Abstract
Calcium and redox signaling both play important roles in the pathogenesis of cardiac disease; although how these signals are integrated in the heart remains unclear. One putative sensor for both calcium and oxidative stress in the heart is CaMKII, a calcium activated kinase that has recently been shown to also be regulated by oxidation. Oxidative activation of CaMKII occurs in several models of cardiac disease, including myocardial injury and inflammation, excessive neurohumoral activation, atrial fibrillation, and sinus node dysfunction. Additionally, oxidative activation of CaMKII is suggested in subcellular domains where calcium and ROS signaling intersect, such as mitochondria. This review describes the mechanism of activation of CAMKII by oxidation, the cardiac diseases where oxidized CaMKII has been identified, and suggests contexts where oxidized CaMKII is likely to play an important role.
Keywords: Ca2+/calmodulin dependent protein kinase II, Reactive oxygen species, Heart failure, Arrhythmia, Calcium, mitochondria
1. Introduction
Oxidative stress plays an important role in the development of cardiac disease [1]. It is unclear, however, how increased oxidative stress is “translated” into deleterious disease phenotypes. One molecule that has recently been implicated to be a sensor of oxidative stress in the heart and lung is the Ca2+/calmodulin dependent protein kinase II (CaMKII). CaMKII is activated in numerous cardiac diseases, and contributes to the development of heart failure, and arrhythmias [2]. These pathways have intersected with the discovery of direct regulation of CaMKII activity by cellular reactive oxygen species (ROS) by direct oxidation of the enzyme’s regulatory domain [3].
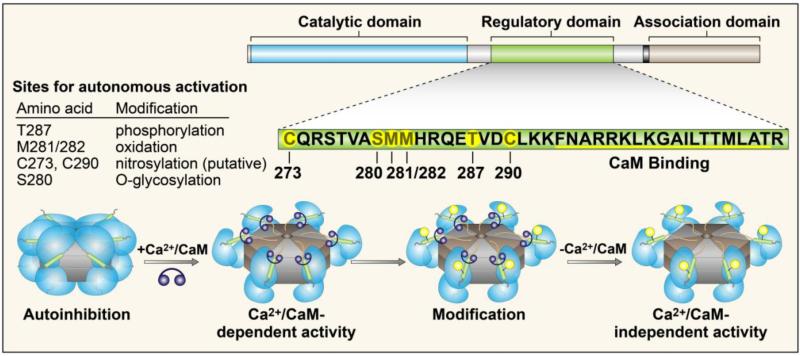
CaMKII functions as a homo- or hetero-multimer consisting of 12 subunits, each consisting of three conserved domains: an amino-terminal catalytic domain, a central autoregulatory domain, and a carboxy-terminal association domain. The catalytic domain contains the ATP and substrate binding pockets, providing the catalytic activity of the protein. The autoregulatory domain contains an inhibitory pseudosubstrate sequence, several sites for post-translational modification (Figure 1), and the calmodulin-binding region. The association domain is responsible for oligomerization of the subunits to form the holoenzyme, and also contains variable regions that are alternatively spliced to form different splice variants of CaMKII [4].
Figure 1.
CaMKII structure and regulation. CaMKII monomers consist of a N-terminal catalytic domain, a central regulatory domain, and a C-terminal association domain. Monomers assemble into the holoenzyme via the association domain. The regulatory domain contains the calmodulin binding site, and several sites for post-translational modification (designated by orange circles). Under resting conditions, the regulatory domain is bound to the catalytic domain, rendering the enzyme inactive. A rise in intracellular Ca2+ stimulates binding of Ca2+/calmodulin to the regulatory domain, releasing the catalytic domain and activating the enzyme. Modification of one or more of the sites listed results in sustained (autonomous) activity in the absence of Ca2+.
CaMKII activity is autoinhibited by its pseudosubstrate region, which resides in the autoregulatory domain. This region binds the catalytic domain and sterically blocks the substrate and ATP binding pockets [5, 6]. Activation of CaMKII occurs upon binding of calcium-activated calmodulin (Ca2+/CaM) to the autoregulatory domain. The binding of Ca2+/CaM displaces the pseudosubstrate region, allowing the substrate and ATP access to the catalytic domain. Sustained activation of the kinase in the presence of ATP results in autophosphorylation across subunits at Thr287 [7]. This phosphorylation event leads to a 1000-fold increase in affinity for CaM, and prevents the reassociation of the catalytic domain resulting in the persistence of enzyme activity even in the absence of Ca2+/CaM [8]. Activation of CaMKII subunits by Ca2+/CaM, and subsequent intra-subunit phosphorylation also stimulates subunit exchange between holoenzymes. This exchange of active subunits leads to further activation of inactive holoenzyme via phosphorylation of neighboring subunits even in the absence of Ca2+/CaM binding [9]. These studies illustrate the complexities of CaMKII activation, and persistent, or autonomous, activity following autophosphorylation.
More recently, our group identified an alternative mechanism for CaMKII to remain active in the absence of Ca2+/CaM. CaMKII can be oxidized at methionines 281 and 282 in the presence of reactive oxygen species. Initial binding of Ca2+/CaM is required to displace the pseudosubstrate region from the catalytic domain before the enzyme can maintain its activated state in the absence of Ca2+/CaM. Mutation of the methionine residues to non-oxidizable valines renders the enzyme insensitive to persistent activity in the presence of ROS. Development of antiserum that specifically recognizes oxidized CaMKII has allowed us to validate that CaMKII is oxidized in vivo [3]. Oxidation of CaMKII at its paired methionine residues appears to act as a sensor of cellular ROS, and not a signal for oxidative damage and subsequent protein degradation. Increased oxidation of CaMKII, determined by immunoblotting, does not correlate with a decrease in the total amount of CaMKII protein present [10-12], but with an increase in kinase activity [3].
Oxidation of CaMKII occurs via ROS produced from variety of sources including NADPH oxidase, and mitochondria. Elimination of either of these pathways via genetic knockout, or targeted ROS scavenging, results in a reduction of CaMKII oxidation [3, 13]. Additionally, oxidation of CaMKII is a reversible event occurring via enzymatic reduction of the methionine residues by methionine sulfoxide reductase A (MsrA) [3]. Chronic CaMKII activiation, and subsequent dysfunction, occurs when the balance of automomously active versus auto-inhibited enzyme is shifted towards increased activity, and its downstream targets become phosphorylated inappropriately. In the case of oxidation, this occurs when the anti-oxidant systems in the heart cannot “keep up” with the oxidation of CaMKII by cellular ROS. This concept is demonstrated in mice with altered MsrA activity. MsrA knockout enhances CaMKII oxidation, cell death, and post-MI mortality in mice [3], while MsrA transgenic over-expression reduces CaMKII oxidation and prevents pathological consequences of aldosterone [10] and angiotensin II [14] in myocardium.
Increased CaMKII expression and activity have been associated with several cardiac diseases. Overexpression of CaMKII in the heart leads to deranged calcium homeostasis and heart failure [15, 16], and arrhythmias [17]. CaMKII activity and expression are also elevated in cardiac injury models, including myocardial infarction (MI) [18, 19] and ischemia-reperfusion (I/R) injury [20, 21]. Oxidation of CaMKII has been directly measured or implicated in all of these conditions, suggesting a critical role for oxidative activation of CaMKII in the pathogenesis of cardiac disease.
2. Myocardial Injury and Inflammation
Inflammation is an innate immune response that is activated early in the wound-healing process, and involves a cascade of signaling events that recruit immune cells to the injury in order to “clean up” the injured tissue. Inflammation occurs following cardiac injury, such as MI or I/R, in order to repair the damaged myocardium. Oxidative stress and ROS production are associated with the inflammatory response, and play a critical role in the pathogenesis of cardiac disease following an injury [22, 23]. Expression of inflammatory genes are differentially regulated following MI in a mouse model of CaMKII inhibition compared to control mice, suggesting that CaMKII is also involved in inflammation, and that oxidation of CaMKII is likely to contribute to the increase in CaMKII activity. Our group tested this hypothesis in an in vitro model of inflammation, where cultured cardiomyocytes are treated with lipopolysaccharide (LPS) to simulate inflammation. In this model, increased expression of inflammatory genes is blunted by CaMKII inhibition [18], while oxidation of CaMKII, and its activity, are enhanced by LPS. Additionally, CaMKII oxidation and activity are reduced following MI in mice lacking a critical mediator of inflammatory signaling, MyD88. These mice have reduced inflammatory cell infiltration, myocyte death, and fibrosis after MI [11]. Oxidized CaMKII can enhance proinflammatory transcriptional signaling by enhancing NF-κB activity [11], a finding that also has implications for asthma [24]. Thus, CaMKII appears to be a nodal signal for connecting the ‘upstream’ signal of elevated ROS to ‘downstream’ inflammatory events by actions at multiple targets.
CaMKII is also activated in the heart following I/R injury [20]. In this model, deletion of the delta isoform of CaMKII results in reduced infarct size and improved functional recovery following injury. These mice also have reduced myocyte death and inflammation in the heart after I/R injury, linking CaMKII signaling to the inflammatory response in this model [25]. I/R injury is also associated with increased oxidative stress [26], suggesting that CaMKII is likely to undergo oxidative activation in this context. These data provide evidence that oxidative activation of CaMKII plays a significant role in the detrimental effects of inflammation following myocardial injury.
3. Neurohumoral signaling
Pathologic activation of neurohumoral signaling pathways, including β-adrenergic receptor, angiotensin II, and aldosterone, contribute to heart failure [27]. For example, elevated angiotensin II and aldosterone are seen in patients with heart failure [28], particularly heart failure related to MI [29, 30]. CaMKII is activated downstream of these signaling pathways, and is a critical mediator of the detrimental effects. These pathways are also associated with increases in ROS production and increased oxidative stress.
Oxidative activation of CaMKII is downstream of increased angiotensin II signaling. Treatment of cultured cardiomyocytes with angiotensin II induces ROS production via an NADPH oxidase dependent mechanism. This increase in oxidative stress leads to myocyte death, and can be blocked by CaMKII inhibition or knock-in replacement of CaMKII with an oxidation resistant mutant (M281/282V) [3]. Additionally, angiotensin II-mediated cell death requires ROS [31], suggesting that it is the oxidative activation of CaMKII that is responsible for this response. Activation of CaMKII via angiotensin II has been shown to be involved in the development of cardiac arrhythmias, including atrial fibrillation [14] and sinus node dysfunction [12] (discussed below).
Chronic treatment of mice with aldosterone stimulates ROS production, leading to CaMKII oxidation and activation. Infusion of aldosterone to approximate levels measured in patients with heart failure after MI significantly increased mortality of mice following MI. This increase in mortality was due to an increase in cardiac rupture, and was reduced by both myocardial CaMKII inhibition and MsrA overexpression. The rupture was mediated by a MEF2- and CaMKII-driven transcriptional increase in MMP9 in myocardium [10]. CaMKII is known to play an important role in the activation of transcription factors in the nucleus including MEF2 [32] and NF-κB [18]. These data add to evidence that oxidized CaMKII promotes pathological transcription in myocardium.
Acute activation of β-adrenergic receptor signaling enhances CaMKII activity, but does not appear to induce oxidation of CaMKII. However, prolonged stimulation of the β-adrenergic receptor results in increased mitochondrial ROS production [33] as well as enhanced CaMKII activity [19]. Inhibition of CaMKII protects mice from the detrimental effects of chronic β-adrenergic activation, such as cardiac hypertrophy and decreased contractile function [19]. Although direct oxidation of CaMKII in a model of chronic β-adrenergic receptor agonist stimulation has not (to our knowledge) been measured, it is likely that oxidative activation is an important mechanism for sustained CaMKII activation under these conditions. Recently, a pathway for CaMKII activation by nitrosylation following β-adrenergic receptor activation was identified. In this study, the authors demonstrated that stimulation of the β-adrenergic receptor increases nitric oxide production, which results in nitrosylation of CaMKII. This nitrosylation event leads to increased CaMKII activity and Ca2+ spark frequency independent of ROS [34]. These data provide evidence for an important role for CaMKII activation via oxidative stress in β-adrenergic receptor signaling.
4. Arrhythmia
Arrhythmia causes most cardiac-related sudden death. Arrhythmias are abnormal changes in heartbeats, and can arise from changes in ion channel properties, loss of normal intracellular Ca2+ homeostasis, or arise as the result of tissue remodeling. Both ROS and CaMKII have been shown to play a role in the development of a variety of arrhythmias [2, 35]. A recent study demonstrated that ROS-induced arrhythmias in isolated myocytes are blocked by inhibiting CaMKII activity [36], suggesting that CaMKII activation by ROS plays a role in generation of arrhythmia.
Aberrant activity of cardiac ion channels can lead to arrhythmia. Both ROS and CaMKII have been shown to regulate the function of several cardiac ion channels. Treatment of cultured cardiomyocytes with H2O2 induces increases L-type calcium current (ICa) [37], late sodium current (INa) [38], and increases diastolic calcium leak from the SR [39], as well as enhances CaMKII oxidation [38]. This increase in ICa can be partially blocked by reducing agents, CaMKII inhibition, and ATP analogues, and more fully blocked by co-treatment with reducing agents in combination with CaMKII inhibition or ATP analogue [37]. Enhancement of late INa with H2O2 treatment results in Na+ and Ca2+ overload, early afterdepolarizations (EADs), delayed afterdepolarizations (DADs), and hypercontracture of cardiac myocytes. Arrhymogenesis in this model also requires CaMKII, suggesting that oxidative activation of CaMKII is responsible for the development of arrhythmia following increased H2O2 treatment of cardiomyocytes [38]. Both ROS and CaMKII can also enhance diastolic Ca2+ leak through the cardiac ryanodine receptor (RyR2) [40-43], which can initiate DADs by increasing inward Na+/Ca2+ exchanger inward current [38]. In addition, preventing phosphorylation of RyR2 by CaMKII reduces arrhythmias in mice [14]. These findings illustrate how CaMKII can transduce ROS into cell membrane hyperexcitability and arrhythmias.
Atrial fibrillation (AF) is the most common sustained arrhythmia in patients [44]. Both CaMKII and oxidative stress have been linked to AF. Markers of oxidative stress are elevated in patients with AF [45], and CaMKII dependent phosphorylation of RyR in humans leads to SR calcium leak that likely contributes to AF [46]. AF is significantly increased in a model of rapid pacing in mice with a genetic gain-of-function mutation in RyR, causing it to be leaky, and CaMKII inhibition reduces the probability of AF induction in this model. Additionally, phosphorylation of RyR by CaMKII at S2814 is observed in mice and patients with AF. Genetic mutation of this site (S2814A) in mice reduced AF inducibility in a pacing model [47]. These data suggest an important role for both RyR and CaMKII activity in the induction of AF. A recent study from our group showed a direct connection between oxidative CaMKII activation and atrial fibrillation. Oxidized CaMKII was increased in atria from patients with AF compared to patients in sinus rhythm, as well as the atria of mice with chronic angiotensin II treatment. In a rapid pacing model of inducible AF, mice treated with angiotensin II had an increased susceptibility to AF compared to saline-treated mice, which was blocked in mice expressing the oxidation-resistant (M281/282V) knock-in mutant CaMKII. Mice expressing a CaMKII-resistant RyR, due to knock in mutation of a CaMKII phosphorylation site (S2814A), also had reduced susceptibility to AF induction in the model, further supporting the idea that oxidized CaMKII is inducing AF via RyR phosphorylation [14].
Sinus node dysfunction (SND) is the result of the inability of the sinoatrial (SA) node to generate a regular heartbeat, and can result in sudden death. SND often occurs in the setting of heart failure and AF, where both oxidative stress [48, 49] and CaMKII signaling are known to be elevated [16]. Oxidized CaMKII is increased in the right atrium of heart failure patients with SND compared to patients in sinus rhythm, as well as in the SA node in a mouse model of SND. In this model, mice are chronically infused with angiotensin II, and develop SND characterized by reduced heart rate, and death of SA nodal cells. Expression of either a CaMKII inhibitor, or deletion of a critical subunit of NADPH oxidase protects against SND in this model [12]. These data provide evidence that oxidative activation of CaMKII plays a critical role in the loss of SA nodal cells, and the development of SND.
5. Diabetes
Diabetes is an important risk factor in the development of heart disease. Diabetes is known to cause increased oxidative stress, which may contribute to increased disease complications in these patients [50]. We recently reported that CaMKII plays a critical role in the increase in mortality in MI in the setting of diabetes. Oxidized CaMKII was elevated in right atrium of diabetic patients compared to non-diabetic patients with MI as well as in the right atrium of diabetic mice. In this model, the diabetic mice have increased SA node cell death and fibrosis, decreased intra-atrial conduction velocity and reduced spontaneous beating rate in the right atrium, similar to the SND observed in our chronic angiotensin II infusion model [12]. In addition to CaMKII inhibition preventing these phenotypes in the diabetic mice, an oxidation resistant CaMKII knock in mutant (M281/281V) also reduces SA node cell death and fibrosis in diabetic mice [13]. These findings support a view that oxidative activation of CaMKII is a critical signal for the development of SND in diabetes as well as chronic Ang II overload.
Oxidative activation of CaMKII in diabetes is not only linked directly to oxidative stress, but is also partly due to hyperglycemia. Hyperglycemia results in increased ROS and cell death in cultured neonatal myocytes, suggesting that hyperglycemic conditions can increase ROS production directly in cardiomyocytes. Myocytes expressing the ox-resistant mutant of CaMKII are resistant to the increase in cell death due to hyperglycemia, suggesting that the excess ROS is responsible for activation of CaMKII and subsequent cell death [13]. A recent study showed that CaMKII is O-glycosylated during hyperglycemia, leading to activation [51]. Both of these modifications may contribute to the detrimental effects of diabetes and hyperglycemia in the heart, but further studies are required to understand if and how ROS and hyperglycemia interact to activate CaMKII. These data illustrate the dynamic regulation of CaMKII activity in disease pathology by multiple mechanisms.
6. Mitochondria
Mitochondria play an integral role in the heart in both calcium- and ROS-mediated signaling. ROS is produced in mitochondria as a byproduct of electron transport and ATP production, and is increased when oxygen consumption becomes uncoupled from ATP production [52]. Mitochondria become uncoupled following dissipation of the negative membrane potential across the inner mitochondrial membrane. Uncoupling can occur after excessive calcium uptake into the mitochondria resulting in the opening of the mitochondrial permeability transition pore (mPTP) [53, 54]. Excessive CaMKII activity results in opening of mPTP and increased ROS production [55]. Treating mice with a mitochondrial-targeted antioxidant, mito-TEMPO, reduces ox-CaMKII and myocyte death in diabetic mouse hearts [13], suggesting that mitochondria are an important source of ROS for oxidative CaMKII activation.
We recently identified CaMKII as a resident protein in mitochondria [56]. In these studies, expression of a mitochondrial-delimited CaMKII inhibitor prevented mPTP opening, and protected from myocyte death following myocardial injury [56]. Additionally, CaMKII phosphorylates the mitochondrial calcium uniporter, resulting in increased calcium currents into mitochondria. This may result in calcium overload, opening of the mPTP, and increased ROS production, further enhancing CaMKII activity. The role of CaMKII in mitochondria is not completely clear, but is likely to play a critical role in mediating cell death pathways and regulation of metabolic pathways and energy production.
7. Conclusions
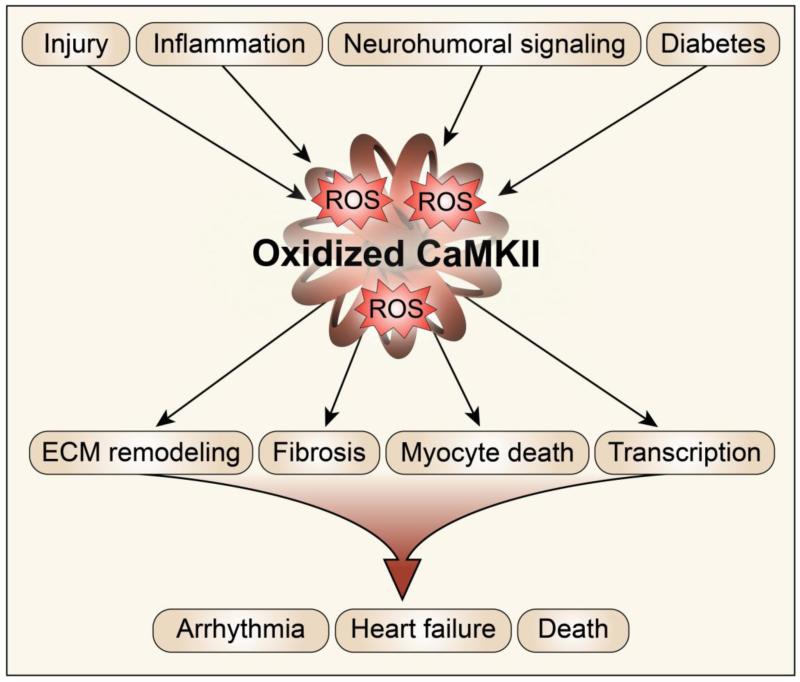
CaMKII is a critical sensor of oxidant stress in the heart, integrating calcium signals and ROS signals in several subcellular locations (Figure 2). The effects of enhancing CaMKII activity via oxidation appear to be complex and widespread in cardiomyocytes. Global anti-oxidant therapies for patients have been largely unsuccessful [57, 58], suggesting that improved understanding of the mechanisms by which ROS-mediated signaling leads to cardiac disease will provide more specific, local targets in the heart, and ultimately improved treatment of cardiac diseases. Recent work by us and by others supports the hypothesis that targeted CaMKII inhibition, via peptides, or anti-oxidants targeted to sub-cellular compartments, can be an effective antioxidant therapy in common cardiovascular [13] and pulmonary diseases [24].
Figure 2.
CaMKII is a ROS sensor. Stress signals in the heart lead to increased intracellular calcium and oxidative stress, which induce CaMKII autonomous activity. Sustained CaMKII activity, and phosphorylation of downstream target proteins lead to detrimental phenotypes that, when combined, lead to cardiac demise.
Highlights.
CaMKII functions as a sensor of cellular ROS via direct oxidation of its regulatory domain.
CaMKII oxidation has been observed in several cardiovascular injury models.
Activation of neurohormonal signaling results in oxidation of CaMKII via increased ROS production.
Excessive CaMKII oxidation leads to cardiac arrhythmias such as atrial fibrillation.
Mitochondrial ROS play an important role in CaMKII oxidation in diabetes.
Acknowledgements
We are grateful for the artistic contributions of Shawn Roach.
Funding
This work was funded in part by US National Institutes of Health (R01HL70250, R01HL079031, R01HL113001, and R01HL096652) and a grant (08CVD01) from the Fondation Leducq as part of the Alliance for CaMKII Signaling in Heart.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
M.E. Anderson is a named inventor on intellectual property claiming to treat myocardial infarction by CaMKII inhibition and is a cofounder of Allosteros Therapeutics, a biotech company aiming to develop enzyme-based therapies.
References
- [1].Tomaselli GF, Barth AS. Sudden cardio arrest: oxidative stress irritates the heart. Nat Med. 2010;16:648–9. doi: 10.1038/nm0610-648. [DOI] [PubMed] [Google Scholar]
- [2].Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–77. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–60. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- [6].Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- [7].Lai Y, Nairn AC, Gorelick F, Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of autophosphorylation sites responsible for generation of Ca2+/calmodulin-independence. Proc Natl Acad Sci U S A. 1987;84:5710–4. doi: 10.1073/pnas.84.16.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- [9].Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, et al. Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife. 2013;3:e01610. doi: 10.7554/eLife.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–8. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012;52:1135–44. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–88. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–74. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, et al. Oxidized CaMKII Triggers Atrial Fibrillation. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–11. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- [16].Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr., Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–9. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- [17].Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–93. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- [18].Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, et al. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest. 2009;119:986–96. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- [20].Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–98. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [21].Salas MA, Valverde CA, Sanchez G, Said M, Rodriguez JS, Portiansky EL, et al. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;48:1298–306. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474–81. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- [24].Sanders PN, Koval OM, Jaffer OA, Prasad AM, Businga TR, Scott JA, et al. CaMKII is essential for the proasthmatic effects of oxidation. Sci Transl Med. 2013;5:195ra97. doi: 10.1126/scitranslmed.3006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ling H, Gray CB, Zambon AC, Grimm M, Gu Y, Dalton N, et al. Ca2+/Calmodulin-dependent protein kinase II delta mediates myocardial ischemia/reperfusion injury through nuclear factor-kappaB. Circ Res. 2013;112:935–44. doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- [27].Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–28. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- [28].Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L, CONSENSUS Trial Study Group Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation. 1990;82:1730–6. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- [29].Gutierrez-Marcos FM, Fernandez-Cruz A, Gutkowska J, Herrero C, Blesa A, Estrada V, et al. Atrial natriuretic peptide in patients with acute myocardial infarction without functional heart failure. Eur Heart J. 1991;12:503–7. doi: 10.1093/oxfordjournals.eurheartj.a059930. [DOI] [PubMed] [Google Scholar]
- [30].Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–10. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- [31].Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, et al. Angiotensin II-Induced Oxidative Stress Resets the Ca2+ Dependence of Ca2+-Calmodulin Protein Kinase II and Promotes a Death Pathway Conserved Across Different Species. Circ Res. 2009;105:1204–12. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- [32].Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca(2)(+) waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol. 2012;590:3291–304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during beta-adrenergic stimulation of cardiac muscle. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt201. [DOI] [PubMed] [Google Scholar]
- [35].Wagner S, Rokita AG, Anderson ME, Maier LS. Redox regulation of sodium and calcium handling. Antioxid Redox Signal. 2013;18:1063–77. doi: 10.1089/ars.2012.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Song YH, Choi E, Park SH, Lee SH, Cho H, Ho WK, et al. Sustained CaMKII activity mediates transient oxidative stress-induced long-term facilitation of L-type Ca(2+) current in cardiomyocytes. Free Radic Biol Med. 2011;51:1708–16. doi: 10.1016/j.freeradbiomed.2011.07.022. [DOI] [PubMed] [Google Scholar]
- [38].Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, et al. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–65. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, et al. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–41. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- [40].Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–40. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [41].Eager KR, Dulhunty AF. Activation of the cardiac ryanodine receptor by sulfhydryl oxidation is modified by Mg2+ and ATP. J Membr Biol. 1998;163:9–18. doi: 10.1007/s002329900365. [DOI] [PubMed] [Google Scholar]
- [42].Eager KR, Roden LD, Dulhunty AF. Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am J Physiol. 1997;272:C1908–18. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- [43].Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–77. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- [45].Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, et al. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935–40. doi: 10.1016/j.hrthm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [46].Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–44. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- [47].Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci (Landmark Ed) 2009;14:3168–87. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- [50].Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013 doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- [53].Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- [54].Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- [55].Odagiri K, Katoh H, Kawashima H, Tanaka T, Ohtani H, Saotome M, et al. Local control of mitochondrial membrane potential, permeability transition pore and reactive oxygen species by calcium and calmodulin in rat ventricular myocytes. J Mol Cell Cardiol. 2009;46:989–97. doi: 10.1016/j.yjmcc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- [56].Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–73. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- [58].MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]