Abstract
One of the most debilitating aspects of schizophrenia is an apparent of interest in or ability to exert effort for rewards. Such “negative symptoms” may prevent individuals from obtaining potentially beneficial outcomes in educational, occupational or social domains. In animal models, dopamine abnormalities decrease willingness to work for rewards, implicating DA function as a candidate substrate for negative symptoms given that schizophrenia involves dysregulation of the dopamine system. We used the Effort-Expenditure for Rewards Task (EEfRT) to assess the degree to which individuals with schizophrenia were wiling to exert increased effort for either larger magnitude rewards or for rewards that were more probable. Fifty-nine individuals with schizophrenia and thirty-nine demographically similar controls performed the EEfRT task, which involves making choices between “easy” and “hard” tasks to earn potential rewards. Individuals with schizophrenia showed less of an increase in effort allocation as either reward magnitude or probability increased. In controls, the frequency of choosing the hard task in high reward magnitude and probability conditions was negatively correlated with depression severity and anhedonia. In schizophrenia, fewer hard task choices were associated with more severe negative symptoms and worse community and work function as assessed by a caretaker. Consistent with patterns of disrupted dopamine functioning observed in animal models of schizophrenia, these results suggest that one mechanism contributing to impaired function and motivational drive in schizophrenia may be a reduced allocation of greater effort for higher magnitude or higher probability rewards.
Introduction
The negative symptoms that plague many individuals with schizophrenia are just as debilitating as psychotic symptoms, and much less effectively treated by our currently available antipsychotic medications (Kirkpatrick, Fenton, Carpenter, & Marder, 2006). Critically, research on negative symptoms has uncovered that anhedonia and amotivation in schizophrenia do not reflect a blunted capacity to enjoy pleasurable experiences (Aghevli, Blanchard, & Horan, 2003; Cohen & Minor, 2010; Kring & Moran, 2008; Llerena, Strauss, & Cohen, 2012). This suggests that the mechanisms leading to impaired motivation in schizophrenia must involve something other than a lack of enjoyment in or “liking” of rewarding outcomes (Barch & Dowd, 2010; Kring & Elis, 2012). There is a growing evidence that people with schizophrenia have deficits in reward learning/prediction that relate to negative symptoms (Gold et al., 2012; Waltz, Frank, Robinson, & Gold, 2007; Waltz, Frank, Wiecki, & Gold, 2011; Waltz & Gold, 2007). However, there is much less work on motivation or effort allocation.
One reason to think that motivation impairments in schizophrenia might involve reduced willingness to exert effort is the large literature on the role of dopamine in computing cost-benefit computations and in modulating the amount of effort allocated to obtain rewarding outcomes (for a review, see (Fervaha, Foussias, Agid, & Remington, 2013). There is a wealth of animal data showing that dopamine function plays a crucial role in constraining the amount of effort than an animal will exert for a given reward (Assadi, Yucel, & Pantelis, 2009; Floresco, St Onge, Ghods-Sharifi, & Winstanley, 2008; Kurniawan, Guitart-Masip, & Dolan, 2011; Salamone & Correa, 2012; Salamone, Correa, Farrar, Nunes, & Pardo, 2009). Numerous studies have shown that a variety of manipulations that block dopamine receptors or down-regulate dopamine function lead animals to be less willing to exert greater effort to obtain high magnitude outcome (Assadi, Yucel, & Pantelis, 2009; Floresco, St Onge, Ghods-Sharifi, & Winstanley, 2008; Kurniawan, Guitart-Masip, & Dolan, 2011; Salamone & Correa, 2012; Salamone et al., 2009) and can modulate their sensitivity to reward information (Floresco, 2013). In healthy humans, stimulating dopamine release via d-amphetamine administration increases willingness to exert effort (Wardle, Treadway, Mayo, Zald, & de Wit, 2011), and those individuals with the greatest increase in dopamine release show the largest increases in effort expenditure (Treadway et al., 2012).
However, people with schizophrenia do not show reduced dopamine release or dopamine receptor availability, at least not in the striatum. Indeed, prior work suggests that both tonic striatal dopamine transmission as well as dopaminergic responses to dopamine-releasing agents such as d-amphetamine may be increased in schizophrenia (Abi-Dargham et al., 2000; Abi-Dargham, van de Giessen, Slifstein, Kegeles, & Laruelle, 2009; Laruelle, Abi-Dargham, Gil, Kegeles, & Innis, 1999). Similarly, individuals at high risk for developing schizophrenia also show greater dopamine release in response to d-amphetamine or stress (Abi-Dargham et al., 2004; Soliman et al., 2008; Woodward et al., 2011). These findings would appear to argue against a role for striatal dopamine as a substrate for negative symptoms related to effort. One explanation for this discrepancy may that decreased motivation can result from an over-expression of post-synaptic D2 receptors rather than attenuated dopamine release. Mice genetically altered to overexpress D2 receptors across the course of development show a reduction in effort allocation in the context of intact hedonic experience (Drew et al., 2007; Ward et al., 2012), though D2 overexpression that only occurs in adulthood does not have the same effect (Trifilieff et al., 2013). Consistent with this mechanism, human imaging studies suggest that schizophrenia may be associated with a modest increase in D2 receptor availability (Fusar-Poli & Meyer-Lindenberg, 2013a, 2013b; Howes et al., 2012).
An additional factor that may help reconcile the preclinical and clinical literatures regarding dopamine levels and effort is the importance of timing for normative dopamine function. In animal models, the reinforcing effects of dopamine on target behaviors has been repeatedly shown to depend on the temporal correspondence between dopamine neuron firing and reward-related cues (Schultz, 2007; Tsai et al., 2009). Consequently, one possibility is that patients with schizophrenia experience erratic burst firing of dopamine neurons (Heinz & Schlagenhauf, 2010). This hypothesis would suggest that schizophrenia is not associated with a steady-state attenuation of dopamine response, but rather a deficit in the appropriate recruitment of dopamine systems when needed for everyday activities. When considering the effort-related functions of striatal dopamine, this model would make the key prediction that individuals with schizophrenia would not necessarily be “less motivated” than controls in terms of overall effort production, but would be unable to optimize the deployment of effort in response to reward information in the environment. Consistent with this hypothesis, there are two previous studies showing sub-optimal allocation of effort for monetary rewards among individuals with schizophrenia (Fervaha et al., 2013; Gold et al., 2013), with a greater impairment in patients with high negative symptoms (Gold et al., 2013) or apathy (Fervaha et al., 2013). Critically, there have been no prior studies looking at how laboratory-based measures of effort expenditure may relate to informant based ratings of motivated behavior in daily life. This is a crucial step in linking translational research and laboratory-based assessments to functional impairments in this disorder.
The goal of the current study was to test the hypotheses that: 1) individuals with schizophrenia would show a reduced willingness to choose tasks that require greater effort even if they come with the possibility of greater reward, and 2) that such deficits would be related to negative symptoms such as amotivation and function in community and work settings. The current study used the Effort-Expenditure for Rewards Task (EEfRT)(Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009), which involves making choices between easy and “hard” tasks (number of button presses) to earn potential rewards. This task has been shown to be related to dopamine function in the striatum and the ventral medial prefrontal cortex, (Treadway et al., 2012), and reduced effort allocation on this task predicts individual differences in anhedonia in a healthy population as well as among depressed individuals (Treadway, Bossaller, Shelton, & Zald, 2012; Treadway et al., 2012; Treadway et al., 2009). We also wished to examine whether or not such deficits in effort allocation could be associated with impaired motor function, so we included a finger tapping test. In addition, we examined the potential influence of antipsychotic medication dosage on effort allocation.
Methods
Participants
Participants were 59 individuals with schizophrenia (N=46) or schizoaffective disorder (N=13) (SCZ) who did not have a major depressive episode in the past year, and 39 healthy controls (CON). These individuals were participating in a larger study on motivation and reward processing in schizophrenia, and the majority of these participants also participated in the study described in (Mann, Footer, Chung, Driscoll, & Barch, in press). Fifty-three of the SCZ were taking antipsychotic medications. Olanzapine equivalents were computed using the method of Gardner et al (Gardner, Murphy, O’Donnell, Centorrino, & Baldessarini, 2010). See Table 1 for demographic and clinical characteristics of the two groups. The Washington University Human Research Protection Office approved the protocol used in this study and participants were compensated for their time and effort.
Table 1.
Participant Demographic, Clinical, and Self-Report Measures
| Characteristics | Group
|
p | |||
|---|---|---|---|---|---|
| Healthy controls (N = 39) | Individuals with schizophrenia (N =59) | ||||
|
| |||||
| M | SD | M | SD | ||
| Demographics | |||||
| Age (years) | 37.4 | 9.2 | 39.3 | 8.12 | 0.27 |
| Sex (% male) | 48.7% | 57.6% | 0.26 | ||
| Ethnicity (% non-Caucasian) | 66.7% | 62.7% | 0.38 | ||
| Education (years) | 13.9 | 2.8 | 12.9 | 2.2 | 0.06 |
| Parental SES | 43.8 | 0.7 | 40.1 | 14.8 | 0.18 |
| Medication status | NA | ||||
| Typical antipsychotics (%) | NA | 5% | |||
| Atypical antipsychotics (%) | NA | 78% | |||
| Typical and atypical (%) | 7% | ||||
| Average Olanzapine | NA | 18.9 | 13.8 | NA | |
| Equivalents (Gardner et al., 2010) | |||||
| Clinical Ratings | |||||
| Positive (SAPS) | 0.03 | 0.2 | 3.9 | 2.8 | <.001 |
| Disorganization (SAPS) | 1.4 | 1.3 | 3.3 | 2.8 | <.001 |
| Negative (SANS) | 1.6 | 2.3 | 8.0 | 3.2 | <.001 |
| Negative (BNSS) | 3.1 | 5.9 | 20.2 | 10.9 | <.001 |
| Personality trait measures | |||||
| Beck Depression Inventory | 2.9 | 4.5 | 11.4 | 11.7 | <.001 |
| Social Anhedonia | 8.3 | 6.2 | 14.9 | 8.0 | <.001 |
| Physical Anhedonia | 10.0 | 5.7 | 18.8 | 9.1 | <.001 |
| Snaith Hamilton Pleasure | 52.8 | 2.9 | 47.2 | 10.0 | .001 |
| TEPS Consummatory | 38.2 | 5.8 | 35.1 | 8.1 | .06 |
| TEPS Anticipatory Pleasure | 49.3 | 5.2 | 46.4 | 8.8 | .046 |
Note. SAPS = Schedule for the Assessment of Positive Symptoms; SANS = Schedule for the Assessment of Negative Symptoms; BNSS = Brief Negative Symptom Scale; The Revised Social Anhedonia Scale is from Chapman et al. (1976), and the Revised Physical Anhedonia Scale is from Eckblad et al. (1982). The Snaith-Hamilton Pleasure Scale is from Snaith et al. (1995). The Temporal Experiences of Pleasure Scale (TEPS) Anticipatory Pleasure is from Gard et al. (2005).
Clinical Ratings
Diagnoses were based on a Structured Clinical Interview for the DSM–IV–TR (First, Spitzer, Gibbon, & Williams, 2001) conducted by one of two Masters level clinicians who participated in regular joint interview and rating sessions involving consensus building discussion to ensure ongoing rating consistency. Exclusion criteria were mental retardation, a major depressive episode or dysthymia within the past year, a substance abuse/dependence disorder within the past 6 months, or a head injury event with neurological sequelae and/or loss of consciousness. Healthy controls were excluded for any personal or family history of psychosis, as well as bipolar disorder. To make contact with the existing literature, clinical symptoms were assessed using the Scales for Assessment of Negative (Andreasen, 1983a) and Positive Symptoms (Andreasen, 1983b), and grouped as follows: positive symptoms (global scores for hallucinations and delusions); disorganization (global scores for bizarre behavior, positive thought disorder, and attention), and the anhedonia and avolition subscales of the SANS. In addition, we also assessed negative symptoms using the more recently developed Brief Negative Symptom Scale (BNSS) (Kirkpatrick et al., 2010; Strauss et al., 2012), with subscales for anhedonia and avolition. To minimize the number of analyses, we z-scored the respective Anhedonia and Avolition scales from the BNSS and the SANS and combined them into a Clinical Anhedonia and a Clinical Avolition scale. The pattern of correlations with clinically rated anhedonia and avolition was essentially identical if we examined associations with the SANS and the BNSS measures separately.
Community Function
We used the Specific Levels of Functioning Scale (SLOF) (Schneider & Struening, 1983) to assess functional status. The SLOF relates to both proxy measures of functional performance and to neuropsychological performance (Bowie et al., 2008). The SLOF assesses the domains of interpersonal relationships, participation in community activities and work skills. We focused on the community and work function subscales, as those seemed most likely to be related to motivation to exert effort. Reports from someone who knows the patient well are more valid than patient reports (Bowie et al., 2007). Thus, we asked an individual with information about the patient’s function to fill out the SLOF (family member, caseworker, therapist). We were able to obtain informant reports for 44 of 59 patients. Patients with and without SLOF informant reports did not differ significantly on age, personal education, parental SES, BNSS or SANS/SAPS scores (all ps>.07, and 9/10 ps > .11) or in the percentage of hard task choices in any of the EEfRT conditions (all ps >.22).
Self-report Measures of Depression, Anhedonia and Pleasure
Participants completed the following individual difference measures: 1) Revised Social (Eckblad, Chapman, Chapman, & Mishlove, 1982) and Physical Anhedonia Scales (Chapman & Chapman, 1978) (higher = more anhedonia); 2) Snaith-Hamilton Pleasure Scale (Snaith et al., 1995) (higher = less anhedonia); 3) Temporal Experience of Pleasure Scale (TEPS) to measure individual differences in anticipatory pleasure and consummatory pleasure (Gard, Germans, Kring, & John, 2006)(higher scores = greater pleasure; 4) The Beck Depression Inventory (A T Beck & Steere, 1987; A. T. Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) to assess depression. We had originally included the Snaith-Hamilton, the TEPS and the Chapman measures of anhedonia to try to assess varied aspects of pleasure and anhedonia. However, when we examined their interrelationships, we were somewhat surprised at the degree of intercorrelation. The TEPS anticipatory and consummatory pleasure scales were strongly correlated (r = .51), and the Snaith-Hamilton was also fairly strongly correlated (r = .36 and r = .46) with the two TEPS measures. As such, to reduce the number of analyses we created two summary scores: 1) an anhedonia measure by Z-scoring and averaging the Chapman Social and Physical Anhedonia measures; and 2) a pleasure measure by Z-scoring and averaging the Snaith-Hamilton Pleasure Scale and the TEPS Anticipatory and Consummatory Pleasure Scales.
EEfRT Task
We used a modification of the Effort-Expenditure for Rewards Task (EEfRT) developed by Treadway and colleagues (Treadway et al., 2012; Treadway et al., 2009; Wardle et al., 2011). On each trial, participants were asked to choose between two different task difficulty levels in order to obtain a monetary award. The easy task was always worth $1, and the hard task was worth between $1.24 and $4.30). The easy task required the participants to make 30 button presses using their non-dominant “pinky” finger within 7 seconds, and the hard task required 100 button presses within 21 seconds. Participants were told that they would not always win the amount of reward associated with the successful completion of their chosen task. Instead, each trial included an indication of the likelihood of “winning” the reward if they completed their task choice successfully. We used two probability levels, either 50% probability or 88% probability. We eliminated the 12% probability level used in the original version of this task because it elicited a relatively low level of hard task choice even in healthy individuals (Treadway et al., 2009). There were an equal number of high and low probability level trials associated with each reward level, and all participants were presented with trials in the same pre-randomized order. Each trial started with a 1-second fixation cross, following by a 5 second “choice” period during which participants were shown information about the probability level associated with that trial and the reward magnitude associated with the hard task. Once participants selected a task, they were shown the word “Ready” for 1 second, and then they completed their task choice (a “counter” on the screen showed their progress). They were then give feedback for 2 seconds as to whether they successfully completed the task, and if so, they were then provided with feedback as to whether they won money for that trial (for 2 seconds). Participants were told ahead of time that two of their trials would be randomly selected for payment at the end of the task, and that they would receive the amount of reward earned on those two trials. Participants completed as many trials as possible in 15 minutes. Participants were provided with standardized instructions and a number of examples of how their choices could influence the amount of money they win and what was meant by variations in probability. All participants experienced trials across all reward magnitude and probability levels.
Finger Tapping Speed
It is possible that an individual’s ability to complete the 100 finger presses within the requisite time might influence their willingness to choose the hard task option. Thus, each participant also completed an assessment of the number of presses they could make with their non-dominant pinky within 15 seconds. Each person completed three trials, with the average used as an estimate of finger tapping speed.
Data Analysis
To simply the analysis of the EEfRT task, the reward levels were grouped into quartiles, with low (<$1.86), medium ($1.96 to < $2.77), high ($2.77 to <$3.58) and highest (>= $3.58) levels. To determine whether there were an adequate number of trials within each cell, we computed percentiles for the number of trials in each of the eight cells (two probability conditions by four reward levels). The 10th percentile for the number of trials in each cell across all conditions and all groups was 6, and the 25th percentile was 6 trials. Thus, we believed we had sufficient trials in each cell. If we were to group reward levels into only low and high, we would have a minimum of 8 trials per cell, with the 10th percentile being 10 trials per cell. Analyses using such a two reward level approach were essentially identical, but we present the analyses using all four reward magnitudes for greater specificity.
The dependent measure for each condition was the percentage of “hard” task choices. The majority of the dependent measures from the EEfRT task were not normally distributed. Thus, we used an Arcsine transformation for the analyses of the percentage of “hard” choices in each condition. These data were analyzed using a mixed model repeated measures ANOVA with group (CON, SCZ) as a between subject factor and both probability (50 and 88%) and reward level (low, medium, high and highest) as within subjects factors. Correlations with clinical and function measures used Pearson’s product moment correlations, as the data were normally distributed.
Results
The groups did not differ in age, gender, ethnicity, or parental education, though as expected the SCZ had lower personal education (Table 1). The SCZ reported significantly greater anhedonia across almost all of the self-report measures. However results for these self-report measures in a largely overlapping subset were previously reported in (Mann et al., in press). The SCZ reported significantly greater levels of depression. Table 1 reports the percentage of individuals taking typical versus atypical antipsychotics, and Supplemental Table 1 provides a detailed breakdown of the percentage of individuals taking each type of antipsychotic medication.
EEfRT Task
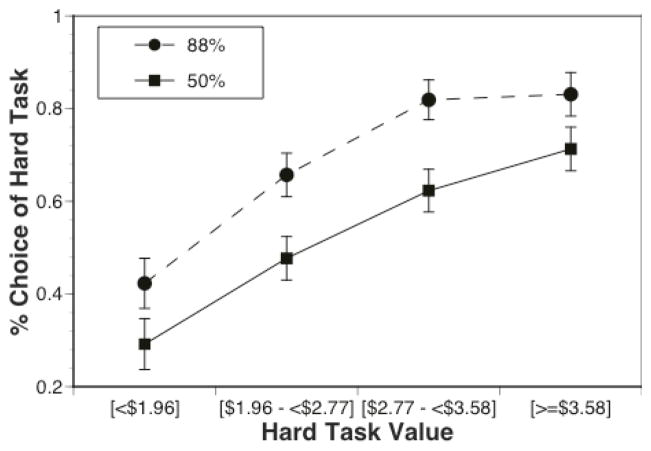
On average, CON choose the hard task on 61% trials (median = 63%, 75 percentile = 77%) and SCZ choose the hard task on 56% of the trials (median 58%, 75 percentile = 67%). Only 3 CON chose the hard task on more than 90% of trials and only 6 SCZ chose the hard task on more than 90% of trials. The repeated measures ANOVA indicated significant main effects of probability (F(1,96)=43.33, p<.001) and reward (F(3,288)=61.87, p< .001), with the likelihood of hard task choices greater in the 88% versus 50% condition and greater in higher reward versus lower reward conditions. There was also a probability by reward interaction (F(3,288)=3.83, p=.01). Posthoc contrasts indicated that, as can be seen in Figure 1, there was a highly significant linear effect of reward in the 50% probability condition (F(1,96)=72.80, p<.001), but only a marginal quadratic effect (F(1,96)=4.23, p=.04). In contrast, there were highly significant linear (F(1,96)=63.65, p<.001), and quadratic effects (F(1,96)=30.71, p<.001) in the 88% condition, reflecting the fact that the likelihood of hard choice selection did not increase from the high to highest reward conditions in the 88% condition, although it did in the 50% condition.
Figure 1.
Percentage of hard task choices as a function of probability and reward magnitude.
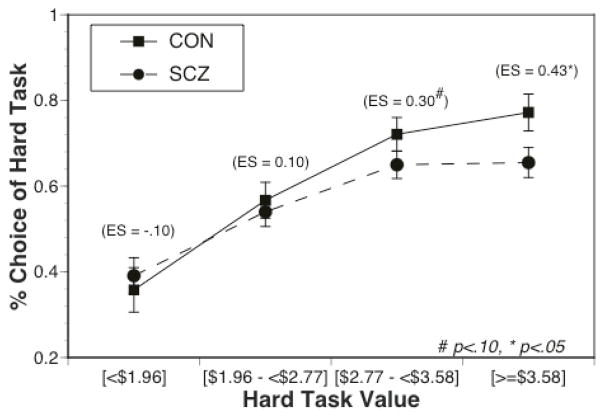
Most importantly, there were also significant interactions between diagnostic group and reward (F(3,288) = 3.30, p < .05) and between diagnostic group and probability (F(1,96)=2.25, p<.05). As can be seen in Figure 2, the effect size of the group difference in hard task choice increased as a function of reward value, with the SCZ (M=.19, SD=.28) showing a significantly smaller increase than CON (M=.28, SD=.21) in hard task choices from the low/medium conditions to the high/highest conditions (F(1,96)=4.45, p<.05). More specifically, individuals with SCZ (M=.65, SD=.25) made significantly fewer hard task choices than CON (M=.75, SD=.22) in the high/highest reward conditions (F(1,96)=4.3, p<.05), but the SCZ (M=.47, SD=.29) did not differ from CON (M=.46, SD=.24) in the low/medium reward conditions (F(1,96)=0.01, p>.90). In addition, the effect size of the group difference in hard task choice is greater in the 88% versus 50% probability condition (see Figure 3), with SCZ (M=.08, SD=.15) showing a significantly smaller increase than CON (M=.16, SD=.20) from 50% to 88% probability (F(1,96)=4.87, p <.05). There was no significant main effect of group (F(1,96)=1.16, p=.28 and no significant three-way interaction (F(3,288)=0.05, p=.98; see Supplemental Figure 1). Of note, both the group by reward and group by probability interactions remained significant if we only included the individuals with schizophrenia (ps < .01 and .05 respectively), and we plot the data separately for individuals with schizophrenia and individuals with schizoaffective disorder in Supplemental Figure 2.
Figure 2.
Percentage of hard task choices as a function of reward magnitude, plotted separately for individuals with schizophrenia and healthy controls. The values in parenthesis are the effect size of the group difference at each reward magnitude.
Figure 3.
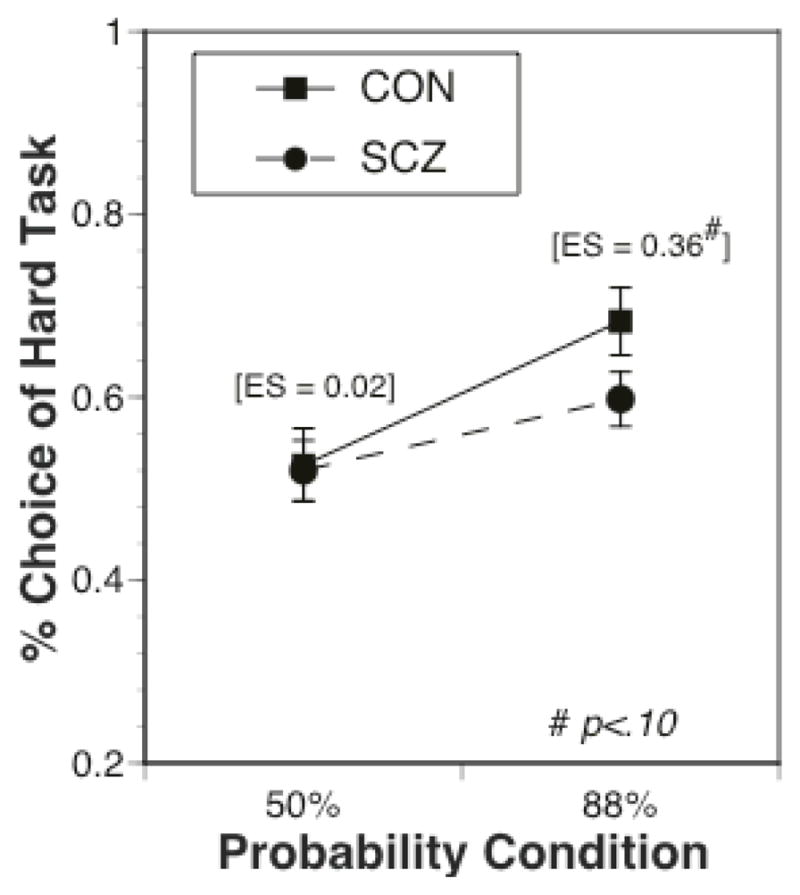
Percentage of hard task choices as a function of reward probability, plotted separately for individuals with schizophrenia and healthy controls. The values in parenthesis are the effect size of the group difference at each reward magnitude. Note, the group X probability interaction was significant, though the group difference at the 88% probability condition was trend level.
The SCZ (M=92.4, SD=11.8) were significantly less likely than CON (M=98.7, SD=2.4) to successfully complete trials (t(96)=3.30, p<.001), though the rate of completion was above 90% for both groups. There was no significant correlation between the number of successfully completed trials and the likelihood of choosing the hard task among CON (r=−.09, p=.60) or SCZ (r =−.05, p=.70). The effects reported above remained significant if outliers in completion rate (e.g., above or below 3 SD from the mean, 2 CON and 6 SCZ) were eliminated from the analysis. SCZ had a significantly slower finger tapping rate than CON (t(1,96)=4.98, p <.001), but there was no significant correlation between finger tapping and the likelihood of choosing the hard task among CON (r=.12, p=.45) or SCZ (r=.07, p=.59).
Correlations with Self-Reported Depression and Hedonic Function
To capture the conditions showing the largest group differences on the EEfRT task, we created the following summary scores: 1) percentage of hard task choices in the 88% probability conditions; 2) percentage of hard task choices in the high and highest reward conditions for both 50% and 88% probability; 3) increase in hard task choices from the 50% to 88% probability conditions; and 4) increase in hard task choices from the low/medium to the high/highest reward conditions. Among CON (see Table 2), the percentage of hard task choices in both the 88% probability conditions and the high/highest reward conditions were strongly negatively associated with depression severity, as was the increase in hard task choice from low/medium to high/highest reward and the increase from 50% to 88% probability conditions. Further, among CON, more severe anhedonia was associated with fewer hard task choices in the high/highest reward conditions and a smaller increase in hard task choices from low/medium to high/highest reward. All but two of these significant correlations survive Bonferroni correction.
Table 2.
Correlations Between Percentage of Hard Task Choices and Self-Report Measures
| 88% Probability | High & Highest Reward | Change from 50% to 88% | Change from Low/Med to High/Highest | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SCZ | CON | SCZ | CON | SCZ | CON | SCZ | |
| Beck Depression Inventory | −.37* | −.05 | −.38** | −.09 | −.30* | −.01 | −.33* | −.09 |
| Chapman Social and Physical Anhedonia | −.24 | .12 | −.46** | .10 | .06 | .11 | −.45** | .05 |
| Pleasure^ | −.08 | .15 | −.16 | −.04 | .06 | .16 | −.13 | .27* |
p<.05;
p<.01
Note: Bold indicates a correlation with significance of p<.05. Underline indicates that the correlation survived Bonferroni correction (p=.05/4 = p<.0125).
Average of Snaith-Hamilton Pleasure Scale, TEPS Anticipatory Pleasure and TEPS Consummatory Pleasure
In SCZ, neither depression nor anhedonia scores were associated with hard task choices. Greater pleasure was associated with a larger increase in hard task choices from the low/medium to high/highest reward conditions, but did not survive Bonferroni correction.
Correlations with Clinician Rated Measures of Symptoms
Among SCZ (Table 3), the severity of Clinical Avolition was negatively correlated with the percentage of hard task choices in the 88% probability condition, and with the increase in hard task choices from 50 to 88% probability conditions, though only the later correlation survived Bonferroni correction. Positive symptoms were also negatively correlated with the increase in hard task choices from 50 to 88% probability conditions, and both positive and disorganization symptoms were negatively correlated with increase in hard task choices from low to high reward values, though these last two correlations did not survive Bonferroni correction. In CON, there was a significant negative correlation between Clinical Avolition the percentage of hard task choices in the high reward level conditions, and a significant negative correlation between Clinical Anhedonia and the change in hard task choices with an increase in reward, though only this last correlation survived Bonferroni correction. The CON showed too few positive and disorganization symptoms to examine correlations for these domains.
Table 3.
Correlations Between Percentage of Hard Task Choices and Clinician Rated Measures
| 88% Probability | High & Highest Reward | Change from 50% to 88% | Change from Low/Med to High/Highest | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SCZ | CON | SCZ | CON | SCZ | CON | SCZ | |
| Clinical Anhedonia& | −.06 | .08 | −.19 | .17 | −.13 | −.09 | −.42** | .13 |
| Clinical Avolition% | −.24 | −.23* | −.32* | −.13 | −.06 | −.31** | −.27 | −.01 |
| SANS/SAPS Disorganization | NA | −.02 | NA | −.12 | NA | −.12 | NA | −.26* |
| SAPS/SANS Positive | NA | −.18 | NA | −.17 | NA | −.36** | NA | −.20# |
p<.10
p<.05;
p<.01
Combination of SANs Global Anhedonia and BNSS Anhedonia
Combination of SANS Global Avolition and BNSS Avolition
Note: Bold indicates a correlation with significance of p<.05. Underline indicates that the correlation survived Bonferroni correction (p=.05/4 = p<.0125).
Correlations with Informant Rated Functional Status
As shown in Figure 4, among SCZ, better community function was associated with more hard task choices in the 88% probability conditions (r=.36, p<.01, passing Bonferroni correction) and nominally with the high/highest reward conditions (r=.29, p<.05), but not with the percent increase scores (rs of .09 and −.05 respectively). Similarly, better work function was associated with more hard task choices in the 88% probability conditions (r=.38, p<.01, passing Bonferroni correction) and nominally the high/highest reward conditions (r=.34, p<.05), but not with the percent increase scores (rs of −.03 and −.06 respectively). Because the function measures were not associated with change scores, we also examined whether they were correlated with the percentage of hard task choices in the 50% probability and/or the low/medium reward conditions. Community function was nominally correlated with hard task choices in the low/medium reward conditions (r=.28, p<.05), with a similar trend for the 50% probability conditions (r=.24, p=.06), Similarly, work function was nominally correlated with hard task choices in the low/medium (r=.32, p<.05) and 50% probability conditions (r=.33, p<.05). Taken together, these results suggest that work and community function were associated with an overall reduction in hard task choices given the similar magnitude of the correlations across both high and low probability conditions and high and low reward magnitude conditions.
Figure 4.
Graphs illustrating the relationships between SLOF community and work function and the percentage of hard task choices in the 88% probability and high reward conditions. Correlation values are on each group, with * = p<.05 and ** p<.01.
Correlations with Antipsychotic Olanzapine Equivalents
There were no significant correlations between olanzapine equivalents and the percentage of hard task choices in the 88% probability condition (r=−.22, p=.10), the high/highest reward conditions (r=.03, p=.84), or in the change in hard task choice from the 50% to 88% condition (r=−.09, p=.70). There was a positive correlation with the percent change in hard task choice from the low/medium to high/highest reward condition (r=.37, p<.05), indicating that a higher antipsychotic olanzapine equivalent dosage was associated with a larger increase in hard task choice in the high/highest reward condition. This finding runs counter to the idea that antipsychotics might blunt modulation of effort and vigor. Further, all of the correlations between negative symptoms, work function and community function remain when covaring for olanzapine equivalents. In addition, analyses comparing just the six unmedicated individuals with schizophrenia to controls also showed a significant diagnostic group X reward magnitude interaction (F(3,129)=4.9, p<.01), with a lower percentage of hard choices in unmedicated patients compared to controls at the two highest reward magnitudes.
Discussion
The goal of the current study was to examine effort allocation in response to different reward magnitude and reward probabilities using the Effort-Expenditure for Rewards Task (EEfRT). On average, individuals with schizophrenia were less likely than healthy participants to choose the harder task when the reward magnitudes were high and when the likelihood of receiving the reward was high. Further, this could not be accounted for by impaired motor function. Consistent with prior literature, among controls, the likelihood of choosing the hard task in the high reward magnitude and high reward probability conditions was strongly negatively associated with depression severity. Further, greater Chapman anhedonia scores in controls were associated with fewer hard task choices in the high reward condition. In patients, worse avolition was associated with a smaller increase in hard task choices as probability of reward increased. Most importantly, among patients, worse community and work function was associated with an overall reduction in effort allocation. The observation of decreased effort allocation during high reward magnitude and high reward probability conditions replicates prior findings (Fervaha et al., 2013; Gold et al., 2013), using both the same task (Fervaha et al., 2013) and a different but conceptually similar task (Fervaha et al., 2013; Gold et al., 2013). Importantly, in both our study and in the study by Gold et al., greater deficits in effort allocation were associated with worse negative symptoms, and in the previous study by Fervaha, there was an association to apathy (Fervaha et al., 2013). This represents a strong degree of replication across studies, and clearly points to an important role for effort allocation in the negative symptoms of schizophrenia. However, in the current study, we found that reduced effort allocation as a function of probability was also associated with worse positive symptoms.
As described in the introduction, our results fit with prior findings of reduced effort allocation in D2 overexpressing mice (Drew et al., 2007; Ward et al., 2012), particularly the finding that poor community and work function was associated with overall reduced effort allocation. In addition, the aberrant salience model predicts that schizophrenia patients may exhibit dysregulated firing of dopamine neurons (Heinz & Schlagenhauf, 2010), which may result in both excessive firing to non-predictive reward cues as well as attenuated activity to relevant information. This hypothesis has been supported by a well-characterized animal model of schizophrenia—the mitotoxin methylazoxymethanol acetate (MAM) model (Lodge & Grace, 2009)—where altered signaling within a hippocampal-VTA circuit drives elevated striatal dopamine release as well as impaired dopaminergic coding of reward-relevant cues (Lodge & Grace, 2008, 2009, 2011). Consistent with this hypothesis, we did not observe a main effect of group across all trials, but observed our strongest effects in the extent to which patients and controls modulated their effort in terms of condition context. Further, the severity of negative symptoms was associated with diminished sensitivity to probability level in terms of effort allocation. Thus, our data are consistent with the hypotheses that several aspects of impaired dopamine function in schizophrenia contribute to negative symptoms and impaired community and work function. It is interesting however that negative symptoms were associated with a diminished responsivity to the increase in likelihood of receiving reward (e.g., probability) but not with sensitivity to changes in reward magnitude. It is not clear why this was so, though it is possible that it reflects a mediating effect of cognitive function, given that negative symptoms are frequently associated with impaired cognitive function (Ventura, Wood, & Hellemann, 2011), and at least some aspects of cognitive function may be particularly relevant to computing probability. Unfortunately, we did not measure cognitive function in the current study and thus this is a hypotheses that will need to be tested in future work. Our results are also potentially consistent with the hypothesis, but forth by Gold and colleagues (Brown et al., 2013; Gold et al., 2013; Gold, Waltz, Prentice, Morris, & Heerey, 2008), that motivational impairments in schizophrenia may reflect abnormalities in the appropriate “valuation” of potentially rewarding outcomes. If so, then in the individuals with schizophrenia may have failed to increase their effort allocation as reward magnitude increased and as reward probability increased because they did not appropriately increase the value associated with changes in these dimensions of the outcomes.
These findings also extended the results of previous studies by showing that effort allocation was related to work and community function in schizophrenia. This result provides important validation of laboratory effort-tasks as measures of real-world motivation, given that intact work and community function require the ability to expend effort in order to seek and maintain a vocation, as well as to participate in a range of community events. Although individuals with schizophrenia enjoy the positive outcomes associated with work (e.g., money) and community function (e.g., social interactions, consumable goods, etc.) difficulties modulating effort allocation in order to obtain those positive outcomes can clearly impede functional status in this illness. It is interesting to speculate as to possible interventions that may help to remediate such impairments in effort allocation, and thus potentially improve work and community function. For example, the work of Kring and colleagues suggests that emotional responses in schizophrenia may not be sustained when stimuli are not present in the immediate environment (Kring, Germans Gard, & Gard, 2011; Ursu et al., 2011). This is in keeping with the proposed impairment of dopaminergic reward coding discussed above. If true, environmental supports or cues that remind individuals with this illness about rewarding outcomes “in-the-moment” may increase the likelihood of increased effort allocation to engage in the behaviors necessary to eventually achieve such goals. While speculative, this hypothesis helps translate these laboratory findings into a novel intervention for amotivational symptoms in schizophrenia. However, this speculation should be moderated by the fact that many of the correlations between hard task choice and clinician rated and self-reported measures of anhedonia and amotivation were non-significant. It is not entirely clear why this was so, as there was adequate range on the clinical and self-report measures in the patient group.
There are a number of potential limitations to our results. The first is that patients completed fewer trials than controls. However, there was no relationship between the number of trials completed and the likelihood of choosing the hard task in either group, and all results held with outliers for completion rate were excluded. Such results reduce the likelihood that differential difficulty of button pressing in patients versus controls might be confounding the findings, but do not rule out this possibility. However, if patients simply found the button pressing more difficult, one might have expected a main effect of overall lower hard task choice, rather than specific interactions with reward magnitude and probability. Second, one might be concerned that the meaning or “value” associated with the amount of money might be different across groups. For example, the fact that the patients had lower personal SES could mean that the same amount of money had a higher value to them. However, this would actually predict the opposite results from what we found, namely a greater tendency to choose the hard task when you have a higher reward magnitude among patients. Another limitation to our findings is the potential effect of D2 blockade by antipsychotic drugs, given evidence in rats that D2 antagonists can reduce willingness to work for rewards (Randall et al., 2012). However, we found no relationship between increased antipsychotic dosage and reduced effort allocation – in fact we found evidence of the opposite relationship.. In addition, we found that our small subset of unmedicated patients showed reduced effort allocation in the high probability and high reward magnitude conditions. Taken together, these findings argue against reduced effort allocation being the result of antipsychotic medications. However, there are numerous issues with using antipsychotic equivalents to assess medication effects, and the absence of a relationship does not rule out the potential influence of antipsychotics Thus, further work in a larger sample of unmedicated patients and/or in unmedicated high risk individuals (e.g., relatives) will be needed to more clearly rule out this potential confound.
In summary, the current study adds to the literature on mechanisms of functional impairment in schizophrenia by demonstrating that impaired work and community function in schizophrenia is related to a reduced ability to allocate effort in order to obtain monetary gain. This deficit is consistent with the animal literature suggesting that motivational deficits in schizophrenia may partially reflect alterations in mesolimbic dopamine systems. Future research will be needed to identify the precise role of dopamine function as a substrate for these symptoms, as well as examine potential methods by which improving effort allocation in schizophrenia may enhance work and community function.
Supplementary Material
Acknowledgments
The authors would like to thank the participants in this study, who gave generously of their time. Funding for this study was provided by NIMH MH066031.
Footnotes
Author DMB had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. DMB consults for Pfizer, Amgen and Roche on projects related to the treatment of cognition and motivation in psychosis.
Contributor Information
Deanna M. Barch, Departments of Psychology, Psychiatry, and Radiology, Washington University
Michael Treadway, Center for Depression, Anxiety and Stress Research, McLean Hospital/Harvard Medical School.
Nathan Schoen, Department of Psychology, Washington University.
References
- Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, O’Flynn K, Koenigsberg HW, Van Heertum R, Cooper T, Laruelle M, Siever LJ. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55(10):1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A, 97. 2000;14:8104–8109. doi: 10.1073/pnas.97.14.8104. 97/14/8104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65(12):1091–1093. doi: 10.1016/j.biopsych.2008.12.007. S0006-3223(08)01591-6 [pii] [DOI] [PubMed] [Google Scholar]
- Aghevli MA, Blanchard JJ, Horan WP. The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Res. 2003;119(3):261–270. doi: 10.1016/s0165-1781(03)00133-1. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) University of Iowa; 1983b. The scale for the assessment of positive symptoms (SAPS) [Google Scholar]
- Assadi SM, Yucel M, Pantelis C. Dopamine modulates neural networks involved in effort-based decision-making. Neuroscience and biobehavioral reviews. 2009;33(3):383–393. doi: 10.1016/j.neubiorev.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. sbq068 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steere RA. Beck Depression Inventory Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. S0006-3223(07)00506-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Twamley EW, Anderson H, Halpern B, Patterson TL, Harvey PD. Self-assessment of functional status in schizophrenia. J Psychiatr Res. 2007;41(12):1012–1018. doi: 10.1016/j.jpsychires.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JK, Waltz JA, Strauss GP, McMahon RP, Frank MJ, Gold JM. Hypothetical decision making in schizophrenia: The role of expected value computation and “irrational” biases. Psychiatry research. 2013 doi: 10.1016/j.psychres.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The revised physical anhedonia scale, unpublished test. Madison: University of Wisconsin; 1978. [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia bulletin. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The revised social anhedonia scale. 1982. [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. Neural substrates underlying effort computation in schizophrenia. Neuroscience and biobehavioral reviews. 2013;37(10 Pt 2):2649–2665. doi: 10.1016/j.neubiorev.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. Journal of psychiatric research. 2013 doi: 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV-TR Axis I disorders. Washington, D. C: American Psychiatric Press; 2001. [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Frontiers in neuroscience. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, affective & behavioral neuroscience. 2008;8(4):375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part I: meta-analysis of dopamine active transporter (DAT) density. Schizophrenia bulletin. 2013a;39(1):22–32. doi: 10.1093/schbul/sbr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophrenia bulletin. 2013b;39(1):33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Germans M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Personality Research. 2006;40:1086–1102. [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American journal of psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative Symptoms of Schizophrenia Are Associated with Abnormal Effort-Cost Computations. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Collins AG, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of general psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophrenia bulletin. 2010;36(3):472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The Brief Negative Symptom Scale: Psychometric Properties. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq059. sbq059 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Elis O. Emotion Deficits in People with Schizophrenia. Annual review of clinical psychology. 2012 doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. Journal of abnormal psychology. 2011;120(1):79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dolan RJ. Dopamine and effort-based decision making. Frontiers in neuroscience. 2011;5:81. doi: 10.3389/fnins.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophrenia research. 2012;142(1–3):65–70. doi: 10.1016/j.schres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotoxicity research. 2008;14(2–3):97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behavioural brain research. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in pharmacological sciences. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM. Spared and impaired aspects of motivated cognitive control in schizophrenia. Journal of Abnormal Psychology. doi: 10.1037/a0033069. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PloS one. 2012;7(10):e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in behavioral neuroscience. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. SLOF: A behavioral rating scale for assessing the mentally ill. Social Work Research Abstracts. 1983;19:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry : the journal of mental science. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Soliman A, O’Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(8):2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: Validation of the Brief Negative Symptom Scale. Schizophrenia research. 2012;142(1–3):88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. Journal of abnormal psychology. 2012;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS one. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. The American journal of psychiatry. 2011;168(3):276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Wood RC, Hellemann GS. Symptom Domains and Neurocognitive Functioning Can Help Differentiate Social Cognitive Processes in Schizophrenia: A Meta-Analysis. Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. S0006-3223(06)01239-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25(1):86–97. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93(1–3):296–303. doi: 10.1016/j.schres.2007.03.010. S0920-9964(07)00120-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, Kandel ER, Balsam PD. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(7):1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, Doop M, Kessler RM, Zald DH. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. The American journal of psychiatry. 2011;168(4):418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.