Abstract
Hydrogen sulfide (H2S) has emerged as an important gaseous signaling molecule that is produced endogenously by enzymes in the sulfur metabolic network. H2S exerts its effects on multiple physiological processes important under both normal and pathological conditions. These functions include neuromodulation, regulation of blood pressure and cardiac function, inflammation, cellular energetics and apoptosis. Despite the recognition of its biological importance and its beneficial effects, the mechanism of H2S action and the regulation of its tissue levels remain unclear in part owing to its chemical and physical properties that render handling and analysis challenging. Furthermore, the multitude of potential H2S effects has made it difficult to dissect its signaling mechanism and to identify specific targets. In this review, we focus on H2S metabolism and provide an overview of the recent literature that sheds some light on its mechanism of action in cellular redox signaling in health and disease. This article is part of a Special Issue entitled: Thiol-Based Redox Processes.
Keywords: Redox, Thiol, Hydrogen sulfide
1. Introduction
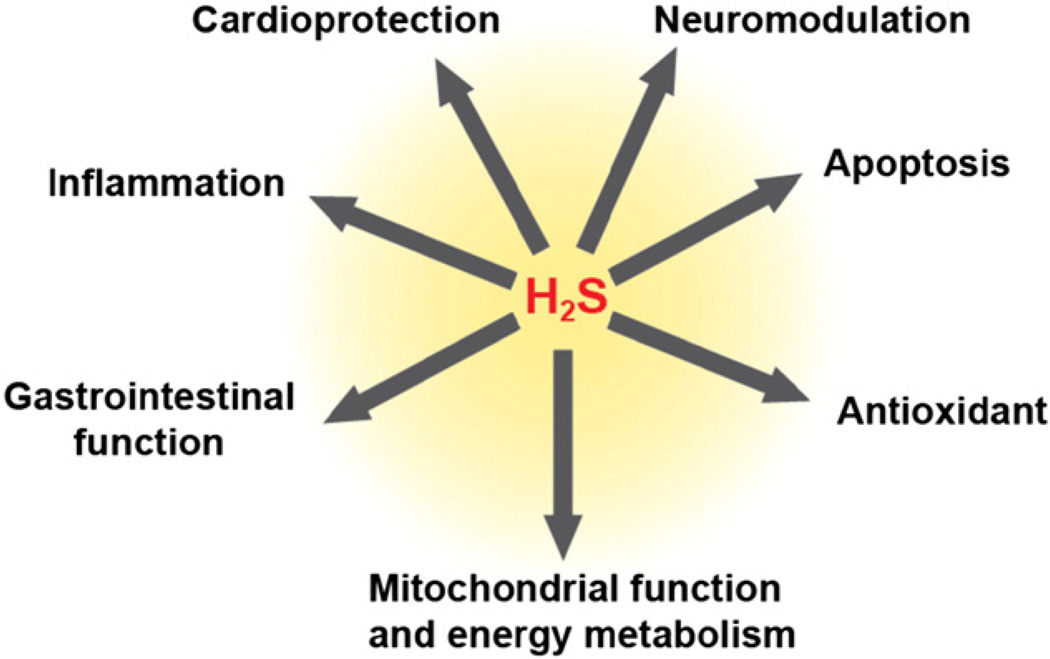
Sulfur-based chemistry is exploited by nature for maintaining cellular redox homeostasis, for redox-based signaling and for neutralizing reactive oxygen and nitrogen species. Reactive cysteine residues in the proteome are important constituents of redox signaling pathways. Reversible changes in the oxidation state of cysteines allow them to function as redox switches in multiple signaling pathways [1] and these residues are often targets of modifications. Additionally, transition metal centers with sulfur ligands can participate in redox-signaling pathways and function as biological redox sensors. Some key messengers used for communication through these redox hotspots are reactive oxygen species (ROS) and reactive nitrogen species (RNS) along with the gaseous signaling molecules such as CO, NO and H2S. Recognition of H2S as a signaling molecule in mammals took longer than NO and CO perhaps due to its long reputation as an environmental toxin and the prevailing view that it was primarily relevant to microbial metabolism. However, since the first report of a physiological role for endogenously produced H2S in mammals [2], there has been an explosion in the literature of its varied biological effects (Fig. 1). Among the signaling mechanisms invoked for H2S, cysteine persulfidation is the one that is most commonly cited [3]. However, the technical problems associated with existing methods for the detection of proteomic persulfidation raise concerns about the validity of the identified targets [4] in addition to raising questions about how target specificity is achieved. The chemical versatility of H2S and the multiplicity of its reported targets suggest that additional mechanisms might be involved in H2S signaling.
Fig. 1.
Overview of the physiological effects elicited by H2S.
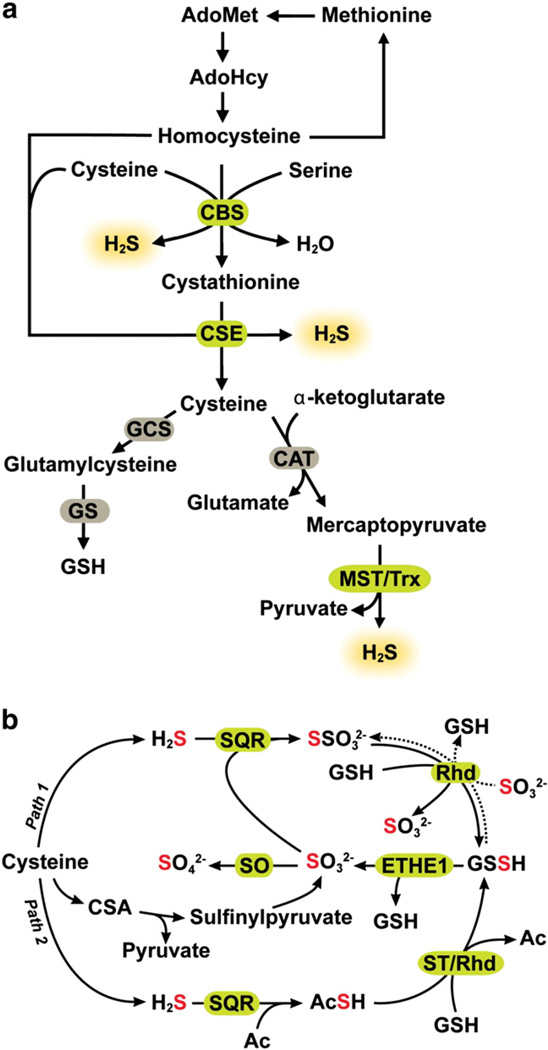
Fundamental gaps in our understanding of how intracellular H2S is regulated hamper in turn, our understanding of its mechanism of action and target selectivity. At least three enzymes, cystathionine β-synthase (CBS), cystathione γ-lyase (CSE) [5,6], and 3-mercaptopyruvate sulfurtransferase (MST) [7,8], contribute to H2S production (Fig. 2a). Housekeeping enzymes produce H2S and it is not known whether signaling by H2S as with NO and CO, can be regulated by increased enzymatic synthesis. It is also not known how H2S biogenesis is selectively regulated relative to the canonical transsulfuration reactions catalyzed by CBS and CSE [9,10]. The low tissue concentration of H2S is a product of both the H2S biogenesis and oxidation fluxes [11].
Fig. 2.
Pathways for sulfide biogenesis and clearance. (a) H2S is synthesized from the amino acids, cysteine and homocysteine via the transsulfuration pathway enzymes, CBS and CSE and by the action of CAT, MST and thioredoxin (Trx) as described in the text. (b) Sulfide oxidation occurs in the mitochondrion. While the first reaction is catalyzed by SQR, the subsequent steps in the pathway remain to be defined. In “path 1” sulfite is shown as the acceptor of sulfane sulfur from SQR while in “path 2”, the acceptor (Ac) is not known and is an intermediate carrier of the sulfane sulfur between SQR and glutathione. The following new abbreviations are used in the figure: Ac (acceptor), GCS (γ-glutamylcysteine synthetase), GS (glutathione synthetase), Rhd (rhodanese), ST (sulfurtransferase).
2. Tissue H2S metabolism
2.1. H2S production
H2S is produced endogenously from cysteine and homocysteine via various reactions catalyzed by CBS and CSE [5,6] and from 3-mercaptopyruvate in a reaction catalyzed by MST [7,8] (Fig. 2a). 3-Mercaptopyruvate is derived via a transamination reaction between cysteine and α-ketoglutarate catalyzed by cysteine aminotransferase (CAT), which is identical to aspartate aminotransferase [12]. MST catalyzes the desulfuration of 3-mercaptopyruvate generating an MST-bound persulfide at an active site cysteine (C248 in the human sequence) and pyruvate [13,14]. The resulting persulfide on MST can be released as H2S in the presence of a reductant. Thioredoxin is the most efficient of the thiophilic acceptors tested for MST and generates H2S in the presence of thioredoxin reductase and NADPH [14,15].
While CBS, CSE and MST catalyze H2S production efficiently at saturating substrate concentrations, their respective tissue distribution, expression levels and efficiency of H2S generation at physiologically relevant substrate concentrations govern their relative intracellular importance to H2S production. Based on murine tissue-specific expression studies of the transsulfuration enzymes, CBS appears to represent the major source of H2S in the murine brain and kidney while CSE is primarily responsible for H2S production in the liver [16]. This is consistent with other reports that CSE is the major source of H2S in the liver and in peripheral tissues while its contribution in the brain is negligible [17,18]. Brain H2S levels are essentially unaffected in the CSE knockout mouse despite a significant decrease in serum H2S and decreased rates of H2S production in the aorta and heart, consistent with the importance of CSE as a source of H2S in peripheral tissues [18]. MST is expressed in the kidney, liver, aorta, and brain [19] and is apparently important for H2S production in the brain [20] and in the vasculature [8]. A detailed kinetic characterization of human MST has been reported recently [14]. The tissue concentrations and contribution of MST to endogenous H2S production in comparison to CBS and CSE await elucidation. The kinetics of H2S generation by CBS, CSE and MST are the subject of several recent reviews and are not covered here [21–23].
The biochemical mechanisms for rapidly modulating H2S levels to induce or ablate H2S-based signaling await elucidation. In addition to H2S production, the transsulfuration pathway enzymes are responsible for the synthesis of cysteine from homocysteine, two substrates used for H2S synthesis (Fig. 2a). Cysteine biogenesis by the transsulfuration pathway supplies a significant portion of cysteine needed intracellularly [9,10]. Although cysteine concentration is 90–100 µM in most tissues [24], pool sizes of metabolites derived from it, i.e. glutathione (~10 mM) and taurine (~20 mM) are high [24–27]. Partitioning of cysteine between multiple metabolic fates is highly regulated [28]. Mechanisms for diverting the transsulfuration enzymes to H2S versus cysteine synthesis remain to be elucidated. Mutations at two conserved residues, Thr257 and Thr260 in human CBS differentially affect the canonical (homocysteine + serine → cystathionine + H2O) versus H2S producing (homocysteine + cysteine → cystathionine + H2S) activities [29]. The Thr257Val mutation results in a ~10-fold decrease in the canonical activity while the H2S generating activity is essentially unaffected. In contrast, introducing Ile at the same position decreases H2S generating activity ~10-fold compared to a ~30-fold decrease in the canonical activity. These results suggest the possibility of independent regulation of H2S generating versus the canonical activities of CBS [29]. The expression levels and activities of CBS and CSE are regulated in multiple ways (reviewed in [22]).
2.2. H2S oxidation
Contrary to the widely varying reports in the literature, tissue H2S is in the low 10–30 nM concentration range [11,30,31] with the exception of aorta, where the concentration is ~20–100-fold higher [32]. The flux of sulfur into H2S in murine liver is comparable to that of glutathione, which is present at steady-state concentrations of 6–10 mM. Hence, the sulfide clearance rate must be high to account for the low steady-state H2S concentrations [11]. Indeed, mammalian tissues harbor a high capacity for H2S oxidation [33,34], which occurs in the mitochondria [35,36]. The oxidation products include persulfide, sulfite, thiosulfate and sulfate . Sulfate constitutes ~90% of total urinary sulfur [37].
The structures and reaction mechanisms of enzymes involved in sulfide oxidation have been discussed in a recent review [23] and only an overview is presented here. The first step of H2S oxidation is catalyzed by a sulfide quinone oxidoreductase (SQR), a flavoprotein localized in the inner mitochondrial membrane (Fig. 2b). SQR catalyzes the two-electron oxidation of sulfide to persulfide and transfers the reducing equivalents to ubiquinone via FAD [38], thus linking H2S oxidation to mitochondrial energy production [35]. The physiological acceptor of the sulfane sulfur bound to mammalian SQR is not known and sulfite is reported to be an efficient acceptor of human SQR under in vitro assay conditions [39] (Fig. 2b, path 1).
Persulfide dioxygenase (the product of the human ethe1 gene) is a non-heme iron protein and catalyzes the oxidation of persulfide to sulfide. Under in vitro assay conditions, glutathione persulfide (GSSH) serves as a substrate for the persulfide dioxygenase [36,40]. Alternatively, rhodanese can catalyze the transfer of the sulfane sulfur from GSSH to sulfite to form thiosulfate [36]. The sulfide oxidation circuitry as presented (Fig. 2b) raises prickly questions for which answers do not currently exist. First, if persulfide dioxygenase is the second enzyme in the sulfide oxidation pathway [36], then what is the proximal acceptor that carries the sulfane sulfur from SQR to GSH (Fig. 2b, paths 1 versus 2)? Human SQR reportedly cannot use GSH as a sulfane sulfur acceptor and sulfide is a poor acceptor, generating hydrogen disulfide, H2S2 [39]. The alternative proposal, that sulfite accepts sulfane sulfur from SQR [39], poses a logical conundrum since sulfite (in the +4 oxidation state) is more oxidized than persulfide (S0 oxidation state) and therefore should be formed downstream in the sulfide oxidation pathway. Hence, an alternative source of sulfite that supports the activity of SQR is needed for the operation of this version of the sulfide oxidation pathway. An alternative route to sulfite does exist in the cysteine catabolic pathway (Fig. 2b) where it is generated by the consecutive actions of cysteine dioxygenase and a transaminase, which generate cysteine sulfinic acid and β-sulfinylpyruvate, respectively; the latter decomposes to give sulfite and pyruvate [41,42]. The contribution of this catabolic pathway to the sulfite pool is, however, expected to be significant only under conditions of excess cysteine, when cysteine dioxygenase is stabilized [43].
Utilization of sulfite by SQR to generate thiosulfate also appears to be at odds with the clinical data on patients with ETHE1 deficiency who exhibit: (a) elevated levels of H2S and thiosulfate, and (b) greatly reduced levels of sulfite [40]. If the role of persulfide dioxygenase is to provide sulfite for SQR, then, the diminished sulfite level in ETHE1 deficiency could be explained by the inactivity of persulfide dioxygenase and possibly, by the exhaustion of a limited sulfite supply that is generated via the cysteine catabolic pathway. However, the increase in the product of the SQR reaction i.e. thiosulfate (Fig. 2b, path 1) and the substrate, H2S, is difficult to explain. The existence of microbial variants in which persulfide dioxygenase and rhodanese are fused suggests utilization of the toxic product of the dioxygenase reaction, i.e. sulfite, by rhodanese. Clearly, a better understanding of the organization of the sulfide oxidation pathway is needed. Increased GSH synthesis by administration of the precursor, N-acetyl cysteine, is an effective treatment for ethylmalonic encephalopathy, caused by mutations in ethe1 and promotes clearance of high levels of H2S by accepting SQR-borne persulfide forming GSSH [44]. These results are consistent with a role for GSH in the sulfide oxidation pathway.
2.3. Bound sulfane sulfur pool
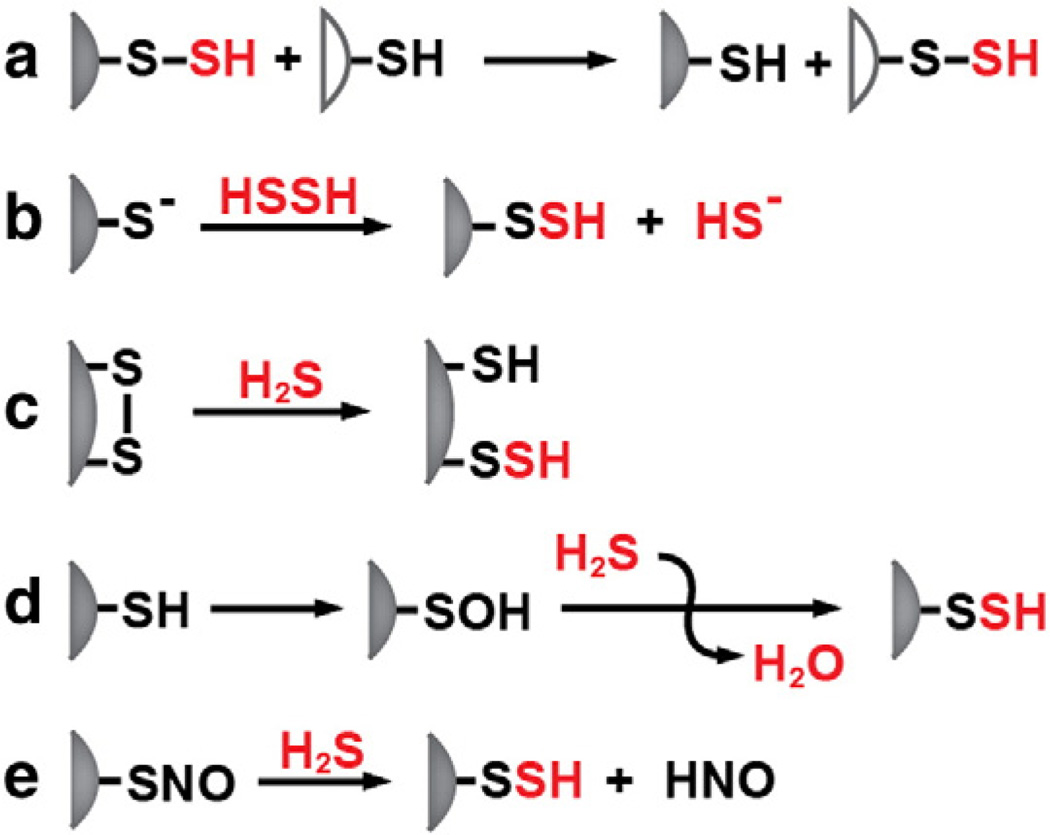
In addition to free H2S, acid labile and protein-bound sulfane sulfur represent two other sulfur pools that are potentially important in H2S metabolism. The acid-labile sulfur pool is associated with iron–sulfur cluster-containing proteins, which are ubiquitous in cells and tissues [45]. Release of sulfide from this pool is unlikely to modulate endogenous H2S levels and is expected to depend on protein turnover. Protein bound persulfides can be formed either by the transfer of a sulfane sulfur group from a protein-bound or small molecule donor to a cysteine acceptor (Fig. 3a,b) or by attack of sulfide on an oxidized cysteine (Fig. 3c–e). H2S can be released from persulfides in the presence of a reductant. The tissue content of cyanolyzable sulfur (i.e. of persulfides) is reported to be ~40, ~324, ~18 and ~34 nmol/g tissue in the rat liver, kidney, heart and spleen [17]. A significant pool of H2S is reportedly stored as bound sulfane in the brain and ~1.5 µmol H2S/g protein was released from mouse brain homogenate by DTT treatment [46]. Gomori-positive granules in periventricular astrocytes are postulated to contain sulfane sulfur [47]. Cyanide treatment decreased the number of these granules in the brain, while treatment with diallyl disulfide, an H2S source, increased their number [48]. While the function of the cytoplasmic Gomori-positive inclusions is not known, their involvement in sulfane sulfur metabolism has been proposed [48]. The bound sulfane sulfur pool is proposed to be important for modulating H2S levels in response to cellular demand [46]. Persulfides are chemically labile and it is not known how a sulfane sulfur pool, if one exists, is protected from continuously leaking H2S in the reducing milieu of the cell.
Fig. 3.
Alternative mechanisms for persulfidation. Persulfidation can occur by transsulfuration in which a small molecule or protein persulfide donor transfers the sulfane sulfur to an acceptor (a), by reaction of a protein thiolate with H2S2 (b), by attack of sulfide on a disulfide (c), by attack of sulfide on cysteine sulfenic acid or (d) by attack of sulfide on cysteine-S-nitrosothiol.
3. Chemical properties of H2S
H2S is a weak acid and readily ionizes in aqueous solution with pKa1 and pKa2 values of 6.9 and > 12 respectively [49]. Therefore at physiological pH, approximately two thirds of total H2S is in the anionic sulfide (HS−) form. Further ionization to S2− requires alkaline conditions. Hence, the concentration of the sulfide dianion is negligible under cellular conditions. H2S is lipophilic and can freely diffuse through membranes [50]. While the electronic configuration of sulfur is similar to that of oxygen, their chemical properties are quite different. Sulfur is less electronegative than oxygen. The more diffuse orbital system and more polarized electron cloud of H2S make it a better nucleophile compared to water at physiological pH. With six valence electrons and a vacant 3d orbital, sulfur can expand its valence shell to accommodate extra electrons resulting in oxidation states ranging from −2 to +6. Sulfane sulfur is in the S0 oxidation state. It is labile and reactive and the terminal or sulfane sulfur atom can be transferred between thiophilic acceptors [51]. Examples of biologically important sulfane sulfur compounds include persulfide (RSSH), thiosulfate , hydropolysulfides (RSnH), polysulfides (RSnR, n > 2), polythionates , and elemental sulfur (S8) that can function in sulfide storage in cells [52].
The sulfur in H2S is in the −2 oxidation state, which represents the most reduced form of sulfur. The two-electron redox potential of H2S (HS− → So + H+ + 2e−) is +0.17 V at pH 7 [53], which makes it a weaker reductant than cysteine and glutathione. The cytoprotective role of H2S has been attributed to its antioxidative capacity [54–58] and a direct role for H2S in scavenging reactive oxygen/nitrogen species such as HOCl [59], ONOO– [60] and •NO [61,62] has been proposed. While the second order rate constant for the reaction of H2S with HOCl, an inflammatory mediator in neutrophils, is large [63], the rate constants for its reaction with other biologically relevant oxidants is small [64,65] and would appear to argue against a significant antioxidant role. However, the products of these reactions, if formed, could be important for cellular function especially under oxidative stress conditions. For example, the two-electron oxidation of H2S by H2O2 would yield sulfenic acid (HSOH), which can react with a second mole of H2S to generate H2S2. Oxidation of H2S by peroxynitrite was reported to generate nitrite and H2S2 in one study [64] and thionitrate (HSNO2) was present predominantly as sulfinyl nitrite (HS(O)NO) in another [65]. It was proposed that unlike thiols, H2S reacts with peroxynitrite through an associative mechanism [65]. Sulfinyl nitrite can act as an •NO donor. One electron oxidation of H2S by peroxynitrite-derived secondary radicals (e.g. •NO2, HO•, ) yields a highly reactive sulfanyl radical (S•−), which can initiate a radical chain reaction in the presence of oxygen forming a range of oxidation products [64]. In addition to its intrinsic ability to modify various cellular targets [66], S•− can react with oxygen to form or with HS− to form HSS•2−. Alternatively S•− can interact with a second mole of S•− to give H2S2 [64]. Other secondary radicals such as [64,67–69] and [70] have been detected using spin trapping experiments. Although the reported cytoprotective effects of H2S are attributed in part to its reactivity towards various oxidant species, the relatively small rate constants for most H2S oxidation reactions together with the low intracellular concentration of H2S versus the more abundant reductants, e.g. glutathione and cysteine, makes a significant role for H2S as an antioxidant scavenger unlikely.
H2S can react with disulfides and oxidized thiols generating persulfide [71]. Persulfides are labile and unless sequestered, are susceptible to reduction or transsulfuration reactions with other acceptors. The sulfane sulfur atom in persulfides is nucleophilic [4]. Since the S–H bond in persulfides is ~22 kcal/mol weaker than the S–H bond in thiols [72], persulfides are stronger acids with pKa values that are ~1–2 units lower compared to the corresponding thiols [73,74]. Persulfide anions are more nucleophilic and better antioxidants than the corresponding thiolates [75] and perthiyl radicals are more stable than thiyl radicals [73]. Reaction of H2S with nitrosothiols forms thionitrous acid (HSNO) adding a new player that might be involved in H2S signaling [76]. Other reactions of H2S involve its reactivity with transition metals and its ability to undergo nucleophilic addition and substitution reactions with unsaturated carbonyl compounds, nitrogen oxides and other biological electrophiles.
4. Mechanisms of H2S-based signaling
4.1. Protein persulfidation
An increasing number of reports suggest the importance of protein persulfidation in H2S-based signaling [77]. In principle, persulfidation can occur by one of at least three mechanisms: (i) nucleophilic attack of a sulfide anion on an oxidized cysteine residue, e.g. sulfenic acid, S-nitrosyl or disulfide, (ii) via transsulfuration from an existing persulfide carried by a small molecule like GSSH or a protein, or (iii) by attack of a cysteine thiol on H2S2 [78] (Fig. 2a,b) or polysulfide [79]. While the inherent lability of the persulfide modification and the lack of obvious mechanisms for achieving target specificity are issues that need to be addressed for establishing persulfidation as a significant mechanism of sulfide-based signaling, examples of persulfidation influencing enzyme activity have been around in the literature for over four decades [80–82]. Although protein sulfhydration was reported to be as prevalent as phosphorylation [77], the inability of the modified biotin switch assay used in this study, to distinguish between persulfides and other sulfur species, especially thiols [4], raises questions about the reported 10–25% prevalence of persulfidation in the liver proteome and the specific proteins that were identified [77]. Assessing the importance of the persulfide modification for H2S signaling and its prevalence in the proteome, await development and application of persulfide-selective reagents together with the direct identification of the persulfide modification by mass spectrometry.
Persulfidation of the active site cysteine in protein tyrosine phosphatase, PTP1B, was demonstrated by mass spectrometry both with purified protein and under cell culture conditions [83]. Persulfidation inactivates PTP1B and leads to accumulation of phosphorylated PERK in response to ER stress. Persulfidation of the p65 subunit of NF-κB in response to TNFα treatment, demonstrated by LC-MS/MS, increases its antiapoptotic function and is dependent on CSE expression [84]. DNA binding of NF-κB and transcriptional activation of antiapoptotic genes was decreased in CSE knockout mice [84]. The modified cysteine in p65 is also a target for S-nitrosylation, which has the opposite functional consequence [84]. Persulfidation of ATP-sensitive K+ channels reportedly elicits channel opening and results in hyperpolarization of endothelial smooth muscle cells and vasodilation, which is impaired in CSE knockout mice [85,86].
4.2. Interaction of H2S with S-nitrosothiols
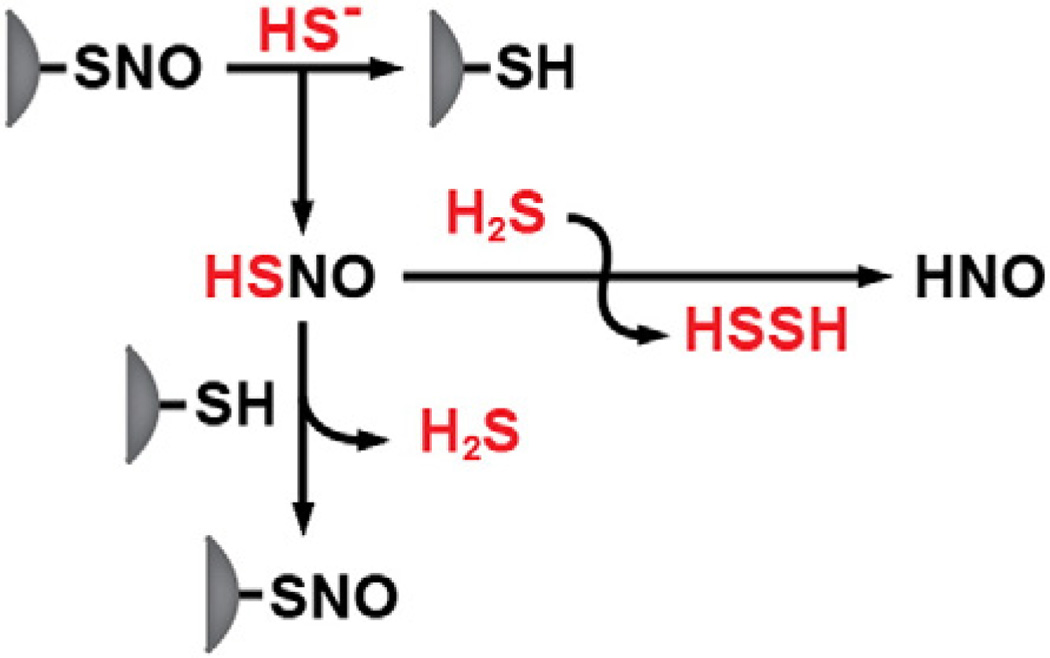
Accumulating evidence for the interaction between •NO and H2S signaling pathways provides a new perspective for potentially understanding the mechanism of H2S action. H2S and •NO show some synergistic physiological effects indicating cross talk between the two signaling systems [87–93]. A molecular mechanism for the interaction between the H2S and •NO signaling pathways is suggested by the identification of nitrosothiol (HSNO) formed by the reaction of H2S and •NO donor compounds in vitro [61]. H2S treatment blocked •NO release from various •NO donor compounds and blocked •NO-dependent increase in cGMP levels in cell culture. Formation of nitrosothiol was inferred by EPR spectroscopy, amperometry and nitrite measurement and the effect on cGMP levels was reversed by Cu2+ consistent with the formation of an adduct between H2S and •NO species [61]. HSNO is also formed by the reaction of H2S with S-nitrosothiols, e.g. GSNO, suggesting a physiologically relevant alternative mechanism for its formation [76] (Fig. 4). In principle, HSNO can generate NO+, NO•, NO− and HNO potentially eliciting diverse specificities and physiological functions [76]. Since HSNO can freely diffuse across membranes [50], it can potentially be used to transnitrosylate proteins at sites remote from the site of •NO synthesis. Despite its known cardioprotective role [94–98], the physiological relevance of HNO as a signaling molecule has been open to question. Formation of HNO in endothelial cells has been demonstrated using a nitroxyl sensitive fluorescence probe [76]. The formation of HNO in a direct displacement reaction of GSNO by GSH had been previously proposed [99,100]. In these studies, nitrous oxide (N2O) and hydroxylamine were used as markers for HNO formation since N2O is generated by decomposition of the HNO dimerization product, hyponitrous acid [100,101].
Fig. 4.
Mechanism for crosstalk between NO and H2S signaling pathways. S-nitrosothiol (HSNO) canbeformed via transnitrosylation and can itself be a donor in anitrosylation reaction or can react with a second mole of H2S generating HNO.
High-level ab initio calculations indicate that the RSNO wave function possesses significant multireference character [102,103] due to its zwitterionic RS+/NO− structure. Nucleophilic attack on the sulfur atom in the ion pair by a thiolate would yield HNO. By analogy, a nucleophilic attack on the sulfur atom of RSNO by HS− would produce HNO and H2S2. Similarly, an attack by a thiolate would produce a persulfide, representing an alternative route to persulfide formation. It has been proposed that HNO mediates at least some of the pharmacological effects of sodium nitroprusside (Na2[Fe(CN)5(NO)]), a potent vasodilator [93,104]. Nitrosylation of thiols [105] and modulation of voltage-dependent K+ channels [106] by HNO have been reported. Importantly, the effect of Angeli's salt, an HNO donor, on myocyte contractility mimics the effect of treatment with sodium nitroprusside and H2S [93].
4.3. Sulfhydration of electrophiles by sulfide
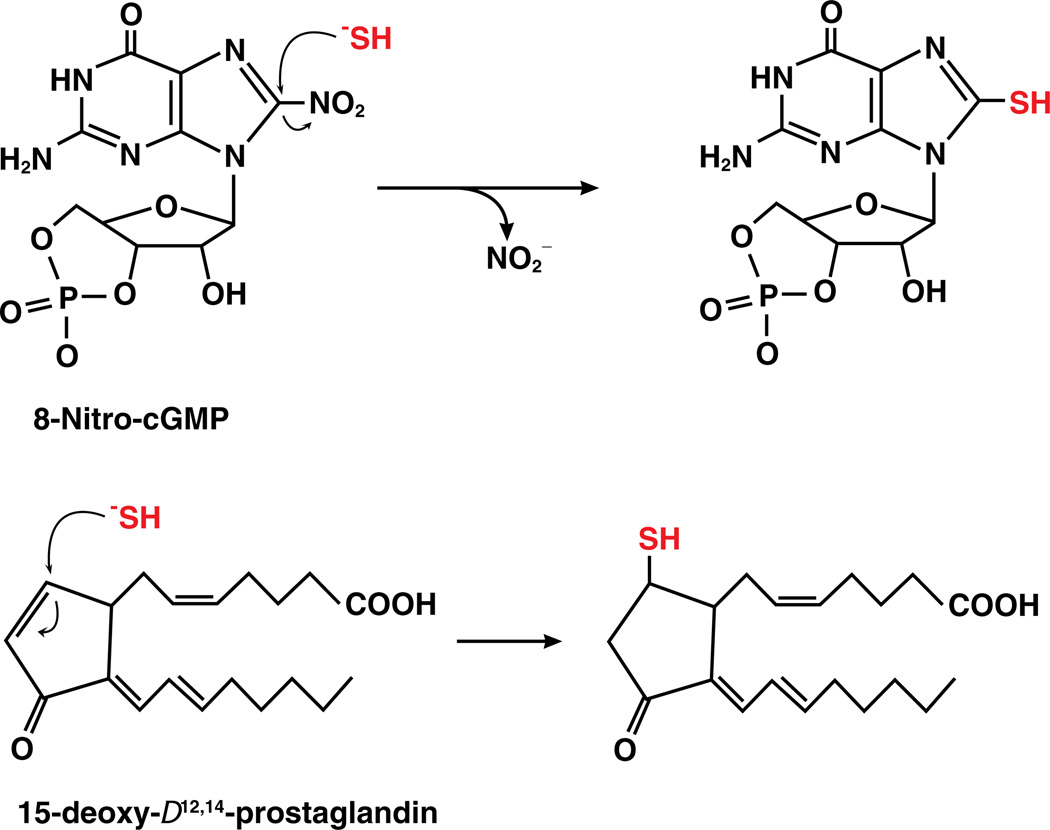
Recently, direct sulfhydration of various cellular electrophilic messengers, such as 8-nitro-cGMP, nitro- and keto- derivatives of unsaturated fatty acids and cyclopentenone prostaglandin, by sulfide has been reported and provides an additional mechanism for H2S-mediated signaling (Fig. 5) [107]. Electrophiles are generated endogenously as byproducts of oxidation reactions and also as byproducts of lipid peroxidation reactions in the presence of ROS and RNS species and primarily react with cysteine residues to initiate signaling [108–111]. S-alkylation of cysteine sulfhydryl groups by electrophilic metabolites such as 8-nitroguanosine 3′,5′-cyclic monophosphate and cyclopentenone prostaglandins and isoprostanes [109,112] mediates various redox-dependent signaling pathways [108] including cGMP-dependent NO signaling [113]. H2S treatment blocks iNOS-dependent S-guanylation (i.e. formation of a cysteine-cGMP adduct) of H-Ras, a modification that activates H-Ras to signal cell senescence in response to stress [107]. The protective role of H2S in myocardial infarction-associated heart failure was proposed to be due in part to increased levels of 8-SH-cGMP and the concomitant loss of H-Ras activation. Protein S-guanylation level was increased by CBS knockdown in various human cell lines [107]. S-guanylation of H-Ras is an example of a system where H2S and •NO elicit opposing functions. Covalent modification of a cysteine in the p50 subunit of NF-κB by 15-deoxy-Δ12,14-prostaglandin J2 (15d–PGJ2), inhibits DNA binding [114], which can be protected by sulfhydration of 15d–PGJ2 [107]. Sulfhydration of the p65 subunit of NF-κB induces its antiapoptotic functions [84]. 15d–PGJ2 also suppresses angiogenesis [115,116]. These findings indicate that H2S could modulate •NO based signaling either via transnitrosylation or competition with targets of •NO. Synergistic effects of H2S and •NO in the endothelial system, where both induce vasorelaxation, might be due to transnitrosylation mediated by H2S.
Fig. 5.
Reaction of H2S with electrophiles. Sulfide can attack electrophiles like 8-nitro-cGMP to form 8-HS-cGMP (top) or fatty acids like 15-deoxy-Δ12,14-prostaglandin to form the thio-adduct.
4.4. Interaction of H2S with metal centers
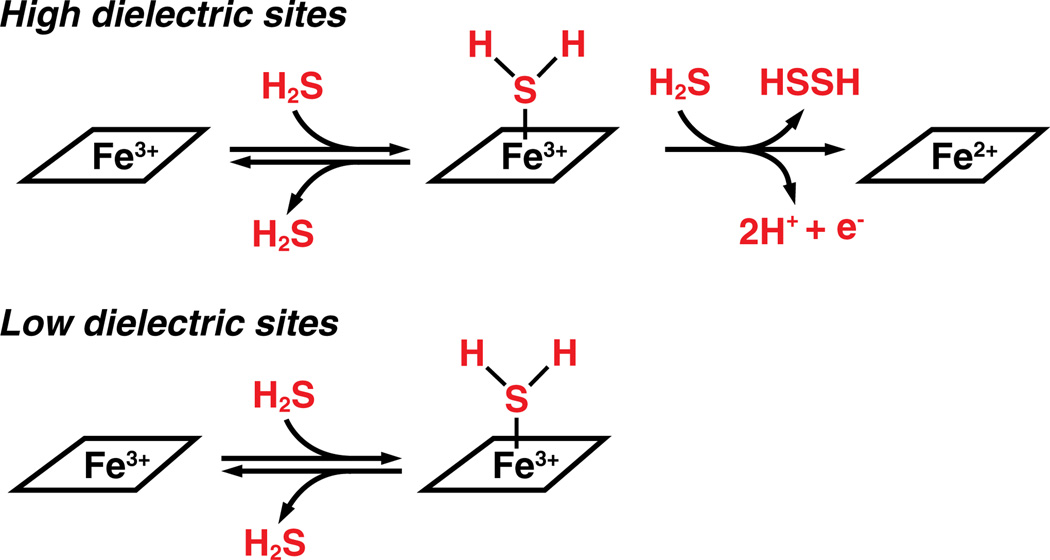
Another potential mechanism for H2S-dependent signaling is its interaction with metal centers. H2S can either reduce the metal center or it can coordinate to it depending on the stereoelectronic characteristics of metal-containing active site. With hemeproteins, polar active sites and high H2S concentrations promote heme reduction by H2S where as non-polar active sites promote formation of a heme–H2S complex (Fig. 6) [117]. With a stronger nucleophilic character, compared to water, sulfide can easily displace water that generally fills empty coordination sites at metal centers in the active sites of proteins. H2S binds to both heme a3 and CuB in the binuclear center [118] and reversibly inhibits cytochrome c oxidase, which results in a drop in the metabolic rate inducing a state of suspended animation [119]. The Ki for sulfide inhibition is 0.2 µM and ~20 µM with purified cytochrome c oxidase [120] and in intact cells [121], respectively. Isolated mitochondria respire maximally in the presence of 10 µM H2S consuming oxygen and generating ATP but the rate decreases with increasing sulfide concentrations due to inhibition of cytochrome c oxidase [122]. Decreased ROS generation is one consequence of inhibition of cytochrome c oxidase by H2S, which is believed to account for the protective effect of H2S donors in reperfusion-associated cardiac injury.
Fig. 6.
The reaction of H2S with heme is dictated by the polarity of the heme pocket. A high dielectric (or polar) site favors reduction to ferrous heme whereas a low dielectric (or non-polar) site favors coordination to ferric heme.
Invertebrates living in sulfide-rich habitats use hemoglobin to transport sulfide to symbiotic bacteria to protect themselves from H2S toxicity [46]. The mollusk, Lucina pectinata, which lives in sulfide-rich mangroves, uses ferric heme to transport H2S. Although it possesses three types of hemoglobin, only hemoglobin I binds H2S [46]. The reactivity of H2S with mammalian hemoglobin and myoglobin varies and is partly determined by the organization of the active site [117]. It is not known whether and if, coordination or reduction of metal centers by H2S is involved in signaling. H2S can react with ferric hemoglobin to form sulfhemoglobin, which might function as a metabolic sink for H2S under some conditions [123].
5. Cellular and physiological processes regulated by H2S
5.1. Vasorelaxation
H2S functions as an endothelial-derived hyperpolarizing factor (EDHF) and its vasorelaxant activity is ascribed primarily to activation of KATP channels [18,62,85–87,124]. The effect of H2S on relaxation of blood vessels is sensitive to the presence of KATP channel inhibitors and believed to involve direct channel activation by H2S [85,86,124,125]. Alternatively, an indirect mechanism can be considered as inhibition of cytochrome c oxidase results in reduced ATP levels, which activate KATP channels [126]. H2S-induced vasorelaxation is independent of the cGMP-mediated pathway. Hyperpolarization by acetylcholine, which is mediated by intermediate- and small-conductance potassium channels, is dependent on CSE expression [86]. CSE knockout mice suffer from hypertension, exhibit endothelial dysfunction and are deficient in inducing endothelial-dependent hyperpolarization, consistent with H2S functioning as an EDHF [18,86]. While CSE−/− mice exhibit decreased H2S levels (~80% lower in the heart and aorta and 50% lower in the serum), they have significantly increased plasma homocysteine and reduced glutathione levels in the aorta and mesenteric artery beds [18], which could also contribute to the observed endothelial dysfunction in these animals [127].
5.2. Neuromodulation
H2S functions as a signaling molecule in the brain and facilitates long-term potentiation in hippocampal neurons by activating NMDA receptors [2] and activates Ca2+ channels increasing Ca2+ influx into glial cells [128]. In addition to signaling, several lines of evidence indicate a neuroprotective role for H2S. Opening of KATP channels by H2S protects hippocampal pyramidal neurons from excitotoxicity and cell death [55] and K+ channel openers are considered to have therapeutic potential for diseases such as epilepsy [129]. Upon exposure to high levels of the neurotransmitter, glutamate, H2S is reported to increase neuronal antioxidant capacity by enhancing intracellular glutathione as a consequence of increasing uptake of cystine and cysteine and activating γ-glutamylcysteine synthase [54]. H2S also protects immortalized mouse hippocampal cells from oxidative glutamate toxicity and in addition to increasing glutathione synthesis, activates KATP and Cl− channels [55]. However the mechanism by which H2S modulates the cystine/glutamate antiporter to increase intracellular cysteine at high extracellular glutamate concentrations is not known. Increased synthesis and redistribution of glutathione to mitochondria is proposed to protect neuronal cells from oxidative stress induced by H2O2 [130]. Overexpression of mitochondrial MST and CAT protects cells from glutamate toxicity suggesting an anti-oxidative role for endogenously produced H2S [130]. Involvement of H2S in neuronal cell differentiation has been reported recently [131]. Cysteine supplementation induced proliferation and differentiation of neuronal stem cells to neurons and astroglia, which was attenuated by siRNA-induced inhibition of CBS expression [131].
5.3. Regulation of ion channels
Ion channel regulation is believed to be an important mechanism for mediating the physiological effects of H2S [132,133]. KATP channels are widely distributed in mitochondrial and plasma membranes and their modulation by H2S is reportedly important for myocardial protection against ischemia/reperfusion injury [134], insulin secretion by pancreatic β-cells [135], protection against glutamate induced neuronal toxicity [55] and regulation of nociception [136] and inflammation [137]. H2S activates KATP channels and brings about relaxation of endothelial smooth muscle cells [85]. Activation of the KATP channel apparently occurs by persulfidation at Cys43 in the pore-forming Kir6.1 subunit [86], which decreases ATP binding and promotes channel binding to phospholipid phosphatidylinositol 4,5-bisphosphate. Mutations at Cys6 and Cys105 in the extracellular loop of the regulatory SUR1 subunit of the rat KATP channel, also impaired H2S-dependent activation suggesting their involvement in H2S-modulation of channel function [138].
H2S has also been implicated as a modulator of other channels including the Ca2+-sensitive K+ channel (BKCa), L-type and T-type Ca2+ channels, chloride channels, members of the TRP (transient receptor potential) family of channels and Na+ channels (reviewed in [133]). Of these, regulation by H2S of the BKCa, important for oxygen sensing, is particularly interesting, since H2S also functions as an oxygen sensor [139,140]. Studies with glomus cells from the rat carotid body and of human channel proteins expressed in HEK293 cells suggested an inhibitory role for H2S with high IC50 values: 670 µM [141] and 275 µM [142]. However, conflicting results have also been reported with H2S activating rather than inhibiting BKCa channels in rat pituitary tumor cells albeit with high EC50 values (169 µM and 2 mM in a biphasic dose dependence response) [143].
H2S regulates low-voltage activated T-type Ca2+ channels, which are responsible for the pacemaker potential in the heart and low-threshold spikes in the central nervous system. H2S increases T-type Ca2+ channel currents in mouse N18TG2 neuroblastoma cells [144]. These channels are involved in epilepsy and chronic pain [145,146]. High voltage L-type Ca2+ channels from rat cerebellar granule neurons are also reportedly activated by H2S. However, cardiac myocyte L-type Ca2+ channels isolated from both spontaneously hypertensive rats and normal rats are reportedly inactivated by H2S [147–149] suggesting tissue/cell specific regulation. Activation of Na+ channels has also been reported by addition of NaSH by a mechanism proposed to be sensitive to reducing agents [133,150] and alkylating agents [133,150] suggesting involvement of cysteine modification. Activation of the TRP family of channels, TRPV1 and TRPA1, is proposed to stimulate neukinin-mediated neurogenic inflammation response and increases intracellular Ca2+ [133,151,152]. Conversely, H2S inhibits chloride channels by decreasing the open probability of the channel [55,133,153,154]. It is important to note however that high concentrations of H2S (up to 1 mM) were used in many of these studies.
5.4. Endoplasmic reticulum (ER) stress
Stresses such as infection, inflammation, and increased synthesis of secreted proteins such as in diabetes or conditions triggering abnormal folding of proteins can result in accumulation of misfolded or unfolded proteins leading to compromised ER function and induction of the unfolded protein response. The latter activates autophosphorylation of an ER resident kinase, PERK, which in turn phosphorylates eukaryotic initiator factor 2α resulting in inhibition of general protein synthesis providing a temporal window for reestablishment of cellular homeostasis. Persulfidation of PTP1B, a tyrosine phosphatase that dephosphorylates PERK, at the active site cysteine is seen in response to ER stress and is dependent on CSE activity [83]. Persulfidation of PTP1B inhibits its activity resulting in higher level of PERK phosphorylation under ER stress conditions. Consistent with these results, CSE expression increases in response to ER stress by a mechanism dependent on ATF4 (activating transcription factor 4) [155], a transcription factor that is activated in response to ER stress. Disruption of the ATF4 gene results in decreased CSE expression and concomitant decrease in cellular glutathione levels. CSE−/− mouse embryonic fibroblast cells are susceptible to ER stress-induced cell death [155]. Together, these results suggest a regulatory role for H2S in the ER stress response.
5.5. Hypoxia
The carotid body is important for oxygen chemoreception and in response to reduced O2 partial pressure, signals to the central nervous system, which initiates an autonomic reflex leading to increased respiratory rate and activation of the sympathetic nervous system, which in turn leads to vasoconstriction resulting in increased blood pressure and heart rate. However, the mechanism by which O2 tension is sensed is not well understood. Carotid body activity is inhibited under normoxia by CO and NO [156]. It has been hypothesized that H2S metabolism is involved in oxygen sensing since hypoxic conditions lead to increased intracellular H2S due to decreased mitochondrial H2S oxidation [157]. H2S treatment of isolated pulmonary arteries produces contractile patterns similar to that induced by hypoxia [157] and in perfused trout gills [158]. H2S administration to specialized chemoreceptor organs of trout resulted in a dose-dependent reduction of heart rate and an increase in ventillatory frequency mimicking hypoxia [139]. Removal of the first pair of gills that contains chemoreceptors analogous to the vertebrate carotid body, inhibited H2S-induced reduction in heart rate consistent with a role for H2S in O2 sensing in this organ [139]. A concentration-dependent activation of murine afferent nerves in the carotid body was observed in response to NaHS [140]. Activation of chemoreceptor afferent nerve by hypoxia was decreased by inhibition of CBS but not CSE. As seen with hypoxia, NaHS inhibited BKCa channel currents and the inhibition was alleviated by a CBS inhibitor [140]. CSE deficiency induced pharmacologically or achieved via gene disruption in mice impaired carotid body response to hypoxia [159]. NaHS has been shown to inhibit BKCa channel activity by decreasing the open state probability, albeit with a very high IC50 value as discussed above (275–670 µM) [141,142]. The mechanism and physiological relevance of BKCa channel modulation by H2S remain to be elucidated.
5.6. Inflammation
Inflammation is a complex process orchestrated by a proinflammatory phase characterized by activation and trafficking of lymphocytes and macrophages to the affected area and expression of pro-inflammatory cytokines followed by a resolution phase characterized by secretion of anti-inflammatory mediators and induction of apoptosis of infiltrating lymphocytes leading to clearance of the affected area. Dysregulation in this process could lead to chronic inflammation. A growing number of reports implicate a regulatory role for H2S in inflammatory processes although mechanistic insights are limited and both pro- and anti-inflammatory effects of H2S have been reported.
H2S treatment reduced leukocyte adherence to rat mesenteric venules, which was reversed by a K(atp) channel blocker or inhibition of CSE [160]. H2S also prevented infiltration of neutrophils and lymphocytes into endothelial walls [160,161], reduced expression of pro-inflammatory cytokines IL-1B, IL-6, TNFα, and prostaglandin E [162,163], and attenuated mucosal injury [164]. Intraperitoneal administration on NaHS in an oleic acid-induced acute lung injury model in rat resulted in decreased expression of the pro-inflammatory cytokines IL-6 and IL-8 and increased levels of the anti-inflammatory cytokine, IL-10 [165].
Oxidized products of LDL, implicated in the inflammatory response, decreased CSE expression and H2S production in macrophages [166]. CSE overexpression in the transformed macrophage cell line, Raw264.7, and in primary macrophages blocked the oxidized LDL-induced increase in TNFα level and subsequent NF-κB activation while CSE knockdown elicited the opposite effect [166]. A negative correlation between blood H2S level and the LDL: HDL ratio has been reported, which could be explained by a positive correlation between H2S and HDL [167]. Inhibition of H2S synthesis resulted in inflammation and colonic mucosal injury in healthy rats, decreased cyclooxygenase-2 (COX-2) expression and reduced prostaglandin E2 production [168]. COX-2 generates anti-inflammatory electrophilic oxo-derivatives of fatty acid [169] while prostaglandin E2 inhibits expression of inflammatory chemokines [170] and production of TNFα [171]. H2S induced polymorphonuclear cell apoptosis, consistent with promoting an inflammatory response during the resolution phase [172]. The high H2S concentration used in this study (1 mM) was explained as being relevant to anaerobic infections such as in chronic periodontitis, frequently associated with microflora that are sulfide-producing [172].
On the other hand, studies suggesting that H2S is a proinflammatory agent, which increases production of pro-inflammatory cytokines [173] and modulates leukocyte trafficking (i.e. rolling and adhesion), have been reported [174,175]. H2S levels increase in LPS-induced rodent models of systemic inflammation and inhibition of H2S production was reported to be protective [174,176–178]. Increased CSE [176,178] and CBS [178] expression was correlated with inflammation in these studies. Inhibition of CSE-dependent H2S synthesis decreased carrageenan-induced paw edema in mouse [179] and increased plasma H2S was seen in rats exposed to hemorrhagic shock [180]. Patients with septic shock [176] or various types of shock [181] displayed increased plasma H2S. The discrepancy in the literature on the effect of H2S in inflammation could be due to the phase of the inflammation that was characterized or alternatively, the varying and often relatively high, concentrations of H2S used [162]. High bolus concentrations of NaHS produce a burst of H2S in a short period of time that may not be physiologically relevant.
5.7. Role of H2S in mitochondrial bioenergetics
The ability to oxidize H2S is an ancient one inherited from prokaryotes that utilize sulfur compounds such as sulfide as electron donors to generate energy. Invertebrates such as clams and worms living in sulfide-rich environments also use sulfide as an inorganic energy source coupling its oxidation to mitochondrial oxidative phosphorylation [35,182]. At higher concentrations, i.e. >20 µM, cytochrome c oxidase is inhibited in intact cells and H2S acts as a poison for the electron transfer chain [126]. Efficient oxidation of sulfide is important for averting its buildup and consequent toxicity [183]. Sulfide oxidation is activated at concentrations as low as 10–20 nM, helping maintain subtoxic concentrations of H2S [184]. Studies on isolated mitochondria and murine hepatoma cells revealed that low concentrations (0.1–1 µM) increase while higher concentrations (3–30 µM) inhibit respiration [185]. The presence of 10–100 nM 3-mercaptopyruvate enhanced mitochondrial electron transport activity, which was reversed by suppression of MST or SQR expression suggesting the potential role for sulfide metabolism in regulating cellular energetics [185].
Reversible inhibition of cytochrome c oxidase decreases metabolic rate [119] and mediates the cytoprotective effects of H2S during myocardial ischemia reperfusion by decreasing production of damaging ROS [186]. It is not known whether and how mitochondrial sulfide metabolism is regulated in response to cellular needs for H2S. The quantitative significance of mitochondrial sulfide oxidation to ATP production also awaits elucidation.
5.8. Apoptosis
Overexpression of CSE in human smooth muscle cells results in significant inhibition of cell growth and in increased apoptosis, which are proposed to be due to an increased endogenous production of H2S [187]. These cells also exhibit increased extracellular signal-regulated kinase (ERK) and MAP kinase activation, induction of p21 and down-regulation of cyclin D1 expression [187]. Smooth muscle cells derived from CSE knockout mice showed enhanced proliferation compared to cells derived from wild-type mice [188]. CSE−/− cells were more susceptible to H2S-induced apoptosis than control cells and displayed decreased phosphorylation of ERK in mesenteric arteries [188]. Exogenously added H2S or overexpression of CSE reduced viability of insulin-secreting pancreatic beta cells [189] and decreased insulin secretion [135]. The effect of H2S on cell fate could be cell-type specific. For example, pancreatic beta cells specialized to express and export high concentrations of insulin, could be sensitive to H2S-mediated modulation of their highly regulated ER function.
Anti-apoptotic effects have also been reported for H2S. Human neuroblastoma SH-SY5Y cells were protected by H2S from 6-hydroxydopamine-induced injury [190] and PC12 cells were protected by H2S against methyl phenylpyridinium-induced cytotoxicity and cell death [191]. H2S protected cells from oxidative glutamate toxicity by stimulating production of glutathione [54,130,192]. The anti-apoptotic action of NF-κB is mediated by H2S-dependent persulfidation and is markedly diminished in CSE knockout mice [84]. Further studies are needed to elucidate the opposing pro- and anti-apoptotic effects of H2S.
5.9. Gastrointestinal physiology
H2S is proposed to play a regulatory role in the gastrointestinal tract by modulating intestinal contractility [193], by affecting colonic ion secretion via involvement of potassium channels [194,195] and by its excitatory action on enteric neuorons [196–198]. Intestinal microflora produces large amounts of H2S by degradation of sulfur-containing organic compounds and by dissimilatory sulfate reduction. Consequently, H2S can reach up to millimolar concentrations [199] and its levels correlate with the dietary protein intake [200]. A significant proportion of intestinal H2S is bound to fecal components and the free H2S content is low [199]. The colonic mucosa has a high capacity for oxidizing sulfide to thiosulfate [201,202], which is accompanied by mitochondrial energization [183], thus protecting against the injurious effects of high sulfide levels. The cecal and colonic mucosa also express high thiol methyltransferase activity, which can generate methanethiol [203] and high steady-state levels of rhodanese are found in the submucosa and crypts of the colon [204].
Imbalance in colonic epithelial cell sulfide oxidation is proposed to be a contributing factor in the pathogenesis of ulcerative colitis, an inflammatory bowel disease characterized by epithelial cell damage [205]. Excess H2S is proposed to inhibit oxidation of butyrate [206], a major energy source for colonic epithelial cells [207]. Inhibition of butyrate utilization could exacerbate cell damage leading to inflammation of colonic crypts. Fecal H2S production is increased in patients with ulcerative colitis [208] although the fecal count of sulfate reducing bacteria in controls versus ulcerative colitis patients was not found to differ [209]. Sulfide oxidation was reportedly ~3-fold lower at sites of mucosal ulceration in hapten-induced colitis in rats versus in healthy controls while H2S production was increased and mirrored in enhanced expression of CSE and MST (10- and 3-fold respectively), and diminished expression of SQR (~3-fold) [210]. Rhodanese expression and activity were diminished in colonic tissues in dextran sulfate sodium-induced colitis in mice, consistent with a role for impaired sulfide clearance in inflammatory bowel disease [211]. Administration of high concentrations of methionine or S-adenosylmethionine 1,4 butane disulfonate to rat colonocytes ablated the effects of sulfide toxicity and was proposed to be due to sulfide methylation [212].
The role of H2S in ulcerative colitis is however controversial. No detectable difference in colonic luminal sulfide levels between ulcerative colitis and control subjects has been reported [213]. In dextran sulfate-induced ulcerative colitis in rats, administration of a sulfide binding compound, bismuth subsalicylate, reduced fecal sulfide release but did not protect against colitis, indicating that elevated sulfide does not play a role in the etiology of colitis, at least in this model [214]. On the other hand, other studies have reported a protective role for H2S in healing of gastric ulcers and colitis [215,216], gastric injury caused by anti-inflammatory nonsteroidal drugs [164] and resolution of colitis [168]. It is important to note that experimental models of ulcerative colitis might be substantially different from the naturally occurring disease and the effect of H2S could be dependent on both the experimental model as well as the stage of colitis.
6. Summary
As fast-moving as the field of H2S biochemistry has become, the pace is tempered by the many gaps in our understanding of the molecular mechanisms underlying sulfide oxidation, H2S homeostasis and H2S signaling targets. These gaps in knowledge also represent opportunities for moving the field forward. The controversies surrounding the sometimes dichotomous effects of H2S (e.g. it is both pro- and anti-inflammatory) highlight the problems associated with interpreting studies performed with a wide concentration range of H2S and the technical challenges with handling a redox-active gas. Given the profound physiological effects attributed to H2S, it is expected that it will continue to enjoy attention particularly in the areas of redox-signaling mechanisms and the enzymology of its biogenesis and decay in the context of health and disease.
Abbreviations
- AdoMet
S-adenosylmethionine
- AdoHcy
S-adenosylhomocysteine
- ATF4
activating transcription factor 4
- BKCa
large conductance calcium-sensitive potassium channel
- CBS
cystathionine β-synthase
- COX-2
cyclooxygenase-2
- EDHF
endothelial-derived hyperpolarizing factor
- ER
endolasmic reticulum
- ERK
extracellular signal-regulated kinase
- ETHE1
persulfide dioxygenase
- GSH
glutathione
- H2S
hydrogen sulfide
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
This article is part of a Special Issue entitled: Thiol-Based Redox Processes.
This work was supported in part by a grant from the National Institutes of Health (HL58984 to R.B.) and the American Heart Association (13SDG17070096 to O.K.).
References
- 1.Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 4.Pan J, Carroll KS. Persulfide reactivity in the detection of protein S-sulfhydration. ACS Chem. Biol. 2013;8(6):1110–1116. doi: 10.1021/cb4001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister A, Fraser PE, Tice SV. Enzymatic desulfuration of beta-mercaptopyruvate to pyruvate. J. Biol. Chem. 1954;206:561–575. [PubMed] [Google Scholar]
- 8.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 9.Beatty PW, Reed DJ. Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch. Biochem. Biophys. 1980;204:80–87. doi: 10.1016/0003-9861(80)90009-0. [DOI] [PubMed] [Google Scholar]
- 10.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 11.Vitvitsky V, Kabil O, Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 2012;17:22–31. doi: 10.1089/ars.2011.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubuka T, Umemura S, Yuasa S, Kinuta M, Watanabe K. Purification and characterization of mitochondrial cysteine aminotransferase from rat liver. Physiol. Chem. Phys. 1978;10:483–500. [PubMed] [Google Scholar]
- 13.Westrop GD, Georg I, Coombs GH. The mercaptopyruvate sulfurtransferase of Trichomonas vaginalis links cysteine catabolism to the production of thioredoxin persulfide. J. Biol. Chem. 2009;284:33485–33494. doi: 10.1074/jbc.M109.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 16.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasawara Y, Isoda S, Tanabe S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994;17:1535–1542. doi: 10.1248/bpb.17.1535. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagahara N, Ito T, Kitamura H, Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998;110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Banerjee R. PLP-dependent H2S biogenesis. Biochim. Biophys. Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2013 doi: 10.1089/ars.2013.5339. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motl N, Yadav PK, Banerjee R. Enzymology of hydrogen sulfide turnover. In: Kimura H, editor. Hydrogen Sulfide and its Therapeutic Applications. 2013. Springer [Google Scholar]
- 24.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Banerjee R. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperho-mocysteinemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R39–R46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi N, Higashi T, Shinya S, Naruse A, Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J. Biochem. 1974;75:93–103. doi: 10.1093/oxfordjournals.jbchem.a130387. [DOI] [PubMed] [Google Scholar]
- 26.Ueki I, Roman HB, Hirschberger LL, Junior C, Stipanuk MH. Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1292–E1299. doi: 10.1152/ajpendo.00589.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitvitsky V, Garg SK, Banerjee R. Taurine biosynthesis by neurons and astrocytes. J. Biol. Chem. 2011;286:32002–32010. doi: 10.1074/jbc.M111.253344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stipanuk MH, Dominy JE, Jr., Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J. Nutr. 2006;136:1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 29.Yadav PK, Xie P, Banerjee R. Allosteric communication between the pyridoxal 5′-phosphate (PLP) and heme sites in the H2S generator human cystathionine beta-synthase. J. Biol. Chem. 2012;287:37611–37620. doi: 10.1074/jbc.M112.414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in is-chemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 32.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 33.Curtis CG, Bartholomew TC, Rose FA, Dodgson KS. Detoxication of sodium 35 S-sulphide in the rat. Biochem. Pharmacol. 1972;21:2313–2321. doi: 10.1016/0006-2952(72)90382-6. [DOI] [PubMed] [Google Scholar]
- 34.Bartholomew TC, Powell GM, Dodgson KS, Curtis CG, Oxidation of sodium sulphide by rat liver. lungs and kidney. Biochem. Pharmacol. 1980;29:2431–2437. doi: 10.1016/0006-2952(80)90346-9. [DOI] [PubMed] [Google Scholar]
- 35.Powell MA, Somero GN. Hydrogen sulfide oxidation is coupled to oxidative phosphorylation in mitochondria of Solemya reidi . Science. 1986;233:563–566. doi: 10.1126/science.233.4763.563. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 37.Beauchamp RO, Jr., Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 38.Cherney MM, Zhang Y, Solomonson M, Weiner JH, James MN. Crystal structure of sulfide:quinone oxidoreductase from Acidithiobacillus ferrooxidans: insights into sulfidotrophic respiration and detoxification. J. Mol. Biol. 2010;398:292–305. doi: 10.1016/j.jmb.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 40.Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 41.Singer TP, Kearney EB. Intermediary metabolism of L-cysteinesulfinic acid in animal tissues. Arch. Biochem. Biophys. 1956;61:397–409. doi: 10.1016/0003-9861(56)90363-0. [DOI] [PubMed] [Google Scholar]
- 42.Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- 43.Dominy JE, Jr., Hirschberger LL, Coloso RM, Stipanuk MH. In vivo regulation of cysteine dioxygenase via the ubiquitin-26S proteasome system. Adv. Exp. Med. Biol. 2006;583:37–47. doi: 10.1007/978-0-387-33504-9_4. [DOI] [PubMed] [Google Scholar]
- 44.Viscomi C, Burlina AB, Dweikat I, Savoiardo M, Lamperti C, Hildebrandt T, Tiranti V, Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat. Med. 2010;16:869–871. doi: 10.1038/nm.2188. [DOI] [PubMed] [Google Scholar]
- 45.Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature's modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 46.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 47.Srebro Z, Slebodzinski A. Periventricular Gomori-positive glia in the hypothalamus of the rabbit. Folia Biol. 1966;14:391–395. [PubMed] [Google Scholar]
- 48.Iciek M, Bilska A, Ksiazek L, Srebro Z, Wlodek L. Allyl disulfide as donor and cyanide as acceptor of sulfane sulfur in the mouse tissues. Pharmacol. Rep. 2005;57:212–218. [PubMed] [Google Scholar]
- 49.Vorobets VS, Kovach SK. G.Y. Kolbasov. Russ. J. Appl. Chem. 2002;75:229–234. [Google Scholar]
- 50.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohwerder T, Sand W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology. 2003;149:1699–1710. doi: 10.1099/mic.0.26212-0. [DOI] [PubMed] [Google Scholar]
- 52.Toohey JI. Sulphane sulphur in biological systems: a possible regulatory role. Biochem. J. 1989;264:625–632. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collman JP, Ghosh S, Dey A, Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 55.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 56.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, Moore PK. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 60.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 61.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 62.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagy P, Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 64.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Moller MN, Folkes LK, Garcia-Bereguiain MA, Gutierrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 65.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M. I. Ivanovic-Burmazovic, Biochemical insight into physiological effects of H(2)S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 66.Wardman P, von Sonntag C. Kinetic factors that control the fate of thiyl radicals in cells. Methods Enzymol. 1995;251:31–45. doi: 10.1016/0076-6879(95)51108-3. [DOI] [PubMed] [Google Scholar]
- 67.Das TN, Huie RE, Neta P, Padmaja S. Reduction potential of the sulfhydryl radical: pulse radiolysis and laser flash photolysis studies of the formation and reactions of •SH and HSSH•− in aqueous solutions. J. Phys. Chem. A. 1999;103:5221–5226. [Google Scholar]
- 68.Mills G, Schmidt KH, Matheson MS, Meisel D. Thermal and photochemical reactions of sulfhydryl radicals: implications for colloid photocorrosion. J. Phys. Chem. 1987;91:1590–1596. [Google Scholar]
- 69.Zhu J, Petit K, Colson AO, DeBolt S, Sevilla MD. Reactions of sulfhydryl and sulfide radicals with oxygen, hydrogen sulfide, hydrosulfide, and sulfide:formation of SO2−, HSSH−, HSS•2− and HSS. J. Phys. Chem. 1991;95:3676–3681. [Google Scholar]
- 70.Stasko A, Brezova V, Zalibera M, Biskupic S, Ondrias K. Electron transfer: a primary step in the reactions of sodium hydrosulphide, an H2S/HS(−) donor. Free Radic. Res. 2009;43:581–593. doi: 10.1080/10715760902977416. [DOI] [PubMed] [Google Scholar]
- 71.Francoleon NE, Carrington SJ, Fukuto JM. The reaction of H(2)S with oxidized thiols: generation of persulfides and implications to H(2)S biology. Arch. Biochem. Biophys. 2011;516:146–153. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Benson SD. Thermochemistry and kinetics of sulfur-containing molecules and radicals. Chem. Rev. 1978;78:23–25. [Google Scholar]
- 73.Everett SA, Folkes LK, Wardman P, Asmus KD. Free-radical repair by a novel perthiol: reversible hydrogen transfer and perthiyl radical formation. Free Radic. Res. 1994;20:387–400. doi: 10.3109/10715769409145638. [DOI] [PubMed] [Google Scholar]
- 74.Newton GL, Dwyer TJ, Kim T, Ward JF, Fahey RC. Determination of the acid dissociation constants for WR-1065 by proton NMR spectroscopy. Radiat. Res. 1992;131:143–151. [PubMed] [Google Scholar]
- 75.Everett SA, Wardman P. Perthiols as antioxidants: radical-scavenging and prooxidative mechanisms. Methods Enzymol. 1995;251:55–69. doi: 10.1016/0076-6879(95)51110-5. [DOI] [PubMed] [Google Scholar]
- 76.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ. I. vanovic-Burmazovic, Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagy P, Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 79.Greiner R, Palinkas Z, Basell K, Becher D, Antelmann H, Nagy P, Dick TP. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Massey V, Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J. Biol. Chem. 1970;245:6595–6598. [PubMed] [Google Scholar]
- 81.Branzoli U, Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J. Biol. Chem. 1974;249:4346–4349. [PubMed] [Google Scholar]
- 82.Hargrove JL. Persulfide generated from L-cysteine inactivates tyrosine amino-transferase. Requirement for a protein with cysteine oxidase activity and gamma-cystathionase. J. Biol. Chem. 1988;263:17262–17269. [PubMed] [Google Scholar]
- 83.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S–induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 88.Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation–a tale of three gases! Pharmacol. Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fago A, Jensen FB, Tota B, Feelisch M, Olson KR, Helbo S, Lefevre S, Mancardi D, Palumbo A, Sandvik GK, Skovgaard N. Integrating nitric oxide, nitrite and hydrogen sulfide signaling in the physiological adaptations to hypoxia: a comparative approach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;162:1–6. doi: 10.1016/j.cbpa.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the is-chemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomaskova Z, Bertova A, Ondrias K. On the involvement of H2S in nitroso signaling and other mechanisms of H2S action. Curr. Pharm. Biotechnol. 2011;12:1394–1405. doi: 10.2174/138920111798281009. [DOI] [PubMed] [Google Scholar]
- 93.Yong QC, Hu LF, Wang S, Huang D, Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc. Res. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- 94.Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 95.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ. Res. 2005;96:234–243. doi: 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- 97.Massion PB, Pelat M, Belge C, Balligand JL. Regulation of the mammalian heart function by nitric oxide. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:144–150. doi: 10.1016/j.cbpb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 98.Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, Wink DA. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid. Redox Signal. 2011;14:1659–1674. doi: 10.1089/ars.2010.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. The chemistry of the S-nitrosoglutathione/glutathione system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14428–14433. doi: 10.1073/pnas.93.25.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong PS, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 101.Arnelle DR, Stamler JS. NO+ NO and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 102.Timerghazin QK, Peslherbe GH, English AM. Resonance description of S-nitrosothiols: insights into reactivity. Org. Lett. 2007;9:3049–3052. doi: 10.1021/ol0711016. [DOI] [PubMed] [Google Scholar]
- 103.Timerghazin QK, Peslherbe GH, English AM. Structure and stability of HSNO, the simplest S-nitrosothiol. Phys. Chem. Chem. Phys. 2008;10:1532–1539. doi: 10.1039/b715025c. [DOI] [PubMed] [Google Scholar]
- 104.Filipovic MR, Eberhardt M, Prokopovic V, Mijuskovic A, Orescanin-Dusic Z, Reeh P. I. Ivanovic-Burmazovic, Beyond H2S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. J. Med. Chem. 2013;56:1499–1508. doi: 10.1021/jm3012036. [DOI] [PubMed] [Google Scholar]
- 105.Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem. Res. Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- 106.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 107.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012;8:714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile–protein reactions. Sci. Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann. N. Y. Acad. Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 110.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 111.Uchida K, Shibata T. 15-Deoxy-delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 2008;21:138–144. doi: 10.1021/tx700177j. [DOI] [PubMed] [Google Scholar]
- 112.Sawa T, Zaki MH, Okamoto T, Akuta T, Tokutomi Y, Kim-Mitsuyama S, Ihara H, Kobayashi A, Yamamoto M, Fujii S, Arimoto H, Akaike T. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 113.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 114.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 115.Fu YG, Sung JJ, Wu KC, Bai AH, Chan MC, Yu J, Fan DM, Leung WK. Inhibition of gastric cancer cells associated angiogenesis by 15d–prostaglandin J2 through the downregulation of angiopoietin-1. Cancer Lett. 2006;243:246–254. doi: 10.1016/j.canlet.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 116.Cho WH, Choi CH, Park JY, Kang SK, Kim YK. 15-Deoxy-(delta12,14)-prostaglandin J2 (15d–PGJ2) induces cell death through caspase-independent mechanism in A172 human glioma cells. Neurochem. Res. 2006;31:1247–1254. doi: 10.1007/s11064-006-9157-0. [DOI] [PubMed] [Google Scholar]
- 117.Pietri R, Lewis A, Leon RG, Casabona G, Kiger L, Yeh SR, Fernandez-Alberti S, Marden MC, Cadilla CL, Lopez-Garriga J. Factors controlling the reactivity of hydrogen sulfide with hemeproteins. Biochemistry. 2009;48:4881–4894. doi: 10.1021/bi801738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hill BC, Woon TC, Nicholls P, Peterson J, Greenwood C, Thomson AJ. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem. J. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 120.Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]
- 121.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim. Biophys. Acta. 2005;1725:201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 122.Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;129:129–137. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 123.Nichol AW, Hendry I, Morell DB. Mechanism of formation of sulphhaemoglobin. Biochim. Biophys. Acta. 1968;156:97–108. doi: 10.1016/0304-4165(68)90108-6. [DOI] [PubMed] [Google Scholar]
- 124.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 125.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 126.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 127.Edwards G, Feletou M, Weston AH. Hydrogen sulfide as an endothelium-derived hyperpolarizing factor in rodent mesenteric arteries. Circ. Res. 2012;110:e13–e14. doi: 10.1161/CIRCRESAHA.111.259309. author reply e15–16. [DOI] [PubMed] [Google Scholar]
- 128.Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 129.Abele AE, Miller RJ. Potassium channel activators abolish excitotoxicity in cultured hippocampal pyramidal neurons. Neurosci. Lett. 1990;115:195–200. doi: 10.1016/0304-3940(90)90454-h. [DOI] [PubMed] [Google Scholar]
- 130.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z, Liu DX, Wang FW, Zhang Q, Du ZX, Zhan JM, Yuan QH, Ling EA, Hao AJ. L-cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H(2)S pathway. NeuroScience. 2013;237:106–117. doi: 10.1016/j.neuroscience.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 132.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens. 2011;20:107–112. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 133.Peers C, Bauer CC, Boyle JP, Scragg JL, Dallas ML. Modulation of ion channels by hydrogen sulfide. Antioxid. Redox Signal. 2012;17:95–105. doi: 10.1089/ars.2011.4359. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can. J. Physiol. Pharmacol. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 135.Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 137.Gade AR, Kang M, Akbarali HI. Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol. Pharmacol. 2013;83:294–306. doi: 10.1124/mol.112.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid. Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 139.Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang G, Wang R, Perry SF. Hydrogen sulfide as an oxygen sensor in trout gill che-moreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- 140.Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- 141.Telezhkin V, Brazier SP, Cayzac S, Muller CT, Riccardi D, Kemp PJ. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009;648:65–72. doi: 10.1007/978-90-481-2259-2_7. [DOI] [PubMed] [Google Scholar]
- 142.Telezhkin V, Brazier SP, Cayzac SH, Wilkinson WJ, Riccardi D, Kemp PJ. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir. Physiol. Neurobiol. 2010;172:169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 143.Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 2010;459:389–397. doi: 10.1007/s00424-009-0737-0. [DOI] [PubMed] [Google Scholar]
- 144.Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S, Nishikawa H. Hydrogen sulfide as a novel nociceptive messenger. Pain. 2007;132:74–81. doi: 10.1016/j.pain.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 145.Nelson MT, Todorovic SM, Perez-Reyes E. The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des. 2006;12:2189–2197. doi: 10.2174/138161206777585184. [DOI] [PubMed] [Google Scholar]
- 146.Swayne LA, Bourinet E. Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing. Pflugers Arch. 2008;456:459–466. doi: 10.1007/s00424-007-0390-4. [DOI] [PubMed] [Google Scholar]
- 147.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc. Res. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 148.Garcia-Bereguiain MA, Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxid. Redox Signal. 2008;10:31–42. doi: 10.1089/ars.2007.1656. [DOI] [PubMed] [Google Scholar]
- 149.Gutierrez-Martin Y, Martin-Romero FJ, Henao F, Gutierrez-Merino C. Alteration of cytosolic free calcium homeostasis by SIN-1: high sensitivity of L-type Ca2+ channels to extracellular oxidative/nitrosative stress in cerebellar granule cells. J. Neurochem. 2005;92:973–989. doi: 10.1111/j.1471-4159.2004.02964.x. [DOI] [PubMed] [Google Scholar]
- 150.Strege PR, Bernard CE, Kraichely RE, Mazzone A, Sha L, Beyder A, Gibbons SJ, Linden DR, Kendrick ML, Sarr MG, Szurszewski JH, Farrugia G. Hydrogen sul-fide is a partially redox-independent activator of the human jejunum Na+ channel Nav1.5. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G1105–G1114. doi: 10.1152/ajpgi.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br. J. Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur. Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 153.Malekova L, Krizanova O, Ondrias K. H(2)S and HS(−) donor NaHS inhibits intra-cellular chloride channels. Gen. Physiol. Biophys. 2009;28:190–194. doi: 10.4149/gpb_2009_02_190. [DOI] [PubMed] [Google Scholar]
- 154.Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 155.Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, Austin RC. Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism. J. Biol. Chem. 2012;287:7603–7614. doi: 10.1074/jbc.M111.304576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respir. Physiol. 1999;115:161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 157.Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006;209:4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- 158.Skovgaard N, Olson KR. Hydrogen sulfide mediates hypoxic vasoconstriction through a production of mitochondrial ROS in trout gills. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R487–R494. doi: 10.1152/ajpregu.00151.2012. [DOI] [PubMed] [Google Scholar]
- 159.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 161.Ekundi-Valentim E, Santos KT, Camargo EA, Denadai-Souza A, Teixeira SA, Zanoni CI, Grant AD, Wallace J, Muscara MN, Costa SK. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br. J. Pharmacol. 2010;159:1463–1474. doi: 10.1111/j.1476-5381.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 164.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 165.Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp. Biol. Med. (Maywood) 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 166.Wang XH, Wang F, You SJ, Cao YJ, Cao LD, Han Q, Liu CF, Hu LF. Dysregulation of cystathionine gamma-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell. Signal. 2013;25:2255–2262. doi: 10.1016/j.cellsig.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 167.Jain SK, Micinski D, Lieblong BJ, Stapleton T. Relationship between hydrogen sulfide levels and HDL-cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis. 2012;225:242–245. doi: 10.1016/j.atherosclerosis.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. (578 e561) [DOI] [PubMed] [Google Scholar]
- 169.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J. Leukoc. Biol. 2003;74:868–879. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 171.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell. Immunol. 2003;223:120–132. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 172.Mariggio MA, Minunno V, Riccardi S, Santacroce R, De Rinaldis P, Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol. Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- 173.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J. Leukoc. Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 174.Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J. Leukoc. Biol. 2007;82:894–905. doi: 10.1189/jlb.0407237. [DOI] [PubMed] [Google Scholar]
- 175.Dal-Secco D, Cunha TM, Freitas A, Alves-Filho JC, Souto FO, Fukada SY, Grespan R, Alencar NM, Neto AF, Rossi MA, Ferreira SH, Hothersall JS, Cunha FQ. Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 inter-nalization: role of ATP-sensitive potassium channels. J. Immunol. 2008;181:4287–4298. doi: 10.4049/jimmunol.181.6.4287. [DOI] [PubMed] [Google Scholar]
- 176.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 177.Hui Y, Du J, Tang C, Bin G, Jiang H. Changes in arterial hydrogen sulfide (H(2)S) content during septic shock and endotoxin shock in rats. J. Infect. 2003;47:155–160. doi: 10.1016/s0163-4453(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 178.Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mok YY, Atan MS, Yoke Ping C, Zhong Jing W, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goslar T, Mars T, Podbregar M. Total plasma sulfide as a marker of shock severity in nonsurgical adult patients. Shock. 2011;36:350–355. doi: 10.1097/SHK.0b013e31822bcfd0. [DOI] [PubMed] [Google Scholar]
- 182.Ouml, Lkel S, Grieshaber M. Sulphide oxidation and oxidative phosphorylation in the mitochondria of the lugworm. J. Exp. Biol. 1997;200:83–92. doi: 10.1242/jeb.200.1.83. [DOI] [PubMed] [Google Scholar]
- 183.Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 184.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid. Redox Signal. 2011;15:379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 185.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 186.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 188.Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc. Res. 2010;86:487–495. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 189.Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 190.Tiong CX, Lu M, Bian JS. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br. J. Pharmacol. 2010;161:467–480. doi: 10.1111/j.1476-5381.2010.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yin WL, He JQ, Hu B, Jiang ZS, Tang XQ. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci. 2009;85:269–275. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 192.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid. Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 193.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J. Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hennig B, Diener M. Actions of hydrogen sulphide on ion transport across rat distal colon. Br. J. Pharmacol. 2009;158:1263–1275. doi: 10.1111/j.1476-5381.2009.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pouokam E, Diener M. Mechanisms of actions of hydrogen sulphide on rat distal colonic epithelium. Br. J. Pharmacol. 2011;162:392–404. doi: 10.1111/j.1476-5381.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 197.Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, Takeuchi K. Stimulation of duodenal HCO(3)(−) secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol. (Oxf.) 2011;201:117–126. doi: 10.1111/j.1748-1716.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 198.Matsunami M, Kirishi S, Okui T, Kawabata A. Hydrogen sulfide-induced colonic mucosal cytoprotection involves T-type calcium channel-dependent neuronal excitation in rats. J. Physiol. Pharmacol. 2012;63:61–68. [PubMed] [Google Scholar]
- 199.Levitt MD, Springfield J, Furne J, Koenig T, Suarez FL. Physiology of sulfide in the rat colon: use of bismuth to assess colonic sulfide production. J. Appl. Physiol. 2002;92:1655–1660. doi: 10.1152/japplphysiol.00907.2001. [DOI] [PubMed] [Google Scholar]
- 200.Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000;72:1488–1494. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- 201.Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Invest. 1999;104:1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem. Pharmacol. 2001;62:255–259. doi: 10.1016/s0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 203.Weisiger RA, Pinkus LM, Jakoby WB. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem. Pharmacol. 1980;29:2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- 204.Picton R, Eggo MC, Merrill GA, Langman MJ, Singh S. Mucosal protection against sulphide: importance of the enzyme rhodanese. Gut. 2002;50:201–205. doi: 10.1136/gut.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Blachier F, Davila AM, Mimoun S, Benetti PH, Atanasiu C, Andriamihaja M, Benamouzig R, Bouillaud F, Tome D. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39:335–347. doi: 10.1007/s00726-009-0445-2. [DOI] [PubMed] [Google Scholar]
- 206.Roediger WE, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroen-terology. 1993;104:802–809. doi: 10.1016/0016-5085(93)91016-b. [DOI] [PubMed] [Google Scholar]
- 207.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Levine J, Ellis CJ, Furne JK, Springfield J, Levitt MD. Fecal hydrogen sulfide production in ulcerative colitis. Am. J. Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- 209.Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Flannigan KL, Ferraz JG, Wang R, Wallace JL. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: a site-specific, pro-resolution mechanism. PLoS One. 2013;8:e71962. doi: 10.1371/journal.pone.0071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Taniguchi E, Matsunami M, Kimura T, Yonezawa D, Ishiki T, Sekiguchi F, Nishikawa H, Maeda Y, Ishikura H, Kawabata A. Rhodanese, but not cystathionine-gamma-lyase, is associated with dextran sulfate sodium-evoked colitis in mice: a sign of impaired colonic sulfide detoxification? Toxicology. 2009;264:96–103. doi: 10.1016/j.tox.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 212.Roediger WE, Babidge W, Millard S. Methionine derivatives diminish sulphide damage to colonocytes-implications for ulcerative colitis. Gut. 1996;39:77–81. doi: 10.1136/gut.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moore J, Babidge W, Millard S, Roediger W. Colonic luminal hydrogen sulfide is not elevated in ulcerative colitis. Dig. Dis. Sci. 1998;43:162–165. doi: 10.1023/a:1018848709769. [DOI] [PubMed] [Google Scholar]
- 214.Furne JK, Suarez FL, Ewing SL, Springfield J, Levitt MD. Binding of hydrogen sulfide by bismuth does not prevent dextran sulfate-induced colitis in rats. Dig. Dis. Sci. 2000;45:1439–1443. doi: 10.1023/a:1005580709390. [DOI] [PubMed] [Google Scholar]
- 215.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 216.Hirata I, Naito Y, Takagi T, Mizushima K, Suzuki T, Omatsu T, Handa O, Ichikawa H, Ueda H, Yoshikawa T. Endogenous hydrogen sulfide is an anti-inflammatory molecule in dextran sodium sulfate-induced colitis in mice. Dig. Dis. Sci. 2011;56:1379–1386. doi: 10.1007/s10620-010-1461-5. [DOI] [PubMed] [Google Scholar]