Abstract
Bisphosphonates have been used for years to suppress bone turnover and reduce fracture risk. Bisphosphonates have recently been associated with atypical femoral fractures, which are catastrophic, low trauma, brittle fractures that appear to occur more frequently than in untreated individuals. Previous work using a dog model has demonstrated bisphosphonate-induced reductions in bone toughness (the inverse of brittleness), yet data are lacking to show this occurs in rodents. The goal of this study was to determine if bisphosphonate-induced alterations in toughness could be quantified in rats. At 26 weeks of age, skeletally mature rats (n=32 total) were given an injection of either zoledronate (100 µg /kg body weight) or vehicle (0.5 mL saline). Five weeks post-injection, both femora were collected and analyzed for geometry and mechanical properties. To assess the effect of testing rate on the biomechanical outcomes, the left femora were broken at 2 mm/min, while the right femora were broken at 20 mm/min. The results showed a significantly lower energy to failure in zoledronate-treated animals compared to vehicle at the slow testing rate (−15%, p < 0.05) with no difference at the faster rate. While there was not a significant interaction between drug and testing rate for toughness to fracture (p = 0.07), toughness between ultimate stress and fracture was significantly lower with zoledronate only at the slow rate (−40%, p < 0.05). These data document that bisphosphonate-induced reductions in energy absorption and toughness can be quantified in rats yet they are highly dependent on testing rate.
Keywords: zoledronate, mechanical testing, atypical femoral fractures, sub-trochanteric fracture
INTRODUCTION
Bisphosphonates have long been used to reduce fracture risk in osteoporotic patients [1]. They act to suppress bone turnover by inhibiting osteoclast-induced bone resorption, thus increasing bone mineral density (BMD) and select bone strength. Recently, atypical femoral fractures have been associated with bisphosphonate treatment [2–4]. Although these fractures are relatively uncommon, they are extremely debilitating and pose a number of problems for both patients and physicians. The 2010 American Society for Bone and Mineral Research task force recently classified these fractures as being caused by low trauma, occurring at the proximal femoral shaft, and having a morphological pattern consistent with a brittle fracture [5]. To date no causal relationship between bisphosphonates and atypical femoral fractures has been established.
Work from our laboratory and others has documented that bisphosphonates cause a reduction in bone toughness, an estimated material-level property related to the amount of energy the matrix can absorb before fracturing [6–10]. Reduced toughness is analogous to increased brittleness, thus making the change consistent with the fracture characteristics of atypical femoral fractures. To date, laboratory studies showing reduced toughness have been conducted exclusively using a dog model. Although dogs have a number of advantages, particularly for studying cortical bone biomechanics, they pose a number of limitations including high experimental costs and long experiment durations [11]. Rats are a well-accepted, FDA-approved model for studying skeletal properties [12], yet there are limited data concerning how bisphosphonates affect cortical bone toughness in rodents [13–15]. If shown to have altered toughness in response to bisphosphonates, rodents could serve as a useful model to more rapidly assess underlying mechanisms and potential preventative options related to atypical femoral fractures. Therefore the goal of this study was to test the hypothesis that zoledronic acid alters cortical bone toughness in rats.
MATERIALS AND METHODS
Animals
Thirty-two skeletally mature retired breeder male rats (6 months old) were purchased from Charles River and housed throughout the experiment in environmentally controlled rooms at Indiana University School of Medicine’s AAALAC accredited facility. Male rats were chosen as a previous study in our laboratory had shown trends for reduced toughness following zoledronate treatment yet the study had insufficient power for biomechanical analyses [16]. All animal procedures were approved prior to the study by the IU School of Medicine Animal Care and Use Committee.
Experimental Design
Following two week of acclimatization, rats were injected subcutaneously with either saline vehicle (0.5 mL, n=16) or zoledronate (100 µg/kg, n=16). This dosage of zoledronate has been shown previously to produce the expected remodeling suppression effects in this age animal [16]. At 31 weeks of age, rats were euthanized with carbon dioxide, and bilateral femora were dissected free, wrapped in gauze with saline solution, and frozen until analysis.
Peripheral Quantitative Computed Tomography (pQCT)
Volumetric bone density and geometry were quantified using a pQCT. Femur length and mid-diaphysis bone diameter (anterior-posterior diameter) was manually measured with calipers and a single CT image slice was obtained at the midshaft. Total bone mineral content (BMC, mg/mm), total volumetric bone mineral density (vBMD, mg/cm3), cortical bone area (BA, mm2), and polar cross-sectional moment of inertia (CSMIp, mm4) were obtained using standard scanner software. Diameter and CSMIp values were calculated in the plane perpendicular to the axis of three-point bending. A second scan was obtained at the distal metaphysis (single slice 6.5 mm from the distal condyle) to assess vBMD of a region rich in trabecular bone.
Biomechanical Testing
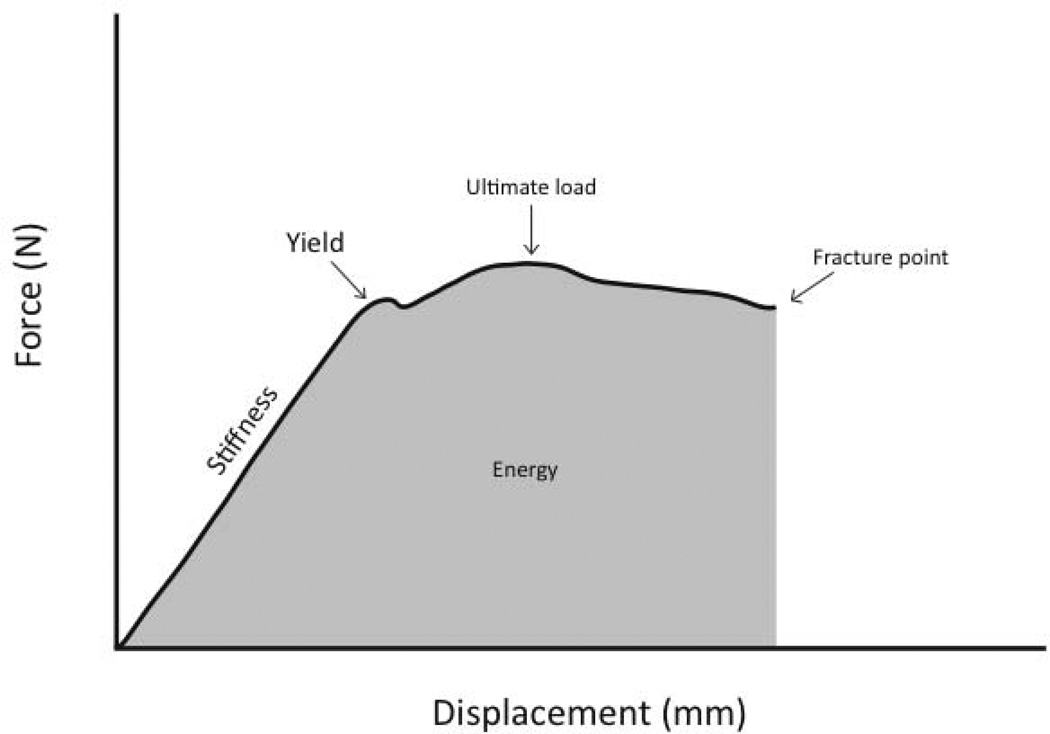
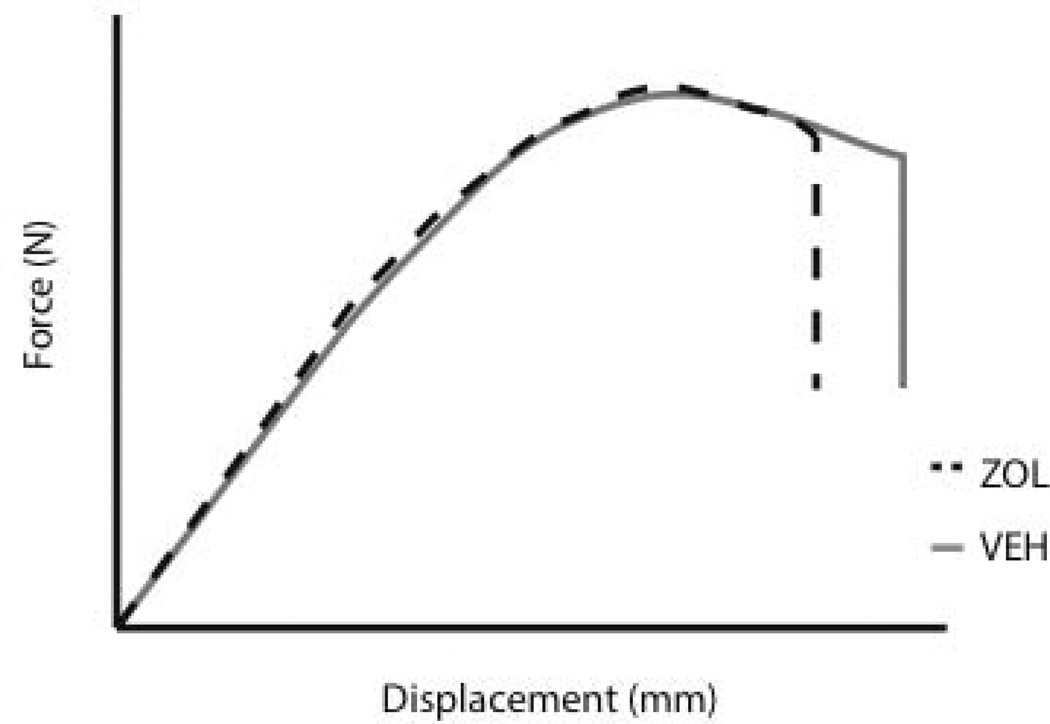
Three-point bending was conducted in accordance with previous studies on rat femora [16, 17]. Briefly, bones were thawed to room temperature and then placed on a three-point bending fixture. The bottom support span measured 19 mm across, and the posterior aspect of the femur faced upwards. In order to determine if the testing rate had any affect on the measured parameters, left femora were tested at 2 mm/min, while the right femora were broken at 20 mm/min. Data were collected at 10 Hz. Structural mechanical properties (ultimate load, stiffness, and energy absorption) were determined from the load–deformation curves (Figure 1). Material-level properties (ultimate stress, modulus, and toughness) were estimated by normalizing the structural parameters using standard equations that include bone diameter and CSMIp [18].
Figure 1.
Force versus deformation curve from a three-point bending test. Key properties from the test include ultimate load (the maximum load achieved during the test), stiffness (the slope of the elastic portion of the curve) and energy (the area under the curve).
Statistics
Statistical tests were performed using SAS software (SAS Institute, Inc.). Parameters were compared between the vehicle and zoledronate groups using 2-way ANOVA followed by least squares means post-hoc tests when appropriate. For all tests, p ≤ 0.05 was used to determine statistical significance.
RESULTS
There was no difference in bone structure or density at the diaphysis between control and zoledronate treated animals, nor between the left and right limbs of animals in each group (Table 1). Total vBMD at distal femur metaphysis was 11% higher in zoledronate-treated animals compared to control (Table 1), confirming the effectiveness of zoledronate dosing.
Table 1.
Peripheral quantitative computed tomography assessed bone density and cortical geometry of the femur for paired samples tested at two different loading rates (20 mm/min=right; 2 mm/min = left).
| 20 mm/min testing rate | 2 mm/min testing rate | Drug | Rate | Drug × Rate |
|||
|---|---|---|---|---|---|---|---|
| Vehicle | Zoledronate | Vehicle | Zoledronate | ||||
| Distal femur total vBMD, mg/cm3 | 660 ± 17 | 732 ± 14 | - | - | 0.001 | - | - |
| Mid-diaphysis BMC, mg/mm | 16.3 ± 0.4 | 16.3 ± 0.5 | 16.6 ± 0.4 | 16.6 ± 0.5 | NS | NS | NS |
| Mid-diaphysis vBMD, mg/cm3 | 1487 ± 4 | 1491 ± 4 | 1490 ± 5 | 1494 ± 4.2 | NS | NS | NS |
| Cortical bone area, mm2 | 11.0 ± 0.3 | 11.0 ± 0.3 | 11.2 ± 0.3 | 11.1 ± 0.3 | NS | NS | NS |
| Polar cross-sectional moment of inertia, mm4 | 39.0 ± 2.3 | 39.2 ± 2.7 | 39.5 ± 2.2 | 39.1 ± 2.4 | NS | NS | NS |
| A-P diameter, mm | 4.0 ± 0.07 | 4.0 ± 0.07 | 4.0 ± 0.06 | 4.0 ± 0.07 | NS | NS | NS |
Data presented as mean ± standard error.
Significant p-values included for two-way ANOVA results. NS, non-significant.
vBMD, volumetric bone mineral density; BMD, bone mineral content; A-P, anterior-posterior.
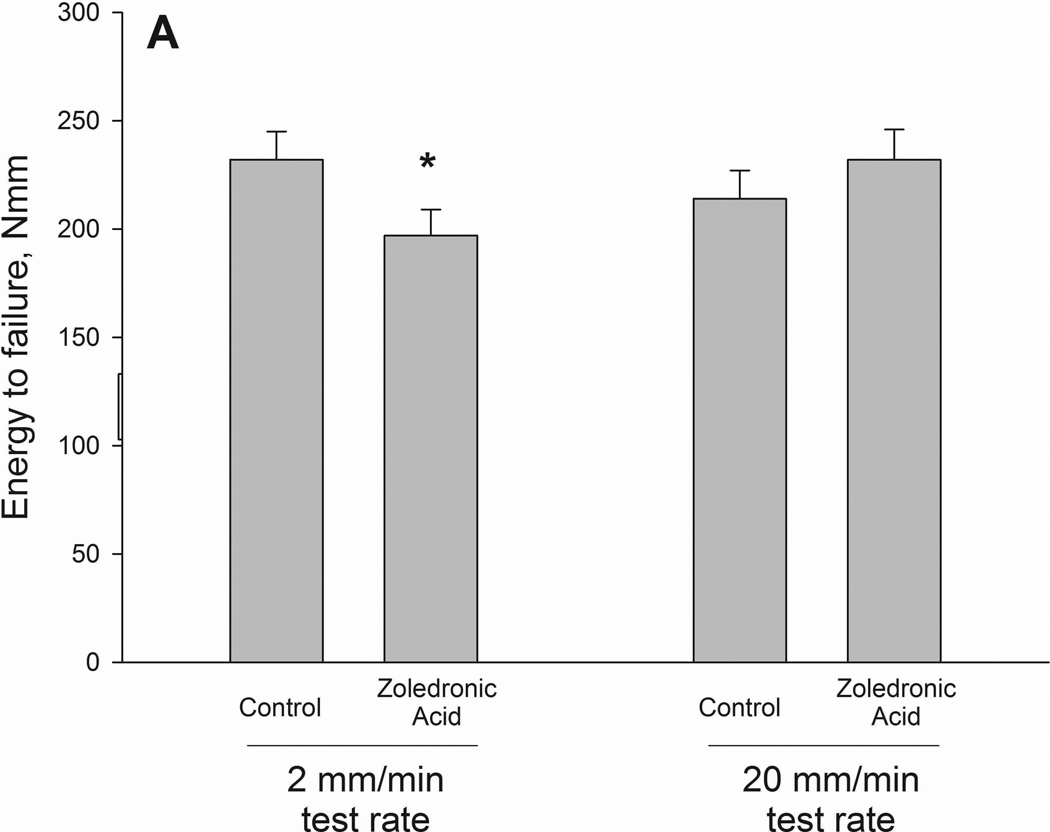
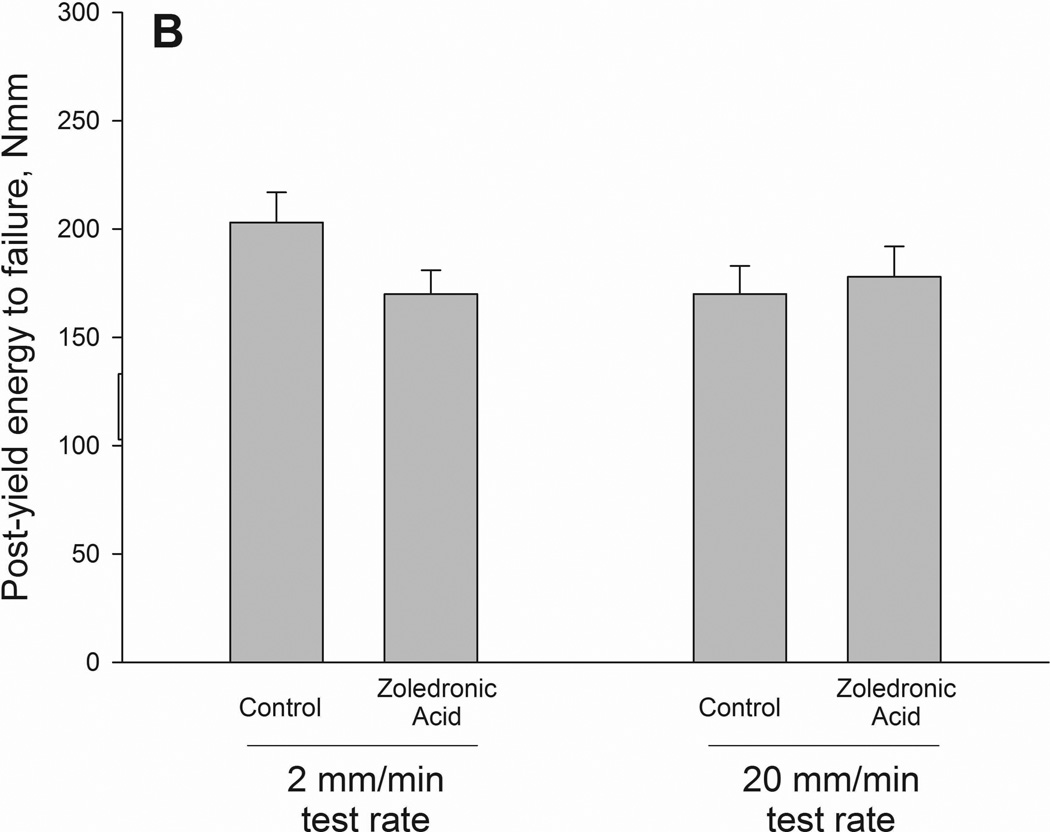
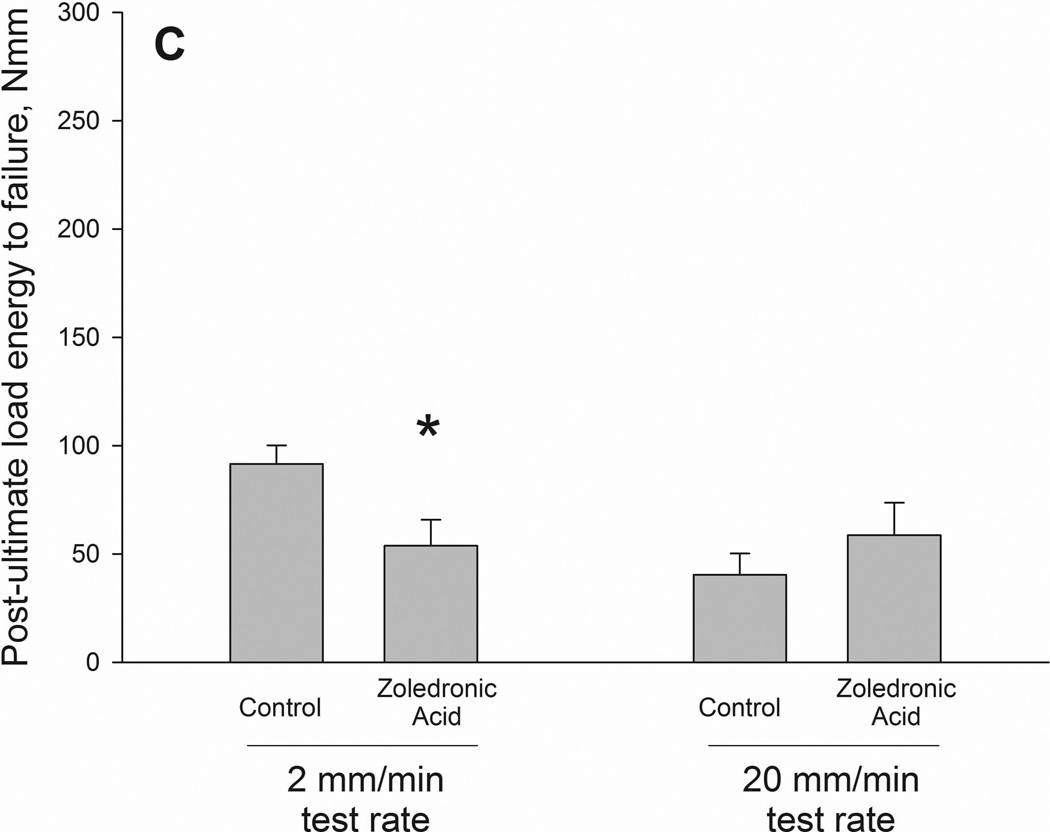
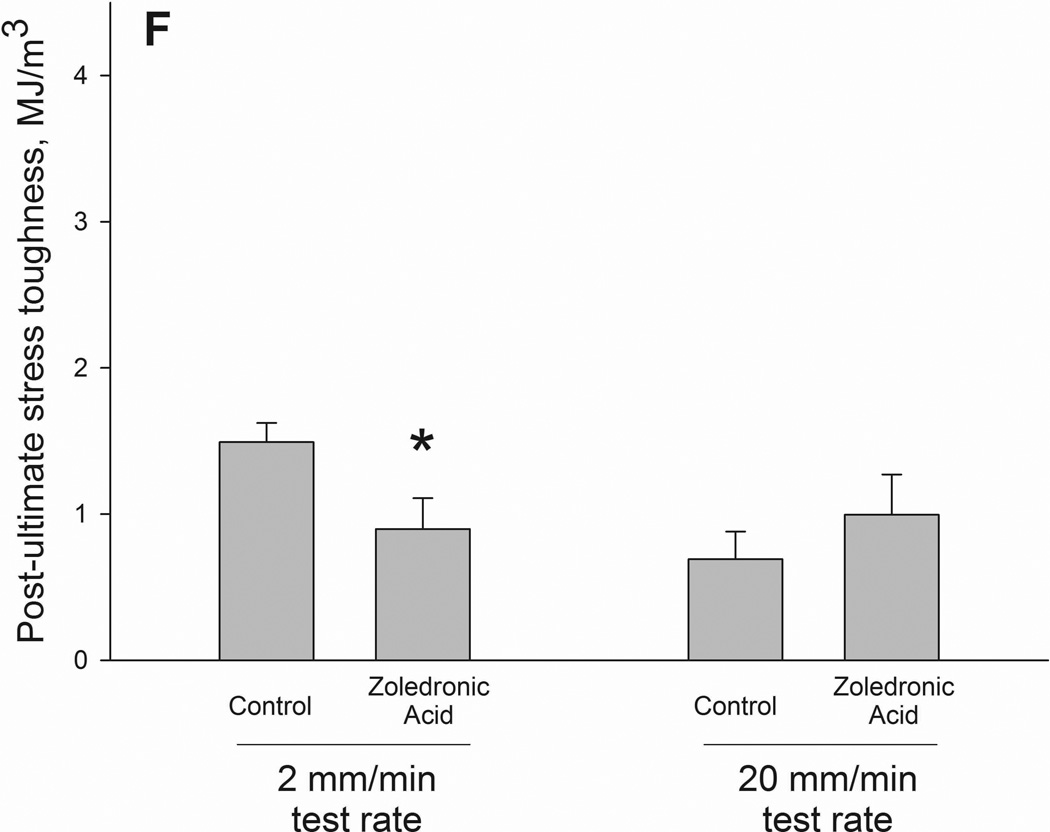
Ultimate load and stiffness were not significantly altered by drug but did show a significant effect of testing rate. When drug treatments were pooled together within test rate, the higher testing rate producing higher properties for both ultimate load and stiffness (Table 2). Energy to failure had a significant interaction between drug and testing rate (p = 0.05)(Figure 2 and 3). Values were significantly lower in zoledronate-treated animals compared to controls at the 2mm test rate (−15%) with no difference at the 20mm rate. The same pattern was noted in energy absorption between ultimate load and fracture (interaction term p = 0.019) with values −41% lower in zoledronate-treated animals compared to control at 2mm test rate. There was a trend toward an interaction for energy absorption between yield and fracture (p=0.12).
Table 2.
Structural and material biomechanical properties of the femoral diaphysis assessed by three-point bending at two different loading rates (20 mm/min=right; 2 mm/min = left).
| 20 mm/min testing rate | 2 mm/min testing rate | Drug | Rate | Drug × Rate |
|||
|---|---|---|---|---|---|---|---|
| Vehicle | Zoledronate | Vehicle | Zoledronate | ||||
| Ultimate Load, N | 297 ± 8 | 393 ± 12 | 260 ± 8 | 263 ± 10 | NS | 0.001 | NS |
| Stiffness, N/mm | 573 ± 16 | 567 ± 23 | 518 ± 10 | 538 ± 14 | NS | 0.014 | NS |
| Energy to failure, Nmm | 214 ± 13 | 232 ± 14 | 232 ± 13 | 197 ± 12 * | NS | NS | 0.05 |
| Post-ultimate load energy to failure, Nmm | 40.3 ± 9.9 | 58.7 ± 15 | 91.6 ± 8.6 | 53.8 ± 12 * | NS | 0.041 | 0.019 |
| Post-yield energy to failure, Nmm | 170 ± 13 | 178 ± 14 | 203 ± 14 | 170 ± 11 | NS | NS | NS |
| Ultimate Stress, MPa | 74 ± 3 | 74 ± 2 | 64 ± 1 | 64 ± 1 | NS | 0.001 | NS |
| Modulus, MPa | 2154 ± 80 | 2129 ± 90 | 1933 ± 82 | 2030 ± 96 | NS | 0.067 | NS |
| Toughness, MJ/m3 | 3.6 ± 0.2 | 3.8 ± 0.3 | 3.8 ± 0.2 | 3.2 ± 0.2 | NS | NS | 0.07 |
| Post-ultimate stress toughness, MJ/m3 | 0.69 ± 0.19 | 1.0 ± 0.3 | 1.49 ± 0.13 | 0.9 ± 0.2 * | NS | 0.078 | 0.033 |
| Post-yield toughness, MJ/m3 | 2.83 ± 0.21 | 2.91 ± 0.25 | 3.29 ± 0.18 | 2.75 ± 0.21 | NS | NS | NS |
Data presented as mean ± standard error.
Significant p-values included for two-way ANOVA results. NS, non-significant;
p < 0.05 versus vehicle within test rate as determined by least squares means post-hoc test.
Figure 2.
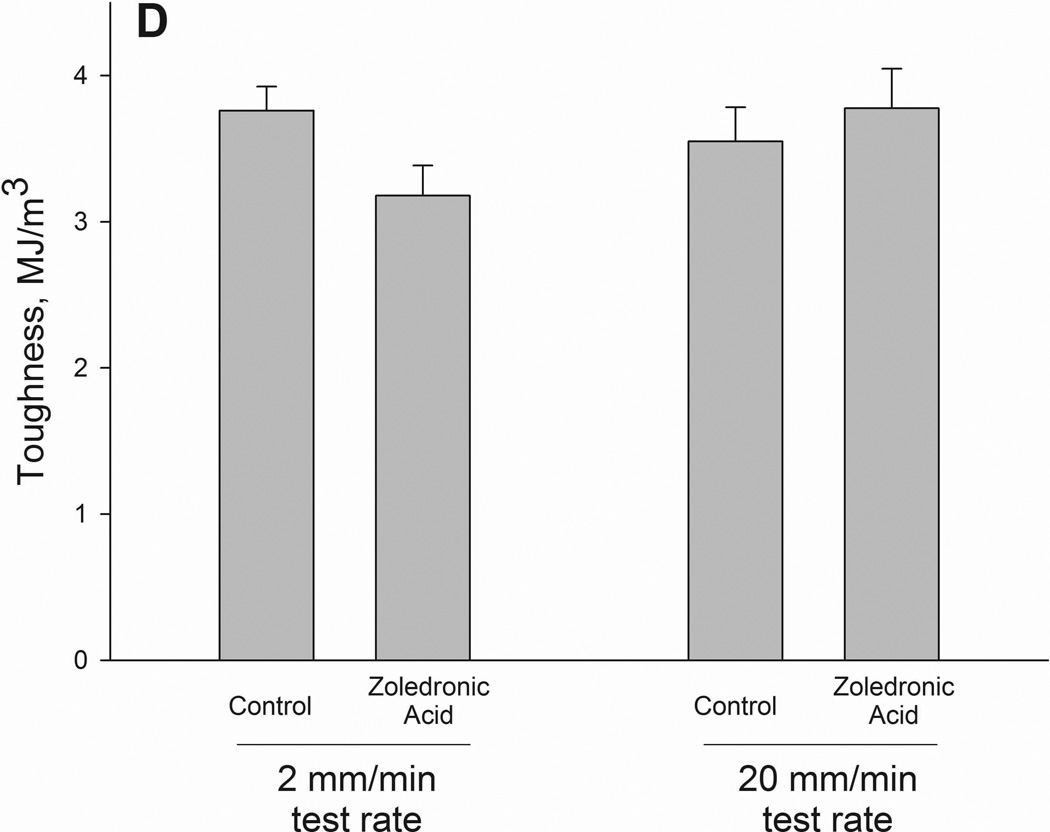
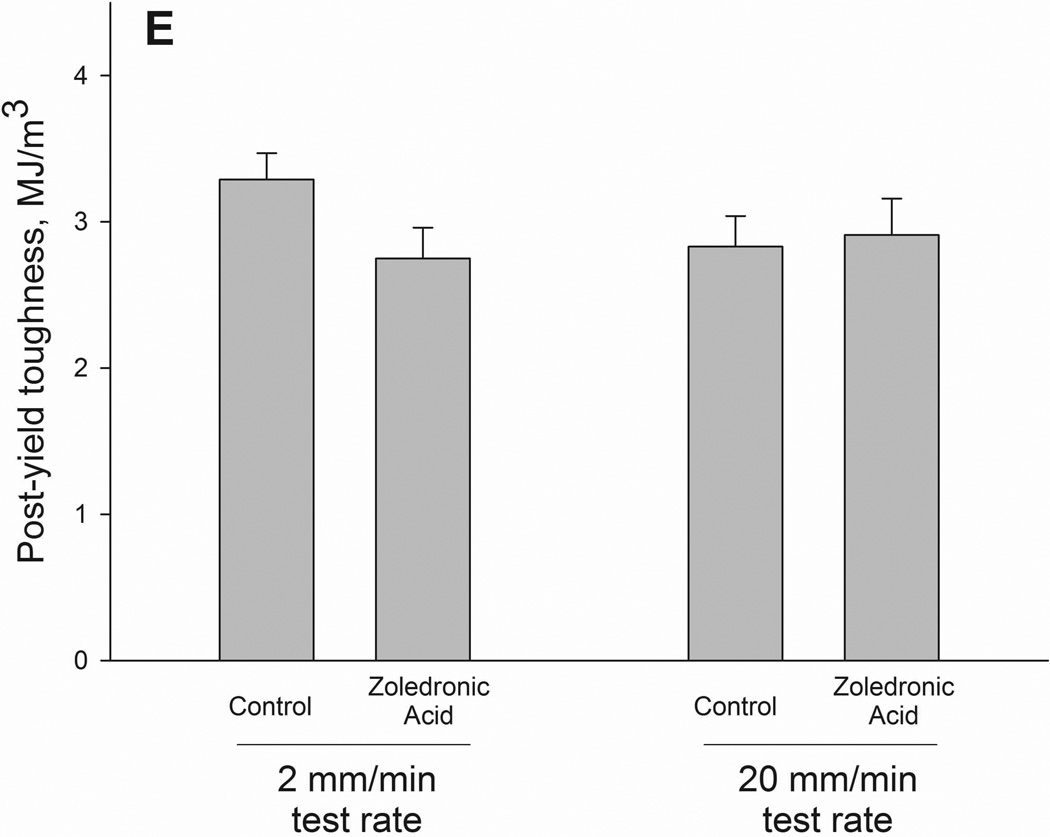
Structural (A–C) and material (D–F) energy absorption properties as determined by three-point bending tests. Whole bone energy absorption to fracture (A) and energy absorption between ultimate load and fracture (C) both displayed significant drug × test rate interaction with ZOL-treated animals having lower values only at the slow test rate (noted by *). Post-yield energy absorption (B) displayed a similar pattern but the effect was not statistically significant (2-way ANOVA p values < 0.15). When normalized for bone geometry to calculate toughness, patterns similar to those of the structure were observed. Only toughness from ultimate stress to fracture was significant (*) while both toughness to fracture (D) and post-yield toughness (E) showed consistent trends (2-way ANOVA p values < 0.15).
Figure 3.
Idealized force versus displacement curve for vehicle and zoledronic acid groups tested at the 2 mm/min rate. Curves were generated using mean values for all animals within each group.
As there were no significant differences in bone geometry among test groups, the intrinsic properties followed similar patterns as the structural properties. Ultimate stress showed a testing rate effect with significantly higher values at the 20mm rate compared to the 2mm rate. Neither toughness nor post-yield toughness had significant interaction terms (p = 0.07 and 0.14, respectively). Post-ultimate stress toughness had a drug by testing rate interaction with values 40% lower in zoledronate-treated animals compared to controls at the 2mm test rate; these effects were not observed at the higher test rate (Figure 2).
DISCUSSION
Previous research in dog models has consistently documented that bisphosphonate treatment leads to reduced toughness, which is the ability of the material to absorb energy prior to fracture [6–10]. These reductions in toughness have been ascribed to the remodeling-reducing effects of bisphosphonates which lead to an increase in mean tissue age [9, 19]. Increasing the mean tissue age results in the tissue having a higher mean degree of mineralization, higher levels of microdamage, and higher amounts of collagen cross-linking. Each of these effects is independently associated with reduced toughness, yet correlation studies have not produced convincing evidence that any of these are independently responsible for the toughness reduction with bisphosphonates [9, 19]. Furthermore, data from a three year experiment using two different doses of bisphosphonates, differing by 5×, showed changes in remodeling, microdamage, mineralization, and collagen were comparable between groups yet toughness was significantly lower in the high dose group [7]. These data suggested that bisphosphonates themselves were affecting bone toughness, or alternatively, having a dose-dependent effect on some other properties of the bone tissue yet to be uncovered.
Bisphosphonate-induced reductions in toughness have been quantified using vertebra, ribs, and long bones, suggesting that the effect occurs in both cortical and trabecular bone. Dogs and rodents undergo trabecular bone remodeling similar to humans, yet remodeling of cortical bone occurs only in dogs and humans, but not rodents. Intracortical remodeling, the process of renewing bone matrix within the cortical shell, does not occur under normal conditions in rodents [20, 21]. While remodeling suppression with bisphosphonates would increase mean tissue age of cortical sites in humans and dogs, it would have no effect on rodents. Bisphosphonates have also been shown to not affect periosteal bone formation [22], thus the only site in rat cortical bone affected by bisphosphonates would be the endocortical surface. Our pQCT data did not note any difference in bone area although the resolution of these scans may not be sufficient to pick up subtle differences. None-the-less, small changes in endocortical bone would not be expected to have a substantial effect on mechanical properties. These data showing that bisphosphonates affect toughness in non-remodeling rat bone further support the idea that bisphosphonates are affecting bone toughness independent of remodeling suppression. These conclusions are limited to cortical bone as we did not assess trabecular bone mechanical properties in this experiment.
Somewhat to our surprise the effects of bisphosphonates on bone toughness were dependent on the testing rate. The effects of displacement rate on bone properties have been studied in detail and in general show that increasing the testing rate produces higher ultimate load and stiffness and lower energy absorption [23–26]. We are not aware of any data in the literature showing an in vivo treatment effect on mechanical properties that is rate-dependent. These results highlight the importance of choosing a test rate for assessing biomechanical properties of rat bone in bisphosphonate experiments.
Although numerous studies have documented the effects of bisphosphonates on mechanical properties in rats, few studies report data on energy absorption and toughness. Neither ibandronate nor risedronate altered femoral fracture toughness in aged (20 month) ovariectomized rats although these data were generated using fracture toughness tests, which provide different measures of toughness compared to estimated toughness from three-point bending tests [14]. Following ten months of alendronate treatment in ovariectomized rats, femoral shaft toughness by three-point bending was significantly higher compared to controls [13]. The testing rate in this study was not reported although testing of the femoral neck was at a rate of 1 mm/sec suggesting the rate of diaphysis testing may have been quite high. Following eight months of treatment in ovariectomized rats, zoledronate and alendronate (each at various doses) resulted in higher toughness compared to control using a test rate of 6 mm/min [15]. These later data are most directly related to the current study as they had a dose of zoledronate that matched ours (100 µg/kg). Whether the discrepancy in findings is due to differences in animal models (OVX females versus intact males), duration of treatment (thirty-three weeks verses five), or rate of testing (6 mm/min versus 2 mm/min) is not clear.
Atypical femoral fractures are a rare but significant type of fracture that have recently been associated with bisphosphonate treatment [2–5] although no causal relationship has been established. The large number of individuals treated with bisphosphonates, as well as other potent remodeling-suppression agents, emphasizes the need to understand the pathophysiology and find ways to reduce the risk of these fractures. Preclinical models have an essential role in this process and the current results showing changes in femoral mechanical properties in rodents provide a springboard for such work. Although reductions in toughness are consistent with characteristics of atypical sub-trochanteric fractures, at this time there is no definitive evidence that it is part of the pathophysiology.
In conclusion, we show that bisphosphonate-induced reductions in toughness can be quantified in rats but that these effects are highly dependent on testing rate. These data highlight the need to carefully consider the testing rate, perhaps even using multiple rates when possible. More importantly, they provide a rodent model to study aspects related to femoral fractures associated with bisphosphonate treatment.
Acknowledgements
This work was supported by the NIH (AR062002 and HL110845). The authors would like to thank Drew Brown and Chris Newman for assistance with the project and Dr. Jason Organ for his input on statistical presentation.
Footnotes
The authors have no conflicts of interest associated with this study.
References
- 1.R. Graham G R. Bisphosphonates: The first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate Use and Atypical Fractures of the Femoral Shaft. New England Journal of Medicine. 2011;364:1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 3.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC. Bisphosphonates and Fractures of the Subtrochanteric or Diaphyseal Femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 5.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O'Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 6.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Allen MR, Reinwald S, Burr DB. Alendronate reduces bone toughness of ribs without significantly increasing microdamage accumulation in dogs following 3 years of daily treatment. Calcified Tissue International. 2008;82:354–360. doi: 10.1007/s00223-008-9131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. Journal of Bone and Mineral Research. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 9.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we don't know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 10.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporosis International. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinwald S, Burr D. Review of nonprimate, large animal models for osteoporosis research. J Bone Miner Res. 2008;23:1353–1368. doi: 10.1359/JBMR.080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone. 1995;17:S125–S133. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 13.Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M. New bone formation with teriparatide [human parathyroid hormone-(1–34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology. 2003;144:2008–2015. doi: 10.1210/en.2002-221061. [DOI] [PubMed] [Google Scholar]
- 14.Shahnazari M, Yao W, Dai W, Wang B, Ionova-Martin SS, Ritchie RO, Heeren D, Burghardt AJ, Nicolella DP, Kimiecik MG, Lane NE. Higher doses of bisphosphonates further improve bone mass, architecture, and strength but not the tissue material properties in aged rats. Bone. 2010;46:1267–1274. doi: 10.1016/j.bone.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasser JA, Ingold P, Venturiere A, Shen V, Green JR. Long-Term Protective Effects of Zoledronic Acid on Cancellous and Cortical Bone in the Ovariectomized Rat. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;23:544–551. doi: 10.1359/jbmr.071207. [DOI] [PubMed] [Google Scholar]
- 16.Allen MR, Chen NX, Gattone V, II, Chen X, Carr AJ, LeBlanc P, Brown DM, Moe S. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporos Int. doi: 10.1007/s00198-012-2103-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs RK, Allen MR, Condon KW, Reinwald S, Miller LM, McClenathan D, Keck B, Phipps RJ, Burr DB. Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporosis International. 2008;19:1331–1341. doi: 10.1007/s00198-008-0602-6. [DOI] [PubMed] [Google Scholar]
- 18.Turner CH, Burr DB. Basic biomechanical measurements of bone - A tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 19.Allen MR, Burr DB. Mineralization, Microdamage, and Matrix: How Bisphosphonates Influence Material Properties of Bone. [4/2/49];BoneKEy. 2007 4:49–60. http://www.bonekeyibms.org/cgi/content/abstract/ibmske. [Google Scholar]
- 20.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 21.Kubek D, Burr DB, Allen MR. Ovariectomy Stimulates and Bisphosphonates Inhibit Intracortical Remodeling in the Mouse Mandible. Orthodontics and Craniofacial Research. 2010;13:214–222. doi: 10.1111/j.1601-6343.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feher A, Koivunemi A, Koivunemi M, Fuchs RK, Burr DB, Phipps RJ, Reinwald S, Allen MR. Bisphosphonates do not inhibit periosteal bone formation in estrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone. 2010;46:203–207. doi: 10.1016/j.bone.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Pal S, Saha S. Effect of deformation rate on the flexural fracture behaviour of long bones. Medical & biological engineering & computing. 1984;22:251–254. doi: 10.1007/BF02442751. [DOI] [PubMed] [Google Scholar]
- 24.Pithioux M, Subit D, Chabrand P. Comparison of compact bone failure under two different loading rates: experimental and modelling approaches. Medical Engineering and Physics. 2004;26:647–653. doi: 10.1016/j.medengphy.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Schaffler MB, Radin EL, Burr DB. Mechanical and morphological effects of strain rate on fatigue of compact bone. Bone. 1989;10:207–214. doi: 10.1016/8756-3282(89)90055-0. [DOI] [PubMed] [Google Scholar]
- 26.Wright TM, Hayes WC. Tensile testing of bone over a wide range of strain rates: effects of strain rate, microstructure and density. Medical & biological engineering. 2012;14:671–680. doi: 10.1007/BF02477046. [DOI] [PubMed] [Google Scholar]