Abstract
Tumor-infiltrating lymphocytes (TIL) in colorectal cancer liver metastases (CLM) have been associated with more favorable patient outcomes, but whether MHC class I (MHC-I) expression on cancer cells impacts prognosis is uncertain. Immunohistochemistry was performed on a tissue microarray of 158 patients with CLM, who underwent partial hepatectomy with curative intent. Using the antibody HC-10, which detects HLA-B and HLA-C antigens and a minority of HLA-A antigens, MHC-I expression was correlated with β-2 microglobulin (β2m) (r=0.7, p<0.001), but not with T cell density (r<0.32). The median follow up for survivors was 9.7 years. High levels of MHC-I expression in tumors concomitant with high T-cell infiltration (CD3, CD4, or CD8) best identified patients with favorable outcomes, compared to patients with one or none of these immune features. The median overall survival (OS) of patients with MHC-IhiCD3hi tumors (n=31) was 116 months compared to 40 months for the others (p=0.001), and the median time-to-tumor recurrence (TTR) was not reached compared to 17 months (p=0.008). By multivariate analysis, MHChiCD3hi was associated with OS and TTR independent of the standard clinicopathologic variables. An immune score that combines MHC-I expression and TIL density may be a valuable prognostic tool in the treatment of patients with CLM.
Keywords: MHC Class I, tumor infiltrating lymphocytes, colorectal cancer, liver metastases, prognosis, immunity
Introduction
Individualizing the care of patients with metastatic colorectal cancer based on tumor biology requires biomarkers that estimate a patient’s outcome better than what standard clinicopathologic variables accomplish currently. Accumulating evidence suggests that the adaptive immune system can influence cancer progression, and the quantification of tumor-infiltrating lymphocytes (TIL) may improve prognostic staging of patients with solid cancers (1). In this regard, primary colorectal cancer has been the most comprehensively studied tumor (2). A pivotal study of 406 primary colorectal patients showed that high intratumoral CD3+ T cell density could identify patients with similar disease-free survival, independent of depth of tumor penetration (T stage) or nodal metastases (N stage) (3). Additionally, the intratumoral T cell density and quality has been inversely correlated with colorectal cancer progression (4, 5). For instance, the primary tumors of patients with metastatic colorectal cancer to distant organs (TNM stage IV, n=86) harbored 2 to 3 times fewer CD8+ T cells and 3 to 5 times fewer granzyme B+ T cells than tumors of patients with only regional lymph node metastases (TNM stage I to III, n=312) (5). Although these findings suggest that metastatic tumor deposits represent immune escape variants, we and others have shown that TIL in colorectal cancer liver metastases (CLM) had prognostic value after complete resection (6, 7) or chemotherapy (8). When considered alone however, TIL density in CLM appears to be only a modest predictor of clinical outcomes.
Partial or total loss of MHC class I (MHC-I) expression is regarded as a common tumor immune escape mechanism, which theoretically can render cancer cells “invisible” to CD8+ T cells. MHC-I loss has been reported at high frequency in solid tumors (9) and in up to 74% of primary colorectal cancers (10). Conversely, the HLA and β-2 microglobulin (β2m) genes, encoding the MHC constituents, are interferon-responsive and their expression can be upregulated in a tumor microenvironment where productive immune recognition occurs. The prognostic value of MHC-I expression is uncertain in primary colorectal cancer (11, 12), but strong MHC-I tumor expression combined with high CD3 TIL density has been associated with modestly longer disease-specific survival compared to patients with either feature alone (72.5, 68.0, and 69.9 months, respectively) (11, 13). The aim of this study was to analyze whether prognostic immune scoring in metastatic colorectal cancer could be improved by assessing MHC-I expression in conjunction with TIL quantification in CLM resected with curative intent.
Methods
Patients
We identified from a prospective database consecutive patients, who underwent resection of CLM with curative intent at our institution between 1998 and 2000 (7). Indications for resectability have been described (7, 14). Institutional Review Board approval was obtained. We previously developed a Clinical Risk Score (14), which estimates postoperative outcome and has been validated by others (15). To calculate the Clinical Risk Score, a point is given for each of the following clinicopathologic characteristics: node-positive primary cancer, disease-free interval (DFI, time between resection of primary and liver recurrence) <12 months, more than 1 liver metastasis, largest liver metastasis >5 cm, and prehepatectomy serum carcinoembryonic antigen (CEA) level >200 ng/ml.
Immunohistochemistry
Following pathologic review for diagnostic confirmation and exclusion of highly fibrotic or necrotic tumors, tissue microarrays (TMA) were constructed from 188 patients as described (7). Cores measuring 0.6 mm in diameter were made in triplicate from paraffin blocks and processed using the ATA-27 automated arrayer (Beecher instruments). TMA blocks were cut to 5 μm sections, deparaffinized, rehydrated in graded alcohol, and stained with biotinylated secondary antibodies and positive or isotype controls. CD3, CD4, CD8, and Fox3 staining and quantification have been reported separately (7). We used a validated mouse anti-human monoclonal antibody that binds to MHC-I heavy chains, preferentially for the HLA-B and HLA–C molecules, and seven HLA-As (HC-10, provided by Hidde L. Ploegh, Whitehead Institute; 1:1000, 1h) (16, 17). The polyclonal rabbit anti-human antibody reacting to light-chain β-2 microglobulin was used (A0072, DAKO; 1:50,000, 1h). Automated staining was done on a Ventana XT with the OmniMap DAB detection system (Roche). Nuclei were counterstained with hematoxylin. High resolution TMA digital images were acquired on a MIRAX SCAN (Carl Zeiss) and quantification done with the Metamorph Image Analysis Software (Molecular Devices) blinded to clinical data. The areas of positive signal and the total area of the tissue core were calculated based on color, where pixels with identical RGB values were grouped together, to calculate a ratio of positive brown staining (moderate to strong) over total staining (all brown and hematoxylin blue) for each core (Fig. S1). Thresholds were set to avoid connective tissue, fat, and necrosis. Mean ± standard error was calculated per tumor replicate. Quantification of MHC-I on full cores was compared to quantification on zones of tumors excluding stromal bands and necrotic areas, and found to be similar and highly correlated (spearman r=.993, p<0.001, Table S1). Patients were excluded from the analysis when at least one tumor core could not be quantified for MHC-I expression.
Statistical analysis
Patient disease status was updated through April 2013. Overall survival (OS) and time-to-recurrence (TTR) were calculated from the time of hepatectomy by the Kaplan Meier method. Groups were compared by the log-rank test. Association between immune parameters and outcome was also evaluated by univariate Cox regression on continuous variables, and using optimal cut-off points selected using the maximally selected chi-square method (R version 2.7, www.r-project.org) to estimate the best separation between groups with p-values corrected for overfitting (7, 18). Forward selection stepwise multivariate Cox regression models were applied to the MHChiCD3hi immune score and significant clinicopathologic prognostic factors. The Spearman r test was used to assess the correlation between continuous variables, and the Pearson Chi-square test for the association between categorical variables. A two-sided p-value of ≤0.05 was considered statistically significant (SPSS version 21).
Results
Clinicopathological features
For this study we have identified 188 patients with CLM, who underwent resection surgery with curative intent in our institution; samples from 158 of these patients were analyzed after those from 20 patients were excluded for lack of quantifiable cores, 8 for inadequate follow up, one for palliative resection, and one for duplicative sample. The median age at hepatectomy was 63 years, and 57% were male. The median follow-up time was 42 months overall and 116 (41–171) months for survivors. At the last follow up, there were 35 patients (22%) alive without disease, 106 patients (67%) had recurred tumors, and of the 114 patients (72%) that died 84% were from the cancer. The median OS was 46 months. The 5 and 10 year predicted survival was 39% and 24%, respectively. The median TTR was 20.4 months. The 5 and 10 year predicted recurrence free survival was 30% and 25%, respectively. Clinicopathologic variables significantly associated with longer OS and TTR (Table 1) were resection margins clear of cancer, and most components of the Clinical Risk Score (14) (c.f. methods). By these conventional criteria, the longest median OS and TTR were 77.4 months and 31.4 months for the 53 patients (34.6%) with the lowest Clinical Risk Scores (0 or 1).
Table 1.
Univariate analysis of clinicopathological and immune characteristics for survival and recurrence
| OS | TTR | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Variables | Cut-off | n | (%) | (months) | p -value | (months) | p -value |
|
|
|
|
|||||
| Clinicopathological | |||||||
| Age | > 63 | 79 | 50 | 40.3 | 0.06 | 17.5 | 0.03 |
| ≤ 63 | 79 | 50 | 50.6 | 18.5 | |||
|
| |||||||
| Gender | Male | 90 | 57 | 42.7 | 0.9 | 18.5 | 0.6 |
| Female | 68 | 43 | 45.6 | 17.7 | |||
|
| |||||||
| Site of primary Ca. | Colon | 101 | 64 | 40.3 | 0.2 | 16.8 | 0.9 |
| Rectum | 57 | 36 | 50.1 | 21.2 | |||
|
| |||||||
| Perioperative chemotherapy | Yes | 139 | 89 | 45.6 | 0.8 | 17.7 | 0.5 |
| No | 17 | 11 | 36.9 | 59.9 | |||
|
| |||||||
| Major resection (≥ 3 segments) | No | 66 | 42 | 55.0 | 0.02 | 16.9 | 0.09 |
| Yes | 92 | 58 | 38.5 | 27.1 | |||
|
| |||||||
| Cancer at resection margin | Negative | 141 | 91 | 49.5 | <0.001 | 18.8 | 0.04 |
| Positive | 14 | 9 | 19.6 | 10.1 | |||
|
| |||||||
| No. of hepatic metastasisa,b | Solitary | 79 | 50 | 53.0 | 0.008 | 22.5 | 0.05 |
| > 1 | 79 | 50 | 37.4 | 15.7 | |||
|
| |||||||
| Size of largets tumor (cm)a | ≤ 5 | 106 | 67 | 53.7 | 0.003 | 21.7 | 0.13 |
| > 5 | 52 | 33 | 33.3 | 15.0 | |||
|
| |||||||
| Preoperative CEA (ng/ml)a | ≤ 200 | 125 | 85 | 50.0 | 0.001 | 19.0 | 0.002 |
| > 200 | 22 | 15 | 26.6 | 10.4 | |||
|
| |||||||
| Node-positive primarya | No | 52 | 33 | 67.8 | 0.005 | 46.0 | 0.002 |
| Yes | 106 | 67 | 41.2 | 15.9 | |||
|
| |||||||
| DFI (months)a | ≥ 12 | 88 | 56 | 53.7 | 0.04 | 22.5 | 0.07 |
| < 12 | 70 | 44 | 39.6 | 15.8 | |||
|
| |||||||
| Clinical Risk Score | 0 or 1 | 53 | 35 | 77.4 | <0.001 | 31.4 | <0.001 |
| 2 | 48 | 31 | 50.6 | 21.4 | |||
| ≥ 3 | 52 | 34 | 26.6 | 10.2 | |||
|
|
|
|
|||||
| Immune featuresc | |||||||
| MHC-I | High | 40 | 25 | 89.0 | 0.13 | 83.7 | 0.04 |
| Low | 118 | 75 | 40.1 | 21.5 | |||
|
| |||||||
| β2m | High | 63 | 40 | 53.4 | 0.64 | 25.3 | 0.44 |
| Low | 94 | 60 | 40.1 | 16.9 | |||
|
| |||||||
| CD3 | High | 36 | 23 | 67.1 | 0.7 | 18.5 | 0.97 |
| Low | 118 | 77 | 43.7 | 18.4 | |||
|
| |||||||
| CD4 | High | 32 | 21 | 110.9 | 0.02 | 76.9 | 0.21 |
| Low | 123 | 79 | 40.3 | 17.0 | |||
|
| |||||||
| CD8 | High | 39 | 25 | 89.7 | 0.09 | 46.0 | 0.38 |
| Low | 116 | 75 | 40.1 | 17.0 | |||
|
|
|
|
|||||
| Combination of immune features | |||||||
| MHC-IhiCD3hi | Yes | 31 | 20 | 115.9 | 0.001 | NR | 0.008 |
| No | 123 | 80 | 40.3 | 17.1 | |||
|
| |||||||
| MHC-IhiCD4hi | Yes | 30 | 19 | 105.7 | 0.001 | NR | 0.001 |
| No | 125 | 81 | 39.6 | 16.9 | |||
|
| |||||||
| MHC-IhiCD8hi | Yes | 31 | 20 | 89.7 | 0.008 | NR | 0.02 |
| No | 124 | 80 | 40.1 | 17.0 | |||
NOTE: Log-rank test, median overall survival and time-to-recurrence.
Clinicopathological features used to calculate the Clinical Risk Score.
Only continuous variable significantly associated with OS and TTR at a level of p≤ .05 (Cox regression).
Optimal cut-off points by maximally selected chi-square method, and p-values corrected for over-fitting.
Abbreviations: β2m, Beta-2 microglobulin; CEA, carcinoembryonic antigen; DFI, disease-free interval; MHC-I, MHC class I; NR, not reached; OS, overall survival; TTR, time to recurrence
Immunological features
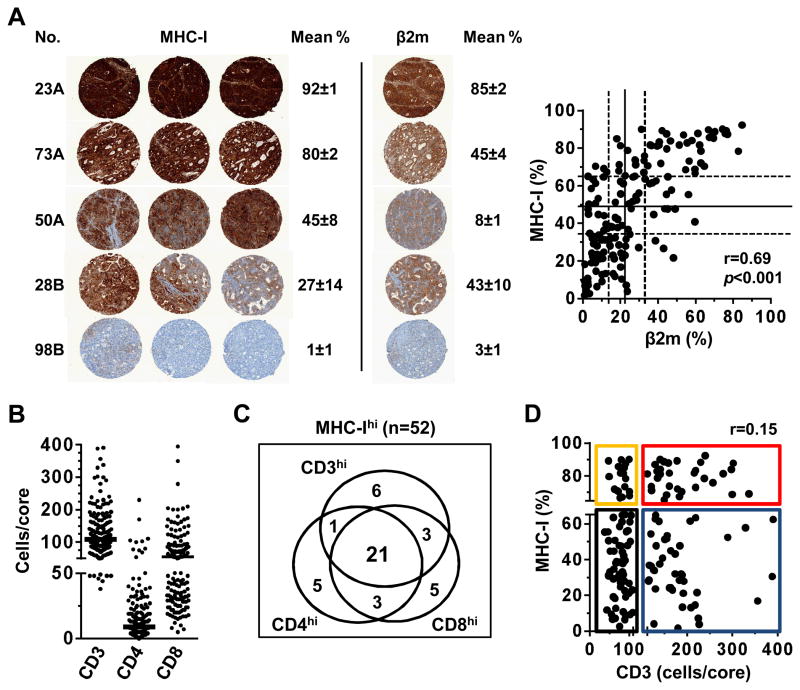
MHC-I and β2m expressions in CLM were quantified to obtain the percent of expression per tumor core (Fig. S1). The distribution of MHC-I expression across CLM ranged uniformly from undetectable to high levels (range 1.4% – 92.3%, median 47.7%, terciles 32.1% and 65.0%) (Fig. 1A). This broad distribution allowed the use of terciles as cutoff points to group patients by null/low, moderate, or high MHC-I expression level. The specificity of MHC-I detection was supported by strong correlation with β2m expression (r=0.69, p<0.001), however the β2m distribution was skewed toward the lower values (range 1.0% to 84.8%, median 20.9%, terciles 13.1% and 32.7%) (Fig. 1A). The imperfect correlation between MHC-I and β2m was consistent with the preferential binding of HC-10 to HLA-B and -C heavy chains free of β2m (16, 17). Enumeration of T cell subsets (CD3, CD4 and CD8) infiltrating CLM yielded distributions also skewed toward the lower values (CD3 range 38 to 390, median 109; CD4 range 0 to 230, median 9; and CD8 range 5 to 502, median 52) (Fig. 1B). MHC-I and β2m expressions did not correlate with the infiltration of the T cell subsets (Table S1, spearman r <0.32), supporting that MHC-I was measured mainly on cancer cells and did not simply reflect intratumoral T cell infiltration.
Figure 1. Quantification of MHC class I, Beta-2 microglobulin, and T-cell subsets.
A. Representative staining in triplicate of MHC class I (MHC-I) expression in 5 colorectal liver metastases, with calculated mean percent surface expression ±standard error. For the same tumors, example of Beta-2 microglobulin (β2m) staining of one of the triplicate core is shown. Correlation of MHC-I and β2m expression is shown. Solid and dotted lines represent median and terciles, respectively (MHC-I, 32%, 48%, and 65%; β2m, 13%, 21%, and 33%).
B. Quantification of intratumoral CD3 (n=154), CD4 (n=155), and CD8 (n=155) T cells. One dot represents one metastasis (cells/core, mean of replicates). Bars represent medians (109, 9, and 52 cells/core, respectively). One value is out of scale (CD8=502).
C. Within the 52 tumors found to express the highest level of MHC-I (upper tercile), 31 displayed high CD3 infiltration, 80.6% of which represented tumors detected to have high CD4 and/or CD8 infiltration (25 of 31, medians used as cut-off points).
D. Dot plot representing the absence of correlation between MHC-I expression and CD3 infiltration. Using the highest tercile as cut-off for high MHC-I expression (broken Y axis, 65%) and the median count for high CD3 infiltration (broken X axis, 109 cells/core), 4 groups are defined: MHC-IhiCD3hi (n=31, red); MHC-IloCD3hi (n=46, blue); MHC-IhiCD3lo (n=19, yellow); MHC-IloCD3lo (n=58, black).
Spearman r used for correlation analysis.
Weak prognostic value of individual immune parameters
Optimal cut-off values that best separated groups of patients by OS and TTR were calculated for MHC-I (71%), β2m (27%), CD3 (174 cells), CD4 (26 cells), and CD8 (89 cells) (Table 1, middle). Individually, MHC-I and CD4 appeared to be the most prognostic immune parameters, but were not robustly associated with both longer OS and TTR. Similar trends were obtained when analyzing immune parameters as continuous variables, but none reached statistical significance at a level of 0.05 (not shown). The prognostic significance of the individual immune parameters thus appeared inferior to the clinicopathologic variables (Table 1).
Combined MHC class-I expression and T cell infiltration defines a subgroup of patients with favorable outcomes
To test the prognostic value of MHC-I expression in CLM in conjunction with intratumoral T cell density and to avoid overfitting results to the studied population, inclusive thresholds were chosen to define groups by immune parameters rather than optimal cutoffs. MHC-Ihi tumors were designated based on the expression above the upper tercile (65%), and high T cell infiltration was defined by a cell count above the median for a given T cell subset.
The longest median OS was 116 months, noted in the 31 patients (20.1%) with MHC-IhiCD3hi tumors compared to 40 months for patients with one or none of these immune features (p=0.001), and the median TTR was not reached for the MHC-IhiCD3hi group compared to 17 months for the others (p=0.008) (Table 1, bottom). The 5 and 10 year OS rate for patients with MHC-IhiCD3hi tumors was 67% and 49%, respectively, compared to 33% and 19% for all other patients (p=0.001). Recurrence was seen in 41.9% of patients with MHC-IhiCD3hi tumors compared to 73.2% for the other patients (p=0.001).
Since MHC-IhiCD3hi tumors captured most of the MHC-IhiCD4hi and MHC-IhiCD8hi tumors (Fig. 1C), similar associations were observed with OS and TTR for these other patient subgroups (Table 1). The prognostic value of β2m expression combined with the infiltration of T cell subsets was better than either β2m, CD3, CD4, or CD8 alone, but it could not discriminate patients with favorable outcomes as well as MHC-I combined with T cell quantification did (not shown).
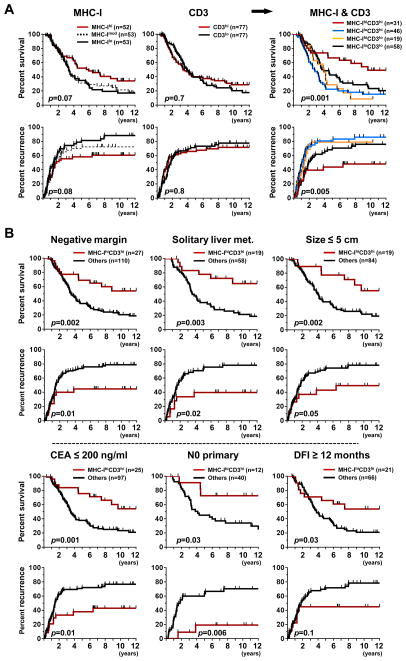
We next assessed the outcomes for the four groups that could be defined based on MHC-I expression and CD3 T-cell infiltration (Fig. 1D). Patients with MHC-IhiCD3hi tumors had the longest median OS and TTR (116 months and not reached, respectively) compared to those of patients with MHC-IloCD3lo tumors (47 and 23 months, respectively), MHC-IloCD3hi (33 and 17 months, respectively), or MHC-IhiCD3lo (47 and 16 months, respectively) (Fig. 2A).
Figure 2. Prognostic impact of MHC class I expression and CD3 T-cell infiltration.
A. Association between MHC-I expression and CD3 infiltration alone and combined with overall survival (upper) and time to recurrence (lower) in the entire patient cohort. For MHC-I alone, outcomes are displayed for groups trichotomized based on tercile. For CD3, outcomes are displayed using the median count as cutoff. For the combination of MHC-I and CD3, 4 groups are displayed and color-coded: MHC-IhiCD3hi (red); MHC-IloCD3hi (blue); MHC-IhiCD3lo (yellow); MHC-IloCD3lo (black).
B. Impact of favorable tumor immune features (MHC-IhiCD3hi, red) on overall survival in patient subgroups according to clinicopathologic features associated with better prognosis (see supplementary Fig. S2 for recurrence).
Log-rank (Mantel Cox). CEA, carcinoembryonic antigen; DFI, disease-free interval, i.e. time between resection of primary tumor and liver recurrence; Met, metastasis; N0 primary, absence of cancer cells in mesenteric lymph nodes draining the primary tumor.
High MHC-I and CD3 is a prognostic factor independent of clinicopathologic characteristics
There was no correlation between MHC-IhiCD3hi tumors and other clinicopathologic variables (Table S2). The MHC-IhiCD3hi immune score stratified patients with better outcomes after grouping by favorable clinicopathologic prognostic factors (Fig. 2B). For example, among the 52 patients with a node-negative (N0) primary tumor, the median OS of patients with MHC-IhiCD3hi liver metastases was not reached compared to 49.5 months for the other patients. Multivariate analysis further supported the MHC-IhiCD3hi immune score as a significant prognostic factor, independent of the clinicopathologic characteristics considered individually, or grouped into the Clinical Risk Score (Table 2). In the 53 patients with a favorable Clinical Risk Score of 0 or 1, patients with MHC-IhiCD3hi tumors fared better with a 10 year overall and recurrence-free survival of 75% and 67%, respectively (Fig. S2). Thus, the MHC-IhiCD3hi immune score still could stratify patients with good prognosis within the Clinical Risk Score, but not those with aggressive disease (Clinical Risk Score ≥ 3), 84% of whom had died and 90% recurred at 5 years after CLM resection. (Fig. S2).
Table 2.
Multivariate analysis of clinicopathological and immune characteristics for survival and recurrence
| Variables | Overall survival
|
Time to recurrence
|
||
|---|---|---|---|---|
| HR (95% CI) | p -value | HR (95% CI) | p -value | |
|
|
|
|||
| Model 1 | ||||
| Minor resection (< 3 segments) | 0.62 (0.39–0.98) | 0.04 | NS | |
| Negative margin | 0.30 (0.15–0.60) | 0.001 | NS | |
| Solitary hepatic metastasis | NS | NS | ||
| Size of largest tumor ≤ 5 cm | 0.47 (0.29–0.76) | 0.002 | NS | |
| Preoperative CEA ≤ 200 ng/ml | 0.50 (0.29–0.85) | 0.01 | 0.46 (0.27–0.78) | 0.004 |
| Node-negative primary | 0.38 (0.24–0.62) | <0.001 | 0.52 (0.33–0.81) | 0.004 |
| DFI > 12 months | NS | NS | ||
| MHC-IhiCD3hi | 0.36 (0.20–0.67) | 0.001 | 0.54 (0.29–0.98) | 0.046 |
|
|
|
|||
| Model 2 | ||||
| Clinical Risk Score <3 | 0.37 (0.25–0.55) | <0.001 | 0.43 (0.28–0.64) | <0.001 |
| MHC-IhiCD3hi | 0.45 (0.26–0.79) | 0.005 | 0.52 (0.29–0.94) | 0.031 |
NOTE: Forward selection stepwise multivariate Cox regression. CEA, carcinoembryonic antigen; DFI, disease-free interval; MHC-I, MHC class I; NS, not significant.
Discussion
Patients with CLM have heterogeneous clinical outcomes. By measuring immune features in CLM resected with curative intent, we identified a subgroup of MHC-IhiCD3hi patients (20% of the cohort) that had a median OS of 9.7 years and a risk of cancer recurrence at 10 years of 48%. The MHC-IhiCD3hi immune score was prognostic of OS and TTR independent of other parameters. This immune score further stratifies outcomes in patients with favorable clinicopatologic features.
Partial or complete loss of MHC-I has been reported in 63% of melanoma, 89% of breast cancer, and 90% of prostate tumors (9). Altered MHC-I expression in primary colorectal tumor was found in 74% of 95 patients (10) but the prognostic value has shown mixed results. Typically, the association between MHC-I expression and the patients’ disease outcomes can be nonlinear, given that both strong expression and complete loss of MHC-I molecules can be associated with better prognosis (11). This apparent paradox may be explained by the particular susceptibility of tumor cells lacking HLA surface molecules to natural killer (NK) cell cytotoxicity mediated by a lack of ligands for the killer-cell inhibitory receptors (“missing-self” hypothesis) (19, 20). It is noteworthy that in addition to T cells, high NK cell infiltration in primary colorectal cancer has been associated with longer overall and disease-free survival in 157 patients (21). Interestingly, we found that patients with MHCloCD3lo CLM tended to have better outcome than patients with MHChiCD3lo or MHCloCD3hi CLM (median OS 46.6 vs 37.4 months (p=0.04) and median TTR 23 vs 14 months (p=0.02)). To our knowledge, no studies have tested the prognostic value of concurrent NK cell infiltration and (lack of) MHC-I expression in colorectal cancer.
Our results indicate that the MHChiCD3hi immune score provides a better discriminatory capacity in CLM compared to its prognostic value when measured in primary colorectal cancer (11, 13). Notably in these studies, 53% of primary tumors assessed in 422 patients had early stage tumors confined to the colon, 33% had nodal positive cancer, and only 12% had metastasis. In contrast to studies in CLM, the prognostic value of T-cells infiltration alone in early-stage colorectal cancer is strong (22) and may simply outweigh significant additional discriminatory value for MHC-I. Alternatively, since metastases theoretically may harbor more immune escape variants than primary tumor, persistence of strong MHC-I expression in CLM could represent a particularly favorable tumor biology, or detection at an earlier time point in disease progression.
Since 89% of patients in our study received perioperative chemotherapy, another interpretation of our results could be that MHChiCD3hi in CLM is a surrogate for favorable response to adjuvant chemotherapy. Indeed, growing evidence suggests that the efficacy of some chemotherapeutic agents can be immune-mediated and may not entirely result from direct cytotoxicity (23). Most studies testing the value of adaptive immune signatures to predict response to chemotherapy have been performed in breast cancer. Based on 89 breast cancer biopsies obtained from women with locally advanced tumors prior to the initiation of neoadjuvant chemotherapy, several immune-related genes, such as CD3 zeta chain, HLA.DPB1, and β2m, were in the top third of genes most closely associated with pathologic complete response (24). Furthermore, MHC-I expression has been associated with improved recurrence-free survival only for breast cancer patients treated with adjuvant chemotherapy (25). Finally, in patients with unresectable CLM, high levels of CD3/CD8/granzyme-B-positive cells at the normal liver/CLM interface had a sensitivity of 79% to predict response to chemotherapy, whereas the absence of such a favorable immune profile was 100% in predicting non-response to chemotherapy (8).
Although our findings are retrospective and require external validation with standardization of MHC-I expression and TIL quantification, they suggest that an immune score that combines MHC-I expression and TIL density may be a valuable prognostic tool in the treatment of patients with CLM. This prognostic tool may also apply to other advanced solid malignancies. Our findings are limited by the fact that the HC-10 antibody detects only a minority of HLA-A alleles. Further studies are necessary to draw firm conclusions about the prognostic value of HLA-A-specific expression. Our results provide an additional rationale to test whether an immune-based signature can predict benefit from adjuvant chemotherapy after complete resection of CLM. As anti-cancer immune-modulation with monoclonal antibodies (e.g., anti-CTLA4, anti-PD1) begins to show efficacy in a variety of metastatic cancers, MHC expression by tumors may also be evaluated as a predictive marker of response to immunotherapy. Conversely, strategies to restore MHC expression may be pursued to expand the number of patients who could potentially benefit from T cell-based cancer immunotherapy.
Supplementary Material
Acknowledgments
Financial support: NIH grant DK068346
We thank Hidde L. Ploegh from the Whitehead Institute for kindly providing and discussing the specificity of the HC-10 antibody. From the Memorial Sloan-Kettering Cancer Center, we are thankful to Mithat Gonen and Joanne Chou from the Department of Biostatistics for help with statistical analyses, Irina Linkov from the Immunohistochemistry Research Core for optimizing and performing the staining, and Yevgeniy Romin from the Molecular Cytology Core for guidance with digital imaging and automated quantification.
Footnotes
Statement of conflict of interest: None
References
- 1.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 6.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524–30. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 7.Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2013;20:946–55. doi: 10.1245/s10434-012-2668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 9.Aptsiauri N, Cabrera T, Garcia-Lora A, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol. 2007;256:139–89. doi: 10.1016/S0074-7696(07)56005-5. [DOI] [PubMed] [Google Scholar]
- 10.Maleno I, Cabrera CM, Cabrera T, Paco L, Lopez-Nevot MA, Collado A, et al. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56:244–53. doi: 10.1007/s00251-004-0692-z. [DOI] [PubMed] [Google Scholar]
- 11.Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 12.Kasajima A, Sers C, Sasano H, Johrens K, Stenzinger A, Noske A, et al. Down-regulation of the antigen processing machinery is linked to a loss of inflammatory response in colorectal cancer. Hum Pathol. 2010;41:1758–69. doi: 10.1016/j.humpath.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Simpson JA, Al-Attar A, Watson NF, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut. 2010;59:926–33. doi: 10.1136/gut.2009.194472. [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 15.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–72. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 16.Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol. 1990;2:113–25. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 17.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–26. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 18.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121–137. [Google Scholar]
- 19.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 20.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 21.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 23.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 24.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 25.de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010;16:1272–80. doi: 10.1158/1078-0432.CCR-09-1844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.