Abstract
In an industrialized society, the increase in obesity incidence has led to an increase in premature morbidity and mortality rates. There is a relationship between body mass index (BMI) and the increased incidence of hypertension, dyslipidemia, type 2 diabetes, and cardiovascular disease, leading to mortality. However, obese individuals with these conditions may have better outcomes than their lean counterparts, thus the term “obesity paradox.” Most studies supporting this paradox are cross-sectional and do not take into account the quantity or type of adiposity, the disease severity and comorbidities. While BMI is an indicator of the amount of body fat, it does not differentiate between adiposity types. Adipocytes that are highly functional and have good fuel storage capacity are different from adipocytes found in visceral obesity, which are poorly functioning, laden with macrophages and causing low grade inflammation. Individuals with high BMI may be physically fit and have a lower mortality risk when compared to individuals with a lower BMI and poorly functioning adiposity. We review the complexity of adipose tissue as well as its location, function, metabolic implications and role in cardiovascular morbidity and mortality. The terminology ‘obesity paradox’ may reflect a lack of understanding of the complex pathophysiology of obesity and the association between adiposity and cardiovascular disease.
Keywords: Obesity, Overweight, Obesity Paradox, Cardiovascular Disease, Diabetes
The incidence of obesity and diabetes is increasing rapidly worldwide. Despite the high incidence of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) in obese individuals, those with a higher body mass index (BMI) may survive longer when compared to their counterparts with a lower BMI, thus the term “obesity paradox”.1, 2 In observational studies, BMI measurements are regularly used to define obesity. However, BMI is not a direct measure of body fat content and certainly is not an indicator of the potential harmful effects that adiposity can cause. Increased adipose tissue can potentially play a protective role (“good adiposity”). More often adipose tissue is harmful (“bad adiposity”), causing metabolic abnormalities and a low inflammatory state, both important components of the metabolic syndrome (MetSyn). It is difficult to determine true adiposity by using BMI measurements alone. The term “obesity paradox” is rooted in the fact that BMI is considered to be a measurement of both obesity and adiposity, but in fact it may not distinguish between “good” and “bad” adiposity. Understanding the complex effects of body fat composition, distribution, type of fat, and function, represents a first step towards appreciating the multifaceted relationship that exists between BMI, adiposity, morbidity and mortality.
WHY MORE OBESITY?
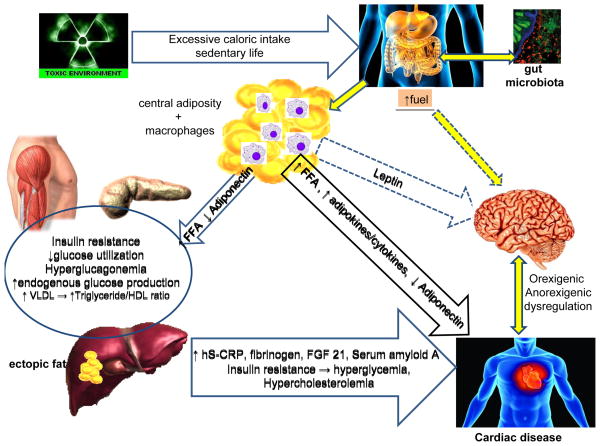
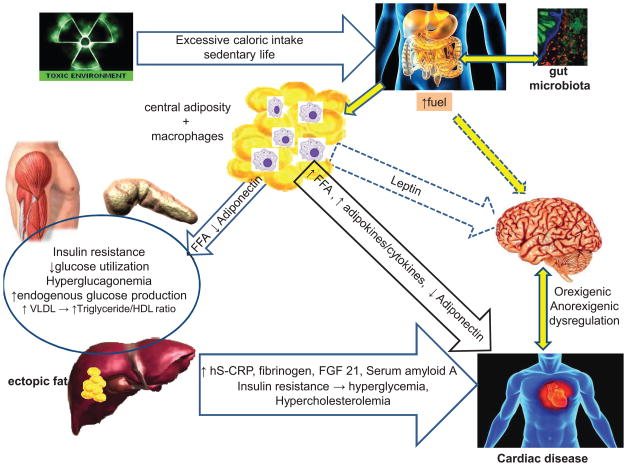
The prevalence of obesity in the United States has continued to increase dramatically as in other developed industrialized societies, a trend that now appears to be leveling off.3 The cause of the increased rate of obesity is multifactorial, driven by an interplay of genetic and environmental factors.4 Genes have a particularly strong impact as evident by the clustering of obesity among certain families and racial groups. An individual with “genetic susceptibility” for obesity who is exposed to a “toxic environment” is more likely to gain weight and be at higher risk for premature morbidity and mortality.4,5 A “toxic environment” is not limited to excessive caloric intake and a sedentary lifestyle. It comprises many other variables that include geographic location (such as certain southern states known as the “obesity belt” or living in neighborhoods with higher rates of obesity),6 socioeconomic status and education,7 stress8, work schedules, air pollutants,9 sleep habits,10,11 and processed foods. The rapid increase in obesity and the high incidence of T2DM affecting a younger population, suggest that epigenetic changes may also have a role. These are characterized by gene expression and/or cellular phenotype caused by mechanisms other than alterations of the DNA sequence. These inheritable modifications contribute to the incessantly high incidence of obesity in a younger population.12,13 In addition, the excessive caloric intake in obese individuals is not well regulated by the brain. As illustrated in Figure 1, the dysfunctional resistance to excessive fuels and hormones such as leptin and insulin prevents the expected satiety and appetite suppression,14 leading to excessive caloric intake and weight gain.14,15
Figure 1. Illustration of complex and multi-organ relationship between excessive nutrients and neuronal dysregulation responsible for obesity and cardiovascular disease.
Excessive caloric intake, along with gut microbiota, play a role in obesity and CVD. Visceral adiposity is characterized by increased adipose mass and macrophages that together produce pro-inflammatory adipokines and cytokines adversely affecting the cardiovascular system. Increased visceral adiposity also causes hyperleptinemia and hyperinsulinemia that are poorly regulated by the brain, perpetuating increased caloric intake. Insulin resistance and the increased FFA flux cause dyslipidemia with increased VLDL, triglycerides and decreased HDL-cholesterol which together with chronic low-grade inflammation results in CVD.
CVD, cardiovascular disease; hS–CRP, highly sensitive C-reactive protein; FFA, free fatty acid; FGF 21, fibroblast growth factor 21; HDL, high density lipoprotein; VLDL, very low density lipoprotein
IS BMI A GOOD INDICATOR OF OBESITY?
Obesity, defined as a condition of excess body fat (adiposity), is detrimental to health.16 For adults, being overweight or obese is defined by using weight and height to calculate a statistical measurement called BMI. This prevalent method is used for population assessment of overweight and obesity and correlates with the overall body adiposity (as measured by hydrodensitometry). Since it only requires height and weight measurements, it is pragmatic, inexpensive, and commonly used. Although BMI includes consideration of body weight, it does not measure the percentage component of body fat, the type of fat, where in the body it is located, nor the degree of metabolic disturbances that it can cause. Because of this, other techniques have been used for estimating body fat and body fat distribution, such as measurements of skinfold thickness, waist circumference, waist-to-hip circumference ratio, and imaging techniques (i.e., ultrasound, computed tomography, and magnetic resonance imaging). These measurements are less practical, some are expensive, and therefore are used mainly for research purposes. It is important to remember that BMI represents just one indicator of potential health risks, and other parameters are necessary to estimate CVD risk. For instance, the National Heart, Lung and Blood Institute guidelines recommend at least two other predictors, such as blood pressure, serum lipid levels, and/or physical activity levels.17
Obesity does not imply good health as the majority of obese individuals have the MetSyn. Also, obese individuals have a high incidence of comorbidities such as T2DM, CVD, and cancer, among others. Obesity is a spectrum of distinct subtypes described in the literature and summarized in Table 1.18–22 It is estimated that approximately 25% of obese individuals can be metabolically healthy,18,23,24 and although they have a high BMI, they are not insulin resistant,19 have normal lipid profiles, and in general, favorable CVD risk.25 Alternatively, approximately 23% of the normal weight adult population is “metabolically obese normal-weight”,24 a term coined nearly 40 years ago.23 The “metabolically obese normal-weight” population characterizes individuals with a normal BMI who are insulin resistant and have dyslipidemia, similar to unhealthy obese individuals. The dyslipidemia of obesity is characterized by elevated triglycerides and low levels of high density lipoprotein (HDL). These individuals tend to have lower physical activity energy expenditure and increased visceral adiposity, and are at high risk for CVD even while having a lower BMI.24,26–29
Table 1.
Characteristics of normal weight, overweight and obese individuals as determined by BMI
| Normal Weight (BMI 18.5–24.9 kg/m2) | Overweight (BMI 25.0–29.9 kg/m2) and Obese (BMI ≥ 30 kg/m2) | ||||
|---|---|---|---|---|---|
| Healthy Normal Weight (HNW) | Metabolically Obese Normal Weight (MONW) | Metabolically Healthy Obese (MHO) | Metabolically Unhealthy Obese (MUHO) | ||
| Metabolic Syndrome | Visceral obesity Hypertension Dyslipidemia Hyperglycemia/Diabetes |
Decreased Decreased No No |
Increased Increased Yes High risk |
Decreased Decreased No High risk |
Increased Increased Yes High risk |
| Adiposity | White/Brown ratio Leptin/Adiponectin ratio Inflammatory Adipokines |
Decreased Decreased Decreased |
Increased Increased Increased |
Decreased Increased Decreased |
Increased Increased Increased |
| Morbidity | Low | Increased | At risk | Increased | |
| Mortality | Low | Increased | Increased | Increased | |
The MetSyn is defined when at least three of the following criteria are present:30 i) waist circumference is ≥102 cm in males and ≥88 cm in females; ii) serum triglycerides are ≥150 mg/dL or receiving drug treatment for elevated triglycerides; iii) serum HDL is <40 mg/dL in men and <50 mg/dL in women or receiving drug treatment for low HDL levels; iv) blood pressure is ≥130/85 mmHg or receiving drug treatment for hypertension; or v) fasting blood glucose (FBG)is ≥100 mg/dL or receiving drug treatment for elevated blood glucose. Because visceral obesity may be phenotypically deceiving, an alternate set of criteria for the MetSyn is used by the International Diabetes Federation. These criteria include ethnicity-specific central or visceral obesity plus two other factors: i) triglycerides >150 mg/dL or receiving treatment for elevated triglycerides; ii) HDL <40 mg/dL in men or <50 mg/dL in women, or receiving treatment for low HDL; iii) blood pressure >130/85 or receiving drug treatment for hypertension; or iv) FBG >100 mg/dL or previously diagnosed T2DM.31 In summary, BMI alone is not a good indicator of CVD risk. The “bad adiposity”, the MetSyn and/or T2DM are all critical conditions that should be considered for the increased CVD morbidity and mortality rates.
ADIPOSITY - THE GOOD AND THE BAD
Elevated BMI alone may not be indicative of the type of adiposity or risk for CVD. As mentioned previously, “good adiposity” is characterized by adipocytes with an efficient fuel storage capacity, and “bad adiposity” is characterized by adipocytes that are inefficient in fuel storage and associated with inflammation. The majority of overweight and obese individuals have “bad adiposity” that plays a crucial role in the development of MetSyn, CVD, and an increased mortality rate.
The adipose tissue has complex endocrine functions and is a major source of pro-inflammatory and anti-inflammatory adipokines, which play key roles in the related comorbidities.32 Further, adipose tissue has important structural and functional roles. For instance, epicardial fat is metabolically active and produces factors that modulate both cardiac structure and function with clinical implications.33 The adipose tissue pool is composed of at least two functionally distinct types, namely white and brown adipose tissue (WAT, BAT). WAT plays an important role in energy storage and in secretion of hormones and cytokines that have an impact on appetite regulation, metabolism, insulin resistance and vascular disease.34 WAT also serves as a thermal insulator and protects organs from mechanical damage. BAT differs in that instead of storing energy, it dissipates chemical energy by non-shivering thermogenesis. This process takes place via mitochondrial uncoupling mediated by expression of tissue-specific, mitochondrial and uncoupling protein 1 (UCP1).34,35 BAT is especially abundant in newborns and hibernating mammals. Until recently it was thought to be scarce in adults, but was incidentally found in the supraclavicular and neck regions while using functional positron emission tomography.36 Brown fat cells can also form “beige cells” or “recruitable brown fat cells”.37 The transformation of WAT to beige cells takes place through Irisin (named after Iris, the Greek messenger goddess), a membrane polypeptide produced by skeletal muscle.38 Beige adipocytes have low basal expression of UCP1 and can respond to cyclic AMP stimulation with increased UCP1 expression.37 Therefore BAT and beige adipocytes are “good fat” that can burn calories and cause weight loss. Transformation of WAT to beige cells may also hold therapeutic potential in reducing obesity and improving glucose homeostasis.
The function of WAT varies by location in the body. In humans, adipose tissue can be found beneath the skin (subcutaneous adipose tissue), around internal organs (visceral or central adipose tissue), in bone marrow (yellow bone marrow), and in breast tissue. Insulin resistance and inadequate fuel storage capacity of the adipocytes cause a “spill-over” effect leading to accumulation of ectopic fat in non-adipose tissues. This aberrant fat accumulation correlates with an unhealthy constellation of abnormalities that are associated with MetSyn.39,40 Visceral obesity is linked to increased cytokine production, elevated free fatty acid (FFA) flux, insulin resistance and gluconeogenesis.41 Insulin resistance and elevated FFA flux in the portal system induce an inappropriate gluconeogenesis as well as increased levels of very low density lipoprotein (VLDL).41 The large VLDL pool found in insulin resistance is affected by cholesteryl ester transfer protein (CETP) promoting transfer of CEs from HDLs to the pro-atherogenic apolipoprotein B. In individuals with CETP deficiency, the increased HDL levels and decreased LDL levels result in longevity. A mirror image takes place in individuals with the MetSyn having a high CETP expression that results in high triglycerides and low HDL levels, with increased CVD disease.
Ectopic fat in liver (intrahepatic triglyceride content) is an important phenotypic marker of adverse metabolic consequences.21,42,43 More recently, epicardial fat has also been used as a reliable marker of visceral adiposity.33 Thus the type of adipocyte (WAT versus BAT), its metabolic activity, production of adipokines, and location has important clinical implications in obesity and supports the concept of healthy “good adiposity” versus unhealthy “bad adiposity.”
ADIPOSE TISSUE AS AN ENDOCRINE ORGAN
Adipose tissue was recognized as a metabolically active endocrine organ soon after the discovery of leptin,34, a hormone that modulates food intake by suppressing orexigenic neuropeptides such as agouti-related protein, neuropeptide Y, and gamma-aminobutyric acid, while at the same time upregulating the anorexigenic neuropeptide α-melanocyte-stimulating hormone. Leptin also serves as a barometer of adiposity levels and provides feedback to the brain. By this mechanism, among others, leptin regulates the dynamic balance between appetite and energy reserves, a function that is lost in individuals with obesity as their central nervous system (CNS) becomes leptin resistant, perpetuating a poorly regulated high caloric intake.
In addition to its CNS regulatory role, leptin has a direct beneficial effect on the heart by decreasing the accumulation of lipid intermediates, that lead to cellular dysfunction and cell death.44 In the ischemic heart, leptin has also been found to reduce infarct size by delaying the opening of mitochondrial permeability,45 a protective pathway proposed in reperfusion injury.46 In contrast, leptin can cause atherogenesis via induction of endothelial dysfunction, upregulation of inflammatory mediators, and proliferation of vascular smooth muscle cells.47 The beneficial effects may be decreased or abolished in individuals with MetSyn. Due to the multiple effects of leptin, it is difficult to dissect the role that leptin plays in CVD. Some believe that it is just a biomarker of vascular dysfunction.48
Adiponectin is another adipokine with an important CVD impact. While only produced by fat, adiponectin levels are inversely related to the volume of fat mass.49 In contrast to leptin, its levels are decreased in proportion to fat mass.50 Adiponectin, being a “good adipokine”, improves insulin sensitivity through activation of AMP protein kinase in the liver and skeletal muscle,51 and reduces hepatic gluconeogenesis.52 It also has important anti-atherogenic properties, such as modulating endothelial inflammatory responses, suppressing expression of macrophage scavenger receptors, and inducing conversion from macrophage to foam-cells.53,54
In addition to leptin and adiponectin, fibroblast growth factor 21 (FGF21), has recently surfaced as an important metabolic hormone. It is predominantly produced by the liver and expressed in adipocytes and in the pancreas. It regulates glucose and lipid metabolism through the CNS and has favorable pleiotropic actions. FGF-21 stimulates gluconeogenesis, fatty acid oxidation, and ketogenesis as an adaptive response to the fasting state, and acts as an autocrine factor in adipocytes during the fed state. It has been shown to confer benefits on insulin sensitivity, blood glucose, lipid profile and body weight in the obese. High circulating levels of FGF21 can be found in obesity, Met-Syn, T2DM, and coronary artery disease, suggesting FGF21 resistance.55 An increased plasma FGF-21 level is especially noted in obese individuals with fatty liver and can be used as a good biomarker of hepatic fat content.56
ADIPOSE TISSUE AND INFLAMMATION
Adipose tissue is a major source of pro-inflammatory and anti-inflammatory adipokines as depicted in Table 2.32 Increased adipose tissue mass and adipocyte hypertrophy leads to macrophage infiltration and inflammation. Adipocytes express a multitude of molecular receptors to which pathogens and inflammatory signals bind. Through activation of nuclear factor-κB, adipocytes induce secretion of inflammatory cytokines.57 In addition, macrophages that are abundant in visceral adiposity act independently or synergistically with adipocytes, producing and regulating inflammatory cytokines.32,58,59 The consequence is a chronic low-grade inflammation that plays an important role in the pathogenesis of insulin resistance, and is clinically detected by elevated circulating levels of highly sensitive C-reactive protein.60 Many other pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, among others, (Table 2) are involved in the inflammatory process and have been shown to play an important role in the pathogenesis of CVD.61
Table 2.
Adiposity and inflammation
| Pro-inflammatory | Anti-inflammatory | |
|---|---|---|
| Hormones/Adipokines/Cytokines | Leptin Nuclear factor –κB Tumor necrosis factor- alpha Interleukin-1β Interleukin-6 Interleukin-8 Interleukin-10 Interleukin-15 Complement factors B, C3, D Haptoglobin Serum amyloid A3 Macrophage migration inhibitory factor Leukemia inhibitory factor Prostaglandin E2 Plasminogen activator inhibitor-1 |
Adiponectin Interleukin-13 Interleukin-4 Janus kinase 1 Fibroblast growth factor 21 |
Thus, a complex and multi-organ relationship exists in obesity consisting of excessive nutrient fuels, hyperleptinemia, hyper-insulinemia, and poor regulation by the brain, perpetuating obesity. The state of insulin resistance increases glucose levels through an inappropriate and excessive hepatic glucose production and decreased insulin-mediated peripheral glucose uptake that can eventually result in diabetes. At the same time, the insulin-resistant state contributes to the abnormal lipid profile. Furthermore, a state of chronic inflammation also plays a role in the proatherogenic process with increased CVD as depicted in Figure 1.
GUT MICROBIOTA
Genes and environmental factors interact by modulating biologic processes and dietary elements (Figure 1). The gut metagenome is critical to this interaction, and changes in the microbial population can lead to alterations in normal metabolism that can potentially promote obesity, MetSyn and T2DM.62 Gut flora consists of approximately 100 trillion microorganism species living in the digestive tract,63 with bacterial metabolic activities resembling those of an individual. Interaction between the genome and microbiome64 leads to production of an array of metabolites that can affect the metabolism of both host and microbiota.65 Obesity and diabetes are associated with an altered gut microbial composition in mice66 and humans.67, 68 Gut microbiota has also been found to modulate lipid metabolism and inflammation 69–71 via lipopolysaccharides and peptidoglycans with potential atherogenesis.72 Emerging data show the importance and interdependency between gut microbiota and diseases, and remains an area of active research.
CAN THE OBESITY PARADOX BE EXPLAINED BY INCREASED BMI WITH ABSENCE OF THE METABOLIC SYNDROME?
Measurement of BMI alone does not provide information regarding the amount or type of adiposity. It is the type of adiposity, its location, function and the resultant degree of inflammation that has deleterious consequences on cardiovascular health. Obese and non-obese individuals who are metabolically healthy have lower levels of inflammatory markers such as complement component 3, C-reactive protein, interleukin-6, tumor necrosis factor-alpha, plasminogen activator inhibitor-1, and higher levels of adiponectin compared with their metabolically unhealthy counterparts.61 Therefore it is the amount of “bad fat,” and not BMI per se that is important.73 In a large study, a BMI of 22.5–25 kg/m2, the so called “sweet spot”, showed a low mortality rate (Figure 2). Above this range, each 5 kg/m2 increase in BMI was associated with an approximate 30% higher all-cause mortality, while individuals with low BMI also had increased mortality,74 thus a “J” or “U-shaped” curve exists for BMI. While the paradox exists in overweight and obese individuals, few studies have examined this in the more severely obese population, such as those with BMI >35 kg/m2. These individuals do not appear to have the obesity paradox; instead they have poor outcomes and a high mortality rate.75,76 In fact, the morbidly obese (defined as BMI 40 – 45 kg/m2) may have about 8–10 years decreased survival rate.74 Therefore, the obesity paradox that has been described in the overweight and obese individuals does not apply to the morbidly obese.
Figure 2. Proposed relationship between body mass index and mortality rate.
The J-shaped curve illustrates increased mortality rate with a high body mass index (BMI) and with a very low BMI. The “sweet spot” depicts an ideal BMI, approximately 21 to 24 kg/m2 in women and 22 to 25 kg/m2 in men. Ethnic, racial and gender variations are not shown. The arrow shows the obesity paradox in individuals with cardiovascular disease. A mitigating effect of a higher BMI on mortality is shown by points A and B. Point “B” conceptualizes a population with higher BMI but lower mortality as compared to matched population shown as point “A” with a lower BMI but higher mortality.
The obesity paradox exists in populations with already established CVD that are overweight or obese as measured by BMI. In these obese individuals, a higher BMI may have a protective or a less harmful effect when compared to individuals with the same chronic conditions and lower BMI76–78 as conceptualized in Figure 2. The paradox has also been reported in a number of epidemiological studies and meta-analyses of patients with heart failure,79 atrial fibrillation80 and sudden cardiac death.81 Decreased mortality has been noted in women with a BMI between 21 and 24 kg/m2 and in men with a BMI between 22 to 25 kg/m2 in men, albeit with important adjustments made for ethnic/racial variations.74 As the BMI increases above these ranges, there is a higher overall mortality rate; the paradox lies in the mitigated mortality rates for individuals with higher BMI as compared to their counterparts with a lower BMI (Figure 2).
CLINICAL STUDIES SUPPORTING THE OBESITY PARADOX
Studies in Coronary Artery Disease
In a large prospective study involving over 250,000 patients with CVD followed for 3.8 years, overweight and obese patients had lower mortality compared to underweight and normal weight individuals.82 Similar findings were reported in a large cross-sectional cohort of ST-elevation myocardial infarction (STEMI) patients,75 and in STEMI survivors.83 The obesity paradox was not observed in individuals with a BMI higher than 35 kg/m2 (morbidly obese), a population that had a high mortality rate. A U-shape curve for the relation between BMI and mortality was also found in a large Swedish study with CAD. Underweight patients (BMI <18.5 kg/m2) had the highest mortality rates, followed by patients with normal weight, while the overweight patients (BMI 26.5–28 kg/m2) had the lowest mortality. All-cause mortality decreased with increasing BMI between 30 and 40 kg/m2, and then began to increase again at a BMI of >40 kg/m2.84
Studies in Heart Failure
The mortality rate in chronic systolic heart failure is inversely related to BMI,85 with a lower mortality risk in the overweight and obese as compared to individuals at a “healthy weight”, again confirming the “obesity paradox”.86,87 Other studies support these findings with a 10% lower mortality rate for every 5 unit increase in BMI. Similar results were found in younger hospitalized individuals, with lower mortality in a near-linear fashion in those with higher BMI, in spite of having a higher prevalence of T2DM.88 In advanced systolic heart failure, every 1% increase in body fat resulted in a 13% independent reduction of CVD events,89 with similar findings in other observational heart failure studies.79 Therefore, there is evidence of a lower mortality rate with a higher BMI in individuals with heart failure.
Studies in Type 2 Diabetes
Several studies have described a decreased mortality rate in obese individuals with T2DM. In a pooled analysis of 5 longitudinal cohort studies, results showed that participants who were normal weight experienced higher total and non-cardiovascular mortality rates compared to those who were overweight or obese.2
All clinical studies show that patients with coronary artery disease, heart failure, and T2DM have a lower mortality with a higher BMI, thus supporting the obesity paradox. It is conceivable that patients with established diseases may garner protective effects from increased “good adiposity” or fewer harmful effects by having less “bad adiposity”, the latter being more likely. The potential benefits of good adiposity have been attributed to higher fuel reserve, and/or the presence of other protective factors.77,90 For instance, in heart failure, adipose tissue secretes soluble tumor necrosis factor-α receptors that neutralize the adverse biological effects of tumor necrosis factor-α.91 Higher levels of circulating lipoproteins in obese patients can bind lipopolysaccharides, decreasing inflammatory cytokine secretion, also used as an explanation for the paradox.77,92 An important potential confounder lies in the fact that obese patients may be diagnosed earlier and treated more aggressively than non-obese patients.77
All these mechanisms supporting the obesity paradox found in clinical studies may be valid but none is well established. In fact, a true paradox may not exist at all. Instead, we may simply be observing heterogeneous populations that have been poorly characterized into obese and non-obese groups by measurements of BMI alone, without factoring-in the type of adiposity, level of inflammation and the metabolic health. Further, the severity of underlying illness and the comorbidities are not adjusted in most of the studies. Patients with catabolic diseases such as cancer or other terminal illness often have abnormally low body weight along with an increased mortality rate, part of the “J” or “U”-shaped mortality curve observed in epidemiological studies. When associated catabolic diseases are ruled out, a “J” curve is still seen for CVD, particularly in severe and terminal heart failure with cardiac cachexia, a wasting syndrome with significant weight loss in the absence of peripheral edema.93 Finally, death is another confounding factor as in non-prospective epidemiological observational studies; individuals with high death rate are not part of the analysis. Thus, the obesity paradox as characterized by BMI alone, without consideration of other confounders, should not be accepted as a true paradox.
TREATMENT
There is a growing body of evidence supporting the positive effects of physical activity and weight loss on health-related outcomes. Recent data from the Look AHEAD (Action for Health in Diabetes) study showed that obese patients with T2DM treated with standard of care medications for T2DM, hypertension and hyperlipidemia, had sustained weight loss that resulted in improved fitness and improved levels of biomarkers such as adiponectin and HDL, but CVD morbidity or mortality were not affected.94,95 Data from the Aerobics Center Longitudinal Study showed that life-style changes had a positive effect on fitness and mortality.96, 97 Independent of corresponding changes in BMI, regular physical activity is effective for improving cardiorespiratory fitness, lipid profile, blood pressure, glucose metabolism, and visceral fat.98,99 Thus, regardless of the BMI, cardiorespiratory fitness lowers the metabolic risk. Certainly, weight gain cannot be recommended as a therapy in order to justify an obesity paradox.
CONCLUSIONS
Being overweight or obese is not healthy. There is a clear relationship between higher weight, as determined by BMI, and the increased incidence of hypertension, dyslipidemia, T2DM, CVD, and increased mortality. The obesity paradox exists in epidemiological observational studies using BMI alone as a marker for obesity, but does not distinguish individuals who may have “bad adiposity” or the MetSyn. Individuals with a higher BMI may be physically fit, with less insulin resistance, better lipid profile and therefore lower mortality. Similarly, individuals with a normal BMI and an adverse metabolic profile may have a false sense of protection from their normal weight; nonetheless they have the MetSyn and an associated high mortality rate. Adipose tissue can have both protective and harmful effects. It is the type of adiposity that can play a role in CVD. The obesity paradox may simply reflect a lack of understanding of the complex pathophysiology of obesity and the association between adiposity and CVD.
Acknowledgments
This work was partially supported by grants from the Diabetes Research and Training Center at Albert Einstein College of Medicine, Bronx, New York (Grant number # P60 DK20541). The authors thank Dr. Norman Fleischer and Dr. Jeffrey Pessin for their critical review of the text.
Footnotes
Contribution from authors: AK, KRN, and JZ had full access to all of the data in the study and take responsibility for the integrity and accuracy of this paper
Conflict of Interest: All authors have not received any support from any organization for the submitted work; JZ has a relationship with Takeda Pharmaceutical North America, Merck, Novo Nordisk, and Janssen Pharmaceuticals
References
- 1.Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: The obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 2.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462:307–314. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- 5.Rokholm B, Silventoinen K, Angquist L, et al. Increased genetic variance of BMI with a higher prevalence of obesity. PloS one. 2011;6:e20816. doi: 10.1371/journal.pone.0020816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. The N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drewnowski A, Specter SE. Poverty and obesity: The role of energy density and energy costs. Am J Clin Nutri. 2004;79:6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obesity Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 9.Madrigano J, Baccarelli A, Wright RO, et al. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environm Med. 2010;67:312–317. doi: 10.1136/oem.2009.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Drewnowski A, Darmon N. The economics of obesity: Dietary energy density and energy cost. Am JClin Nutr. 2005;82:265S–273S. doi: 10.1093/ajcn/82.1.265S. [DOI] [PubMed] [Google Scholar]
- 12.Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011;69:41–49. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slomko H, Heo HJ, Einstein FH. Minireview: Epigenetics of obesity and diabetes in humans. Endocrinol. 2012;153:1025–1030. doi: 10.1210/en.2011-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyenet SJ, Schwartz MW. Clinical review: Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97:745–755. doi: 10.1210/jc.2011-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 18.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 19.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 20.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 21.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Prevalence of uncomplicated obesity in an Italian obese population. Obes Res. 2005;13:1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 23.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutri. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 24.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 25.Succurro E, Marini MA, Frontoni S, et al. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 2008;16:1881–1886. doi: 10.1038/oby.2008.308. [DOI] [PubMed] [Google Scholar]
- 26.Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–2344. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- 27.Conus F, Allison DB, Rabasa-Lhoret R, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab. 2004;89:5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Ha HS, Park YJ, et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: The Chungju Metabolic disease Cohort (CMC) study. Clin Endocrinol (Oxf) 2011;75:475–481. doi: 10.1111/j.1365-2265.2011.04085.x. [DOI] [PubMed] [Google Scholar]
- 29.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 31.Saely CH, Rein P, Drexel H. The metabolic syndrome and risk of cardiovascular disease and diabetes: Experiences with the new diagnostic criteria from the International Diabetes Federation. Horm Metab Res. 2007;39:642–650. doi: 10.1055/s-2007-985822. [DOI] [PubMed] [Google Scholar]
- 32.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 33.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 34.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Argyropoulos G, Harper ME. Uncoupling proteins and thermoregulation. J Appl Physiol (Bethesda 1985) 2002;92:2187–2198. doi: 10.1152/japplphysiol.00994.2001. [DOI] [PubMed] [Google Scholar]
- 36.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bostrom P, Wu J, Jedrychowski MP, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 40.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 41.Orchard TJ. Dyslipoproteinemia and diabetes. Endocrinol Metab Clinics N Am. 1990;19:361–380. [PubMed] [Google Scholar]
- 42.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterol. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y, Naseem RH, Duplomb L, et al. Hyperleptinemia prevents lipotoxic cardiomyopathy in Acyl COA synthase transgenic mice. Proc Natl Acad Sci U S A. 2004;101:13624–13629. doi: 10.1073/pnas.0405499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CC, Dixon RA, Wynne AM, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–1270. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 47.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Schinzari F, Tesauro M, Rovella V, et al. Leptin stimulates both endothelin-1 and nitric oxide activity in lean subjects but not in patients with obesity-related metabolic syndrome. J Clin Endocrinol Metab. 2013;98:1235–1241. doi: 10.1210/jc.2012-3424. [DOI] [PubMed] [Google Scholar]
- 49.Hu E, Liang P, Spiegelman BM. Adipoq is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 50.Lonnqvist F, Nordfors L, Jansson M, et al. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J Clin Invest. 1997;99:2398–2404. doi: 10.1172/JCI119422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 52.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 53.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 54.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 55.Bobbert T, Schwarz F, Fischer-Rosinsky A, et al. Fibroblast growth factor 21 predicts the metabolic syndrome and type 2 diabetes in caucasians. Diabetes Care. 2013;36:145–149. doi: 10.2337/dc12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannini C, Feldstein A, Santoro N, et al. Circulating levels of FGF-21 in obese youth: Associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiology Endocrinol Metab. 2004;287:E1178–1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 58.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 59.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Ghanim H, Aljada A, Daoud N, et al. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia. 2007;50:278–285. doi: 10.1007/s00125-006-0508-9. [DOI] [PubMed] [Google Scholar]
- 61.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 62.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 63.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Ann Rev Pathol. 2012;7:99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- 64.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 68.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 70.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vrieze A, Holleman F, Zoetendal EG, et al. The environment within: How gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–613. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 73.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 74.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2011;58:2642–2650. doi: 10.1016/j.jacc.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 77.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 78.Oreopoulos A, McAlister FA, Kalantar-Zadeh K, et al. The relationship between body mass index, treatment, and mortality in patients with established coronary artery disease: A report from approach. Eur Heart J. 2009;30:2584–2592. doi: 10.1093/eurheartj/ehp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oreopoulos A, Padwal R, Kalantar-Zadeh K, et al. Body mass index and mortality in heart failure: A meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: Yet another obesity paradox. Am J Med. 2010;123:646–651. doi: 10.1016/j.amjmed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 81.Choy B, Hansen E, Moss AJ, et al. Relation of body mass index to sudden cardiac death and the benefit of implantable cardioverter-defibrillator in patients with left ventricular dysfunction after healing of myocardial infarction. Am J Cardiol. 2010;105:581–586. doi: 10.1016/j.amjcard.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 82.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ness AR, Gunnell D, Hughes J, et al. Height, body mass index, and survival in men with coronary disease: Follow up of the diet and reinfarction trial (DART) J Epidemiol Community Health. 2002;56:218–219. doi: 10.1136/jech.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angeras O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: A report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. doi: 10.1093/eurheartj/ehs217. [DOI] [PubMed] [Google Scholar]
- 85.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: Body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 86.Osman AF, Mehra MR, Lavie CJ, et al. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36:2126–2131. doi: 10.1016/s0735-1097(00)00985-2. [DOI] [PubMed] [Google Scholar]
- 87.Horwich TB, Fonarow GC, Hamilton MA, et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 88.Fonarow GC, Srikanthan P, Costanzo MR, et al. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: The obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 90.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 91.Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 92.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 93.Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/s0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 94.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 97.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutri. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 98.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 99.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]