Abstract
Background
Congenital diaphragmatic hernia (CDH) is a frequently lethal birth defect and despite advances, extracorporeal life-support (ECMO) is commonly required for severely affected patients. Published data suggest that CDH survival after 2 weeks on ECMO is poor. Many centers limit duration of ECMO support.
Study Design
A single institution retrospective review of 19 years of CDH patients treated with ECMO, designed to evaluate which factors affect survival and duration of ECMO, and to define how long patients should be supported.
Results
Of two hundred and forty consecutive CDH patients without lethal associated anomalies, 96 were treated with ECMO and 72 (75%) survived. Eighty required a single run of ECMO and 65 survived (81%), 16 required a second ECMO run and 7 survived (44%). Of patients still on ECMO at 2 weeks, 56% survived, at 3 weeks 46% survived, and at 4 weeks, 43% of patients still on ECMO survived to discharge. After 5 weeks of ECMO survival had dropped to 15%, and after 40 days of ECMO support there were no survivors. Apgar-1, Apgar-5, and CDH Study Group Predicted Survival all correlated with survival on ECMO, need for second ECMO, and duration of ECMO. LHR also correlated with duration of ECMO. All survivors were discharged breathing spontaneously with no support other than nasal cannula oxygen if needed.
Conclusions
In patients with severe CDH, improvement in pulmonary function sufficient to wean from ECMO may take 4 weeks or longer, and may require a second ECMO run. Pulmonary outcomes in these CDH patients can still be excellent, and the assignment of arbitrary ECMO treatment durations less than 4 weeks should be avoided.
Introduction
Congenital diaphragmatic hernia (CDH) is a severe birth defect with a broad spectrum of severity. Fortunately many patients are born with low and moderate severity defects and are cared for with standard ventilatory techniques. Patients with more severe CDH, however, often require extracorporeal life-support (ECMO) to maximize survival potential.1,2 While gentle ventilatory techniques have proven to be a necessary component of successful therapeutic strategies3, advances in ventilatory support, protocolized care, and wider use of nitric oxide and other pulmonary vasodilators have been unsuccessful in eliminating the need for ECMO in CDH patients.4–7
In CDH patients who require ECMO, survival rates are lower and complication rates are higher, which is intuitive given that patients who require ECMO represent more severe disease than those who do not. ECMO support is associated with a myriad of additional risks including bleeding, renal dysfunction, embolic phenomena, and compromised neurologic outcome to name a few8–10. Based on published and center-specific data, it is not uncommon for centers to limit the total time of ECMO support in CDH patients that do not improve within assigned time frames. Tiruvoipati and Peek found only an 18% survival rate in CDH patients supported on ECMO beyond two weeks11, and in discussion with other centers we have found 2 weeks to be a common reference point regarding CDH survival on ECMO.
We were early adopters of the gentle ventilatory techniques taught by Wung12 and Stolar13, and helped define the negative effects of hyperventilation14. These ventilation techniques form the foundation of our treatment strategy, and we have offered ECMO to patients who could not be adequately supported with these techniques, regardless of the severity of the underlying CDH physiology.
In this study we review 19 consecutive years of CDH treatment at our institution, to evaluate which patient factors affect survival and duration of ECMO, to evaluate the success of second ECMO runs when needed, to define the ECMO duration required for more severe CDH patients to recover, and to ask if there is a point at which further ECMO support becomes futile?
Methods
This is a retrospective review of consecutive patients with Congenital Diaphragmatic Hernia treated at the UF Health and Shands Hospital for Children between September 1992 and December 31, 2011. A total of 268 CDH patients were identified from the cross reference of two separate medical record queries with operative records, autopsy records, a divisional database, and 2 prenatal evaluation databases. Patients with Morgagni CDH, diaphragmatic eventration, and patients in whom the diagnosis of CDH was missed and delayed more than 48 hours after delivery were not included. All patients were symptomatic in the first 6 hours of life Of 268, 28 (10%) were judged to have lethal associated anomalies15. One hundred and five patients of the total were treated with ECMO. Nine of those were eventually judged to have associated lethal anomalies and are reported here for completeness and transparency, but are not analyzed further. Ninety-six CDH patients, without lethal associated anomalies, were treated with ECMO and comprise the subjects of this study, which was approved by the University of Florida Institutional Review Board.
Clinical Care
The majority of patients were prenatally diagnosed and counseled at our facility. Termination of CDH pregnancies did not occur in patients with non-lethal associated anomalies, regardless of severity, and as such terminations did not affect the survival results reported here. All patients were treated with strict limitation of ventilation pressures, avoidance of hyperventilation, and use of mild sedation as previously described14. Medical oversight throughout the series was uniform, leading to a high degree therapeutic consistency.
ECMO was used for standard indications (OI> 40) and only when available modalities to avoid it (pressors, nitric oxide, steroids, milrinone) had failed. Either veno-venous (VV) or veno-arterial (VA) ECMO was used, and patients who declined on VV ECMO were converted to VA. As the experience matured, VA ECMO was used preferentially for those patients judged by anatomy, LHR, and initial blood gas values to have more severe pulmonary hypoplasia.
Management on ECMO was not considered different from standard. Nitric oxide if started before ECMO was weaned off within 24 hours of initiating ECMO. Patients on HFOV were generally transitioned to conventional ventilation. Ultrafiltration and dialysis were strictly avoided. Representative resting ventilator settings were IMV 30, inspiratory time of 0.6 seconds, PEEP of 6 cm H2O, PIP of 22 cm of H2O, and FiO2 of 0.4. Desired standards for discontinuing ECMO were satisfactory blood gases on IMV 60 or less and FiO2 of 0.50 or less. Patients successfully weaned from ECMO but who later declined and again met ECMO criteria were considered for a second run of ECMO. During the first 6 years of the study patients were decannulated at ECMO cessation, and recannulated if a second ECMO run was necessary. Since then we have perfused the ECMO cannulas with heparinized saline for 48 – 72 hours after cessation of ECMO before removing them, in case a second run of ECMO is needed. Second run ECMO was also either VV or VA.
Patients were removed from ECMO when they met discontinuation criteria, when complications or additional organ failure made the likelihood of recovery appear dismal, but not for arbitrarily defined time endpoints.
Data collected and used for this analysis include gestational age, birth weight, apgar scores at 1 and 5 minutes, CDH study group predicted survival, side of defect, and liver position for left CDH. Use and type of ECMO, timing of ECMO and CDH repair, duration of ECMO, number of ECMO runs, and condition at discharge were also collected. Fifty-eight patients of 96 patients had prenatal lung-to-head ratio (LHR) measured and LHR observed to expected (LHR o/e) ratios calculated (http://www.perinatology.com/calculators/LHR.htm). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Florida.16
Analysis
Survival results were analyzed for the 96 patients in aggregate, and stratified by anatomic definitions of right, left liver-down, and left liver-up. Gestational age, birthweight, Apgar at 1 minute (Apgar-1) and Apgar at 5 minutes (Apgar-5), and CDH Study Group Predicted Survival17 (http://nicutools.org) were tested for affects on survival. To evaluate duration of ECMO required to improve related to patient severity and anatomic variables, 4 patients who died early From ECMO complications (cannulation complications, oxygenator failure, and intra-cranial hemorrhage) were removed from the duration analysis only. Patients who required a second run of ECMO were also tested for associations with patient risk factors. Survivors only were then analyzed to define time required to improve on ECMO, and evaluated for correlations with patient severity factors, anatomical subsets, and CDH Study Group Predicted survival.
Statistical Analysis
We used the R statistical software package (Vienna, Austria; V.3.0.2) to conduct all analyses and create all graphics. We used logistic regression to model survival as a function of time on ECMO. We used Spearman’s correlation or Mann-Whitney tests to evaluate the associations between ECMO duration and birthweight, Apgar-1 and 5, LHR, observed vs. expected LHR, and type of initial ECMO (VV vs. VA). We used t-tests or Mann-Whitney tests, as appropriate, to evaluate the associations between survival on ECMO and severity indices, and between the need for second ECMO and severity indices. We used analysis of variance (ANOVA), with the natural log of ECMO duration as the outcome, to evaluate the association between ECMO duration and anatomy. We used Fisher’s exact test to evaluate the association between anatomy and survival and between anatomy and need for second ECMO. Finally, to facilitate visual comparison of expected ECMO durations by anatomy and by treatment sequence, we fit gamma distributions to the data using a best-fit algorithm (the “fitdistr” function in the R package MASS) and plotted these distributions side by side.
Results
Of 268 consecutive CDH patients, 210 survived for an overall survival of 78%. 105 (39%) were supported with ECMO at some time before their discharge or death. Congenital diaphragmatic hernia study group predicted survival for these patients was 54 patients (51.5%,) and 72 survived (69%, p =0.016).
Twenty-eight of the 268 were judged to have lethal associated anomalies, and survival of the remaining 240 was 210 (88%). Of the 28 defined as having lethal associated anomalies, nine had received some support from ECMO, often only briefly while the full extent of the anomalies was assessed. These included 4 patients with bilateral CDH, 2 patients with severe combined vertebral and chest wall anomalies, two with cardiac defects (severe tricuspid valve anomaly and complete heart block) and one with hepato-pulmonary fusion complicated by AV canal.
Ninety-six (40%) of 240 consecutive CDH patients without lethal associated anomalies were treated with ECMO at some point in their clinical course and are analyzed further. Sixtynine (72%) were inborn. Included patients did have associated anomalies which were considered non-lethal, and these included ASD (8), VSD (5), coarctation of the aorta (1), sacral dysgenesis requiring colostomy (1), congenital CMV infection (1), and multiple patients with chromosomal anomalies including inversion 13, 10q deletion, XXX 16q+, unbalanced translocation with partial trisomy 2, and partial trisomy 19 with a ring chromosome.
Seventy-two of 96 (75%) treated with ECMO survived to discharge. All patients were discharged home breathing spontaneously and on no support other than nasal cannula oxygen.
Surgical Timing Relative to ECMO
Table 1 reports the surgical timing related to ECMO and survivals.
Table 1.
Operative Status of CDH/ ECMO patients
| n | Survived | |
|---|---|---|
| Repaired before ECMO | 30 | 27 (90%) |
| Repaired during ECMO | 34 | 23 (68%) |
| Repaired after ECMO | 25 | 22 (88%) |
| Never Repaired | 7 | 0 (0%) |
Proportion Surviving versus Time
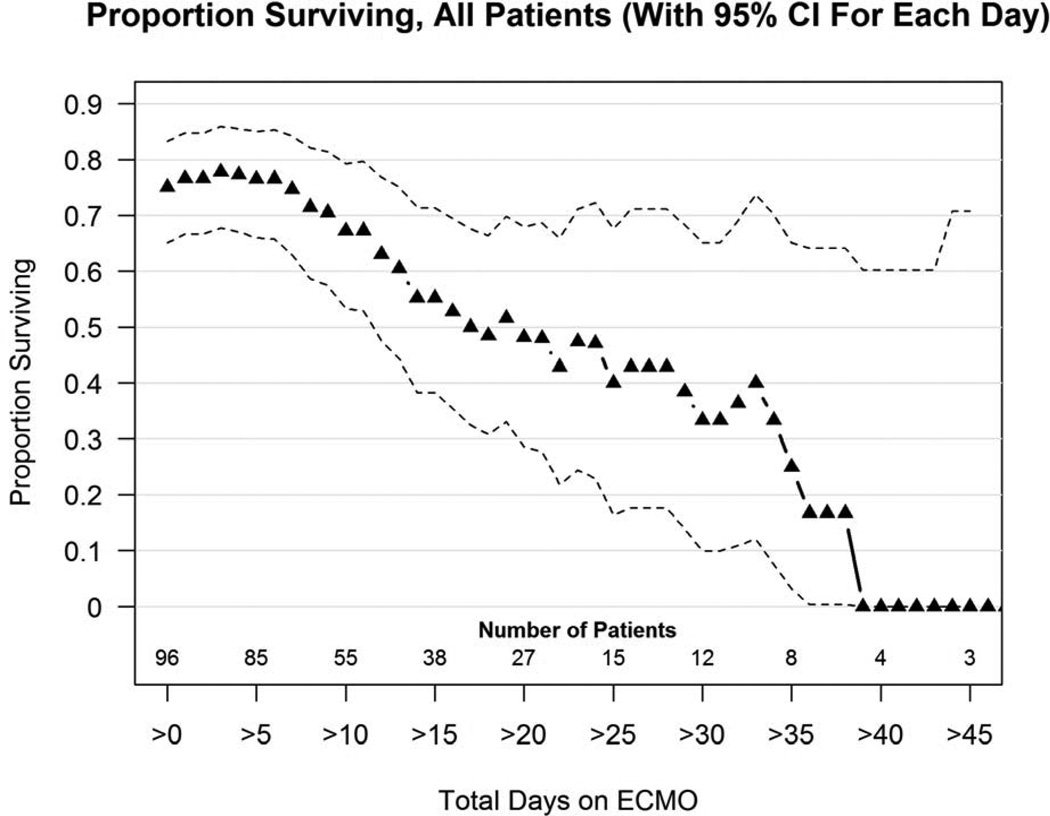
Figure 1 shows the proportion surviving versus days on ECMO for all 96 patients. Of patients who required more than 2 weeks of ECMO support, 56% survived, at 3 weeks 46% survived, and at 4 weeks, 43% of patients still on ECMO survived to discharge. After 5 weeks of ECMO survival had dropped to 15%, and after 40 total days of ECMO support there were no survivors. All survivors of ECMO runs longer than 3 weeks underwent CDH repair prior to 3 weeks.
Figure 1.
Proportion surviving versus days on ECMO curve for 96 consecutive CDH patients without lethal associated anomalies treated with ECMO. This plot includes both first and second run ECMO patients. Survival after 2 weeks on ECMO is 56%, after 3 weeks on ECMO is 46%, after 4 weeks on ECMO is 43%, and after 5 weeks on ECMO is 25%. Number of patients still on ECMO is shown along the bottom of the graph. Triangles represent the percent of patients still on ECMO at any time point who survive to discharge. Dotted lines represent confidence intervals.
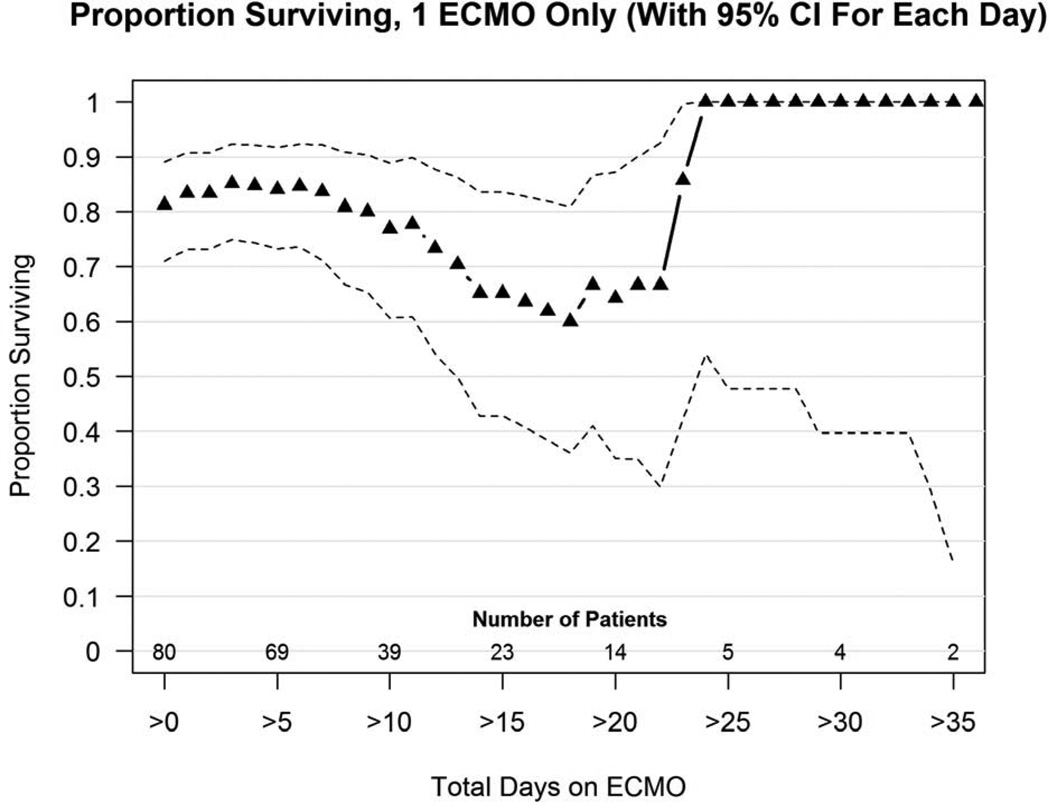
Eighty of 96 patients (83%) received one ECMO run only and 65 survived (81%). A proportion surviving plot for these patients (Figure 2a) shows that at 3 weeks, survival of patients still on ECMO is 62%, and at 4 weeks 100% of patients still on ECMO survived to discharge, and 2 patients still on ECMO at 5 weeks survived.
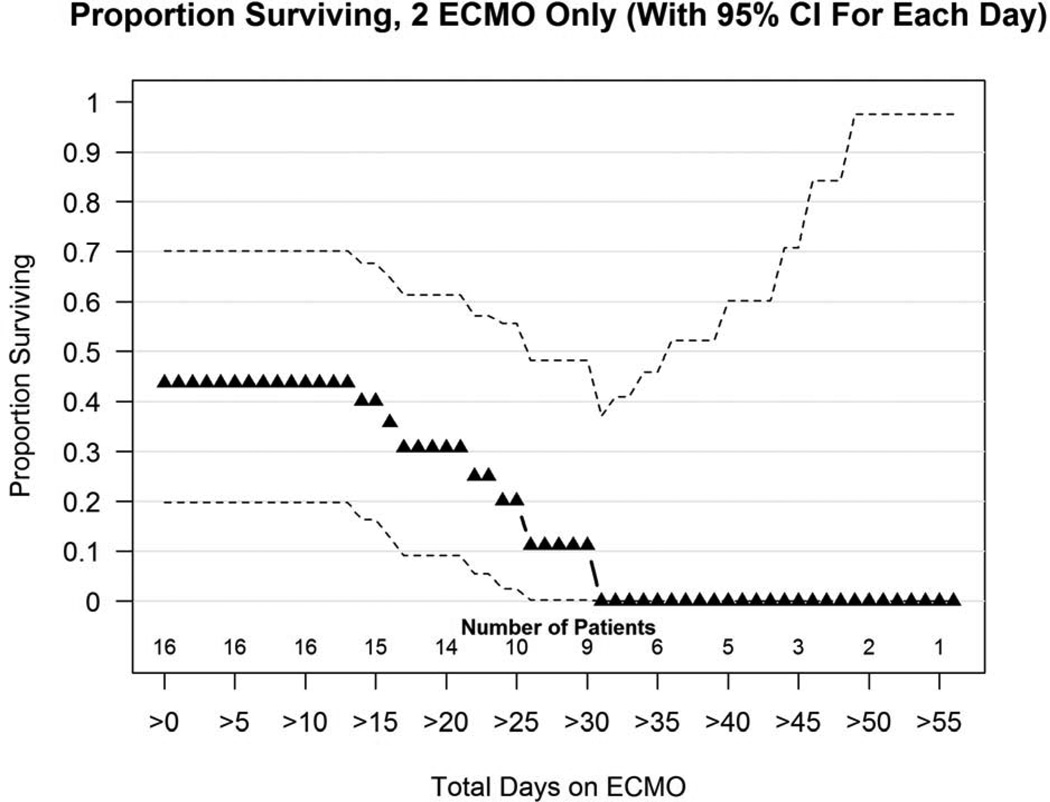
Figure 2.
a and 2b: (a). Proportion surviving versus days on ECMO for 80 CDH patients who required just 1 ECMO run. (b) Proportion surviving versus days on ECMO for 16 patients treated with a second ECMO run. Number of patients still on ECMO is shown along the bottom of the graph. Triangles represent the percent of patients still on ECMO at any time point who survive to discharge. Dotted lines represent confidence intervals.
Sixteen patients worsened after their first ECMO and were treated with a second run of ECMO (17%), and 7 survived to discharge (44%). Figure 2b shows the proportion surviving versus days on ECMO plot for second run ECMO. At 2 weeks survival was 40%, at 3 weeks survival was 4 of 11 (31%), by 4 weeks survival had dropped to 1 of 10 (10%). After 31 days of total ECMO in patients who had 2 runs, there were no survivors out of 9 (0%).
Patient Severity Factors Affecting Survival on ECMO
Birth weight and gestational age did not correlate with survival on ECMO, but Apgar-1, Apgar-5, and CDHSG predicted survival were all strongly associated with survival on ECMO. Not all patients had an LHR measured (58 of 90, 60%). The LHR and LHR o/e was higher in the patients treated with ECMO who survived, but the difference was not statistically significant. (Table 2)
Table 2.
Correlation between Indices of Severity and Survival on ECMO
| Total, n=96 | Survivors, n=72 | Non-survivors, n=24 |
p Value | |
|---|---|---|---|---|
| Gestational age | 37.6 +/−1.8 | 37.7 +/−1.8 | 37.4 =/−1.9 | 0.563 |
| Birthweight | 2891 +/−475 | 2903 +/−490 | 2852 +/−435 | 0.635 |
| Apgar-1 (n= 95) | 3.24 +/−2.1 | 3.61+/−2.1 | 2.1+/−1.5 | 0.002* |
| Apgar-5 (n= 93) | 5.70 +/−2.1 | 6.0 +/−2.1 | 4.7 +/−1.9 | 0.011* |
| LHR (n=58) | 1.15 +/−0.5 | 1.20 +/−0.51 | 1.01 +/−0.48 | 0.210 |
| LHR o/e (n=58) | 29.4 +/−12.0 | 29.71 +/−11.1 | 28.4 +/−14.6 | 0.132 |
| CDHSG Pred Survival | 52 (54%) | 42 (58%) | 10 (42%) | 0.004* |
| Observed Survival | 72 (75%) | 0.001* |
Significant.
Factors Affecting Need for a 2nd Run of ECMO
Gestational age and birth weight did not correlate with need for a second run of ECMO, Apgar-1 showed a marginal association, but Apgar-5 and CDHSG Predicted Survival showed a strong relationship with need for a second run of ECMO. Although the number of patients receiving prenatal LHR was a fraction of the total, LHR showed a marginal relationship with need for a second ECMO run (Table 3).
Table 3.
Relationship between Indices of Severity and Need for Second ECMO
| Total, 1 run n=78 |
Total, 2 runs n=16 |
p Value | |
|---|---|---|---|
| Birthweight | 2906 +/−485 | 2814 +/−429 | p= .435 (ns) |
| Apgar-1 (n=93) | 3.4 +/−2.1 | 2.3 +/−1.7 | p=.061 (marginal) |
| Apgar-5 (n= 93) | 5.9 +/−2.1 | 4.5 +/−2.1 | p=.034 |
| CDHSG Pred Survival (n=93) | 56.7+/−21.2 | 40.7 +/−20.6 | p= .016 |
| LHR (n=58) | 1.2 +/−0.5 | 0.90 +/−0.3 | p=.068 (marginal) |
| LHR o/e (n=58) | 30.6 +/−12.6 | 23.6 +/−6.9 | p= .106 (ns) |
Patients with associated lethal anomalies are not included.
Duration of ECMO relative to Severity Markers
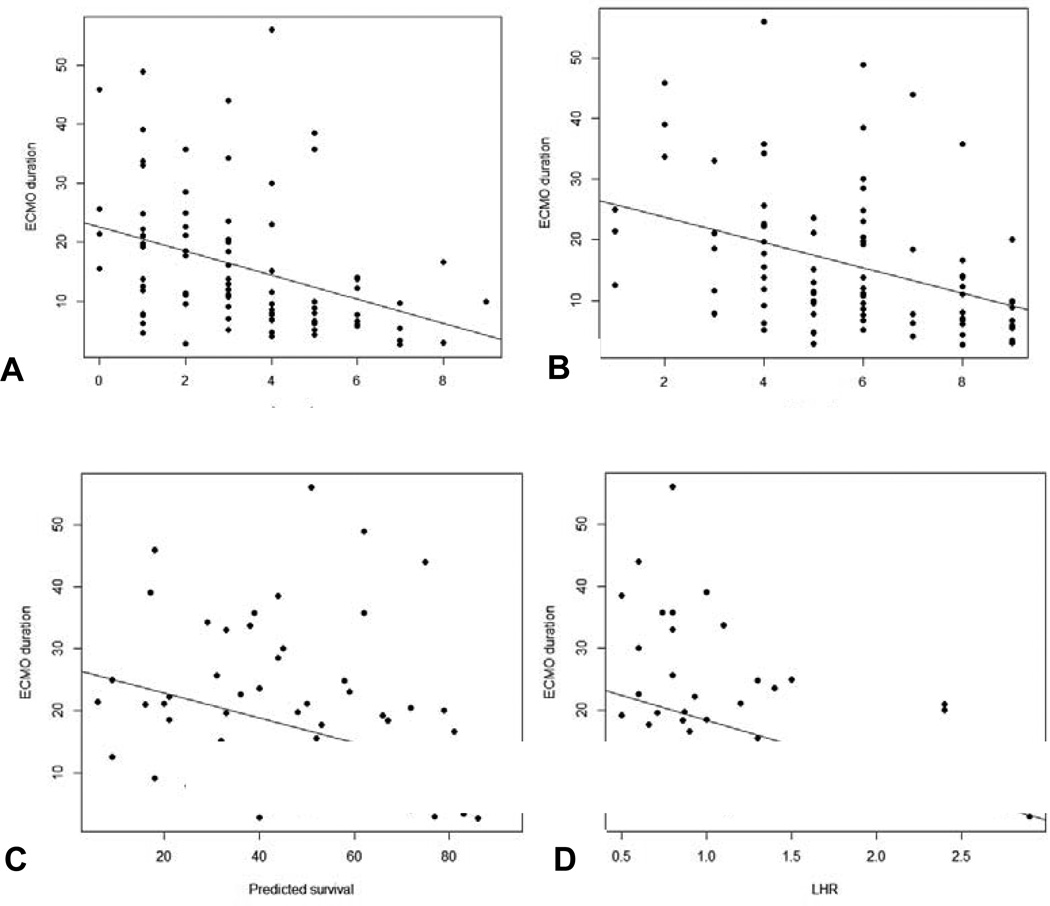
Duration of ECMO was not related to gestational age and birth weight, but was strongly related Apgar-1, Apgar-5, CDHSG Predicted Survival, and LHR. (Figure 3). Linear regression defines the magnitude of effect that changes in each variable has on expected ECMO duration (Figure 3).
Figure 3.
a–d: Relationship between severity markers and duration of ECMO run. (a) Apgar-1: It is estimated that ECMO duration increases by 2.0 days for each unit decrease in Apgar-1 (95% CI=[−3.11, −.964], p<.001). The plot below shows the data with a line of best fit. (b): Apgar-5: It is estimated that ECMO duration increases by 2.1 days for each unit decrease in Apgar-5 (95% CI=[−3.16, −1.00], p<.001). The plot below shows the data with a line of best fit. (c): CDH SG Predicted Survival: It is estimated that ECMO duration increases by 2.0 days for each 10 unit decrease in survival score (95% CI=[−0.306, −0.097], p<.001). The plot below shows the data with a line of best fit. (d): LHR: It is estimated that ECMO duration increases by 0.8 days for each 0.1 decrease in LHR (95% CI=[−1.37, −.234], p=.006). The plot below shows the data with a line of best fit.
Duration of ECMO relative to Anatomy
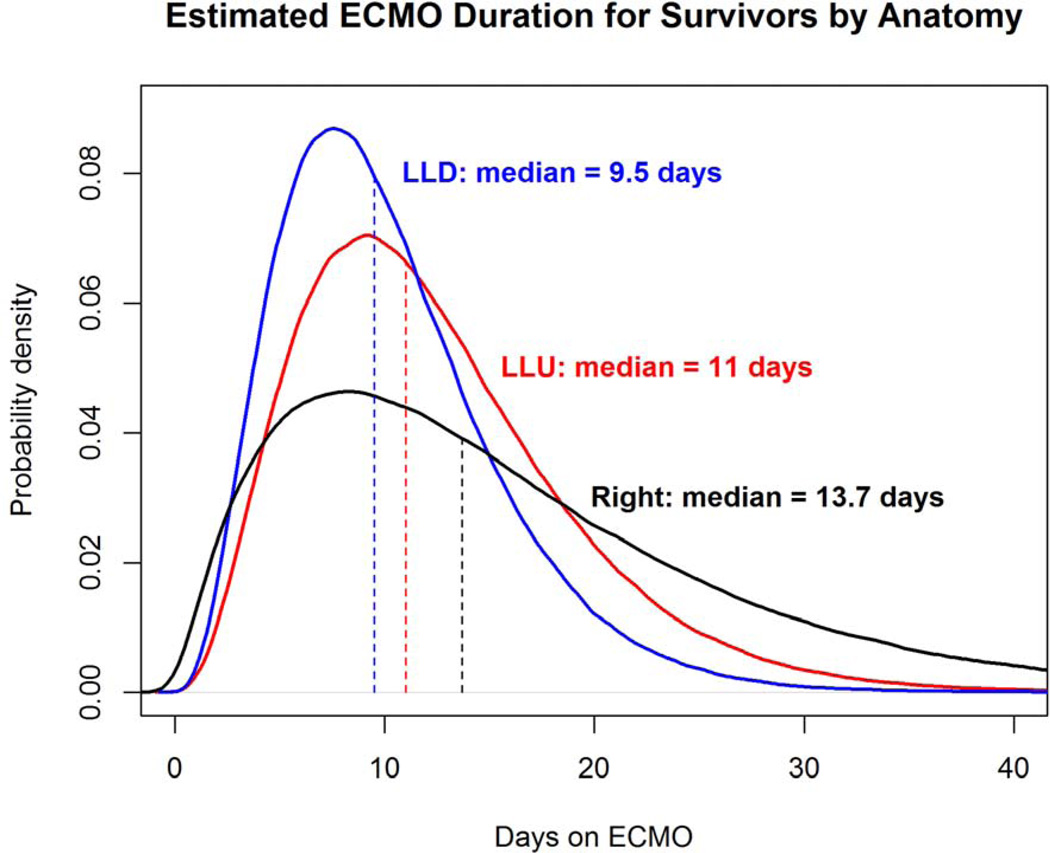
Left liver-down CDH ECMO runs (n=11, surv=10) were the shortest and had a mean length of 10.1 days with a median of 9.5 days. Right CDH (n= 23, surv=19) and left liver-up CDH (n=62, surv=43) runs were longer and similar in length, with a mean of 16.2 days and a median of 13.7 days, compared to a mean of 17.1 days and a median of 12.2 days, respectively. This relationship did not reach statistical significance (overall ANOVA, p=.213)
Analysis of deaths on or after ECMO
24 patients treated with ECMO died before discharge, 15 after the first ECMO run and 9 after the second. Based on the number of days until death and the cause of death, we categorized the timing of those deaths as early (related to early complications, generally less than 10 days), midcourse (complications related to repair on ECMO or failure to reach repair, generally 35 days of life or less), or late (after successful repair but failure to improve, generally greater than 35 days) (Table 4).
Table 4.
Causes of Death and Death Timing in CDH ECMO Patients
| Cause | Total, n (%) |
1st Run, n (%) |
ECMO days |
Death DOL | Days after ECMO stopped |
2nd Run, n (%) |
ECMO Days | Death DOL |
Days after ECMO stopped |
|---|---|---|---|---|---|---|---|---|---|
| Early (5) | |||||||||
| ECMO Complication | 2 (8.3) | 2 (13) | 0 | 5 +/−4 | 0 | 0 | |||
| ICH | 2 (8.3) | 2 (13) | 5 +/−3 | 6 +/−4 | 0 | 0 | |||
| Never Stabilized-VA | 1 (4.2) | 1 (6.6) | 2.9 | 3 | 0 | 0 | |||
| Mid (9) | |||||||||
| Surgical-Bleeding | 2 (8.3) | 2 (13) | 16 +/−8 | 28 +/−19 | 11 +/−11 | 0 | |||
| Surgical-Compartment | 4 (17) | 1 (6.6) | 19 | 22 | 0 | 3 (33) | 32 +/−5 | 35 +/−6 | 1 +/−1 |
| Never Stabilized-VV | 3 (13) | 3 (20) | 22 +/−2 | 23 +/−3 | 0 | 0 | |||
| Late (10) | |||||||||
| Sepsis | 4 (17) | 1 (6.6) | 22 | 38 | 0 | 3 (33) | 45 +/−5 | 58 +/−4 | 4 +/−4 |
| Pulm hypoplasia/HTN | 6 (25) | 3 (20) | 15 +/−9 | 124 +/−70 | 107 +/−67 | 3 (33) | 43 +/−12 | 59 +/−27 | 4 +/−4 |
| Total | 24 | 15 | 9 |
First run ECMO included 5 early deaths, 6 midcourse deaths, and 4 late deaths. Early deaths occurred from 2 technical complications and 2 intra-cranial hemorrhages. One patient never stabilized on VA ECMO and died early. 3 patients failed to stabilize sufficiently on VV ECMO and despite conversion to VA ECMO died unrepaired around 3 weeks. 3 patients who failed to wean from ECMO underwent repair on ECMO beyond 2 weeks and had complications of bleeding and abdominal compartment syndrome that truncated their ECMO courses. They died soon after. There were 4 late deaths due to failure of pulmonary hypertension to improve (3) and fungal sepsis (1).
Nine second run ECMO deaths occurred. Three died mid-course related to abdominal compartment syndrome issues following repairs done after failing to wean from ECMO after 3 weeks. Three died due to late infections after prolonged courses on ECMO, and 3 died after long ECMO runs due to inability to improve their pulmonary hypertension. These are summarized in Table 4.
Survival Related to Initial Type of ECMO
Forty-two of 56 patients who started on VV ECMO survived (75%) and 30 of 40 patients that started with VA ECMO survived (75%). Univariate analysis by chi-square shows no survival advantage related to initial ECMO type (p=1). Multivariate regression analysis controlling for CDH SG predicted survival shows no survival advantage to initial ECMO type (p=.467)
Defining Length of Time to Survive on ECMO (ECMO duration in survivors only)
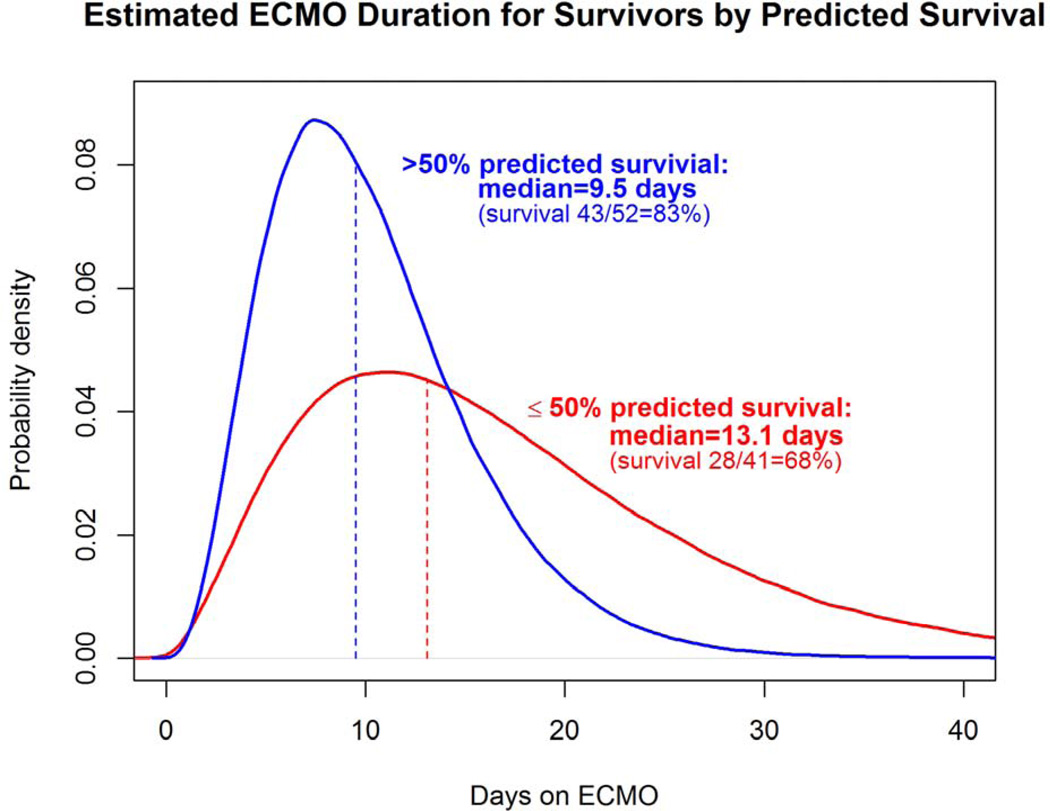
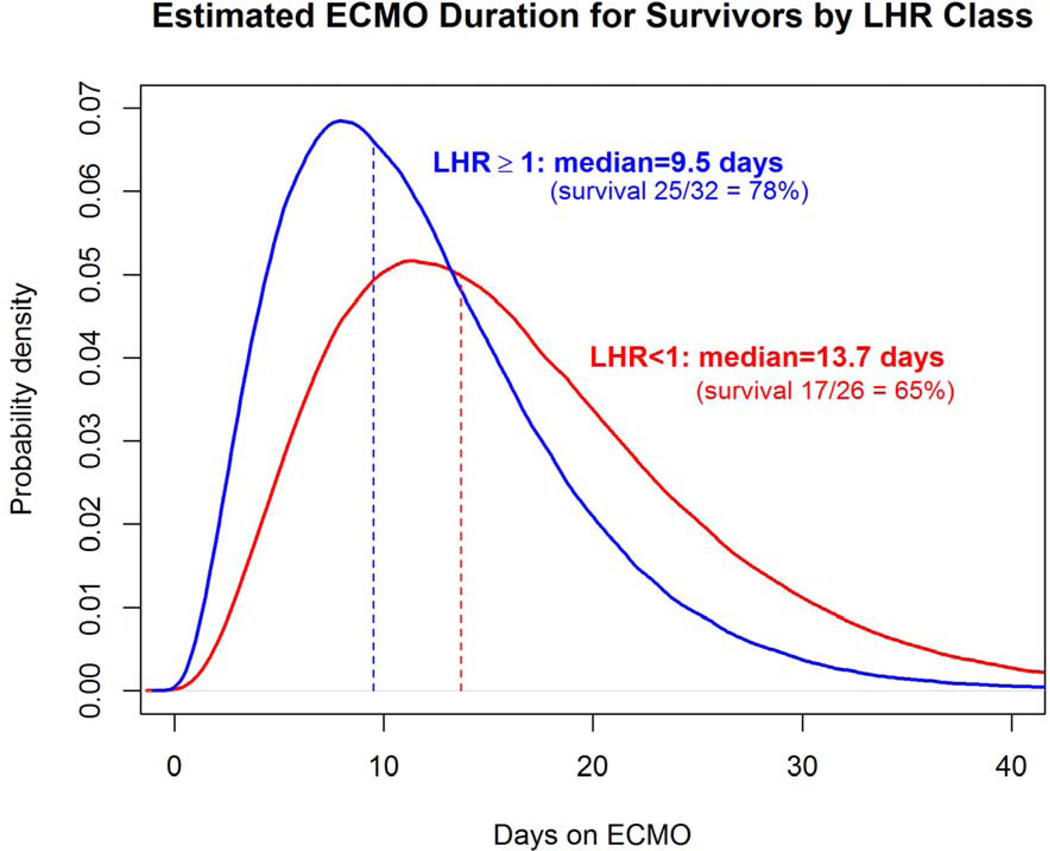
To visually demonstrate the distribution of time on ECMO for survivors, probability density curves based on the data from this series were created for each anatomic subset, for CDHSG predicted survival greater than and less than 50%, and for LHR less than or greater than or equal to 1.0 (Figures 4a–c). The median length of ECMO duration to survival was shortest in left liver-down, intermediate in left liver-up, and longest in right CDH, but ANOVA was not statistically significant (p=0.44). However, median ECMO duration to survival was significantly longer for patients with CDH predicted survival of greater than 50% versus less than or equal to 50% (13.1 days versus 9.5 days, p=0.006) and for initial LHR less than 1.0 versus greater than or equal to 1.0 (13.7 days versus 9.5 days, p=0.012)
Figure 4.
a–c: Comparison of ECMO duration in survivors based on anatomy, CDH Predicted Survival, and LHR. (a); Distribution curve of estimate ECMO duration for patients with CDH anatomy of Left liver-down versus Left liver-up versus Right CDH.(p=0.448) (b): Predicted Survival: Distribution curve of estimated ECMO duration in patients with CDH Predicted Survival greater than 50% compared to less than or equal to 50% (p=0.006). (c): LHR: Distribution curve of Estimated ECMO duration for patients with LHR greater than or equal to 1 versus less than 1. (p=0.012) All curves based on data in this series.
Discussion
This report focuses on the ECMO duration and survival outcomes 96 patients that were part of a large consecutive series of CDH. While patients deemed to have associated lethal anomalies are excluded from this analysis, no patients were excluded due to severity alone, and ECMO was used inclusively in attempt to rescue patients that were not known to have other anomalies that would preclude survival. That 72% were inborn, 89% were either left liver-up or right CDH, and the aggregate CDH SG predicted survival was 54%, all attest to the overall clinical severity of this series.
This study demonstrates several important findings. First, severity markers including Apgar-1, Apgar-5, and CDHSG Predicted survival correlated strongly with survival on ECMO, need for second ECMO run, and duration of ECMO. The CDH study group predicted survival correlated the most strongly of the severity markers. Seetharamaiah previously showed that Apgar-5 correlated with survival in CDH patients treated with ECMO, but in our series we found no outcome associations with gestational age nor birth weight18. While the correlations between known markers of severity and ECMO outcomes may seem intuitive, their clear demonstration allows the clinician to paint an increasingly granular picture of CDH severity and to better understand how that severity will affect the ECMO run.
Lung to head ratio (LHR) measurements were not available in all patients, and this limitation hampered the ability to test associations with outcome measures. However, LHR values varied inversely and strongly with duration of ECMO, clarifying that native lung mass influences time on ECMO. This is important, suggesting that ECMO for CDH patients not only allows time for reactive pulmonary hypertension to resolve, a process that would be expected to be similar in duration in patients regardless of severity, but that additional time is required for smaller lungs to accomodate. From these data we can only postulate what that accommodation consists of, but suggest that multiple processes with a combined longer time course than simply the resolution of reactive pulmonary hypertension are operative. It is tempting to suggest that some degree of lung growth may be occurring, but this was not directly tested here.
In patients with LHR less than 1.0, representing severe pulmonary hypoplasia, Survival to discharge was seventeen of 26 (65%), significantly higher than many reports would suggest.19–21 We could postulate that the LHR measurements were inaccurate, but given the large size and inclusive nature of this series, the distribution of patients with LHR less than 1.0 is consistent with other series22. The survival of two-thirds of these patients is likely related to treatment variables, of which duration of ECMO as reported here, and repair of liver-up CDH before ECMO are treatment factors which may have contributed to the survival obtained in these severely affected patients. Optimal lung adaptation and accommodation in patients with severe pulmonary hypoplasia is likely facilitated by repair, and repair before ECMO in liver-up CDH was associated with improved survival in our recent report15.
These data show that patients with more severe CDH, as defined by a variety of patient specific and reproducible markers have higher mortality, higher risk for second run ECMO, but also need a longer duration of ECMO to survive. To optimize survival in these most severe patients, centers must be willing to invest significant time in their recovery on ECMO. However, it is not as simple as just investing more time, as the more severe CDH patients are less tolerant of complications and missteps, as noted in our own analysis of deaths. It is not an accident that the severity markers correlate so strongly with risk of death and risk of second ECMO, and this clarifies the necessity of exacting care in order to maximize the recovery potential in these infants.
This concept applies to the patient who is weaning from ECMO as well. Although the numbers in the series get smaller as the days on ECMO increase, the message of the first and second run ECMO survival curves suggest it is likely better to invest enough time to come off of ECMO in optimal condition, versus not. Controlling for number of days on ECMO, survival with first run ECMO was always better than second run ECMO. This may help inform decision making when a choice arises between investing more time on ECMO, versus coming off under less than optimal circumstances due to ECMO specific issues. The less severe patient, as defined by risk markers, may tolerate discontinuing ECMO early, but the more severe patient will likely not. Based on these data, we infer the importance of optimization of clinical status before discontinuing ECMO whenever possible, to minimize the need for a second ECMO run and an associated decreased chance of survival.
Similarly, we interpret these data to show reason for patience as patients wean from ECMO. Efforts to get patients off ECMO sooner by escalating ventilation strategies may ultimately compromise the patients chance of recovery as higher ventilator settings may increase the long term risk of unresolved pulmonary hypertension.
To better understand the issues about CDH survival and duration on ECMO, we felt it was important to define when and why patients died. We learned that 10 (42%) of 24 patients died of late issues of infection and unresolved pulmonary hypertension, but that nearly 60% died of early and mid-course issues that are potentially remediable. Although the severity markers show that even those dying in mid-course are much sicker than those who do not, these patients went on ECMO unrepaired and failed to improve for 2 or more weeks resulting in decisions to repair on ECMO. Techniques such as early repair on ECMO8 or repair before ECMO in patients who afford such opportunity15 may provide better options for salvaging these specific high-risk patients. The unsolved challenge is defining who these patients are early in the treatment course so that severity specific treatment algorithms can be developed and applied.19,24
We interpret the totality of the data presented here to show that while many patients with will be weanable from ECMO in 1 – 2 weeks, those patients with more severe CDH will simply take longer, and the discontinuation of ECMO based solely on arbitrary times on ECMO should be abandoned where practiced. In patients who die, the majority will die from complications that are obvious and the need to discontinue ECMO will be clear.
The major weakness of this study is that despite its size, the number of patients still on ECMO at 1 month is small, limiting the strength of observation at that point. Second, this study suffers from the weaknesses of a retrospective review, but as a single institution series with consistent therapeutic leadership, it has significant strengths as well. The ability to analyze deaths in a granular manner is a strength not applicable to multicenter databases. Third, the survival attained in this series is high, and the outcomes related to specific durations of ECMO may not be directly applicable to all centers, although the general points should be. Fourth, some might question if using ECMO in 40% of CDH patients is representative, but our approach is inclusive, and this usage is in-line with other contemporary reports1,4,8, and this rate has not changed from our previous report14. Finally, survival in CDH is only part of the story and quality of outcomes is an essential piece. Many authors have documented the neurodevelopmental challenges faced by this group of patients(17–20), and we are currently evaluating the imaging and neurodevelopmental outcome of our patients for subsequent reporting.
In summary, despite advances in the care of CDH patients, ECMO support remains an important tool in the care of many of these infants. Patients who are more severe as measured by a variety of standard markers are at higher risk of dying, but also require more time on ECMO to recover. Survival is maximized by optimizing first-run ECMO. Patients who decline after a first run of ECMO should be offered a second run unless complications intervene. At our center survival at 4 weeks of ECMO is 43%, and at 5 weeks is 25%, but we had no survivors of second run ECMO past 30 days and no survivors of first run ECMO past 40 days. All patients treated with more than 3 weeks of ECMO and ultimately surviving were repaired before that time. The reasons for death on ECMO are usually visible and definable, and we caution against defining arbitrary lengths of ECMO shorter than 4 weeks in stable patients who have been successfully repaired, especially in those more severely affected patients who as described here need more time for lung adaptation and accommodation.
Acknowledgment
The authors would like to thank Dan Neal for his outstanding statistical help and guidance. We would also like to thank Andrew Kays for his excellent work in developing the REDCap database that houses the data this report is written from. The authors are indebted to the Greek Orthodox Ladies Philoptocos Society for providing grant funding for this project.
This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064. The Greek Orthodox Ladies Philoptocos Society provided grant funding for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 125th Annual Meeting, Hot Springs, VA, December 2013.
Reference List
- 1.Downard CD, Jaksic T, Garza JJ, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–732. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- 2.Fallon SC, Cass DL, Olutoye OO, et al. Repair of congenital diaphragmatic hernias on Extracorporeal Membrane Oxygenation (ECMO): does early repair improve patient survival? J Pediatr Surg. 2013;48:1172–1176. doi: 10.1016/j.jpedsurg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Garcia A, Stolar CJH. Congenital diaphragmatic hernia and protective ventilation strategies in pediatric surgery. Surg Clin North Am. 2012;92 doi: 10.1016/j.suc.2012.03.003. 659–668– ix. [DOI] [PubMed] [Google Scholar]
- 4.Antonoff MB, Hustead VA, Groth SS, Schmeling DJ. Protocolized management of infants with congenital diaphragmatic hernia: effect on survival. J Pediatr Surg. 2011;46:39–46. doi: 10.1016/j.jpedsurg.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 5.Logan JW, Rice HE, Goldberg RN, Cotten CM. Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies. J Perinatol. 2007;27:535–549. doi: 10.1038/sj.jp.7211794. [DOI] [PubMed] [Google Scholar]
- 6.Garriboli M, Duess JW, Ruttenstock E, et al. Trends in the treatment and outcome of congenital diaphragmatic hernia over the last decade. Pediatr Surg Int. 2012;28:1177–1181. doi: 10.1007/s00383-012-3184-5. [DOI] [PubMed] [Google Scholar]
- 7.Sebald M, Friedlich P, Burns C, et al. Risk of need for extracorporeal membrane oxygenation support in neonates with congenital diaphragmatic hernia treated with inhaled nitric oxide. J Perinatol. 2004;24:143–146. doi: 10.1038/sj.jp.7211033. [DOI] [PubMed] [Google Scholar]
- 8.Dassinger MS, Copeland DR, Gossett J, et al. Early repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. 2010;45:693–697. doi: 10.1016/j.jpedsurg.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46:630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin JR, Gustafson KE, Smith PB, et al. Perinatal factors associated with poor neurocognitive outcome in early school age congenital diaphragmatic hernia survivors. J Pediatr Surg. 2013;48:730–737. doi: 10.1016/j.jpedsurg.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiruvoipati R, Vinogradova Y, Faulkner G, et al. Predictors of outcome in patients with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation. J Pediatr Surg. 2007;42:1345–1350. doi: 10.1016/j.jpedsurg.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Wung JT, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76:488–494. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=4047792&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 13.Boloker J, Bateman DA, Wung JT, Stolar C. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002 doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 14.Kays DW, Langham MR, Ledbetter DJ, Talbert JL. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg. 1999;230:340–348. doi: 10.1097/00000658-199909000-00007. discussion 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kays DW, Islam S, Larson SD, et al. Long-term maturation of congenital diaphragmatic hernia treatment results: toward development of a severity-specific treatment algorithm. Ann Surg. 2013;258:638–644. doi: 10.1097/SLA.0b013e3182a53c49. discussion 644–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36(1):141–145. doi: 10.1053/jpsu.2001.20032. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11150453&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 18.Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB Congenital Diaphragmatic Hernia Study Group. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2009;44:1315–1321. doi: 10.1016/j.jpedsurg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Odibo AO, Najaf T, Vachharajani A, et al. Predictors of the need for extracorporeal membrane oxygenation and survival in congenital diaphragmatic hernia: a center's 10-year experience. Prenat Diagn. 2010;30:518–521. doi: 10.1002/pd.2508. [DOI] [PubMed] [Google Scholar]
- 20.Laudy JAM, Van Gucht M, Van Dooren MF, et al. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diagn. 2003;23:634–639. doi: 10.1002/pd.654. [DOI] [PubMed] [Google Scholar]
- 21.Lipshutz GS, Albanese CT, Feldstein VA, et al. Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 1997;32:1634–1636. doi: 10.1016/s0022-3468(97)90471-1. [DOI] [PubMed] [Google Scholar]
- 22.Kattan J, Godoy L, Zavala A, et al. Improvement of survival in infants with congenital diaphragmatic hernia in recent years: effect of ECMO availability and associated factors. Pediatr Surg Int. 2010;26:671–676. doi: 10.1007/s00383-010-2624-3. [DOI] [PubMed] [Google Scholar]
- 23.Datta J, Phillips SE, Yang EY. Association of high ventilator pressures with the development of chronic pulmonary hypertension in congenital diaphragmatic hernia patients requiring ECMO. Pediatr Surg Int. 2012;28:977–982. doi: 10.1007/s00383-012-3132-4. [DOI] [PubMed] [Google Scholar]
- 24.Coleman AJ, Brozanski B, Mahmood B, et al. First 24-h SNAP-II score and highest PaCO2 predict the need for ECMO in congenital diaphragmatic hernia. J Pediatr Surg. 2013;48:2214–2218. doi: 10.1016/j.jpedsurg.2013.03.049. [DOI] [PubMed] [Google Scholar]