Abstract
Despite fluctuations in dietary iron intake and intermittent losses through bleeding, the plasma iron concentrations in humans remain stable at 10–30 μM. While most of the iron entering blood plasma comes from recycling, appropriate amount of iron is absorbed from the diet to compensate for losses and maintain nontoxic amounts in stores. Plasma iron concentration and iron distribution are similarly regulated in laboratory rodents. The hepatic peptide hepcidin was identified as the systemic iron-regulatory hormone. In the efferent arc, hepcidin regulates intestinal iron absorption, plasma iron concentrations, and tissue iron distribution by inducing degradation of its receptor, the cellular iron exporter ferroportin. Ferroportin exports iron into plasma from absorptive enterocytes, from macrophages that recycle the iron of senescent erythrocytes, and from hepatocytes that store iron. In the more complex and less well understood afferent arc, hepatic hepcidin synthesis is transcriptionally regulated by extracellular and intracellular iron concentrations through a molecular complex of bone morphogenetic protein receptors and their iron-specific ligands, modulators and iron sensors. Through as yet undefined pathways, hepcidin is also homeostatically regulated by the iron requirements of erythroid precursors for hemoglobin synthesis. In accordance with the role of hepcidin-mediated iron redistribution in host defense, hepcidin production is regulated by inflammation as well. Increased hepcidin concentrations in plasma are pathogenic in iron-restrictive anemias including anemias associated with inflammation, chronic kidney disease and some cancers. Hepcidin deficiency causes iron overload in hereditary hemochromatosis and ineffective erythropoiesis. Hepcidin, ferroportin and their regulators represent potential targets for the diagnosis and treatment of iron disorders and anemias.
Keywords: Iron overload, iron deficiency, anemia
1. Overview
In the last decade, molecular understanding of systemic iron regulation greatly expanded on the physiological analysis of these processes worked out in the preceding fifty years. This review is focused on the iron regulatory hormone hepcidin and its role in systemic iron homeostasis. For a detailed discussion of the related subjects of iron transport through membranes, intracellular iron transport and the connection between iron and erythropoiesis the reader is referred to many excellent reviews dedicated to those areas. Although the primary emphasis is on molecular mechanisms, we could not ignore the clinical roots of this work and the inspiration provided by its diagnostic and therapeutic applications. The references are selective rather than encyclopedic, and reflective of the authors’ view of this area.
2. Iron homeostasis
2.1. Iron is conserved, recycled and held in storage
In response to the poor bioavailability of iron in many environments, humans and animals have evolved highly efficient mechanisms for iron conservation [1]. The daily losses of iron, 1–2 mg in adults, represent less than 0.1% of the 3–4 g of total iron in the human body, and must be replaced from dietary sources to maintain iron balance. Daily dietary iron requirements are about 8 mg for adult men and 18 mg for adult women with menstrual iron losses [2]. Nonmenstrual iron losses occur predominantly through desquamation of epithelial cells in the intestine and the skin, and through minor bleeding. Importantly, the losses of iron cannot substantially increase through physiologic mechanisms, even if iron intake and stores become excessive. Most of the iron in the body is in hemoglobin of red cells which contain about 1 mg of iron per ml of erythrocytes, or about 2–3 g of iron total. In contrast, blood plasma contains only 2–3 mg of iron, bound to transferrin, the plasma iron carrier that is the exclusive source of iron for erythropoiesis. The life span of human erythrocytes is about 120 days, so every day the oldest 1/120 of erythrocytes are degraded by macrophages and their iron content is returned to plasma transferrin. The recycling of erythrocytes generates a stream of 20–25 mg of iron a day, causing plasma iron to turn over every two hours or so. The iron-transferrin is mostly destined for erythrocyte production in the bone marrow. Other cells contain and require much less iron, and some are able to utilize non-transferrin bound iron as well. In the average adult male, about 1 g of iron is held in storage mostly in the hepatocytes and macrophages in the liver and red pulp macrophages in the spleen but the amount stored is much lower in most women of reproductive age, in part due to blood losses from menstruation and parturition. Hepatocyte and macrophage iron is stored in cytoplasmic ferritin and is readily mobilized during period of high iron demand.
2.2 Species differences in iron metabolism
Other vertebrate species have similar distribution of body iron, but the relative proportion of daily iron absorption, recycling and losses may differ from those observed in humans. These variations are of particular importance in species used as models for studying iron metabolism. In laboratory mice, for example, dietary iron absorption and losses seem to be proportionally far greater than those in humans. The average life span of mouse erythrocytes is close to 40 days [3] and the adult mouse has about 0.6–1 ml of packed erythrocytes (assuming blood volume of 7% of 20–30 g adult mouse weight and hematocrit of 45–50% [4]) or about 0.6–1 mg of iron in hemoglobin. Each day about 15–25 μg of iron is recycled and used for the production of new RBCs. Regarding the daily losses, when mice are placed on iron-deficient diet (~4 ppm Fe) for 2 weeks, at least 200–250 μg Fe is depleted from their iron stores [5], indicating that they normally lose ~15–20 μg Fe per day, i.e. an amount similar to their daily erythropoiesis needs. Thus on an iron-sufficient diet, similarly high amount of iron will be absorbed each day. In contrast, daily iron absorption and losses in humans represent 5–10% of the iron recycling amount. Furthermore, standard mouse chow has high iron content (about 350 ppm or about ten times the daily dietary requirement [6]), and leads to significant iron loading even in healthy mice, potentially confounding studies of iron regulation. Therefore, studies in animal models of iron homeostasis and its disorders need to consider the species differences in iron stores and fluxes, and take into account the disproportionately strong effect of diet on iron homeostasis in mice. The iron turnover parameters in mice as compared to humans are summarized in Table 1.
Table 1.
Comparative iron turnover parameters in adult humans vs. mice
| Approximate adult normal values | Human | Mouse |
|---|---|---|
| Erythrocyte lifespan | 120d | 40d |
| Total erythrocyte iron | 2000–3000 mg | 0.6–1 mg |
| Daily erythrocyte iron turnover (DEIT) | 17–25 mg | 15–25 μg |
| Daily iron absorption | 1–2 mg 5–10% of DEIT |
15–20 μg ~100% of DEIT |
| Daily dietary Fe requirement | 8–18 mg 50–100% of DEIT |
140 μg (35ppm x 4g) 5–10X DEIT |
| Normal dietary iron source | Heme and nonheme Omnivore |
Nonheme Herbivore |
2.3 Consequences of impaired iron homeostasis
Both iron deficiency and iron excess cause cellular and organ dysfunction. Low plasma iron concentrations (hypoferremia) restrict iron uptake by erythrocyte precursors, limiting hemoglobin synthesis and causing anemia. In nonerythroid cell types, the synthesis of other ferroproteins may be compromised affecting muscle performance and the maintentance of epithelia undergoing rapid turnover. At the other extreme, high plasma iron concentrations that exceed the iron-binding capacity of transferrin generate complexes with other plasma proteins as well as with organic anions such as citrate. The non-transferrin bound iron (NTBI) is avidly taken up by hepatocytes and other parenchymal cells by as yet poorly understood pathways. In humans, rapid and excessive accumulation of intracellular iron causes cell and tissue damage, presumably by iron-catalyzed generation of reactive oxygen species, with specific tissue toxicities dependent on both the rate and the extent of iron accumulation. Cardiac and endocrine tissue damage is characteristic of rapid iron accumulation while slower iron accumulation predominantly targets hepatocytes. Laboratory rodent models, with the exception of the Mongolian gerbil [7], appear resistant to iron toxicity. The molecular basis of these differential toxicities is not known.
2.4 Iron regulation and host defense
Iron is essential for nearly all microbes, and microbial pathogens utilize multiple and often complex iron uptake mechanisms to obtain it. Disruption of these uptake mechanisms attenuates microbial viability and pathogenicity [8]. Multicellular hosts limit iron availability to microbes by coupling it to protein carriers (e.g. ferritin, transferrin, lactoferrin, ovotransferrin) or utilizing it in ferroproteins, all forms of iron not readily accessible to most invading microbes. Further targeting microbial vulnerability to iron deprivation, the host rapidly responds to microbial invasion by decreasing total iron concentration in extracellular fluids, presumably to slow down microbial proliferation. The response appears to be evolutionarily ancient as infection-related mechanisms of iron sequestration have been described not only in humans, mice and other vertebrates but also in invertebrates including echinoderms [9]. As will become clear, the molecular mechanisms of these responses are closely tied to homeostatic iron regulation.
3. Hepcidin and its receptor ferroportin control systemic iron homeostasis
3.1 Hepcidin
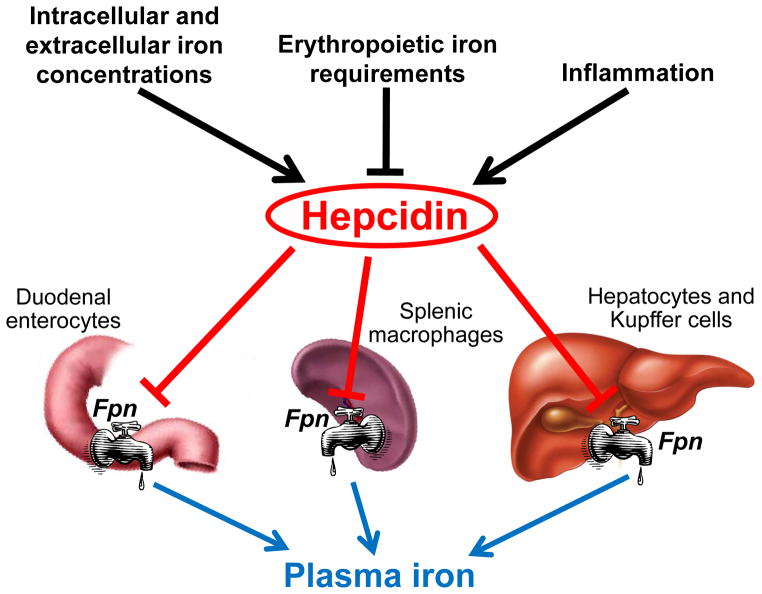
Hepcidin [10,11] is a 25 amino acid peptide hormone that inhibits iron entry into the plasma compartment from the three main sources of iron: dietary absorption in the duodenum, the release of recycled iron from macrophages and the release of stored iron from hepatocytes (Figure 1). Multiple signals reflecting systemic iron stores and concentrations, erythropoietic activity and host defense converge to regulate hepcidin production and thereby affect iron homeostasis. Hepatocytes have evolved as the predominant producers of the iron-regulatory hormone hepcidin, perhaps because of their location astride the portal venous system that delivers iron absorbed in the intestine, because of their involvement in iron storage, or because of their proximity to Kupffer cells that sense pathogens and recycle erythrocytes. The production of hepcidin is regulated by iron, so that more hepcidin is produced by hepatocytes when iron is abundant, limiting further iron absorption and release from stores. When iron is deficient, hepatocytes produce less or no hepcidin, allowing more iron to enter plasma. Both diferric plasma transferrin and stored iron in hepatocytes can stimulate hepcidin synthesis, by distinct mechanisms [5].
Figure 1. Hepcidin has a central role in maintenance of iron homeostasis.
Hepcidin synthesis is regulated at the transcriptional level by multiple stimuli. Intracellular and extracellular iron concentrations increase hepcidin transcription, as does inflammation, whereas increased erythropoietic activity suppresses hepcidin production. In turn, hepcidin regulates plasma iron concentrations by controlling ferroportin concentrations on iron-exporting cells including duodenal enterocytes, recycling macrophages of the spleen and liver, and hepatocytes.
In addition to iron, hepcidin is homeostatically regulated by the erythropoietic requirement for iron [12]. During active erythropoiesis hepcidin production is suppressed, making more iron available for hemoglobin synthesis. The nature of the suppressive signal is unknown but there is evidence that it could be a circulating factor produced by erythroid precursors in the bone marrow (the erythroid factor).
Apart from hepatocytes which are the main source of circulating hepcidin, other cell types such as macrophages [13] and adipocytes [14] express hepcidin mRNA, but at a much lower level. The relevance of the extrahepatic hepcidin production is still unclear, but it could have a role in local regulation of iron fluxes.
Hepcidin has been found only in vertebrates, and may be missing in avians [15]. It is likely that invertebrates and avians have alternative regulatory mechanisms that control iron absorption and systemic iron distribution but these have not yet been identified.
3.2 Iron delivery to plasma is dependent on the cellular iron exporter ferroportin
Iron that is absorbed from the diet by intestinal enterocytes, or recycled by macrophages or stored in hepatocytes must ultimately transit from the cytoplasm of the cells, across their cell membrane, to the plasma iron carrier protein, transferrin. Although it is not yet known how iron reaches the cell membrane before export, it appears that elemental iron can only exit from cells via the iron exporter ferroportin [16], a multipass transmembrane protein. Ferroportin does not bear any significant structural similarities to other membrane transport proteins, but is highly conserved and has been identified in mammals, other vertebrates, as well as in species as distant as Caenorhabditis elegans and Arabidopsis thaliana. Ferroportin topology and mechanism of iron transport are far from understood, and are important unresolved questions in iron biology. In humans, ferroportin is abundant in duodenal enterocytes, splenic and hepatic macrophages, and to a lesser extent in hepatocytes, all cells known to export iron. Ferroportin is also found in the lung [17,18], in renal tubules [19] and in erythrocyte precursors in the bone marrow [20] where its function is less obvious. Ferroportin expression on most other cell types is minimal and they do not appreciably export iron. With the exception of cells that are shed from the body, the iron from cells lacking ferroportin is probably recovered after they undergo cell death and are recycled by macrophages.
3.3 Hepcidin-induced loss of ferroportin decreases iron transfer to plasma and causes hypoferremia
A single injection of synthetic hepcidin into mice exerts a prolonged hypoferremic effect lasting up to 48 hours, despite the clearance of hepcidin from circulation within a few hours [21]. Experiments with cells engineered to express ferroportin [22], and primary cells or cell lines naturally expressing ferroportin [23] show that hepcidin inhibits ferroportin-dependent iron efflux. In vivo, hepcidin-induced block of iron efflux from macrophages, hepatocytes and enterocytes into plasma explains how hepcidin causes hypoferremia, as the small plasma iron pool is rapidly depleted by the consumption of iron, predominantly for hemoglobin synthesis by erythrocyte precursors.
Hepcidin inhibits iron efflux by directly binding to ferroportin [22], presumably inducing a conformational change, and triggering the endocytosis of both molecules, with consequent lysosomal degradation. The prolonged effect of hepcidin may reflect the time required to resynthesize ferroportin and deliver it to the cell membranes.
Hepcidin binding to ferroportin is dependent on the extracellular loop of ferroportin containing the amino acid cysteine (C) in position 326. Cells expressing the C326S mutant ferroportin export iron normally but fail to bind radioactive hepcidin and continue exporting iron in the presence of hepcidin [24]. Affected members of a family carrying a heterozygous C326S mutation developed severe iron overload [25], indicating that the hepcidin-resistant ferroportin acts dominantly. Several other natural autosomal dominant mutations were described that allow hepcidin binding but appear to interfere with internalization of the ligand-receptor complex resulting in similar hepcidin-resistant phenotype. The specifics of the hepcidin-induced conformational change and the molecular pathways mediating the internalization of ferroportin are an area of active study.
In the duodenum, ferroportin is located on the basolateral membrane of enterocytes but dietary iron absorption is dependent on iron uptake on their apical surfaces. Indeed, the expression of the apical iron transporter DMT1 (divalent metal transporter 1) is highly regulated by systemic iron status [26]. To coordinate apical absorption of iron with the basolateral transfer of iron to plasma, the effect of hepcidin on basolateral ferroportin must be communicated to the apical iron absorption mechanisms to decrease apical uptake. Three potential mechanisms may be involved: in the first, cellular iron accumulation, caused by diminished iron export after hepcidin binding to ferroportin, inactivates iron-regulatory proteins (IRP-1 and IRP-2) that bind the 3′ iron-regulatory element (IRE) of DMT1 mRNA, thereby destabilizing the mRNA and decreasing the synthesis of apical DMT1 [26]. In the second mechanism, cellular iron, a co-factor of oxygen-sensing prolyl hydroxylases, induces the hydroxylation of hypoxia-inducible factor HIF-2α leading to its degradation, and removing its stimulatory effect on the transcription of DMT-1 [27]. The third mechanism involves the activation of ubiquitin ligases triggered by the binding of hepcidin to ferroportin, the diffusion of the ubiquitin ligases in the cytoplasm, and the transubiqitination and proteasomal degradation of DMT1 and perhaps other apical transporters [28]. The relative role of these or other mechanisms in the regulation of apical ferrous or heme iron transport by hepcidin remains to be elucidated.
3.4 Hepcidin deficiency increases iron transfer to plasma, causing systemic iron overload
When hepcidin production is inadequate due to autosomal recessive mutations in the hepcidin gene or genes encoding hepcidin regulators [29–31], the hemochromatosis gene (HFE, most commonly mutated in hereditary hemochromatosis in populations of European ancestry), transferrin receptor 2 (TfR2) or hemojuvelin, the iron exporter ferroportin is overexpressed on the basolateral membranes of duodenal enterocytes and increases dietary iron absorption. Macrophages also overexpress cell membrane ferroportin and avidly export iron, leading to relative depletion of macrophage intracellular iron. Greater influx of iron into plasma raises plasma iron concentration, saturating transferrin with iron, and generating NTBI. NTBI is rapidly taken up by hepatocytes, cardiac myocytes and endocrine cells causing tissue damage and organ failure. Considering that hepatocytes do express ferroportin, hepcidin deficiency would be expected to increase also export of hepatocyte iron. Paradoxically, the liver is the primary organ affected by chronic iron overload. This is likely a consequence of avid uptake of NTBI by hepatocytes and their more limited capacity for iron export, resulting in the net accumulation of excess iron.
3.5 Structural determinants of the hepcidin-ferroportin interaction
Hepcidin is a bent β-hairpin stabilized by four disulfide bonds [11]. The loosely structured N-terminus appears essential for activity, as the removal of 5 N-terminal amino acids essentially ablates the bioactivity of the peptide, measured as the ability to induce ferroportin endocytosis [32]. The appearance of N-terminally truncated 20-amino acid form in human plasma and urine suggests that this inactivation pathway may be biologically relevant. More detailed hepcidin mutagenesis studies indicate that the two phenylalanine side chains, F4 and F9, are also important for bioactivity [33]. In fact, the first nine N-terminal amino acids of hepcidin are sufficient to cause ferroportin internalization [33]. With some modifications, this minihepcidin can even match or exceed the potency of native hepcidin [33].
Mutagenesis of ferroportin confirmed the importance of C326, Y333 and F324 for hepcidin binding. Modeling of the hepcidin-ferroportin interaction indicated that F4-Y333 and F9-F324 may make important contacts, and that C326 is involved in a thiol-dependent interaction with hepcidin, perhaps involving the disulfide framework of hepcidin [33]. It was previously reported that a 20 amino acid peptide based on the ferroportin segment involved in hepcidin binding could mimic the interaction of the whole ferroportin molecule with its ligand [34]. However, the interaction of this putative “hepcidin-binding domain (HBD) peptide” with hepcidin was entirely nonspecific [33] suggesting that hepcidin-binding loop on ferroportin must have a specific conformation for the binding to occur, or that additional areas of ferroportin may interact with hepcidin and stabilize the binding.
4. Hepcidin regulation
4.1 Hepcidin production by hepatocytes is transcriptionally regulated by iron
Like other hormones, hepcidin is feedback-regulated by the substance whose concentration it controls, iron. In principle, the feedback requires molecules that function as intracellular or extracellular iron sensors coupled to one or more transduction pathways that regulate hepcidin synthesis or secretion by hepatocytes. Genetic and biochemical evidence suggests that the two transferrin receptors, TfR1 and TfR2, together with the membrane protein HFE that interacts with both receptors, may serve the function of holotransferrin (diferric transferrin) sensors [35,36]. HFE is structurally related to MHC class I molecules. Its binding to TfR1 is competitively inhibited by holotransferrin. With increasing concentrations of holotransferrin, HFE is displaced from the complex with TfR1, as the binding site of HFE overlaps with that of holotransferrin. Free HFE interacts with TfR2, which itself is stabilized by holotransferrin binding. The FeTf/HFE/TfR2 complex then stimulates hepcidin expression through an incompletely understood pathway. It has been proposed that the complex potentiates BMP and/or MAPK pathway signaling [36,37] but further work is needed to delineate the molecular interactions involved.
4.2 The BMP pathway regulates hepcidin transcription
The BMP pathway with its canonical signaling system utilizing cytoplasmic Smads is the key pathway for the regulation of hepcidin transcription [38]. The BMP pathway regulates many other processes, including embryonic morphogenesis, bone development and remodeling and tissue repair. In the liver, this pathway appears to have been specifically adapted for iron regulation through a combination of factors, including a membrane-anchored coreceptor hemojuvelin [38,39] and, in mice, an iron-specific ligand BMP6 [40,41]. Hemojuvelin is not required for other, iron-unrelated BMP functions, and the nonredundant function of BMP6 in bone development appears to be minor [42]. In contrast, BMP6 and hemojuvelin are essential for normal iron homeostasis in mice as their loss [40,41,43,44] ablates the hepcidin response to acute iron loading and impairs the response to chronic iron loading [5,45]. In humans, hemojuvelin mutations result in severe hepcidin deficiency and have been shown to be the main cause of juvenile hemochromatosis [39] but the lack of similarly pathogenic human mutations in BMP6 raises the question of species differences in these mechanisms. BMPs other than BMP6, including BMP2, 4, 5, 7, 9, are also able to induce hepcidin expression in vitro [46], but their physiological role in iron regulation remains to be determined. Although the cellular source of BMPs that affect hepatocyte hepcidin production is not known with certainty, analysis of BMP mRNAs in murine liver cell subpopulations suggests that sinusoidal epithelial cells and stellate cells in the liver could be significant producers [47].
BMP receptors are tetramers of serine/threonine kinase receptors, usually with two type I and two type II subunits. Recent data indicate that the BMP receptor involved in iron regulation utilizes both Alk2 and Alk3 as type I subunits [46,48], and ActRIIA or possibly BMPRII as type II subunits [46]. It is not clear whether certain combinations of subunits fulfill specialized functions or act redundantly. Downstream, liver-specific disruption of Smad4 not only ablated hepcidin production [49] but also induced hepatic inflammation and premature mortality, presumably through the loss of anti-inflammatory activity of TGF-β which also depends on Smad4 signaling.
It needs to be clarified how BMP receptors and hemojuvelin, neither known to sense iron directly, interact with iron-sensing molecules. Two other membrane proteins, the receptor neogenin and a serine protease, matriptase-2 (MT-2, also called transmembrane protease serine 6 or TMPRSS6) also influence hepcidin synthesis, likely by modulating hemojuvelin concentration on the cell membrane [50,51]. The concentration of MT-2 on hepatocytes is acutely increased by iron deficiency [47] and the corresponding mRNA slowly induced by iron overload [52] but it is not yet clear how much each of these apparently contradictory responses contribute to regulation of hepcidin peptide concentration.
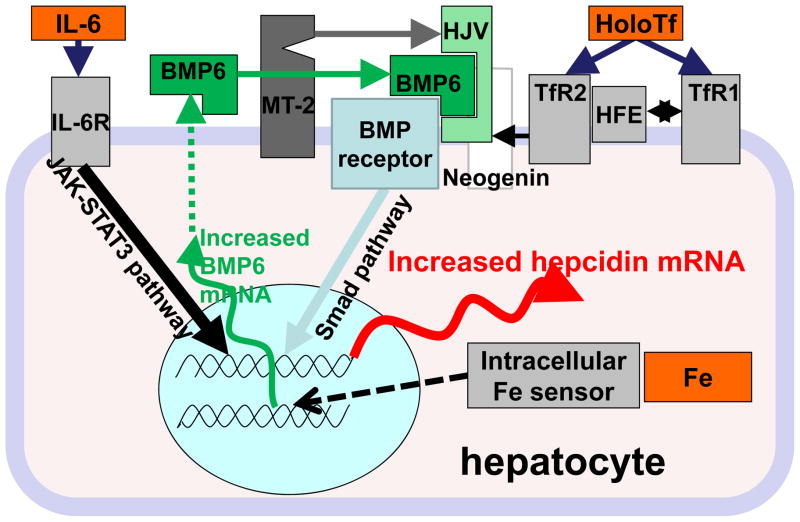
Along with stimulation of hepcidin synthesis by holotransferrin, hepatocytes can also increase hepcidin synthesis in response to stored intracellular iron. The relevant intracellular sensors and mechanisms are not known, but iron-dependent ubiquitin ligases [53] and prolyl hydroxylases [54] are candidates because of their dependence on iron and their association with hypoxia-related regulatory processes. In contrast, iron regulatory proteins IRP1 and IRP2 involved in posttranscriptional regulation of many iron- and erythrocyte-related proteins do not directly regulate hepcidin [55]. Further downstream, BMP6 mRNA was shown to increase in the liver of mice subjected to iron loading suggesting that BMP6 concentrations could reflect the hepatocyte intracellular iron levels [56]. However, hepcidin increase after chronic iron loading is still observed in BMP6-deficient mice [5] so other pathways for hepcidin stimulation by intracellular iron must exist. Figure 2 summarizes our current understanding of hepcidin regulation by iron.
Figure 2. Current understanding of molecular mechanisms of hepcidin regulation.
The iron-regulated pathway and the inflammatory pathway are the two well-studied regulators of hepcidin. The erythroid regulator pathway (not shown here) also exerts a strong effect on hepcidin expression but its molecular components are not yet known. In the iron-regulated pathway, extracellular iron in the form of holotransferrin binds to its two receptors (TfR1 and TfR2) which communicate with each other via iron-specific adaptor HFE and sensitize the BMP receptor to its ligands such as BMP6. Membrane-linked coreceptor hemojuvelin also potentiates the BMP receptor activation, which then controls hepcidin transcription via the SMADs. BMP signaling is also modulated by MT-2 protease, which cleaves hemojuvelin, and by neogenin, which may augment or stabilize membrane hemojuvelin. In hepatocytes, intracellular iron is also sensed, possibly through a mechanism that enhances the expression of BMP6 mRNA and protein, eventually leading to activation of the BMP receptor. Alternative ligands with BMP6-like activity must exist because mice that lack BMP6 still increase hepcidin mRNA in response to iron stores. Hepcidin transcription is prominently increased by inflammation, predominantly through the activity of IL-6, its receptor and its canonical JAK-STAT3 pathway.
4.3 Hepcidin synthesis by hepatocytes is suppressed by erythropoietic activity
Erythropoietic precursors in the bone marrow are the main consumers of iron from holotransferrin, and erythropoiesis is wholly dependent on this source of iron, as illustrated by the profound effect of transferrin deficiency on erythropoiesis, most recently reviewed by Bartnikas et al. [57]. Appropriately, expansion of a precursor population in response to bleeding or the administration of erythropoietin suppresses hepcidin, possibly through a mediator released by the bone marrow which exerts its effect on hepatocytes [12]. The suppressive effect of erythropoiesis on hepcidin is particularly prominent in diseases with ineffective erythropoiesis where erythrocyte precursors massively expand but mostly undergo apoptosis rather than mature into erythrocytes. This mechanism is responsible for low hepcidin [58,59] in β-thalassemia. The BMP family member growth differentiation factor GDF-15 [60], released by erythroid precursors and other cell types during cellular stress or apoptosis, may contribute to the pathological suppression of hepcidin seen in β-thalassemia and other anemias with expanded but ineffective erythropoiesis [61]. However, GDF-15 is not responsible for the physiologic hepcidin suppression in response to hemorrhage-induced stress erythropoiesis [62], and the physiologic erythroid suppressor of hepcidin is not yet known.
4.4 Inflammation increases hepcidin synthesis through IL-6 and other mediators
Hepcidin synthesis by hepatocytes is transcriptionally regulated by IL-6 [63] through the STAT-3 signaling pathway [64–66]. This and possibly other mechanisms increase hepcidin production and blood hepcidin concentrations during infections and systemic inflammatory diseases. Inflammation-induced hepcidin increase causes the hypoferremia that develops early during infections or inflammatory diseases. Although the efficacy of this mechanism in host defense against specific microbes remains to be shown, increased susceptibility of patients with even relatively mild forms of hereditary hemochromatosis to certain infections (e.g. [67]) suggests that this pathological response likely evolved to limit the multiplication of iron-dependent extracellular microbes. As with other host defense mechanisms, there is a price to be paid: iron sequestration and hypoferremia due to inflammation-related hepcidin increase may limit the availability of iron for erythropoiesis, and contribute to anemia of inflammation (also known as anemia of chronic disease).
4.5 Impaired renal clearance of hepcidin leads to its accumulation
Recent studies indicate that hepcidin in blood plasma may circulate in association with α2-macroglobulin [68]. However, the affinity of the binding protein for hepcidin is relatively low (about 200–300 nM) so that a substantial proportion of hepcidin will be unbound at physiologic concentrations. Due to its small size (2.7 kD), hepcidin readily passes through the glomerular membrane but like other small proteins is then taken up and degraded in the proximal tubule. A small fraction of the filtered hepcidin passes intact into urine where it is readily detectable. Chronic kidney diseases impair the clearance of hepcidin leading to its accumulation in plasma [69] where it may contribute to iron sequestration in macrophages and limit the availability of iron for erythropoiesis. This mechanism may contribute significantly to anemia of chronic kidney diseases. Hepcidin is efficiently cleared during hemodialysis suggesting that iron utilization could be improved by more frequent or more effective hemodialysis [70].
4.6 Hepcidin-independent regulation of ferroportin
Although hepcidin has only been found in vertebrates [15], ferroportin is present already in simple invertebrates such C. elegans, as well as in plants. Invertebrate ferroportins generally lack the extracellular cysteine (C326 in humans) shown to be essential for hepcidin binding, indicating that the two molecules coevolved to interact. Consistent with its earlier evolutionary history, ferroportin expression is also regulated in hepcidin-independent cell-autonomous manner by heme and iron [71]. Similarly to the mechanism of regulation of heme oxygenase-1, heme causes a rapid increase in ferroportin transcription by promoting the nuclear accumulation of the transcriptional regulator Nrf2, leading to the displacement of Bach1 transcriptional repressor from the Maf recognition element (MARE)/antioxidant responsive element (ARE) sequence in the ferroportin promoter. This mechanism is important for iron export from macrophages after erythrophagocytosis. Iron controls Fpn translation through the iron-regulatory protein/iron-regulatory element (IRE/IRP) system. One of the two splice forms of ferroportin mRNA contains an IRE in the 5′ untranslated region. When intracellular iron levels decrease, IRP1/2 bind to the 5′ IRE blocking the translation of ferroportin. This mechanism likely prevents excessive depletion of intracellular iron which would be detrimental to cellular functions. Conversely, when intracellular iron levels increase, such as after erythrophagocytosis and heme degradation in macrophages, increased ferroportin translation will ensure the export of recycled iron from macrophages. It is still unknown to what extent different levels of regulation contribute to determining the ultimate concentrations of ferroportin protein. The ferroportin splice variant lacking the 5′ IRE is a relatively minor component of total ferroportin mRNA in most tissues but it makes up 25% of total FPN mRNA in the duodenum and about 40% in the bone marrow, where it is particularly abundant in erythroid precursors [20]. This form is not translationally repressed in iron-deficient cells and, during severe iron deficiency, may promote the altruistic release of iron from duodenal enterocytes and erythroid precursors to meet the minimal iron needs of deprivation-sensitive cells in other organs.
5. Iron overload disorders due to hepcidin deficiency or resistance to hepcidin
5.1 Hereditary hemochromatosis
Hereditary hemochromatosis is a group of primary genetic disorders of iron homeostasis in which hyperabsorption of dietary iron leads to iron accumulation in tissues, iron-mediated injury and organ dysfunction [31]. Iron accumulates because humans and many animals lack compensatory mechanisms that would significantly increase iron excretion in response to iron excess. Although the absorption of iron takes place in the duodenum, mouse models have demonstrated that the primary cause of most forms of hereditary hemochromatosis is insufficient production of hepcidin by hepatocytes [72], resulting in ineffective regulation of duodenal iron absorption and excessive delivery of iron to plasma. The severity of the hepcidin deficiency determines the rate of progression of iron overload and the clinical course. In the juvenile forms of the disease, due to mutations in the hepcidin gene or the hemojuvelin gene, little or no hepcidin is detectable in plasma [69]. Juvenile hemochromatosis is highly penetrant, both genders are equally affected, and clinical problems, including endocrinopathies and cardiomyopathy, develop in late childhood or early adulthood. In the less severe, adult forms of the disease hepcidin synthesis is partially responsive to acute and especially chronic iron loading but hepcidin concentrations are insufficient relative to iron load. As iron is lowered to normal levels by venesections (each ml of packed erythrocytes contains about 1 mg of iron), frank hepcidin deficiency becomes manifest. The adult forms of the disease include a rare form due to autosomal recessive mutations in transferrin receptor 2, and the most common form due to autosomal recessive mutations in the HFE gene. HFE mutations are highly prevalent in populations of northern European ancestry, but the disease is incompletely penetrant and affects men more frequently and more severely than women [73]. The majority of genetically affected individuals are identified because of laboratory abnormalities or family history rather than overt disease. Factors such as alcohol intake, obesity, other modulating genes, and possibly the heme content of the diet may co-determine the rate of progression. If untreated, a small percentage of affected individuals will develop cirrhosis sometimes progressing to liver cancer.
Although iron depletion through phlebotomy is effective treatment, it eventually worsens hepcidin deficiency, increasing iron absorption and necessitating additional therapeutic phlebotomies. Furthermore, phlebotomy is not suitable for all patients because of poor vascular access, adverse physiological responses to phlebotomy, the inconvenience of travel to phlebotomy centers or other burdens. In the future, therapeutic correction of hepcidin deficiency may offer additional treatment options for patients suffering from hereditary hemochromatosis.
5.2 Iron–loading anemias
A severe iron overload disorder develops in most patients with β-thalassemia, even if they do not receive blood transfusions. Unless effectively treated by iron chelators, iron overload is the major cause of serious morbidity and mortality in this disease. In β-thalassemia, defective β-globin production in erythroid precursors causes excess α-chains to precipitate, leading to the apoptosis of precursors during their maturation in the marrow. The resulting anemia stimulates erythropoietin production, which in turn causes massive expansion of erythroid precursors in the marrow and elsewhere, but fails to correct the anemia because the precursors undergo apoptosis. In the absence of transfusions, iron overload is due to the hyperabsorption of dietary iron. Like in hereditary hemochromatosis, low hepcidin is the cause of iron hyperabsorption [74]. Recent studies implicated two members of the BMP family, growth differentiation factor (GDF) 15 and Twisted Gastrulation (TWSG1), as candidate bone marrow-derived hepcidin suppressors in β-thalassemia [60,75], although additional hepcidin-suppressing erythroid factor(s) may exist.
In β-thalassemia patients treated with regular transfusions, iron overload is primarily the consequence of the treatment, and hepcidin levels are normal or even increased, although still deficient considering the iron overload [74]. Transfusions partially correct the anemia, acutely and chronically decreasing erythropoietin secretion [76]. The stimulus to erythroid expansion is diminished, as is presumably the production of hepcidin-suppressive mediators, thus allowing hepcidin levels to rise. There is early evidence that circulating hepcidin concentrations affect the distribution of iron between the macrophage storage compartment (favored by higher hepcidin concentrations) and parenchymal cells including cardiac myocytes and hepatocytes (favored by low hepcidin) [74]. The balance of iron between these two compartments may have implications for the rate of progression of cardiomyopathy. Other iron-loading anemias such as congenital dyserythropoietic anemias [77] display similar iron pathology but are much less common.
Iron overload in β-thalassemia is treated by chelators. These can have serious adverse effects and patient compliance is frequently a problem. In the future, hepcidin supplementation may become an alternative or addition to the chelation therapy, at least in untransfused patients.
Hepcidin therapy may also have an unexpected beneficial effect on ineffective erythropoiesis. It was recently reported that moderate overexpression of hepcidin in a mouse model of β-thalassemia not only decreased iron overload, but also improved erythropoiesis [78]. Although the mechanism is still being elucidated, hepcidin-mediated reduction in plasma iron may prolong the lifespan of erythrocytes in β-thalassemia by reducing heme production in erythroid precursors, consequently limiting the α globin/heme aggregate formation and associated ROS production.
6. Iron-restrictive disorders due to hepcidin excess or ferroportin deficiency
6.1 Iron-refractory iron deficiency anemia
The pathological effects of pure hepcidin excess were first shown in transgenic mouse models of hepatic hepcidin overexpression. The hepcidin-overproducing mice displayed severe hypoferremia and microcytic anemia that was resistant to oral iron administration and difficult to treat even with parenteral iron [79]. The phenotype is mirrored in two human disorders: rare hepatic adenomas that overexpress hepcidin [80], and more common familial iron-refractory iron deficiency anemia (IRIDA), an autosomal recessive disorder caused by mutations ablating or inactivating a negative regulator of hepcidin, the membrane protease MT-2 (also called TMPRSS6) [81,82]. Despite severe iron deficiency which would be expected to physiologically suppress hepcidin production, patients with IRIDA show high normal or even increased serum hepcidin. Recent studies demonstrated that matriptase-2 cleaves membrane hemojuvelin [51]. In the absence of matriptase-2, hemojuvelin presumably accumulates on hepatocyte membranes acting as a coreceptor of the BMP pathway and driving hepcidin transcription, even in the presence of iron deficiency.
6.2 Anemia of inflammation
Chronic inflammatory disorders including infections, rheumatologic disorders and inflammatory bowel disease are associated with hypoferremia and anemia, the latter classically mild to moderate and normocytic but sometimes microcytic and hypochromic, and resistant to iron therapy. Although systematic studies of blood hepcidin concentrations in these diseases have not yet been published, small studies indicate that hepcidin is increased in many patients with these disorders [83]. Inflammatory stimulation of hepcidin production through IL-6 and other pathways is well documented, and would be expected to produce a clinical picture of anemia of inflammation. Indeed, a mouse model of moderate hepcidin overproduction results in mild microcytic anemia and resistance to erythropoietin [84]. Unlike in primary disorders of hepcidin overproduction, such as those due to matriptase-2 mutations or in the transgenic mouse models of hepcidin excess, hypochromia and microcytosis are seen only in a minority of patients with anemia of inflammation, perhaps because the latter condition is generally less severe, of shorter duration, and may have a fluctuating course. It is likely that in individual disease states other effects of inflammation contribute, including shortened erythrocyte survival due to macrophage activation or opsonization of erythrocytes, erythropoietic suppression due to the direct effect of cytokines on erythrocyte precursors, and partial inhibition of erythropoietin production.
6.3 Anemia of chronic kidney diseases
Historically, the anemia of CKD was solely attributed to decreased erythropoietin production by the diseased kidneys. Later measurements showed that erythropoietin levels are increased in most patients with anemia of CKD but that this increase may not be adequate for the severity of anemia. Arguing against the role of simple erythropoietin deficiency, many patients with CKD require high doses of erythropoiesis-stimulating agents to maintain acceptable hemoglobin concentrations. Moreover, the administration of high doses of parenteral iron potentiated the effect of erythropoiesis-stimulating agents even in patients with apparently adequate iron stores as indicated by serum ferritin [85,86]. These observations suggest that CKD patients commonly suffer from an iron-restrictive disorder. More recent studies indicate that CKD patients have high circulating hepcidin levels [87], likely due to the decreased renal clearance of the peptide augmented by the hepcidin induction by inflammation related to the underlying disease process or hemodialysis. High circulating hepcidin would be expected to cause iron sequestration in macrophages, restrict iron flow to the erythropoietic marrow, and contribute to the pathogenesis of anemia.
6.4 Anemia of cancer
Anemia accompanies some malignancies at diagnosis but becomes much more common as the disease progresses, or as a result of chemotherapy and radiation. Depending on the specifics of the disease process, blood loss, malnutrition, infiltration of erythropoietic bone marrow by tumor and cytotoxic injury to the erythropoietic precursors may contribute to anemia. The anemia of some malignancies, including Hodgkin’s disease [88] and multiple myeloma [89,90], resembles anemia of inflammation and is accompanied by increased hepcidin production stimulated by inflammatory cytokines. In other cancers, the contribution of inflammation, increased hepcidin and iron restriction to the pathogenesis of anemia is less well characterized and is likely to vary depending on the type and size of the tumor, specific sites of involvement and the individual host’s inflammatory response to the malignant process.
6.5 Ferroportin disease
Ferroportin disease [91] is a syndrome of severely increased serum ferritin, often normal or even low plasma transferrin saturations, and iron accumulation predominantly in macrophages. It is caused by many distinct autosomal dominant missense mutations in ferroportin, resulting in impaired macrophage iron export either due to decreased synthesis or membrane trafficking of ferroportin, or due to the impairment of its iron transport function. The lack of association with nonsense mutations suggest that the missense mutations have a dominant negative effect. Unless exacerbated by factors such as alcoholic liver disease or hepatitis C, the disorder is often clinically silent. Treatment of ferroportin disease with phlebotomy can lead to anemia even before iron stores are depleted, revealing an iron-restrictive disorder, i.e. impairment of the maximal iron export capacity of macrophages involved in recycling of iron from senescent erythrocytes.
7. Diagnostic and therapeutic potential of hepcidin
7.1 Measurement of hepcidin in blood and other fluids
Two types of hepcidin assays are coming into research use: immunoassays based on anti-hepcidin antibodies that use a reference standard of synthetic hepcidin [69,92], and mass spectrometric assays that detect the characteristic mass of the active 25 amino acid hepcidin species or its fragments, and quantify the intensity of the peak(s) relative to a spiked internal standard [93–96]. Serum or plasma measurements may be preferable to the urinary measurements which, although less invasive, require a creatinine measurement to correct for the varying concentrating activity of the kidneys, thus potentially introducing a source of additional error. Despite discrepancies in the absolute values and normal ranges measured by the various assays, they generally correlate very well [97]. The differences between assays are probably due to the troublesome tendency of hepcidin to aggregate and adhere to surfaces, affecting the peptide standards. All reported studies found very low hepcidin levels in iron deficiency, β-thalassemia intermedia, or juvenile forms of hereditary hemochromatosis, and somewhat higher levels in adult forms of hereditary hemochromatosis. They also detected diurnal variations manifested by the lower morning and the higher afternoon hepcidin concentrations, and observed the gender difference, with lower hepcidin concentrations in women than in men. Patients with secondary iron overload from transfusions have high hepcidin values unless they have exuberant ineffective erythropoiesis as is the case in some patients with β-thalassemia major. Patients with iron-refractory iron deficiency anemia have high normal or even high hepcidin values, despite often severe iron deficiency. Inflammatory disorders, multiple myeloma, Hodgkin disease and many cancers also show increased serum hepcidin values. Hepcidin is markedly elevated in infections where it may play a role in host defense by decreasing circulating iron concentrations, and limiting the availability of this essential nutrient to microbes.
7.2 Hepcidin agonists and antagonists
Because of the key role of hepcidin deficiency or hepcidin excess in the pathogenesis of various iron disorders, agonists or antagonists of hepcidin would be expected to improve the treatment of patients with these disorders. Hepcidin agonists should be useful for preventing or treating iron overload in most forms of hereditary hemochromatosis and in β-thalassemia. Minihepcidins, small peptide hepcidin analogs, have proven effective in controlling plasma iron concentration or preventing iron overload in mouse models [33], but their safety and efficacy in humans remain to be established. BMP6 or other BMPs were shown to stimulate hepcidin synthesis [98], but it remains to be determined if they can be used without activating other BMP-dependent pathways.
Hepcidin antagonists should be useful for the treatment of iron-restrictive anemias with elevated hepcidin concentrations, and for potentiating the effect of erythropoiesis-stimulating agents by increasing iron availability for erythropoiesis. In principle, hepcidin could be antagonized by targeting its synthesis, its interaction with ferroportin, or ferroportin endocytosis. Among agents decreasing hepcidin production, anti-IL6 receptor antibody showed considerable activity in reversing anemia of inflammation in monkeys [99], but it remains to be seen whether the toxicity profile of this agent will allow its widespread use in anemia. Interfering with the BMP pathway using soluble hemojuvelin or a small-molecule inhibitor dorsomorphin, reversed the anemia in the rat model of anemia of inflammation [100]. As mentioned before, the usefulness of the BMP pathway inhibitors will depend on how selective they are for the hepcidin-regulatory pathway as compared to other important related pathways. Hepcidin-neutralizing monoclonal antibodies were shown to reverse anemia in a mouse model of inflammation [101], and most recently, an antibody targeting ferroportin has been described [102], which raises serum iron in monkeys presumably by interfering with hepcidin binding to ferroportin.
8.0 Conclusion
The interaction of hepcidin with ferroportin constitutes the key control step in systemic iron homeostasis. Although much progress has been made in the short time since the molecules were discovered, the molecular regulation and interaction of the two partners is an active area of investigation, with important implications for human and other vertebrate biology and therapeutics.
Contributor Information
Tomas Ganz, Email: TGanz@mednet.ucla.edu.
Elizabeta Nemeth, Email: ENemeth@mednet.ucla.edu.
Reference List
- 1.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 2.TRUMBO PAUL, YATES AA, SCHLICKER SAND, POOS MARY. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Journal of the American Dietetic Association. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 3.Russell ES, Bernstein SE. In: Blood and Blood Formation. Green EL, editor. McGraw-Hill Book Company, Biology of the Laboratory Mouse; 1966. [Google Scholar]
- 4.Biology of the Laboratory Mouse. McGraw-Hill Book Company; 1966. [Google Scholar]
- 5.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E, Ganz T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53:1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorbie J, Valberg LS. Iron balance in the mouse. Lab Anim Sci. 1974;24:900–904. [PubMed] [Google Scholar]
- 7.Kaiser L, Davis JM, Patterson J, Johnson AL, Bohart G, Olivier NB, Schwartz KA. Iron sufficient to cause hepatic fibrosis and ascites does not cause cardiac arrhythmias in the gerbil. Transl Res. 2009;154:202–213. doi: 10.1016/j.trsl.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg ED. Iron availability and infection. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Beck G, Ellis TW, Habicht GS, Schluter SF, Marchalonis JJ. Evolution of the acute phase response: iron release by echinoderm (Asterias forbesi) coelomocytes, and cloning of an echinoderm ferritin molecule. Developmental & Comparative Immunology. 2002;26:11–26. doi: 10.1016/s0145-305x(01)00051-9. [DOI] [PubMed] [Google Scholar]
- 10.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 11.Jordan JB, Poppe L, Haniu M, Arvedson T, Syed R, Li V, Kohno H, Kim H, Schnier PD, Harvey TS, Miranda LP, Cheetham J, Sasu BJ. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J Biol Chem. 2009;284:24155–24167. doi: 10.1074/jbc.M109.017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XB, Nguyen NB, Marquess KD, Yang F, Haile DJ. Regulation of hepcidin and ferroportin expression by lipopolysaccharide in splenic macrophages. Blood Cells, Molecules, and Diseases. 2005;35:47–56. doi: 10.1016/j.bcmd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben A, Saint-Paul IMC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Hilton KB, Lambert LA. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene. 2008;415:40–48. doi: 10.1016/j.gene.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Haile DJ, Wang X, Dailey LA, Stonehuerner JG, Ghio AJ. Apical location of ferroportin 1 in airway epithelia and its role in iron detoxification in the lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L14–L23. doi: 10.1152/ajplung.00456.2004. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Wang X, Haile DJ, Piantadosi CA, Ghio AJ. Iron increases expression of iron-export protein MTP1 in lung cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L932–L939. doi: 10.1152/ajplung.00114.2002. [DOI] [PubMed] [Google Scholar]
- 19.Wolff NA, Liu W, Fenton RA, Lee WK, Thevenod F, Smith CP. Ferroportin 1 is expressed basolaterally in rat kidney proximal tubule cells and iron excess increases its membrane trafficking. J Cell Mol Med. 2011;15:209–219. doi: 10.1111/j.1582-4934.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 23.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes A, Preza GC, Phung Y, De Domenico I, Kaplan J, Ganz T, Nemeth E. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114:437–443. doi: 10.1182/blood-2008-03-146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sham RL, Phatak PD, West C, Lee P, Andrews C, Beutler E. Autosomal dominant hereditary hemochromatosis associated with a novel ferroportin mutation and unique clinical features. Blood Cells, Molecules, and Diseases. 2005;34:157–161. doi: 10.1016/j.bcmd.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- 27.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 Cotransporter Is Down-regulated by Hepcidin via Proteasome Internalization and Degradation. Gastroenterology. 2011;140:1261–1271. doi: 10.1053/j.gastro.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 31.Pietrangelo A. Hereditary hemochromatosis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006;1763:700–710. doi: 10.1016/j.bbamcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011 doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Domenico I, Nemeth E, Nelson JM, Phillips JD, Ajioka RS, Kay MS, Kushner JP, Ganz T, Ward DM, Kaplan J. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8:146–156. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94:765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 39.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 40.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 41.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry MJ, McDougall KE, Hou Sc, Tobias JH. Impaired growth plate function in bmp-6 null mice. Bone. 2008;42:216–225. doi: 10.1016/j.bone.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 43.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54:273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111:5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang AS, Anderson SA, Wang J, Yang F, DeMaster K, Ahmed R, Nizzi CP, Eisenstein RS, Tsukamoto H, Enns CA. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2011;117:1687–1699. doi: 10.1182/blood-2010-06-287292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinbicker AU, Bartnikas TB, Lohmeyer LK, Leyton P, Mayeur C, Kao SM, Pappas AE, Peterson RT, Bloch DB, Yu PB, Fleming MD, Bloch KD. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011 doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Lee DH, Zhou LJ, Zhou Z, Xie JX, Jung JU, Liu Y, Xi CX, Mei L, Xiong WC. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood. 2010;115:3136–3145. doi: 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meynard D, Vaja V, Sun CC, Corradini E, Chen S, Lopez-Otin C, Grgurevic L, Hong CC, Stirnberg M, Gütschow M, Vukicevic S, Babitt JL, Lin HY. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118:747–756. doi: 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–726. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salahudeen AA, Bruick RK. Maintaining Mammalian iron and oxygen homeostasis: sensors, regulation, and cross-talk. Ann N Y Acad Sci. 2009;1177:30–38. doi: 10.1111/j.1749-6632.2009.05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 56.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 57.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117:630–637. doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adamsky K, Weizer O, Amariglio N, Breda L, Harmelin A, Rivella S, Rachmilewitz E, Rechavi G. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124:123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 60.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 61.Tanno T, Miller JL. GDF15 expression and iron overload in ineffective erythropoiesis. Rinsho Ketsueki. 2011;52:387–398. [PubMed] [Google Scholar]
- 62.Tanno T, Rabel A, Lee YT, Yau YY, Leitman SF, Miller JL. Expression of growth differentiation factor 15 is not elevated in individuals with iron deficiency secondary to volunteer blood donation. Transfusion. 2010;50:1532–1535. doi: 10.1111/j.1537-2995.2010.02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 65.Verga Falzacappa MV, Vujic SM, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 66.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank KM, Schneewind O, Shieh WJ. Investigation of a researcher’s death due to septicemic plague. N Engl J Med. 2011;364:2563–2564. doi: 10.1056/NEJMc1010939. [DOI] [PubMed] [Google Scholar]
- 68.Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E, Huang ML, Rahmanto YS, Richardson DR, Vyoral D. Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood. 2009;113:6225–6236. doi: 10.1182/blood-2009-01-201590. [DOI] [PubMed] [Google Scholar]
- 69.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 70.Zaritsky J, Young B, Gales B, Wang HJ, Rastogi A, Westerman M, Nemeth E, Ganz T, Salusky IB. Reduction of serum hepcidin by hemodialysis in pediatric and adult patients. Clin J Am Soc Nephrol. 2010;5:1010–1014. doi: 10.2215/CJN.08161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411:123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 72.Spasic MV, Kiss J, Herrmann T, Kessler R, Stolte J, Galy B, Rathkolb B, Wolf E, Stremmel W, Hentze MW, Muckenthaler MU. Physiologic systemic iron metabolism in mice deficient for duodenal Hfe. Blood. 2007;109:4511–4517. doi: 10.1182/blood-2006-07-036186. [DOI] [PubMed] [Google Scholar]
- 73.Laine F, Jouannolle AM, Morcet J, Brigand A, Pouchard M, Lafraise B, Mosser J, David V, Deugnier Y. Phenotypic expression in detected C282Y homozygous women depends on body mass index. J Hepatol. 2005;43:1055–1059. doi: 10.1016/j.jhep.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 74.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 75.Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kearney SL, Nemeth E, Neufeld EJ, Thapa D, Ganz T, Weinstein DA, Cunningham MJ. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 77.Casanovas G, Swinkels DW, Altamura S, Schwarz K, Laarakkers CM, Gross HJ, Wiesneth M, Heimpel H, Muckenthaler MU. Growth differentiation factor 15 in patients with congenital dyserythropoietic anaemia (CDA) type II. J Mol Med. 2011 doi: 10.1007/s00109-011-0751-5. [DOI] [PubMed] [Google Scholar]
- 78.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC, Nemeth E, Follenzi A, An X, Mohandas N, Ginzburg Y, Rachmilewitz EA, Giardina PJ, Grady RW, Rivella S. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 81.Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K, Andrews NC, Fleming MD. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT, Wroblewski VJ, Wurz E, Datz C, Weiss G. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 84.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis. 2009;16:94–100. doi: 10.1053/j.ackd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR and the DRIVE Study Group, . Ferric Gluconate Is Highly Efficacious in Anemic Hemodialysis Patients with High Serum Ferritin and Low Transferrin Saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 87.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, Ganz T, Rivera S, Nissenson AR, Salusky IB. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hohaus S, Massini G, Giachelia M, Vannata B, Bozzoli V, Cuccaro A, D’Alo’ F, Larocca LM, Raymakers RA, Swinkels DW, Voso MT, Leone G. Anemia in Hodgkin’s lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28:2538–2543. doi: 10.1200/JCO.2009.27.6873. [DOI] [PubMed] [Google Scholar]
- 89.Sharma S, Nemeth E, Chen YH, Goodnough J, Huston A, Roodman GD, Ganz T, Lichtenstein A. Involvement of Hepcidin in the Anemia of Multiple Myeloma. Clin Cancer Res. 2008;14:3262–3267. doi: 10.1158/1078-0432.CCR-07-4153. [DOI] [PubMed] [Google Scholar]
- 90.Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys L, Rivera S, Vanderkerken K, Lichtenstein A, Ganz T. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood. 2010;116:3635–3644. doi: 10.1182/blood-2010-03-274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pietrangelo A. The ferroportin disease. Blood Cells, Molecules, and Diseases. 2004;32:131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Busbridge M, Griffiths C, Ashby D, Gale D, Jayantha A, Sanwaiya A, Chapman RS. Development of a novel immunoassay for the iron regulatory peptide hepcidin. Br J Biomed Sci. 2009;66:150–157. doi: 10.1080/09674845.2009.11730263. [DOI] [PubMed] [Google Scholar]
- 93.Kemna E, Tjalsma H, Laarakkers C, Nemeth E, Willems H, Swinkels D. Novel urine hepcidin assay by mass spectrometry. Blood. 2005;106:3268–3270. doi: 10.1182/blood-2005-05-1873. [DOI] [PubMed] [Google Scholar]
- 94.Murphy AT, Witcher DR, Luan P, Wroblewski VJ. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110:1048–1054. doi: 10.1182/blood-2006-11-057471. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Rose MJ, Tran L, Zhang J, Miranda LP, James CA, Sasu BJ. Development of a method for the sensitive and quantitative determination of hepcidin in human serum using LC-MS/MS. J Pharmacol Toxicol Methods. 2009;59:171–180. doi: 10.1016/j.vascn.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Bansal SS, Halket JM, Fusova J, Bomford A, Simpson RJ, Vasavda N, Thein SL, Hider RC. Quantification of hepcidin using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1531–1542. doi: 10.1002/rcm.4033. [DOI] [PubMed] [Google Scholar]
- 97.Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, Hider RC, Koliaraki V, Mamalaki A, Olbina G, Tomosugi N, Tselepis C, Ward DG, Ganz T, Hendriks JC, Swinkels DW. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–1752. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corradini E, Schmidt PJ, Meynard D, Garuti C, Montosi G, Chen S, Vukicevic S, Pietrangelo A, Lin HY, Babitt JL. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139:1721–1729. doi: 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30:917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 100.Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, Babitt JL, Hong CC, Menhall T, Gearing P, Lin HY, Weiss G. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 102.Leung DDR, Luan P, Manetta JV, Tang Y, Witcher DR. ELI LILLY AND COMPANY; 2011040797 2011. ANTI-FERROPORTIN 1 MONOCLONAL ANTIBODIES AND USES THEREOF. 2008 May 12;