:257–262. doi: 10.2174/187152810793358804. [DOI] [PubMed] [Google Scholar]
- 44.Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, Porcel-Carreno SL, Jimenez-Timon S, Hernandez-Arbeiza FJ. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130:1200–1202. doi: 10.1016/j.jaci.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104:496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugnanam KK, Collins JT, Smith PK, Connor F, Lewindon P, Cleghorn G, Withers G. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy. 2007;62:1257–1260. doi: 10.1111/j.1398-9995.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 47.Paquet B, Begin P, Paradis L, Drouin E, Des Roches A. Variable yield of allergy patch testing in children with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:613. doi: 10.1016/j.jaci.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 48.Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, Ogier V, Petit N, Proust B, Moneret-Vautrin DA, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006;118:250–256. doi: 10.1016/j.jaci.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 49.Asero R, Mistrello G, Roncarolo D, de Vries SC, Gautier MF, Ciurana CL, Verbeek E, Mohammadi T, Knul-Brettlova V, Akkerdaas JH, et al. Lipid transfer protein: a pan-allergen in plant-derived foods that is highly resistant to pepsin digestion. Int Arch Allergy Immunol. 2001;124:67–69. doi: 10.1159/000053671. [DOI] [PubMed] [Google Scholar]
- 50.Ocmant A, Mulier S, Hanssens L, Goldman M, Casimir G, Mascart F, Schandene L. Basophil activation tests for the diagnosis of food allergy in children. Clin Exp Allergy. 2009;39:1234–1245. doi: 10.1111/j.1365-2222.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- 51.Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006;6:226–233. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 52.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, Putnam PE, Rothenberg ME. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1328–1336. doi: 10.1016/j.cgh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. doi: 10.1016/j.cgh.2009.03.022. e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(-) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–1332. doi: 10.1016/j.jaci.2009.09.048. e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol. 2011;187:3111–3120. doi: 10.4049/jimmunol.1101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, A., and Immunology. 2009. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, Liacouras CA. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 59.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 60.Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet. 1982;2:675–678. doi: 10.1016/s0140-6736(82)90709-7. [DOI] [PubMed] [Google Scholar]
- 61.Onbasi K, Sin AZ, Doganavsargil B, Onder GF, Bor S, Sebik F. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35:1423–1431. doi: 10.1111/j.1365-2222.2005.02351.x. [DOI] [PubMed] [Google Scholar]
- 62.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rayapudi M, Mavi P, Zhu X, Pandey AK, Abonia JP, Rothenberg ME, Mishra A. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2010;88:337–346. doi: 10.1189/jlb.0110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramirez RM, Jacobs RL. Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol. 2013;132:503–504. doi: 10.1016/j.jaci.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 65.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796–797. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]