Abstract
Monoaminergic neurotransmitter (serotonin, norepinephrine and dopamine) mechanisms of disease dominated the research landscape in the pathophysiology and treatment of major depressive disorder (MDD) for more than 50 years and still dominate available treatment options. However, the sum of all brain neurons that use monoamines as their primary neurotransmitter is <20 %. In addition, most patients treated with monoaminergic antidepressants are left with significant residual symptoms and psychosocial disability not to mention side effects, e.g., sexual dysfunction. In the past several decades, there has been greater focus on the major excitatory neurotransmitter in the human brain, glutamate, in the pathophysiology and treatment of MDD. Although several preclinical and human magnetic resonance spectroscopy studies had already implicated glutamatergic abnormalities in the human brain, it was rocketed by the discovery that the N-methyl-D-aspartate receptor antagonist ketamine has rapid and potent antidepressant effects in even the most treatment-resistant MDD patients, including those who failed to respond to electroconvulsive therapy and who have active suicidal ideation. In this review, we will first provide a brief introduction to glutamate and its receptors in the mammalian brain. We will then review the clinical evidence for glutamatergic dysfunction in MDD, the discovery and progress-to-date with ketamine as a rapidly acting antidepressant, and other glutamate receptor modulators (including proprietary medications) for treatment-resistant depression. We will finally conclude by offering potential future directions necessary to realize the enormous therapeutic promise of glutamatergic antidepressants.
Keywords: Major depressive disorder, Glutamate, Glutamate receptor, NMDA receptor antagonist, Ketamine
Introduction
The monoaminergic hypothesis for depressive disorders arose in the wake of the serendipitous discovery that tricyclic antidepressants and monoamine oxidase inhibitors had beneficial effects on mood, anxiety and neurovegetative symptoms via monoamine neurotransmitter (dopamine, serotonin and norepinephrine) reuptake, degradation and receptor dynamics. However, several recent large clinical studies have uncovered the limitations of our current armamentarium of psychotropic medications for major depression [STAR*D (Gaynes et al. 2009; Rush et al. 2006), STEP-BD (Perlis et al. 2006; Sachs et al. 2003), and CO-MED (Rush et al. 2011; Sung et al. 2012)].
Beyond monoamines, the glutamate system contributes to the pathophysiology of major mood disorders including major depressive disorder (MDD). Glutamate-based research from our group and others has provided novel insights into the pathogenesis of MDD, facilitated the development in new diagnostic tools and, most importantly, offered effective alternative treatment options for even the most refractory patients (Mathews et al. 2012), including those with electroconvulsive therapy (ECT) resistant symptoms (Ibrahim et al. 2011) and active suicidal thoughts (DiazGranados et al. 2010; Price et al. 2009). Additionally, our improved understanding of glutamate’s role in the pathogenesis and pathophysiology of MDD may allow for an increasingly rational approach to drug development for these common, disabling illnesses. The following review will briefly outline the (extremely complex) physiology and pharmacology of glutamate and its receptors in normal brain and in MDD and progress-to-date in glutamate receptor modulators in the treatment of MDD. In this process, we will highlight specific areas of interest to clinical neuroscience and drug discovery.
Basic neuroscience of glutamate and its receptors
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. Glutamate is metabolized from glucose-derived tricarboxylic acid (TCA) cycle intermediates and branched chain amino acids. Glutamate is then packaged into synaptic vesicles via vesicular glutamate transporters (vGLuTs) (Takamori 2006) to be released into the synaptic cleft on neuronal depolarization. Glutamate release critically involves calcium influx (Sudhof 2012) and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and adapter proteins (Rizo and Sudhof 2012) to bridge the synaptic vesicle to the presynaptic membrane for exocytosis. Stress and glucocorticoids have been demonstrated to alter the expression and/or activity of these vesicular proteins in glutamate neurotransmission (Popoli et al. 2012), which can be affected by traditional antidepressants (Musazzi et al. 2013) and has more recently been studied with ketamine (Muller et al. 2013), which will be described in more detail (see below).
Astrocytic end-feet abutting synaptic endings are critical for the reuptake of glutamate and its conversion to glutamine, which will be discussed in greater detail below. In addition to its role as a neurotransmitter, glutamate also serves as a metabolic precursor to the major inhibitory neurotransmitter in the brain, γ-aminobutyric acid (GABA) and as a component of various amino acid-based derivatives, e.g., the antioxidant glutathione. Consistent with glutamate’s key role in multiple aspects of brain physiology, metabolic studies have determined that almost all the glucose that enters the brain is ultimately converted to glutamate (Shen et al. 1999).
After traversing the synaptic cleft, glutamate binds to cognate receptors on the postsynaptic membrane. These have historically been divided into two classes based on their mechanism of activation: ionotropic and metabotropic (Niciu et al. 2012). Glutamate receptor subclasses can also be categorized based on their synaptic localization, i.e., intra- vs. extrasynaptic, which has significant implications for activity (Hardingham and Bading 2010). Ionotropic receptors flux the small cations Na+ and Ca2+ from the extracellular space into the cytosol. There are three major classes of ionotropic receptors: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate (KA) receptors. AMPA/kainate receptor activation mediates the fast (due to Na+ influx) component (Palmer et al. 2005) while NMDA receptor activation produces a more delayed and prolonged excitation (via Ca2+ influx).
NMDA receptors have the highest affinity for glutamate (EC50 1 μM). Three families of NMDA receptor subunits have been identified: (1) NR1, (2) NR2A-D and (3) NR3A-B (Fig. 1) that form tetrameric complexes. AMPA receptor subunits are GluR1-4, and all AMPA receptors are also tetrameric heteromers with critical RNA editing of the GluR2 subunit that controls calcium permeability. AMPA receptors are also associated with several classes of transmembrane auxiliary proteins—transmembrane AMPA receptor-associated regulatory proteins (TARPs), cornichon proteins (CNIH-2, -3) and synapse differentially induced gene-1 (SynDIG-1)—that regulate subunit folding, surface assembly/clustering, and pharmacological sensitivity (Cokic and Stein 2008; Diaz 2010; Rogawski 2011). Kainate receptor subunits are GluR5-7 and KA1-2 (Fig. 1). There are also several classes of metabotropic glutamate receptors, whose natural ligands are also glutamate and its analogs. Metabotropic glutamate receptors have historically been classified into three categories: type I—mGluR1 and 5, type II—mGluR2 and 3, and type III—mGluR4, 6, 7 and 8 (Fig. 1). As expected, extracellular ligand binding couples to G-protein related second messenger systems, leading to molecular and cellular pathway activation or inhibition.
Fig. 1.

Glutamate receptor classes. Glutamate receptors are first stratified functionally—ionotropic receptors flux cations from the extracellular milieu into the cytosol when activated while metabotropic receptors exert their physiological effects via the indirect stimulation of intracellular second messenger/signal transduction cascades. Ionotropic receptors are then divided based on pharmacodynamics activation—NMDA, AMPA, and kainate. Metabotropic receptors have been classified into three groups based on structural and functional similarity—group I (mGluR1/5), group II (mGluR2/3), and group III (mGluR4/6/7/8). NMDA N-methyl-D-aspartate, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, mGluR metabotropic glutamate receptor
In addition to the endogenous ligand glutamate, NMDA receptors are tightly regulated by co-agonists. At least six binding sites have been identified that regulate the probability of ion channel opening, e.g., sites for two obligatory co-ligands glutamate and either glycine or D-serine (D-serine > glycine affinity at the NMDA receptor “glycine” site), polyamines and cations (Mg2+, Zn2+ and H+). NMDA receptor ligands are short-chain dicarboxylic amino acids. Glutamate and several competitive antagonists, i.e., D-2-amino-5-phosphonopentanoic acid (D-AP5) and 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (2R-CPPene) bind to the NR2 subunit of the tetrameric receptor complex. In contrast, glycine binds to a site on the NR1 subunit (Dingledine et al. 1999; Kleckner and Dingledine 1988). Extracellular Mg2+ acts as an open-channel, voltage-dependent “pore blocker” (Nowak et al. 1984) (interestingly, Zn2+, also a divalent cation, does not block the pore of the NMDA receptor).
Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder (MDD)
Neuroimaging: MRS and PET
Alterations in the glutamate system have been identified in MDD by protein biochemistry (immunoblotting and immunohistochemistry) and neuroimaging [positron emission tomography (PET) and magnetic resonance spectroscopy (MRS)]. As two examples of altered glutamate receptor expression, first, there is decreased mGluR5 expression in MDD brain based on PET (with the mGluR5-selective radioligand [11C]ABP688) and immunoblotting in postmortem samples (Deschwanden et al. 2011). Second, in another postmortem immunoblot study, there was reduced prefrontal NR2A and NR2B expression and the excitatory postsynaptic density protein, PSD-95, relative to well-matched psychiatrically healthy subjects (Feyissa et al. 2009). MRS studies, however, have yielded differing results in glutamate and/or glutamine levels in different brain regions, i.e., increased glutamate/glutamine in the occipital cortex (Sanacora et al. 2004a), but decreased glutamate/glutamine levels in the prefrontal cortex (Hasler et al. 2007; Michael et al. 2003a), anterior cingulate cortex (Auer et al. 2000; Zhang et al. 2013b) and left amygdala (Michael et al. 2003b) [decreased frontal and hippocampal glutamate has also been identified at baseline at 9.4-T in a congenital rodent model of depression (Schulz et al. 2013)]. Several groups that identified decreased prefrontal Glx (a mixture of MRS-detectable glutamine and glutamate at 3T and lower field strengths) in unipolar depression reported subsequent normalization with successful antidepressant treatment, i.e., either ECT (Pfleiderer et al. 2003; Michael et al. 2003a, b; Zhang et al. 2013b) or ECT + antidepressant medications (Michael et al. 2003b). Glx levels also normalized after recovery in the occipital cortex of both unipolar and bipolar depressed patients (Bhagwagar et al. 2007). Occipital cortical Glx, however, was increased in healthy subjects treated for 7 days with the selective serotonin reuptake inhibitor (SSRI) citalopram, but no change was seen with the selective nor-epinephrine reuptake inhibitor reboxetine (Taylor et al. 2008). This effect of citalopram, however, was not observed in the frontal cortex of another sample of healthy volunteers also treated for 1 week (Taylor et al. 2010).
MRS-detectable Glx has also been studied with rapid-acting antidepressant interventions/medications. Total sleep deprivation, which has rapid but transient antidepressant effects, has also been associated with increased Glx levels in the dlPFC of male depressed patients and melancholic depression (Murck et al. 2009). There was no change in occipital cortical amino acid levels at three time points [baseline, 3 h and 48 h post-infusion] in ten unipolar depressed subjects who received the rapidly acting antidepressant ketamine, a non-selective NMDA receptor antagonist (Valentine et al. 2011). No change was also observed in anterior cingulate cortex (ACC) Glx levels in healthy volunteers receiving a subanesthetic dose of ketamine. However, our group observed a negative correlation between pretreatment Glx/glutamate ratio (a surrogate marker of glutamine levels) and ketamine’s antidepressant effects in the dorsomedial-dorsal anterolateral prefrontal cortex (DM/DA-PFC) by 1H-MRS (Salvadore et al. 2011).
None of the aforementioned studies have analyzed acute changes in Glx during ketamine infusion in either healthy volunteers or depressed patients; hence, these studies might have missed a critical window that sets in motion a cascade leading to downstream alterations in second messenger cascades leading to some of the cellular and molecular alterations observed in preclinical models, e.g., long-term potentiation (LTP)-like synaptogenesis (Duman and Aghajanian 2012; Kavalali and Monteggia 2012). In addition, all studies to date have used 1H-MRS at low-field magnetic strength; high-field strengths facilitate more sensitive and specific detection of brain neurochemicals including glutamate. Finally, all MRS studies to date have detected proton (1H) signals; 13C would permit the detection of glutamine/glutamate cycling in neurons and astrocytes, which may provide novel insights as astrocyte cell loss is present in both preclinical models and MDD (see below for additional details).
Genetics
There has been scant literature support for genetic associations of glutamate-related genes in MDD in smaller candidate gene and larger genome-wide association studies, most likely due to the inherent diagnostic heterogeneity based purely on clinical symptoms. Nonetheless, glutamate transporter downregulation has been observed in postmortem neocortical MDD microarrays, e.g., SLC1A2, SLC1A3, and L-glutamate-ammonia ligase (Choudary et al. 2005). In the locus coeruleus, glial-expressed high-affinity glutamate transporters SLC1A2, SLC1A3, and GLUL are downregulated; while the neuronal presynaptic vesicular glutamate transporters SLC17A6/VGLUT2 and postsynaptic glutamate receptors GRIA1, GRIK1, GRM1, and GRM5 are upregulated in MDD relative to bipolar disorder (BD) and non-depressed healthy volunteers (Bernard et al. 2011), alluding to both regional and diagnostic specificity. In the most recent study of 178 treatment-resistant MDD subjects, 612 non-refractory depressed patients and 779 healthy controls, several single nucleotide polymorphisms (SNPs) in the NR2B NMDA receptor gene, GRIN2B, were sequenced, and a single SNP, rs1805502, conferred susceptibility to TRD relative to non-TRD depressed patients and healthy volunteers (Zhang et al. 2013a).
Astrocytes and synaptic dysfunction
As discussed above, under physiological conditions, synaptic glutamate is removed from the synaptic cleft by specific astrocyte transporters. The astrocytic excitatory amino acid transporter (EAAT)2 [glial glutamate transporter (GLT-1) in rodents] reuptakes glutamate from the extracellular space where it is intracellularly converted to glutamine for synaptic recycling (Niciu et al. 2012). Excessive extrasynaptic glutamate receptor stimulation initiates apoptosis, and the physiological activity of EAAT2 theoretically reduces excitotoxicity from synaptic spillover in human brain (Hardingham and Bading 2010). Activated astrocytes also secrete several neurotrophic factors, e.g., glial-derived neurotrophic factor (GDNF). In addition to its neuroprotective role, serum GDNF levels are reduced in major depression [including adolescent (Pallavi et al. 2013) and late-life depression (Diniz et al. 2012)] and has been improved by antidepressant treatment in both preclinical (Liu et al. 2012a) and clinical studies (Zhang et al. 2008; Zhang et al. 2009) [although a recent report noted no change in peripheral GDNF levels by ketamine in bipolar depression (Rybakowski et al. 2013)].
Although initially considered to be merely supportive “glue”, in the past two decades, astrocyte dysfunction has been implicated in MDD. Postmortem studies reveal decreased astroglial cell density in several brain regions, including orbitofrontal cortex, dorsolateral PFC (dlPFC) (Rajkowska et al. 1999), ACC (Cotter et al. 2001), and amygdala (Bowley et al. 2002). An age-dependent reduction in GFAP-immunoreactive astrocyte density has also been observed in the PFC of younger individuals with MDD (Miguel-Hidalgo et al. 2000), but not in the sup-ragenual ACC in later-life depression (Khundakar et al. 2011). There is also a significant reduction in aquaporin-immunoreactive astrocytic end-feet contacting gray matter vessels in MDD PFC, which also implicates blood–brain barrier abnormalities in MDD (Rajkowska et al. 2013).
The data are inconsistent on diagnostic specificity, e.g., decreased GFAP expression in the MDD but not BD amygdala (Altshuler et al. 2010), and in the MDD but not schizophrenic cerebellum (Fatemi et al. 2004). Adding additional complexity, there is increased GFAP expression in subcortical brain structures in schizophrenia and MDD relative to non-psychiatric controls (Barley et al. 2009). Finally, serum levels of glial-derived S100β are increased in MDD, and these levels are not affected by antidepressant treatment (Schroeter et al. 2008).
Inflammation and glutamate
There have been recent preclinical advances in our understanding of inflammatory and glutamate cross-talk in depressive disorders (McNally et al. 2008). Secreted inflammatory mediators, i.e., cytokines, activate the kynurenine pathway, and the two major end products of this pathway bind to NMDA receptors. Kynurenic acid and quinolinic acid are NMDA receptor antagonist and agonist, respectively (de Carvalho et al. 1996; Stone 1993). Plasma kynurenic acid levels are increased in MDD patients with a history of suicide attempt relative to MDD patients without a history of suicide and non-psychiatric healthy volunteers (Sublette et al. 2011). In a study of recently hospitalized medication-free suicide attempters in Sweden, quinolinic acid-to-kynurenic acid and interleukin-6 levels (Lindqvist et al. 2009) were increased in cerebrospinal fluid (CSF), and quinolinic acid levels correlated with increased scores on the Suicidal Intent Scale (Erhardt et al. 2013). CSF quinolinic acid levels then normalized in a smaller cohort of suicide completers who returned for a 6-month follow-up lumbar puncture (Erhardt et al. 2013).
Although the link between inflammation and glutamate appears to be a promising line of research, a central role for inflammation in glutamatergic neurotransmission awaits further translation in humans.
Glutamatergic medications in the treatment of major depressive disorder
Ketamine
Berman et al. (2000) made the serendipitous discovery that a single subanesthetic (0.5 mg/kg) dose of ketamine rapidly improved symptoms in major depression (both unipolar and bipolar depression). Although small (n = 7 completers), this initial study also reported a moderate-to-large effect size. This initial finding was replicated in several larger samples with single (Zarate et al. 2006a; Valentine et al. 2011; Murrough et al. 2013a) and repeated (aan het Rot et al. 2010; Messer et al. 2010) ketamine administration. Ketamine also has rapid antidepressant effects in MDD patients resistant to electroconvulsive therapy (ECT) (Ibrahim et al. 2011) and rapidly resolves suicidal ideation (DiazGranados et al. 2010). This latter finding has also been extended to acutely suicidal patients in the emergency department (Larkin and Beautrais 2011) as well as a reduction in both explicit and implicit measures of suicidal cognition (Price et al. 2009). In MDD, ketamine had a moderate-to-large effect size with response rates of ~70 % and remission rates of ~30 % at 24 h post-infusion and sustained for up to 1 week. Non-intravenous ketamine preparations such as oral (Irwin and Iglewicz 2010; McNulty and Hahn 2012) and intramuscular (Zanicotti et al. 2012; Cusin et al. 2012) have also shown antidepressant efficacy. Intranasal ketamine is also of great clinical interest in MDD owing to its high CNS penetrance and ease of administration.
In the randomized, controlled trials cited above, the antidepressant response to a single subanesthetic ketamine infusion lasted, on average, 1 week. In the only randomized, placebo-controlled extension trial after a single subanesthetic infusion, approximately 27% of ketamine responders maintained efficacy during the 28-day follow-up period (average time to relapse = 13.2 days; standard error of the mean = 2.2) (Ibrahim et al. 2012b). Given the growing evidence for ketamine’s potent—although transient—antidepressant effects, the interest in sustaining these effects has naturally increased. The most obvious method of maintaining response involves multiple infusions, and this has proven efficacious in several reports (Murrough et al. 2011, 2013b; Blier et al. 2012). These results have led some groups to propose ketamine maintenance therapy and/or “boosters” upon early detection of clinical deterioration similar to ECT.
Several alternative strategies have been investigated to augment and/or extend ketamine’s efficacy with unfortunately only modest and/or inconsistent results: the glutamatergic modulator riluzole (Mathew et al. 2009; Ibrahim et al. 2012b) and ECT (Ostroff et al. 2005; Kranaster et al. 2011; Okamoto et al. 2010; Wang et al. 2012; Krystal et al. 2003a; Abdallah et al. 2012; Jarventausta et al. 2013; Loo et al. 2012). Future studies must explore novel pharmacological relapse prevention strategies. Indeed, numerous alternative treatment strategies to extend antidepressant efficacy are currently under investigation. These include traditional antidepressants, mood stabilizers [because both ketamine and lithium are glycogen synthase kinase-3 (GSK-3) inhibitors (Liu et al. 2013)], antipsychotics [because of their efficacy as monotherapy in bipolar depression (Frye 2011) and as augmenting agents in MDD (Nelson and Papakostas 2009)], and evidence-based psychotherapies.
Other NMDA receptor antagonists and glutamate-based medications
Owing to ketamine’s dissociative and other side effects, abuse liability, and potential long-term toxicity, the field has sought more selective NMDA receptor antagonists that replicate ketamine’s antidepressant effects with reduced risk of adverse sequelae. Toward this end, the NMDA receptor antagonist memantine, which is approved for the treatment of moderate-to-severe Alzheimer’s-type dementia, has been studied in unipolar major depression. The first clinical report in MDD was an 8-week, placebo-controlled trial that demonstrated a lack of efficacy of memantine monotherapy (5–20 mg/day) (Zarate et al. 2006b). Another small, placebo-controlled, 12-week trial in depressed older patients who had recently suffered a disabling medical event confirmed memantine’s lack of efficacy on depressive symptoms and functional outcomes (Lenze et al. 2012). Subsequently, a case report explored the antidepressant efficacy of repeat-dose ketamine followed by memantine; although this patient was eventually on many psychotropic medications, she remained in remission for at least approximately 3 months on memantine (Kollmar et al. 2008). Another randomized, non-placebo-controlled trial of memantine (20 mg/day) versus escitalopram (20 mg/day) in patients with MDD comorbid with alcohol dependence found that memantine had antidepressant and anxiolytic effects, improved psychosocial functioning, and decreased alcohol consumption (Muhonen et al. 2008a, b).
The antitussive, dextromethorphan, is also an NMDA receptor antagonist, that, like ketamine, has abuse liability and other concerns (Zhou et al. 2011) but also theoretical potential as a rapid-acting antidepressant (Lauterbach 2011, 2012): there have been no randomized controlled trials of dextromethorphan as monotherapy for the treatment of depressive disorders [although it has been studied in a randomized, placebo-controlled trial add-on to valproic acid in bipolar disorder (Lee et al. 2012)]. A randomized controlled trial of combination dextromethorphan–quinidine, which, under the trade name Nudexta®, has been approved for the treatment of pseudobulbar affect, is currently being investigated in treatment-resistant unipolar depression (ClinicalTrials.gov identifier: NCT01882829). There is also a positive case report of dextromethorphan–quinidine in a single case of a depressed patient with emotional lability (Messias and Everett 2012).
There have also been studies with several proprietary NMDA receptor antagonists (Table 1). A single intravenous infusion of the NR2B-selective NMDA receptor antagonist CP-101,606 had rapid antidepressant effects, within several days (Preskorn et al. 2008). Unfortunately, further development of this compound ceased due to cardiovascular toxicity (QTc prolongation). The oral NR2B-selective antagonist MK-0657, when administered daily to patients with treatment-resistant MDD, had antidepressant effects as assessed by the clinician-administered Hamilton Depression Rating Scale (HDRS) and the self-reported Beck Depression Inventory (BDI) but not the clinician-administered Montgomery–Åsberg Depression Rating Scale (MADRS), which was used to assess efficacy as the primary outcome; no serious or dissociative adverse effects were noted (Ibrahim et al. 2012a). Next, a single infusion of the low-trapping, non-selective NMDA receptor antagonist AZD6765 had rapid antidepressant effects in treatment-resistant unipolar depression (Zarate et al. 2013a). Although antidepressant response occurred on a time scale similar to that of ketamine, no psychotomimetic effects were observed with this compound, likely because of AZD6765’s rapid association/dissociation at the NMDA receptor (memantine is likewise believed to be devoid of psychotomimetic side effects owing to similar low-trapping).
Table 1.
Predictors (“Biomarkers”) of ketamine’s antidepressant response in major depression
| Study | Finding(s) |
|---|---|
| Genetics/BDNF | |
| No change in peripheral BDNF levels without enrichment | |
| Duncan et al. (2012) | Δ in SWA from baseline + Δ in peripheral BDNF → > antidepressant response |
| Laje et al. (2012) | Greater antidepressant response in BDNF val66val66 > met haplotype |
| Phelps et al. (2009), Luckenbaugh et al. (2012) | Family history of alcohol dependence → > antidepressant response |
| Functional neuroimaging/Electrophysiology | |
| Salvadore et al. (2009) | ↑ pretreatment rostral ACC activity with fearful faces (MEG) → > antidepressant response |
| Salvadore et al. (2010) | Preteatment pregenual ACC–amygdala desynchronization (MEG) → > antidepressant response |
| Salvadore et al. (2010) | ↓ baseline pregenual ACC activity (MEG) → > antidepressant response |
| Duncan et al. (2012) | ↑ baseline slow-wave delta sleep activity (EEG) → > antidepressant response |
| Cornwell et al. (2012) | ↑ baseline to post-treatment somatosensory-evoked potential (MEG) → > antidepressant response |
| Valentine et al. (2011) | Baseline to post-treatment GABA and glutamate in occipital cortex (MRS) not correlated with antidepressant response |
| Salvadore et al. (2012) | Pretreatment PFC GABA and glutamate (MRS) not correlated with antidepressant response (MRS) |
| Salvadore et al. (2012) | ↓ baseline PFC Glx/glutamate (MRS) → > antidepressant response |
| Other | |
| Denk et al. (2011) | Antidepressant response correlates with ↑ peripheral mTOR expression (single patient) |
| Zarate et al. (2012) | ↑ [Hydroxynorketamine-4a,c] within 230 min post-infusion → < antidepressant response in bipolar depression |
| Niciu et al. (2013) | ↑ body mass index predicts hyperacute and acute, family history of alcohol use disorder predicts acute and sustained, and no history of suicide attempts predicts sustained antidepressant improvement |
| Luckenbaugh et al. (2013) | Dissociation but not psychotomimetic or sympathomimetic side effects predicts improved antidepressant response |
| Permoda-Osip et al. (2013) | ↑ baseline vitamin B12 → > antidepressant response in bipolar depression |
| Sos et al. (2013) | ↑ BPRS total score increase peri-infusion predicts improved antidepressant response in unipolar depression |
ACC anterior cingulate cortex, BDNF brain-derived neurotrophic factor, BPRS Brief Psychiatric Rating Scale, EEG electroencephalography, GABA γ-aminobutyric acid, MEG magnetoencephalography, MRS magnetic resonance spectroscopy, mTOR mammalian target of rapamycin, PFC prefrontal cortex, SWA slow-wave activity
Finally, other glutamatergic modulators have been investigated in MDD (Table 1). First, in an open-label 6-week trial of 19 treatment-resistant unipolar depressed patients, depressive symptom improvement was noted in weeks 3–6 with riluzole (Zarate et al. 2004). After an initial case report of efficacy as an augmentation strategy (Sanacora et al. 2004b), a 12-week open-label add-on riluzole course had both antidepressant and anxiolytic properties in ten treatment-resistant unipolar depressed subjects (Sanacora et al. 2007). As a caveat, riluzole efficacy as either monotherapy or adjunctive strategy has yet to be reported in a randomized, double-blind, placebo-controlled trial. This is currently underway in two independent studies across the US (ClinicalTrials.gov Identifiers: NCT01204918 and NCT01703039). D-Cycloserine (DCS), a historical broad-spectrum antibiotic primarily used for treatment-resistant tuberculosis, is a partial agonist at the NMDA receptor’s glycine site, and, at doses ≥100 mg/day, acts as a functional NMDA receptor antagonist (Millan 2002). In an initial 6-week, placebo-controlled, cross-over trial of 250 mg/day as an add-on in treatment-resistant MDD, DCS provided depressive symptom reduction but did not separate from placebo (p = 0.51) due to a burst in improvement in the placebo group in the final week of the study (Heresco-Levy et al. 2006). A larger trial of 26 treatment-resistant MDD patients (by the same group and with the same design) used escalating doses of adjuvant DCS up to 1,000 mg/day (Heresco-Levy et al. 2013). In this trial, higher-dose DCS had an improved antidepressant potency as measured by the clinician-administered HDRS (p = 0.005) and self-reported BDI (p = 0.046). Interestingly, 54 % of the patients randomized to high-dose DCS had a ≥50 % reduction in the HDRS at the end of the 6-week trial. Next, N-acetylcysteine (NAC) is a potent anti-inflammatory/antioxidant via its cysteine moiety, which is the rate-limiting substrate for glutathione production in the mammalian brain (De Rosa et al. 2000; Dringen and Hirrlinger 2003). It generates cystine, which, via a specific exchanger on the surface of synaptically localized astrocytes, increases glutamate to act presynaptically on metabotropic glutamate receptor II/III suppressing glutamate secretion (Moran et al. 2005). NAC was an effective MDD augmentation strategy in two MDD patients (Carvalho et al. 2013). A proprietary partial agonist at the glycine binding site, GLYX-13, has ketamine-like antidepressant effects in several preclinical models of despair and induces similar cellular and molecular mechanisms, e.g., AMPA dependence, increased postsynaptic density protein expression and hippocampal long-term (synaptic) potentiation, without adverse properties, e.g., ketamine-like sedative, rewarding or sensory gating abnormalities. GLYX-13 has been studied in treatment-resistant MDD. A single infusion phase II trial in treatment-resistant unipolar depression has been completed (ClinicalTrials.gov identifier: NCT01234558), but the results yet to be published. Another is underway and actively recruiting (ClinicalTrials.gov identifier: NCT01684163).
In sum, our group and others have identified several medications with antidepressant potential that are believed to be glutamatergic modulators. However, as due to the lack of validated target-engagement biomarkers in major depression, we are presently unclear if these medications actually affect glutamatergic neurotransmission in the mammalian brain. Such biomarkers could be invasive, e.g., a PET ligand with high sensitivity and specificity for an NMDA receptor subunit, or non-invasive, e.g., ketamine-sensitive auditory mismatched negativity (MMN), P300 event-related potentials on electroencephalography (EEG) and slow-wave changes on sleep EEG (Oranje et al. 2000; Watson et al. 2009; Umbricht et al. 2000; Knott et al. 2011). Target-engagement biomarkers are critical for rational drug design and development, and, when such biomarkers are available, are now routinely incorporated into clinical trial design to determine if a drug penetrates the brain parenchyma and has an expected molecular signature.
Cellular and molecular mechanisms of ketamine’s rapid antidepressant effects
mTOR, p70S6K, 4E-BP1
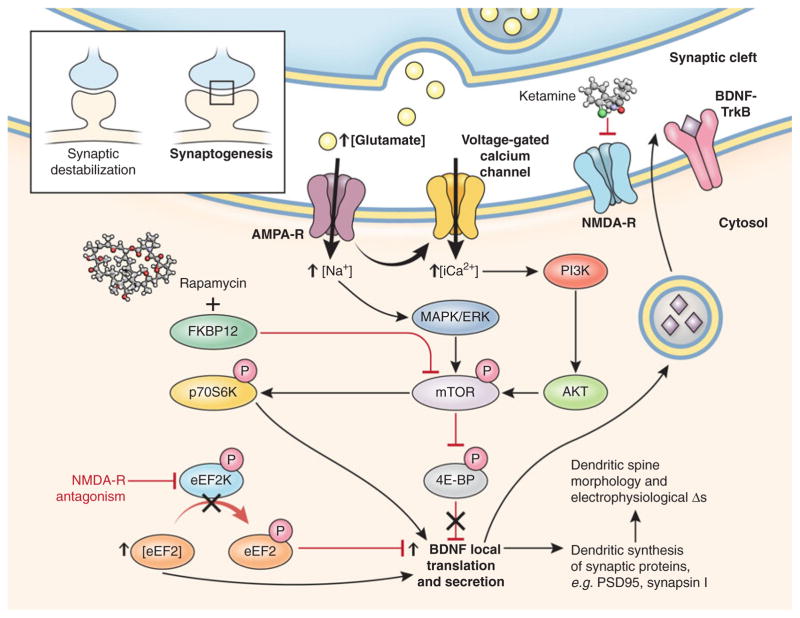
The numerous clinical studies demonstrating ketamine’s rapid antidepressant properties generated preclinical interest in elucidating its mechanism of action in ways presently inaccessible in clinical populations. Ketamine and other NMDA receptor antagonists increase mature synaptic protein expression; promote synaptogenesis; increase the number, morphology, and activity of spine synapses; and reduce depressive-like behaviors in rodent models of depression (Fig. 2) (Li et al. 2010, 2011). Ketamine increased expression of several postsynaptic density proteins (Arc, PSD-95, GluR1, and synapsin I) in PFC synaptosomal preparations approximately 1 to 2 h postinjection. Protein levels remained elevated for up to 72 h, consistent with the time course for the induction of new spine synapses. Induction of synaptosomal PSD-95, GluR1, and synapsin I was abrogated by pretreatment with rapamycin, which selectively inhibits mammalian target of rapamycin (mTOR). Ketamine also increased mTOR, p70 S6 kinase (p70S6K), and 4E-binding protein 1 (4E-BP1) phosphorylation within 1 h of infusion; interestingly, all these proteins stimulate the transcription of target genes involved in synaptogenesis. At 24 h post-infusion, ketamine increased the number of mature-appearing “mushroom” spines and increased the frequency and amplitude of excitatory postsynaptic currents induced by serotonin (from cortico-cortical synapses) and hypocretin (from apical thalamo-cortical synapses) in PFC pyramidal neurons [which is particularly exciting because excitatory synaptic loss was recently identified as a major pathophysiological insult in rodent models of depression (Seese et al. 2013)]. Again, ketamine’s effects were prevented by rapamycin pretreatment. Low-dose (10–20 mg/kg) but not high-dose (80 mg/kg, which is an anesthetic dose) ketamine rapidly reversed despair-like phenotypes, i.e., impairments on the learned helplessness, forced swimming, and novelty-suppressed feeding tests, all of which were blocked by rapamycin pretreatment. Intracerebroventricular pretreatment with the mitogen-activated protein kinase/extra-cellular signal-regulated kinase (MAPK/ERK) inhibitor U0126 and the phosphoinositide-3 kinase (PI3K)/Akt inhibitor LY294002 also negated ketamine’s antidepressant effects. These biochemical, electrophysiological, and behavioral antidepressant-like effects were replicated in a rodent model of chronic (21-day) unpredictable stress. Taken together, the results suggest that mTOR activation via known intracellular second messenger/signal transduction mediators, e.g., MAPK/ERK and PI3K, is necessary for ketamine’s rapid antidepressant-like effects in rodents (Duman and Voleti 2012). Although these exciting preclinical results await translation, one case report found that peripheral mTOR phosphorylation correlated with ketamine’s antidepressant response in a single patient with treatment-resistant MDD (Denk et al. 2011).
Fig. 2.
Synaptic and intracellular events stimulated by the rapid-acting antidepressant ketamine. Preliminary preclinical and unpublished clinical data suggest that postsynaptic NMDA receptor antagonism increases presynaptic glutamate release (i.e., glutamate “surge”). Glutamate is then hypothesized to increase AMPA/NMDA receptor flux. AMPA channel opening in the CNS increases sodium and, indirectly, calcium, stimulating the PI3K cascade to phosphorylate mTOR through Akt. Activated mTOR then phosphorylates p70S6K, increasing translation of downstream postsynaptic targets (notably, mTOR activity can be inhibited by rapamycin through the formation of an inhibitory complex with FKBP12). Activated mTOR also inhibits 4E-BP to relieve inhibition upon translation. NMDA receptor activation also inhibits eEF2K, which increases levels of dephosphorylated eEF2. Dephosphorylated eEF2 relieves inhibition upon BDNF translation in dendritic spines and promotes local secretion, which, in turn, binds to cognate TrkB receptors to activate intracellular mTOR and its downstream targets. In sum, the translational activation induced by acute NMDA receptor blockade increases the expression of several neuromodulatory proteins involved in, among other effects, postsynaptic scaffolding, neurotransmitter dynamics, and dendritic spine morphogenesis from immature thin filopodia-to-mature mushroom-shaped spines (see inset), which form the morphological substrate for antidepressant-like behavioral effects. Through release of inhibition upon local translation of BDNF, ketamine increases excitatory postsynaptic currents in prefrontal cortical and hippocampal neurons. 4E-BP 4E-binding protein, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, AMPA-R AMPA receptor, BDNF brain-derived neurotrophic factor, CNS central nervous system, eEF2 eukaryotic elongation factor 2, eEF2K eEF2 kinase, ERK extracellular signal-regulated kinase, FKBP FK506 binding protein, MAPK mitogen-activated protein kinase, mTOR mammalian target of rapamycin, NMDA N-methyl-D-aspartate, NMDA-R NMDA receptor, p70S6K p70 S6 kinase, PI3K phosphoinositide-3 kinase, TrkB neurotrophic tyrosine kinase, type 2
Several authors have proposed that the antidepressant effects of ketamine may depend on a rapid (within 30 min of administration) presynaptic glutamate “surge” and AMPA/NMDA receptor throughput in critical brain circuitry (Maeng et al. 2008). In 13C-MRS ex vivo studies of rats, subanesthetic (30 mg/kg), but not anesthetic (80 mg/kg) ketamine significantly increased the percentage of 13C-detectable glutamate, glutamine, and GABA in the medial PFC (Chowdhury et al. 2012). As detected by microdialysis, a medial PFC glutamate surge occurs with infusion of subanesthetic (10–30 mg/kg), but not higher-dose (50 and 200 mg/kg) ketamine on a time course consistent with mTOR activation (peaks within 30–60 min and returns to baseline at approximately 2 h) (Moghaddam et al. 1997). As mentioned above, no difference was observed in occipital cortical Glx at three and 24 h after ketamine infusion (Valentine et al. 2011), which might have missed the window to detect this rapid glutamate surge.
A recent report from indicates that subanesthetic-dose ketamine increases connectivity between the prefrontal cortex and the hippocampus, therefore circuitry-level response to ketamine should continue to be investigated as a potential mediator of ketamine’s antidepressant response in clinical studies (Gass et al. 2013).
eEF2K/eEF2/BDNF
In a second landmark preclinical mechanistic study, (Autry et al. 2011) reported that ketamine’s rapid antidepressant effects depended on desuppression of BDNF translation in the hippocampus. On the forced swim test, an inducible BDNF knockout mouse was minimally responsive to the antidepressant effects of ketamine. These effects were reduced by the translational inhibitor anisomycin, but not by the RNA polymerase/transcriptional inhibitor actinomycin D. Consistent with prior studies (Li et al. 2010, 2011), the antidepressant effects of ketamine could be prevented by pretreatment with NBQX, a 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid (AMPA) receptor antagonist. Ketamine decreased the activity of eukaryotic elongation factor 2 kinase (eEF2K) [or calcium-calmodulin dependent protein kinase III (CaMKIII)], thereby reducing eEF2 phosphorylation and desuppressing BDNF translation. These results were replicated using another NMDA receptor antagonist, MK-801, as well as more specific eEF2K inhibitors; interestingly, BDNF conditional knockout mice were insensitive to eEF2K inhibition. In Autry et al. (2011), ketamine-induced mTOR phosphorylation was not increased, and the antidepressant-like effects were not blocked with rapamycin. [Potential explanations for the differences in these two important mechanistic studies are presented in the excellent review by (Duman et al. 2012)].
GSK-3
Like lithium, ketamine inhibits GSK-3 by increasing its phosphorylation. Phospho-GSK-3 interacts with a “destruction complex” of other proteins that target it for degradation in lysosomes, thereby allowing the transcription factor β-catenin to translocate from the cytosol to the nucleus and transcribe target genes (Stamos and Weis 2013). Conversely, a recent rodent study found that intra-cerebroventricular infusion of GSK-3 attenuated ketamine’s psychotomimetic-like effects (Chan et al. 2012). The GSK-3 inhibitor lithium, as well as more selective GSK-3β inhibitors, potentiated mTOR signaling, synaptogenesis, and antidepressant-like effects of lower-dose ketamine (<10 mg/kg) (Ghasemi et al. 2010; Liu et al. 2013). Because GSK-3 inhibition reduces both depressive and psychotic symptoms, it is possible that small molecule inhibitors may ultimately be effective mood stabilizers in cases of BDep and MDD that include psychotic features. Indeed, psychotically depressed patients have been excluded from all ketamine depression trials to date over concerns of worsening psychosis, which has been observed in healthy volunteers (Krystal et al. 1994) and individuals with schizophrenia (Lahti et al. 1995a, b) exposed to subanesthetic doses of ketamine.
Other
In addition to the effects on second messenger/signal transduction proteins, it was recently reported that low-dose ketamine (15 mg/kg) has acute effects on the presynaptic exocytotic proteins in the rat hippocampus (Muller et al. 2013). There was an overall reduction in SNARE proteins accumulation in synaptosomal preparations 1, 2 and 4 h after ketamine administration, which was reflected by decreased synaptotagmin I and increased syntaxin I. Additionally, ketamine reduced the phosphorylation of aCAMKII, thereby decreasing its association with syntaxin I (interestingly, in this study, ketamine appeared to have no effect on GSK-3 expression and activity). As mentioned above, Duman and colleagues have demonstrated that synaptogenesis in the prefrontal cortex (as evidenced by biochemical, morphological and electrophysiological correlates) is critical to ketamine’s rapid antidepressant effects (Li et al. 2010; Liu et al. 2012b), and decreased presynaptic machinery in hippocampal synaptosomes suggests that a reduction in neurotransmitter release seems inconsistent. There might be several reasons for this discrepancy including the area of the brain (prefrontal cortex vs. hippocampus) and timing (24 h vs. 1, 2 and 4 h post-ketamine). Nevertheless, there is an evidence that both reduction in neurotransmitter release and postsynaptic strengthening have mechanistic antidepressant implications, which will require further preclinical experimentation and translation into humans.
Biomarkers of ketamine’s treatment response
The identification of baseline predictor and treatment response biomarkers has been a major focus of investigation by our group and others. Although all the biomarkers described below require validation and qualification before any firm conclusions are drawn, several promising avenues (Table 2) have emerged. Several major positive findings have involved brain-derived neurotrophic factor (BDNF). This is hardly surprising because the discovery that low peripheral BDNF levels respond to effective antidepressant treatment is one of the best-replicated findings in MDD, which is especially impressive considering the clinical heterogeneity of MDD (Shimizu et al. 2003; Kim et al. 2007). In an admittedly small sample, our group did not observe a correlation between peripheral BDNF levels and ketamine’s antidepressant response (Machado-Vieira et al. 2009). Subsequent studies found that changes in peripheral BDNF (a major inducer of synaptic plasticity) were directly proportional to change from baseline slow-wave sleep electroencephalography (a surrogate marker of synaptic plasticity) in ketamine responders (Duncan et al. 2012). Laje et al. (2012) identified the BDNF val66met rs6265 SNP as a predictor of ketamine response; in that study, BDNF val/val homozygotes (n = 41) had a better antidepressant response to ketamine than the met haplotype (n = 21). Smaller hippocampal volumes were observed in BDep patients with the met allele (Chepenik et al. 2009), and knockin of the BDNF met allele in rodents led to basal prefrontal cortex (PFC) synaptic deficits and decreased synaptic strengthening in response to subanesthetic ketamine (Liu et al. 2012b).
Table 2.
Glutamate-based antidepressants as either monotherapy or adjunctive therapy in trials for the treatment of major depressive disorder (MDD)
| Medication | Mechanism of action | Studies | Dose | Route of administration |
|---|---|---|---|---|
| Ketamine | NMDA receptor antagonist (moderate affinity) | Single infusions | ||
| Berman et al. (2000) | 0.5 mg/kg × 40 min | Intravenous | ||
| Zarate et al. (2006a, b) | ||||
| Valentine et al. (2011) | ||||
| Murrough et al. (2013a, b) | Intravenous | |||
| Repeated infusions | ||||
| Murrough et al. (2013a, b) | 0.5 mg/kg × 40 min | |||
| Memantine | NMDA receptor antagonist (low-trapping) | Monotherapy | ||
| Zarate et al. (2006a, b) | 5–20 mg/day | Oral | ||
| CP-101,606/Traxoprodil | NR2B-selective antagonist | Adjunctive therapy | ||
| Preskorn et al. (2008) | 1st 7 patients: 0.75 mg/kg × 1.5 h, 0.15 mg/kg × 6.5 h. 23 patients: 0.5 mg/kg × 1.5 h | Intravenous | ||
| MK-0657 | NR2B-selective antagonist | Monotherapy | ||
| Ibrahim et al. (2012a, b) | 4–8 mg/day | Oral | ||
| AZD6765 | NMDA receptor antagonist (low-trapping) | Monotherapy | ||
| Zarate et al. (2012) | 150 mg over 1 h | Intravenous | ||
| Riluzole | Decreased glutamate release, increased astrocyte GLT-1 expression | Monotherapy | ||
| Zarate et al. (2006a, b) | 100–200 mg/day | Oral | ||
| Adjunctive therapy | ||||
| Sanacora et al. (2007) | 100 mg/day | Oral | ||
| D-Cycloserine (DCS) | NMDA receptor glycine partial agonist | Adjunctive therapy | ||
| Heresco-Levy et al. (2006) | 250 mg/day | Oral | ||
| Heresco-Levy et al. (2013) | 1,000 mg/day | Oral |
Clinical series and case report-level evidence are excluded in this table
Several studies have shown that a key predictor biomarker of ketamine’s antidepressant efficacy is a first-degree relative with a history of an alcohol use disorder. In both MDD (Phelps et al. 2009) and BDep (Luckenbaugh et al. 2012), subjects with a positive family history of alcoholism had a more robust and sustained antidepressant response, which is consistent with the differential effects of ketamine in recently detoxified alcoholics (Krystal et al. 2003b) and in healthy volunteers with a positive family history of alcohol dependence (Petrakis et al. 2004). Genetic polymorphisms in glutamate genes may have contributed to this effect. For instance, the BDNF val66met rs6265 A haplotype, which is overrepresented in subjects with comorbid depression and alcoholism, had a better antidepressant response to sertraline (Su et al. 2011).
Non-invasive neuroimaging and neurophysiological techniques have also been used to probe the neural correlates of ketamine’s antidepressant response (Stahl 2010). For instance, treatment-resistant MDD subjects with increased pretreatment rostral anterior cingulate cortex reactivity to fearful faces had an augmented antidepressant response to ketamine (Salvadore et al. 2009). A magnetoencephalography study administered a spatial working memory task at baseline, preketamine, and post-ketamine and found that performance on this task predicted antidepressant response (Salvadore et al. 2010). Increased baseline slow-wave sleep activity—particularly the delta sleep ratio as defined by the delta wave intensity between the first non-rapid eye movement and second non-rapid eye movement periods (decreased in many patients with MDD; improvement correlates with maintenance of remission) (Kupfer et al. 1990)—positively correlated with ketamine’s antidepressant effects (Duncan et al. 2013). Next, increased tactile stimulus-evoked somatosensory cortical response from baseline to post-ketamine infusion was also correlated with increased antidepressant effects (Cornwell et al. 2012). Finally, a PET study of 20 patients with treatment-resistant MDD measured regional cerebral glucose metabolism at baseline and following ketamine infusion. Although whole-brain metabolism did not change significantly following ketamine, regional metabolism decreased significantly in the habenula, insula, and ventrolateral and dorsolateral prefrontal cortices of the right hemisphere. Metabolism increased post-ketamine in bilateral occipital, right sensorimotor, left parahippocampal, and left inferior parietal cortices. Improvement in depression ratings correlated directly with change in metabolism in right superior and middle temporal gyri. Conversely, clinical improvement correlated inversely with metabolic changes in the right parahippocampal gyrus and temporoparietal cortex (Carlson et al. 2013) [in contrast with bipolar depression, in the nucleus accumbens/ventral striatum, the metabolism of 18F-fluorodeoxyglucose (18FDG) correlates with change in MADRS score in the post-infusion period, and greater pregenual ACC levels post-placebo infusion predicted a more robust antidepressant response to ketamine; also, ketamine decreased radiolabel in the left hippocampus (Nugent et al. 2013)].
Because ketamine increases glutamate in the rodent PFC, acute and/or chronic changes in glutamate have been hypothesized to contribute to ketamine’s antidepressant response. One study of MDD patients found that changes in glutamate, its precursor glutamine, and GABA did not correlate with ketamine’s antidepressant efficacy in the occipital cortex (Valentine et al. 2011). In the MDD PFC, pretreatment glutamate and GABA did not correlate with antidepressant response to ketamine (Salvadore et al. 2012). However, baseline Glx [combination of magnetic resonance imaging (MRS)-detectable glutamine + glutamate]/glutamate ratio was lower in patients with greater antidepressant response, and pretreatment glutamate levels were increased in patients whose anxiety symptoms improved with ketamine (Salvadore et al. 2012). In a healthy volunteer study, subanesthetic-dose ketamine caused no acute changes in Glx or glutamate (Taylor et al. 2012); however, that study used a 3-T magnet, and many authors believe that reliably measuring glutamate at this magnetic strength by 1H-MRS is not possible (Yuksel and Ongur 2010). Although this sample was small, several groups are concurrently investigating ketamine’s effect on amino acid neurotransmitters in both healthy volunteers and depressed patients, therefore additional data are forthcoming.
We also performed a correlational analysis of ketamine metabolite levels in MDD and BDep with nosology, antidepressant response, and acute adverse effects (Zarate et al. 2012). Interestingly, higher levels of hydroxynorketamine-4a and hydroxynorketamine-4c were associated with nonresponse to ketamine in patients with BDep within 230 min. Increased levels of several hydroxylated ketamine metabolites were also associated with lower psychotomimetic side effects. No association was found between several cytochrome P450 genes (responsible for ketamine metabolism) and antidepressant efficacy.
Several other clinical and laboratory assessments have predicted ketamine’s antidepressant efficacy by our group and others (Table 2). First, we pooled our subject-level data from our studies of ketamine in both unipolar and bipolar depression and, using both uni- and multivariate linear regression, we correlated ketamine’s hyperacute (230 min post-infusion), acute (1 day post-infusion), and sustained (7 days post-infusion) antidepressant effects with numerous clinical and demographic features (Niciu et al. 2013). Increased body mass index correlated with ketamine’s hyperacute and acute antidepressant effects, a family history of an alcohol use disorder predicted ketamine’s acute and sustained antidepressant response and no prior history of suicide attempt predicted only the sustained antidepressant response. At day seven, these three features alone predicted more than 1/3 of the variance in ketamine’s antidepressant response. We also have preliminary evidence that dissociative side effects but not psychotomimetic or sympathomimetic side effects correlate with ketamine’s antidepressant effects (Luckenbaugh et al. 2013).
Taken together, the studies described indicate that ketamine likely increases neural connectivity by enhancing synaptic plasticity in several brain regions implicated in depression, e.g., PFC, anterior cingulate cortex, amygdala, and hippocampus. These neuromodulatory effects may improve our existing descriptive and clinically heterogeneous nosology, allow quantification of pretreatment antidepressant probability of efficacy, and provide more reliable measures than those provided by clinical assessments alone. Nevertheless, these promising biomarkers of treatment response require replication/validation in larger studies to advance to surrogate endpoint status (Zarate et al. 2013b).
Ketamine and inflammation
Subanesthetic-dose ketamine has anti-inflammatory effects in both preclinical and clinical studies. A single subanesthetic dose of ketamine inhibited carragenenan-sensitized and endotoxin-induced shock—including the suppression of the pro-inflammatory cytokines TNFα and IL-6—as well as mild-to-moderate hypothermia in rats (Koga et al. 1994; Taniguchi et al. 2001, 2003, 2004). Ketamine also suppressed hepatotoxicity in endoxin-administered rats with concomitant decreases in the pro-inflammatory mediators cyclooxygenase-2 (COX2) and inducible nitric oxide synthase (iNOS) with purported effects via the transcription factor nuclear factor kappa B (NF-κB) (Suliburk et al. 2005). In conjunction with the last cited study, also using electrophoretic mobility shift assays, ketamine inhibited NFkB and another pro-inflammatory transcription factor, activator protein 1 (AP-1) in human neutrophils and a leukocyte-like transformed cell line, which was not dependent on activation of NMDA or opioid receptors (Welters et al. 2010). These transcription factors and selected cytokines play pivotal roles not only in inflammation but also in neurogenesis, neuronal cytoskeletal reorganization, and synaptic plasticity (Albensi and Mattson 2000; Gutierrez and Davies 2011). In the clinical cardiosurgical literature, ketamine also has anti-inflammatory effects at subanesthetic doses. First, in a randomized, double-blind trial of low-dose ketamine vs. inert placebo added onto perioperative fentanyl anesthesia on the day of cardiopulmonary bypass and for 6 days post-operatively, serum IL-6 were suppressed by low-dose (0.25 mg/kg) ketamine during and after coronary artery bypass graft (CABG) (Roytblat et al. 1998). In another randomized, placebo-controlled trial with two doses of ketamine (0.25 or 0.5 mg/kg) vs. placebo in on-pump CABG patients during general anesthesia cardiac induction, both ketamine doses suppressed the elaboration of several pro-inflammatory markers/cytokines—C-reactive protein (CRP), IL-6, and IL-10 (but not IL-8)—with concomitant sympathomimetic effects (Bartoc et al. 2006). Yes, in off-pump CABG, ketamine did not decrease serum levels of pro-inflammatory cytokines—CRP, IL-6, tumor necrosis factor-alpha (TNFα), and cardiac enzymes—in the perioperative period (Cho et al. 2009). To date, there have not been any studies examining levels of pro-inflammatory mediators in response to subanesthetic-dose ketamine for major depression, but this is an area of active investigation by many groups studying ketamine as a rapidly acting anti-depressant in both the preclinical and clinical realms.
Conclusions
We have provided a basic introduction to glutamate and its receptors, then reviewed the clinical literature in the pathophysiology and treatment of MDD. Although initially overshadowed by monoamines, the identification of glutamate-based mechanisms of disease and treatment options, particularly with the rapid-acting antidepressant ketamine, has kindled both academic and public fervor in alternative strategies for treatment-resistant MDD. Although promising, ketamine and related glutamatergic compounds cannot be recommended at this time for routine clinical practice outside of a research milieu due to the lack of multisite, randomized, placebo (and active placebo)-controlled trials with much larger samples (n > 100) to better assess efficacy, safety and tolerability. However, ketamine may be useful late in a treatment algorithm on a case-by-case basis for (temporary) symptom relief and/or a bridge to alternative therapies particularly in specialized treatment-resistant depression centers.
Future research should focus on the modulation of specific glutamatergic circuitry, e.g., GABAergic cortical interneurons-to-glutamatergic cortical outflow (pyramidal) neurons. The manipulation of critical glutamatergic circuitry may occur either pharmacologically, i.e., more selective glutamatergic medications, or technologically, i.e., transcranial magnetic or deep brain stimulation.
Acknowledgments
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health, and Department of Health and Human Services (IRP-NIMH-NIH-DHHS) and by a NARSAD Independent Investigator and the Brain and Behavior Mood Disorders Research Award (CAZ).
Footnotes
Conflict of interest Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government, but will share a percentage of any royalties that may be received by the government.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT. 2012;28:157–161. doi: 10.1097/YCT.0b013e31824f8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, Hellemann G, Vinters HV. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12:541–549. doi: 10.1111/j.1399-5618.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bartoc C, Frumento RJ, Jalbout M, Bennett-Guerrero E, Du E, Nishanian E. A randomized, double-blind, placebo-controlled study assessing the anti-inflammatory effects of ketamine in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2006;20:217–222. doi: 10.1053/j.jvca.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Blier P, Zigman D, Blier J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry. 2012;72:e11–e12. doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Manji HK, Zarate CA, Jr, Drevets WC. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Macedo DS, Goulia P, Hyphantis TN. N-acetylcysteine augmentation to tranylcypromine in treatment-resistant major depression. J Clin Psychopharmacol. 2013;33:719–720. doi: 10.1097/JCP.0b013e31829839c6. [DOI] [PubMed] [Google Scholar]
- Chan MH, Chiu PH, Lin CY, Chen HH. Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr Res. 2012;136:96–103. doi: 10.1016/j.schres.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, Gelernter J, Blumberg HP. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JE, Shim JK, Choi YS, Kim DH, Hong SW, Kwak YL. Effect of low-dose ketamine on inflammatory response in off-pump coronary artery bypass graft surgery. Br J Anaesth. 2009;102:23–28. doi: 10.1093/bja/aen325. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokic B, Stein V. Stargazin modulates AMPA receptor antagonism. Neuropharmacology. 2008;54:1062–1070. doi: 10.1016/j.neuropharm.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA., Jr Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cusin C, Hilton GQ, Nierenberg AA, Fava M. Long-term maintenance with intramuscular ketamine for treatment-resistant bipolar II depression. Am J Psychiatry. 2012;169:868–869. doi: 10.1176/appi.ajp.2012.12020219. [DOI] [PubMed] [Google Scholar]
- de Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem Int. 1996;28:445–452. doi: 10.1016/0197-0186(95)00091-7. [DOI] [PubMed] [Google Scholar]
- De Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, Mitra D, Watanabe N, Nakamura H, Tjioe I, Deresinski SC, Moore WA, Ela SW, Parks D, Herzenberg LA, Herzenberg LA. N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest. 2000;30:915–929. doi: 10.1046/j.1365-2362.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck CW. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiatry. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. Eur J Neurosci. 2010;32:261–268. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Miranda AS, Talib LL, Gattaz WF, Forlenza OV. Circulating Glial-derived neurotrophic factor is reduced in late-life depression. J Psychiatr Res. 2012;46:135–139. doi: 10.1016/j.jpsychires.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2012;16(2):301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Jr, Selter J, Brutsche N, Sarasso S, Zarate CA., Jr Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord. 2013;145:115–119. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S, Gottesman II. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res. 2004;69:317–323. doi: 10.1016/j.schres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA. Clinical practice. Bipolar disorder—a focus on depression. N Engl J Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, Zheng L, Meyer-Lindenberg A, Weber-Fahr W. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.290. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-kappaB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, Kremer I. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006;93:239–243. doi: 10.1016/j.jad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16:501–506. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA., Jr Rapid decrease in depressive symptoms with an N-methyl-D-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, Potter WZ, Zarate CA., Jr A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012a;32:551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012b;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13:903–908. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarventausta K, Chrapek W, Kampman O, Tuohimaa K, Bjorkqvist M, Hakkinen H, Yli-Hankala A, Leinonen E. Effects of s-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J ECT. 2013;29:158–161. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Khundakar AA, Morris CM, Oakley AE, Thomas AJ. Cellular pathology within the anterior cingulate cortex of patients with late-life depression: a morphometric study. Psychiatry Res. 2011;194:184–189. doi: 10.1016/j.pscychresns.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Lee SW, Yoon D, Han C, Kim DJ, Choi SH. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Millar AM, McIntosh JF, Shah DK, Fisher DJ, Blais CM, Ilivitsky V, Horn E. Separate and combined effects of low dose ketamine and nicotine on behavioural and neural correlates of sustained attention. Biol Psychol. 2011;88:83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Koga K, Ogata M, Takenaka I, Matsumoto T, Shigematsu A. Ketamine suppresses tumor necrosis factor-alpha activity and mortality in carrageenan-sensitized endotoxin shock model. Circ Shock. 1994;44:160–168. [PubMed] [Google Scholar]
- Kollmar R, Markovic K, Thurauf N, Schmitt H, Kornhuber J. Ketamine followed by memantine for the treatment of major depression. Aust N Z J Psychiatry. 2008;42:170. doi: 10.1080/00048670701787628. [DOI] [PubMed] [Google Scholar]
- Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci. 2011;261:575–582. doi: 10.1007/s00406-011-0205-7. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, 3rd, Falcone G, Coffey CE. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003a;15:27–34. doi: 10.1176/jnp.15.1.27. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, Boutros NN, Trevisan L, Charney DS. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003b;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. NeuroReport. 1995a;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995b;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C., Jr Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. Dextromethorphan as a potential rapid-acting antidepressant. Med Hypotheses. 2011;76:717–719. doi: 10.1016/j.mehy.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Med Hypotheses. 2012;78:693–702. doi: 10.1016/j.mehy.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, Tzeng NS, Wang CL, Lee IH, Yeh TL, Yang YK, Lu RB. The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. J Affect Disord. 2012;138:295–300. doi: 10.1016/j.jad.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27:974–980. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhu HY, Li B, Wang YQ, Yu J, Wu GC. Chronic clomipramine treatment restores hippocampal expression of glial cell line-derived neurotrophic factor in a rat model of depression. J Affect Disord. 2012a;141:367–372. doi: 10.1016/j.jad.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012b;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affect Disord. 2012;142:233–240. doi: 10.1016/j.jad.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, Cassarly C, Zarate CA., Jr Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2013 doi: 10.1016/j.jad.2014.02.017. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA., Jr Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antide-pressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, Aan Het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2009;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72:1313–1333. doi: 10.2165/11633130-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- McNulty JP, Hahn K. Compounded oral ketamine. Int J Pharm Compd. 2012;16:364–368. [PubMed] [Google Scholar]
- Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- Messias E, Everett B. Dextromethorphan and quinidine combination in emotional lability associated with depression: a case report. Prim Care Companion CNS Disord. 2012;14 doi: 10.4088/PCC.12l01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003a;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003b;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Millan MJ. N-methyl-D-aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia: a critical review. Curr Drug Targets CNS Neurological Disord. 2002;1:191–213. doi: 10.2174/1568007024606258. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Sinclair D, Lonnqvist J, Alho H. Treatment of alcohol dependence in patients with co-morbid major depressive disorder—predictors for the outcomes with memantine and escitalopram medication. Subst Abuse Treat Prev Policy. 2008a;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lonnqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008b;69:392–399. doi: 10.4088/jcp.v69n0308. [DOI] [PubMed] [Google Scholar]
- Muller HK, Wegener G, Liebenberg N, Zarate CA, Jr, Popoli M, Elfving B. Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res. 2013;47:892–899. doi: 10.1016/j.jpsychires.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H, Schubert MI, Schmid D, Schussler P, Steiger A, Auer DP. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression—an MR spectroscopy study. J Psychiatr Res. 2009;43:175–180. doi: 10.1016/j.jpsychires.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry. 2011;72:414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013a;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013b;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry. 2013;73:1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2013 doi: 10.4088/JCP.13m08698. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Drevets WC, Zarate CA., Jr Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2013 doi: 10.1111/bdi.12118. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Ostroff R, Gonzales M, Sanacora G. Antidepressant effect of ketamine during ECT. Am J Psychiatry. 2005;162:1385–1386. doi: 10.1176/appi.ajp.162.7.1385. [DOI] [PubMed] [Google Scholar]
- Pallavi P, Sagar R, Mehta M, Sharma S, Subramanium A, Shamshi F, Sengupta U, Qadri R, Pandey RM, Mukhopadhyay AK. Serum neurotrophic factors in adolescent depression: gender difference and correlation with clinical severity. J Affect Disord. 2013;150:415–423. doi: 10.1016/j.jad.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A, Dorszewska J, Bartkowska-Sniatkowska A, Chlopocka-Wozniak M, Rybakowski JK. Vitamin B12 level may be related to the efficacy of single ketamine infusion in bipolar depression. Pharmacopsychiatry. 2013;46:227–228. doi: 10.1055/s-0033-1349861. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry. 2013;73:613–621. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices—guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11:56–63. doi: 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roytblat L, Talmor D, Rachinsky M, Greemberg L, Pekar A, Appelbaum A, Gurman GM, Shapira Y, Duvdenani A. Ketamine attenuates the interleukin-6 response after cardiopulmonary bypass. Anesth Analg. 1998;87:266–271. doi: 10.1097/00000539-199808000-00006. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR. Combining medications to enhance depression outcomes (COMED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A. Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol. 2013;28:87–90. doi: 10.1002/hup.2271. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, Ibrahim LA, Luckenbaugh DA, Shen J, Drevets WC, Zarate CA. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2011;15(8):1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, Ibrahim LA, Luckenbaugh DA, Shen J, Drevets WC, Zarate CA., Jr An investigation of aminoacid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15:1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004a;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. Am J Psychiatry. 2004b;161:2132. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J Affect Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Schulz D, Smith D, Yu M, Lee H, Henn FA. Selective breeding for helplessness in rats alters the metabolic profile of the hippocampus and frontal cortex: a 1H-MRS study at 9.4 T. Int J Neuropsychopharmacol. 2013;16:199–212. doi: 10.1017/S1461145711001994. [DOI] [PubMed] [Google Scholar]
- Seese RR, Chen LY, Cox CD, Schulz D, Babayan AH, Bunney WE, Henn FA, Gall CM, Lynch G. Synaptic abnormalities in the infralimbic cortex of a model of congenital depression. J Neurosci. 2013;33:13441–13448. doi: 10.1523/JNEUROSCI.2434-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34(4):287–293. [PubMed] [Google Scholar]
- Stahl SM. Psychiatric stress testing: novel strategy for translational psychopharmacology. Neuropsychopharmacology. 2010;35:1413–1414. doi: 10.1038/npp.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Su N, Zhang L, Fei F, Hu H, Wang K, Hui H, Jiang XF, Li X, Zhen HN, Li J, Cao BP, Dang W, Qu Y, Zhou F. The brain-derived neurotrophic factor is associated with alcohol dependence-related depression and antidepressant response. Brain Res. 2011;1415:119–126. doi: 10.1016/j.brainres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–1278. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliburk JW, Helmer KS, Gonzalez EA, Robinson EK, Mercer DW. Ketamine attenuates liver injury attributed to endotoxemia: role of cyclooxygenase-2. Surgery. 2005;138:134–140. doi: 10.1016/j.surg.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Sung SC, Haley CL, Wisniewski SR, Fava M, Nierenberg AA, Warden D, Morris DW, Kurian BT, Trivedi MH, Rush AJ. The impact of chronic depression on acute and long-term outcomes in a randomized trial comparing selective serotonin reuptake inhibitor monotherapy versus each of 2 different antidepressant medication combinations. J Clin Psychiatry. 2012;73:967–976. doi: 10.4088/JCP.11m07043. [DOI] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Shibata K, Yamamoto K. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology. 2001;95:928–932. doi: 10.1097/00000542-200110000-00022. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Takemoto Y, Kanakura H, Kidani Y, Yamamoto K. The dose-related effects of ketamine on mortality and cytokine responses to endotoxin-induced shock in rats. Anesth Analg. 2003;97:1769–1772. doi: 10.1213/01.ANE.0000085634.72426.ED. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Kanakura H, Takemoto Y, Yamamoto K. The antiinflammatory effects of ketamine in endotoxemic rats during moderate and mild hypothermia. Anesth Analg. 2004;98:1114–1120. doi: 10.1213/01.ANE.0000100740.07331.A2. [DOI] [PubMed] [Google Scholar]
- Taylor M, Murphy SE, Selvaraj S, Wylezinkska M, Jezzard P, Cowen PJ, Evans J. Differential effects of citalopram and reboxetine on cortical Glx measured with proton MR spectroscopy. J Psychopharmacol. 2008;22:473–476. doi: 10.1177/0269881107081510. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Norbury R, Murphy S, Rudebeck S, Jezzard P, Cowen PJ. Lack of effect of citalopram on magnetic resonance spectroscopy measures of glutamate and glutamine in frontal cortex of healthy volunteers. J Psychopharmacol. 2010;24:1217–1221. doi: 10.1177/0269881109105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT. 2012;28:128–132. doi: 10.1097/YCT.0b013e31824d1d02. [DOI] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters ID, Hafer G, Menzebach A, Muhling J, Neuhauser C, Browning P, Goumon Y. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth Analg. 2010;110:934–941. doi: 10.1213/ANE.0b013e3181c95cfa. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med. 2012;15:400–403. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006a;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006b;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013a;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013b;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Z, Xie C, Xi G, Zhou H, Zhang Y, Sha W. Effect of treatment on serum glial cell line-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:886–890. doi: 10.1016/j.pnpbp.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Z, Sha W, Xie C, Xi G, Zhou H, Zhang Y. Electroconvulsive therapy increases glial cell-line derived neurotrophic factor (GDNF) serum levels in patients with drug-resistant depression. Psychiatry Res. 2009;170:273–275. doi: 10.1016/j.psychres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, Hong W, Yuan C, Wang Z, Yu S, Xu Y, Xu L, Xiao Z, Fang Y. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl) 2013a doi: 10.1007/s00213-013-3297-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zhang J, Narr KL, Woods RP, Phillips OR, Alger JR, Espinoza RT. Glutamate normalization with ECT treatment response in major depression. Mol Psychiatry. 2013b;18:268–270. doi: 10.1038/mp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wu J, Yang C. Concerns about dextromethorphan as a potential rapid-acting antidepressant. Med Hypotheses. 2011;77:309–310. doi: 10.1016/j.mehy.2011.05.013. [DOI] [PubMed] [Google Scholar]