Abstract
Previous research demonstrating age-related deficits in selective attention have not included old-old adults, an increasingly important group to study. The current investigation compared event-related potentials (ERPs) in 15 young-old (65–79) and 23 old-old (80–99) subjects during a color-selective attention task. Subjects responded to target letters in a specified color (Attend) while ignoring letters in a different color (Ignore) under both low and high load. There were no group differences in visual acuity, accuracy, reaction time, or latency of early ERP components. The old-old group showed a disruption in bottom-up processing, indexed by a substantially diminished posterior N1 (smaller amplitude). They also demonstrated markedly decreased modulation of bottom-up processing based on selected visual features, indexed by the posterior selection negativity (SN), with similar attenuation under both loads. In contrast, there were no group differences in frontally-mediated attentional selection, measured by the anterior selection positivity (SP). There was a robust inverse relationship between the size of the SN and SP (the smaller the SN, the larger the SP), which may represent an anteriorly-supported compensatory mechanism. In the absence of a decline in top-down modulation indexed by the SP, the diminished SN may reflect age-related degradation of early bottom-up visual processing in old-old adults.
Keywords: attention, aging, event-related potentials, visual processing
Introduction
Selective visual attention reflects a set of operations that enables individuals to differentially process stimuli based on task relevance, thereby conserving capacity-limited resources and improving processing efficiency (Desimone & Duncan, 1995; Lavie et al., 2004; Lavie, 2005; Sawaki & Katayama, 2008; Rutman et al., 2010). As people age, limiting one’s attention to only relevant information becomes increasingly difficult. Although older individuals demonstrate a well-preserved ability to focus attention on task-relevant visual stimuli (Kok, 2000; Curran et al., 2001), they show substantial deficits in inhibiting the processing of information that is task-irrelevant (Hasher & Zacks, 1988; McDowd & Filion, 1992; West, 1999; Milham et al., 2002; Gazzaley et al., 2005; Lustig et al., 2007; Park & Reuter-Lorenz, 2009).
Most research dedicated to understanding age-related differences in selective attention has compared processing in young vs. old adult subjects. Such studies often define old age as ranging from 60 to 80 years old. Despite the fact that individuals over the age of 80 constitute the fastest growing segment of the population in many nations (Kinsella & He, 2009), little research has been devoted to the investigation of cognitive processing in this old-old group (Hartley & Kieley, 1995; Baltes & Mayer, 1999; McLaughlin et al., 2010; Daffner et al., 2011). To the best of our knowledge, no research has been dedicated to studying early visual selective attention in old-old age.
The limited literature on old-old adults suggests that there may be accelerated declines in cognitive functioning beyond the age of 80 (Newson et al., 2003; Singer et al., 2003; Daffner et al., 2011). For example, the Berlin Aging Study (Singer et al., 2003) found an increased rate of cognitive deterioration in old-old subjects (78 – 100 years old) on measures of perceptual speed, memory, and fluency. Decline in cognitive performance in healthy old-old adults has been linked to decreases in working memory capacity, slowing of perceptual processing, and impairments in peripheral or central visual sensory processing (Singer et al., 2003; Wang et al., 2009; Daffner et al., 2011).
The current work investigated processing that underlies age-related changes in selective visual attention in very old adults. Using temporally sensitive event-related potential (ERP) measures of visual processing, we compared a sample of cognitively high-performing young-old individuals (65–79 years old) to a well-matched sample of healthy old-old adults (80–99 years old). Electrophysiological activity was recorded during performance on a color-selective attention task, under both low and high memory load. Subjects were shown a series of red and blue letters, with specific letters designated as targets. They were told to respond to target letters in a specified color and to ignore stimuli in the other color. The results from prior research on young adults participating in this color-selective attention task (Daffner et al., 2012a; Daffner et al., 2012b) were used as a guide for identifying early ERP components that could serve as indices of bottom-up and top-down operations, with the P1 and N1 components reflecting bottom-up processing, the anterior selection positivity (SP) indexing top-down processing, and the posterior selection negativity (SN) reflecting the intersection between top-down and bottom-up processing, as reviewed below.
The posterior P1 and N1 components index early cortical processing and initial visual discrimination (Johannes et al., 1995; Hillyard et al., 1998a; Vogel & Luck, 2000; Martinovic et al., 2011) that in many contexts are not modulated by selective attention to non-spatial features like color (Hillyard & Munte, 1984; Hillyard & Anllo-Vento, 1998). Among young adults who have participated in this paradigm, the earliest component to exhibit a differential response to stimuli based on task relevance was the anterior SP. The SP has been interpreted as a frontally-mediated index of the motivational salience of a stimulus based on task relevance or as a marker of a detection process sensitive to stimulus features that have been specified as significant (Luck & Hillyard, 1994; Potts & Tucker, 2001; Riis et al., 2009; Daffner et al., 2012b). The SP is measured as the difference in electrophysiological activity between Attend and Ignore conditions around 200 ms after stimulus onset at anterior electrode sites (see Figure 1, regions of interest (ROIs) CF, LAL, RAL, LAM, and RAM). In the current study, the anterior SP served as a measure of top-down selective attentional operations mediated by the prefrontal cortex (van der Stelt et al., 2001; Jonkman et al., 2004; Potts, 2004).
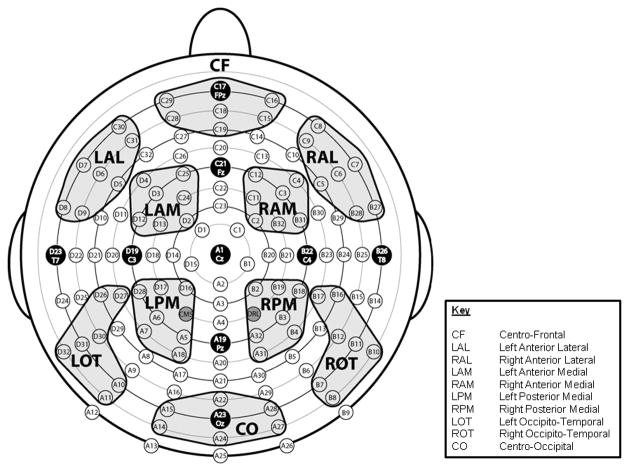
Figure 1.
Montage illustrating the location of 128 electrode sites and the 10 designated regions of interest (ROIs).
The posterior SN reflects enhancement of sensory-perceptual processing of relevant stimulus dimensions compared to irrelevant ones in the feature-selection areas of the extrastriate cortex (Harter & Aine, 1984; Hillyard & Anllo-Vento, 1998; Kopp et al., 2007). As one of the first components to demonstrate a difference in activity between attend and ignore conditions, it likely signals early selection and not post-selection processing (Daffner et al., 2012b) (but see Kenemans et al., 1995; Smid et al., 1999; Zanto et al., 2010 for an alternative hypothesis). The SN is measured as the difference in electrophysiological activity between Attend and Ignore conditions around 300 ms after stimulus onset at occipitotemporal electrode sites (see Figure 1, ROIs LOT and ROT). In the current study, the posterior SN was used as an index of the effect of top down modulation on bottom-up, posterior visual processes. We hypothesized that the SN component would be sensitive to the changes in either processing stream. Because the posterior SN reflects the intersection between top-down and bottom-up processing, interpretation of age-related differences in the SN component would depend on the company they keep.
Theories about cognitive aging have differed in their emphasis on impairment in top-down processes, deterioration in bottom-up processes, or a common cause underlying alterations in both top-down and bottom-up processes. These competing hypotheses about age-related changes in selective attention lead to alternative expectations about what would be found in old-old age. One dominant theory, the frontal deficit hypothesis, suggests that cognitive aging primarily reflects deterioration in top-down, goal-orientated control mechanisms (West, 1996; Kemps & Newson, 2006), which orchestrate differential responses to relevant vs. irrelevant visual stimuli (Gazzaley et al., 2005). Older adults appear to have fewer resources available for working memory and executive control, which may be due to disproportionate age-related decline in prefrontal cortex and its connections (Goh, 2011).
An alternative hypothesis argues that age-related impairments in performance on tests of selective attention are mainly due to a deterioration of bottom-up sensory-perceptual processing, which delivers degraded information upon which to make selections (Grady et al., 1994; Baltes & Lindenberger, 1997; Lindenberger et al., 2001; Park et al., 2004; Tay et al., 2006; Davis et al., 2008; Reuter-Lorenz & Cappell, 2008; Park & Reuter-Lorenz, 2009). For example, studies have found that the functional degradation of early visual processing leads to difficulties with encoding and discrimination that are independent from decline in the peripheral sensory systems (Schmolesky et al., 2000; Yu et al., 2006; Goh, 2011).
Finally, according to the classic version of the common cause hypothesis, neither bottom-up nor top-down processing is disproportionately affected by aging. Rather, there is age-related deterioration in neurophysiological functioning across all levels of the nervous system, including ones that mediate peripheral sensory-motor functions (Lindenberger & Baltes, 1994; Baltes & Lindenberger, 1997; Christensen et al., 2001). This hypothesis states that a common, biologically-based factor is responsible for most of the variance in cognitive, sensory, and motor decline observed in old age.
The theories about cognitive aging reviewed here have largely stemmed from the study of young-old adults (65–80). Presumably, similar explanations would apply to adults over 80 years old, who are products of the aging process at an even more advanced stage. These theories generate different predictions about age-related changes in the pattern of ERP responses among old-old adults. The frontal deficit hypothesis which emphasizes impairment in control mechanisms, would lead to the expectation of an age-related decline among old-old subjects in the SP and SN, which are modulated by top-down processes, but not the P1 and N1, which index bottom-up processes (West, 1996; Kemps & Newson, 2006). It would also predict that the magnitude of age-related changes in the SN and SP would be greater under the high than the low load task because of the depletion of top-down control resources under more demanding conditions (de Fockert et al., 2001; Lavie et al., 2004; Lavie, 2005). In contrast, theories that emphasize impairment in bottom-up processing would lead to the prediction of an age-associated decline in P1, N1 and SN, but not the SP. Finally, the common cause hypothesis would generate an expectation that the magnitude of age-associated changes among old-old subjects would be similar across all components (P1, N1, SP, and SN).
Methods
Participants
Subjects were recruited through community announcements in the Boston metropolitan area, including the Harvard Cooperative Study on Aging. All subjects underwent informed consent procedures approved by the Partners Human Research Committee and an evaluation that included a medical, neurological, and psychiatric history; a formal neurological examination; neuropsychological testing; and completion of questionnaires surveying mood and socioeconomic status.
To be included in the study, participants needed to be 65 years of age or older. All subjects were free of CNS diseases, major psychiatric disorders based on DSM-IV criteria (American Psychiatric Association, 1994), or focal abnormalities on neurological examination consistent with a lesion in the CNS, had a Geriatric Depression Scale (Yesavage et al., 1982) score of ≤ 10, were able to distinguish between the color red and blue, and reported normal or corrected-to normal vision. Subjects were paid for their time.
To ensure that observed differences in neural activity between groups are due to age and not to differences in executive capacity, it is essential to match groups on cognitive abilities (Daselaar & Cabeza, 2005). Executive capacity was emphasized here because of the thesis that selective attention is dependent on top-down executive control mechanisms (Gazzaley et al., 2008; de Fockert et al., 2009; Rissman et al., 2009; Zanto et al., 2011). We selected tests that had well-established norms across a wide range of ages. Tests of executive functions included: 1) Digit Span Backward subtest of the Wechsler Adult Intelligence Scale-IV (WAIS-IV (Wechsler, 2008)), which measures maintenance and manipulation operations of working memory; 2) Controlled Oral Word Association Test (COWAT (Ivnik et al., 1996)), which indexes initiation, self-generation, and monitoring; 3) WAIS-IV Letter-Number Sequencing, which assesses maintenance, monitoring, and manipulation; 4) WAIS-IV Digit-Symbol Coding, which assesses sustained attention/persistence, cognitive speed, and efficiency; 5) Trail-Making Test Parts A and B (Reitan & Wolfson, 1985), which measures planning/sequencing, set shifting, and inhibition. Participants also were assessed for estimated intelligence quotient (AMNART IQ (Ryan & Paolo, 1992)), and performance on the Mini-Mental State Exam (MMSE (Folstein et al., 1975)).
The two age groups were matched on executive capacity, which was defined as the average (composite) percentile performance on all tests of executive function. Consistent with suggestions in the aging literature, the groups were matched according to performance relative to age-appropriate norms (i.e., percentile scores), rather than absolute scores (Daselaar & Cabeza, 2005; Daffner et al., 2006; Daffner et al., 2007; Riis et al., 2008). For the current study, our focus was on older adults with high executive capacity, as defined by a composite score in the top third (≥ 67th percentile) relative to age-appropriate norms.
Because there are currently very limited normative neuropsychological data for adults in their late 80s and 90s, we did not use percentile performance on neuropsychological tests as criteria for defining high capacity in subjects over the age of 87 (n = 5). It has been estimated that only approximately one third of individuals in this age range are free of mild cognitive impairment or dementia (Unverzagt et al., 2007; Corrada et al., 2008). Consistent with previous work (Daffner et al., 2011), participants over the age of 87 were considered to be of high capacity if they did not meet criteria for either mild cognitive impairment (Petersen et al., 1999) or clinical dementia based on DSM-IV guidelines (American Psychiatric Association, 1994). The 87+ subjects did not differ from the 65–79 or 80–86 year olds in regard to years of education, estimated IQ, or MMSE. Percentile performance was calculated for these subjects by using standard norms for the oldest available group.
Experimental Methods
A selective attention task was administered under high and low memory load. In both tasks, subjects were shown physically identical sets of stimuli, which consisted of individual letters presented in either the color red or the color blue. The low load task required subjects to respond by button press to one specific target letter. The high load task required subjects to respond to four specific target letters. Subjects were instructed to pay attention to letters appearing in the designated color while ignoring letters appearing in the other color, and to respond to target letters appearing in the designated color only. Subjects were asked to respond as quickly and as accurately as possible to target letters. Practice trials preceded each set of experimental runs. All subjects participated in both tasks. The hand used for the target response was counterbalanced across subjects.
Each task included 800 stimulus trials divided into 8 blocks. Memory load remained consistent for the duration of the 8 blocks and the order of tasks was counterbalanced. In both the high load and low load tasks, stimuli appeared one at a time within a fixation box that remained on the screen at all times and subtended a visual angle of ~ 3.5° × 3.5° at the center of a high-resolution computer monitor. Half of the stimuli appeared in the color red and half in the color blue, in randomized order. Target stimuli (7.5% in attend color; 7.5% in ignore color) were designated upper case letters and standard stimuli (70% overall; 35% in each color) were any non-target upper case letters. Fillers accounted for the remainder of the stimuli presented. Visual stimuli subtended an angle of 2.5° along their longest dimension and were presented for 250 ms. The inter-stimulus interval (ISI) varied randomly between 815–1015 ms (mean ~ 915 ms) (see Figure 2). For analytic purposes, trials were categorized in terms of whether the stimuli presented were in the attend or the ignore color. The “Attend condition” consisted of all stimuli in the designated color; the “Ignore condition” consisted of all stimuli in the non-designated color.
Figure 2.
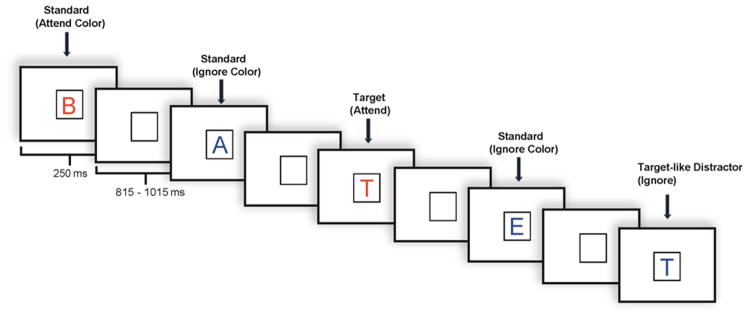
Illustration of an experimental run.
ERP Recordings
An ActiveTwo electrode cap (Behavioral Brain Sciences Center, Birmingham, UK) was used to hold to the scalp a full array of 128 Ag-AgCl BioSemi (Amsterdam, The Netherlands) “active” electrodes whose locations were based on a pre-configured montage. Electrodes were arranged in equidistant concentric circles from the 10–20 system position Cz. In addition to the 128 electrodes on the scalp, 6 mini bio-potential electrodes were placed over the left and right mastoid, beneath each eye, and next to the outer canthi of the eyes to check for eye blinks and vertical and horizontal eye movements. EEG activity was digitized at a sampling rate of 512 Hz.
Data Analysis
Median reaction time (RT) and accuracy rates were measured. A response was considered a hit if it occurred between 200–1000 ms after stimulus presentation. Target stimuli correctly responded to (Target Hits) and stimuli incorrectly identified as targets (False Alarms) were measured in order to determine an overall accuracy score (Percent Target Hits – Percent False Alarms). Performance under the two tasks was compared using repeated measures analyses of variance (ANOVA), with memory load as the within-subject variable and age group as the between-subject variable.
EEG data were analyzed using ERPLAB (www.erpinfo.org/erplab) and EEGLAB (Delorme & Makeig, 2004; http://sccn.ucsd.edu/eeglab) toolboxes that operate within the MATLAB framework. Raw EEG data were resampled to 256 Hz and referenced off-line to the algebraic average of the right and left mastoids. EEG signals were filtered using an IIR filter with a bandwidth of 0.03–30 Hz (12 dB/octave roll-off). Eye artifacts were removed through an independent component analysis. Individual bad channels, identified through visual inspection that revealed a consistently different pattern of activity from all of the surrounding channels, were corrected with the EEGLAB interpolation function. EEG epochs across the two conditions (Attend and Ignore) were averaged separately. The sampling epoch for each trial lasted for 1200 ms, including a 200 ms pre-stimulus period that was used to baseline correct the averaged ERP epochs. Trials were discarded from the analyses if they contained baseline drift or movement artifacts greater than 90 μV. ROIs across the scalp were designated and labeled centro-frontal (CF), left anterior lateral (LAL), right anterior lateral (RAL), left anterior medial (LAM), right anterior medial (RAM), left posterior medial (LPM), right posterior medial (RPM), left occipito-temporal (LOT), right occipito-temporal (ROT) and centro-occipital (CO) (see Figure 1). Each region reflected a cluster of 7 electrode sites. Electrophysiological responses to standards and targets were measured separately. Only ERPs to standard stimuli were analyzed here to avoid the potential confounding influence of motor components associated with responses to target stimuli under the Attend but not the Ignore condition.
The latency of the P1 was measured as the local positive peak latency between 50–150 ms and the latency of the N1 was measured as the local negative peak latency between 100–250 ms at the posterior ROIs LOT and ROT in response to standard stimuli. The size of the P1 and N1 was derived from the mean amplitude of the 30 ms interval centered at the mean peak latency for each component.
The latency of the SP was measured as the positive local peak latency for the Attend – Ignore difference waves between 125–275 ms at anterior ROIs CF, LAL, RAL, LAM, and RAM. The latency of the SN was measured as the negative local peak latency for the Attend – Ignore difference waves between 200–350 ms in response to standard stimuli at lateral posterior ROIs LOT and ROT. The size of the SP and SN were derived from differences between Attend and Ignore conditions of the mean amplitude of the 100 ms interval centered at the mean peak latency for each component.
ERPs were analyzed using ANOVA, with condition, memory load, and ROI as within-subject variables, and age group as the between-subject variable. Analyses that yielded significant interactions between age, condition, load, or ROI resulted in planned contrasts between the levels of the variable. The Greenhouse-Geisser correction was applied for all repeated measures with greater than 1 degree of freedom. Correlation analyses were used to explore the relationships between markers of sensory-perceptual encoding and selective attention.
Results
Participants
Thirty-eight subjects with high executive capacity participated in this study. There were 15 young-old subjects between the ages of 65 and 79 and 23 old-old subjects between the ages of 80 and 99. An additional 3 young-old and 3 old-old subjects participated in the experiment but were excluded due to excessively noisy ERP data. Demographic information, visual acuity, and neuropsychological test scores for each group are presented in Table 1. Of note, the two groups did not differ in terms of years of education, estimated IQ, MMSE, visual acuity, or percentile performance on tests of executive function. In terms of raw scores, the young-old group performed better than the old-old group on Letter-Number Sequencing (t(36) = −3.55, p = .001), Digit-Symbol Coding (t(36) = −3.25, p = .002), and Trail-Making Test Part A (t(36) = −2.45, p = .02).
Table 1.
Demographic, visual and neuropsychological data (mean (SD))
| Young-Old | Old-Old | |
|---|---|---|
| Age (years)* | 73.93 (3.67) | 84.83 (4.55) |
| Male:Female | 6:9 | 9:14 |
| GDS | 2.73 (2.91) | 3.74 (2.70) |
| Years of Education | 16.47 (3.72) | 16.00 (3.10) |
| Visual Acuity | .75 (.17) | .63 (.18) |
| AMNART | 121.13 (8.52) | 122.78 (7.12) |
| MMSE | 29.53 (.74) | 29.13 (1.29) |
| Executive Capacity (%ile) | 81.50 (7.54) | 80.31 (14.96) |
effect of age group: p < .001
GDS = Geriatric Depression Scale;
Visual Acuity1 = based on Snellen 10 feet model wall chart
AMNART = American National Adult Reading Test (estimated intelligence quotient);
MMSE = Mini-Mental State Exam;
Executive Capacity = Average (composite) percentile performance relative to age-appropriate norms on the following tests: Digit Span Backward, Controlled Oral Word Association Test, Letter-Number Sequencing, Trail-Making Test Parts A and B, and Digit-Symbol Coding.
Behavior
An ANOVA revealed an effect of memory load on accuracy (F(1,36) = 8.25, p = .007), but no effect of age group (F(1,36) = 1.82, p = .19), or load x age group interaction (F(1,36) = .008, p = .93). Accuracy was higher on the low load task (M = 95.43%, SD = 6.14%) than the high load task (M = 91.42%, SD = 8.25%). An ANOVA of median RTs also revealed an effect of memory load (F(1,36) = 144.23, p < .001), but no effect of age group (F(1,36) = .21, p = .65), or load x age group interaction (F(1,36) = .13, p = .72). Subjects responded faster in the low load task (M = 521 ms, SD = 66 ms) than the high load task (M = 623 ms, SD = 72 ms). Accuracy and median RTs are presented in Table 2.
Table 2.
Accuracy and Median RT (mean (SD))
| Young-Old | Old-Old | |
|---|---|---|
| Low Load: | ||
| Accuracy (%) * | 96.96 (2.63) | 94.44 (7.51) |
| Median RT (ms) * | 516 (63) | 523 (69) |
| High Load: | ||
| Accuracy (%) * | 93.09 (4.97) | 90.32 (9.77) |
| Median RT (ms) * | 615 (57) | 628 (81) |
effect of memory load (all p values < .01). No age x load interaction (all p values > .13)
ERPs
Main effects or interactions that are not significant or that did not include age group as a factor are not presented, unless of particular theoretical interest.
Posterior P1 Component
Latency
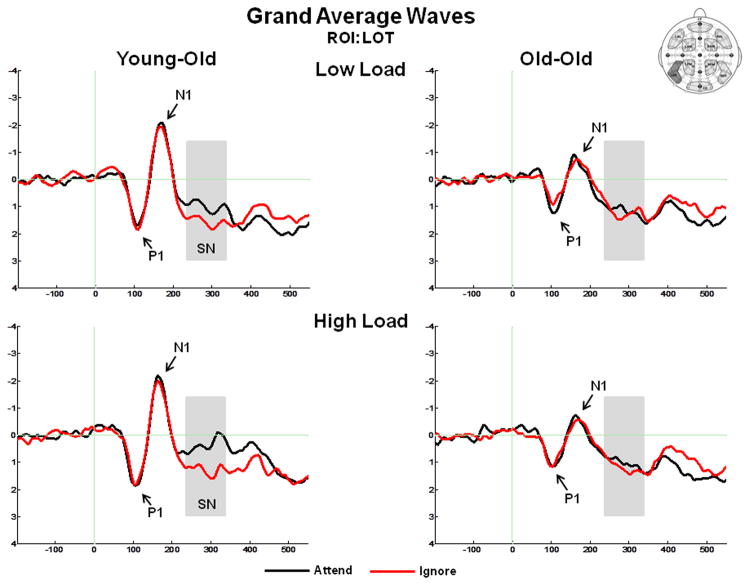
Figure 3 presents the grand average ERPs at ROI LOT, and illustrates the P1 component, N1 component, and the SN. The mean positive local peak latency of the posterior P1 at ROIs LOT and ROT was 111 ms (SD = 11 ms). There was no effect of age group, condition, memory load, or ROI.
Figure 3.
Grand average ERPs in response to standard stimuli at the left occipito-temporal (LOT) ROI, illustrating the P1 component, N1 component, and the selection negativity (SN). The SN is measured as the difference between the ERP response under Attend vs. Ignore conditions (A–I).
Amplitude
The amplitude of the P1 was measured as the mean value between 95–125 ms in response to standard stimuli at ROIs LOT and ROT. There was an effect of ROI (F(1,36) = 15.40, p < .001), which was present because the P1 amplitude was larger over right than left occipito-temporal sites. The magnitude of this effect was similar across conditions (no ROI x condition interaction (F(1,36) = .25, p = .62)) and age groups (no ROI x age group interaction (F(1,36) = .53, p = .47)). There was no effect of condition (F(1,36) = 2.62, p = .11), memory load (F(1,36) = 1.16, p = .29), or age group (F(1,36) = 1.48, p = .23).
Posterior N1 Component
Latency
The mean negative local peak latency of the posterior N1 at ROIs LOT and ROT was 165 ms (SD = 19 ms). There was no effect of age group, condition, memory load, or ROI.
Amplitude
The amplitude of the N1 was measured as the mean value between 150–180 ms in response to standard stimuli at ROIs LOT and ROT. There was an effect of age group (F(1,36) = 5.45, p = .03) such that the N1 amplitude was smaller (less negative) for the old-old group than the young-old group (see Figure 3). There was an effect of ROI (F(1,36) = 10.50, p = .003), which was present because the N1 amplitude was larger over left than right occipital-temporal sites. The magnitude of this effect was similar across age groups (no ROI x age group interaction (F(1,36) = .37, p = .55)). There was no effect of condition (F(1,36) = .08, p = .78) or memory load (F(1,36) = .03, p = .86).
Anterior Selection Positivity (SP)
Latency
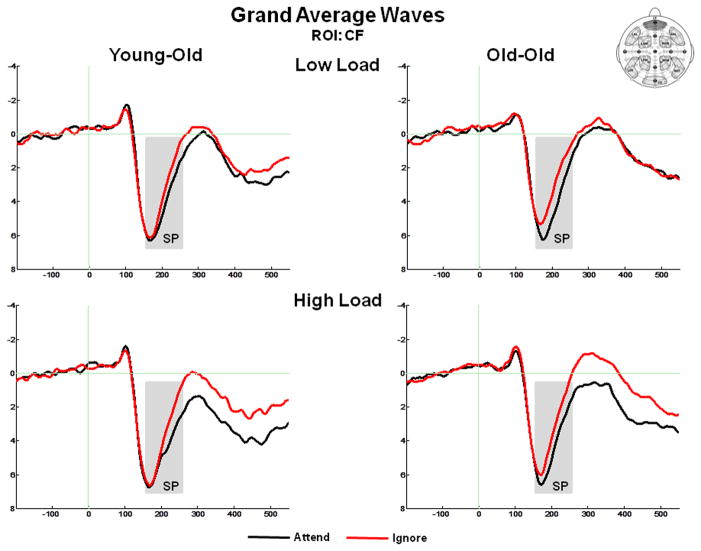
Figure 4 presents the grand average ERPs at the anterior ROI CF, and highlights the temporal interval of the anterior SP. Figure 5 presents the surface potential maps of the Attend – Ignore difference waves representing the SP, which illustrate that the most salient differences between conditions occurred at anterior scalp regions. The mean positive peak latency of the SP difference waves at anterior ROIs (CF, LAL, RAL, LAM, RAM) was 203 ms (SD = 24 ms). There was a marginal effect of memory load (F(1,36) = 3.81, p = .06), due to the SP peaking slightly later (~12 ms) in the high load task. There were no differences between age groups (F(1,36) = .06, p = .81).
Figure 4.
Grand average ERPs in response to standard stimuli at the centro-frontal (CF) ROI, illustrating the selection positivity (SP). The SP is measured as the difference between the ERP response under Attend vs. Ignore conditions (A–I).
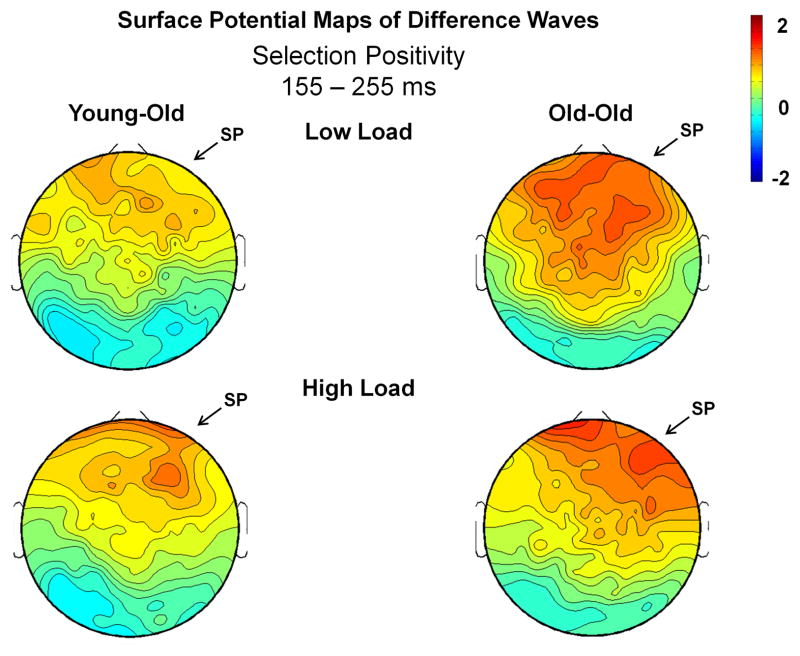
Figure 5.
Surface potential maps of the Attend – Ignore differences waves for the SP interval (155–255 ms). Arrows illustrate the presence of the SP (the difference in electrophysiological activity between Attend and Ignore conditions) in both the young-old and old-old groups.
Amplitude
The amplitude of the anterior SP was measured as the mean value between 155–255 ms at the 5 anterior ROIs. An ANOVA revealed an effect of condition (F(1,36) = 23.51, p < .001), which was due to the mean amplitude being larger (more positive) under Attend than under Ignore conditions, consistent with the presence of the SP. The effect of condition was not modulated by age group (no age group x condition interaction (F(1,36) = 84, p = .37)) or memory load (no condition x memory load interaction (F(1,36) = .02, p = .89)), but was modulated by ROI (condition x ROI interaction (F(4,144) = 4.82, p = .005)). This interaction was due to the condition effects at ROIs LAL and LAM being smaller than at the other three anterior ROIs. Of note, there was a robust condition effect at each of the five anterior ROIs (all p values < .001). There was an effect of memory load (F(1,36) = 5.11, p = .03) due to larger responses under high load, but this effect was not modulated by age (no age group x memory load interaction (F(1,36) = .30, p = .56)). Finally, no effect of age group was observed (F(1,36) = .01, p = .93).
Posterior Selection Negativity (SN)
Latency
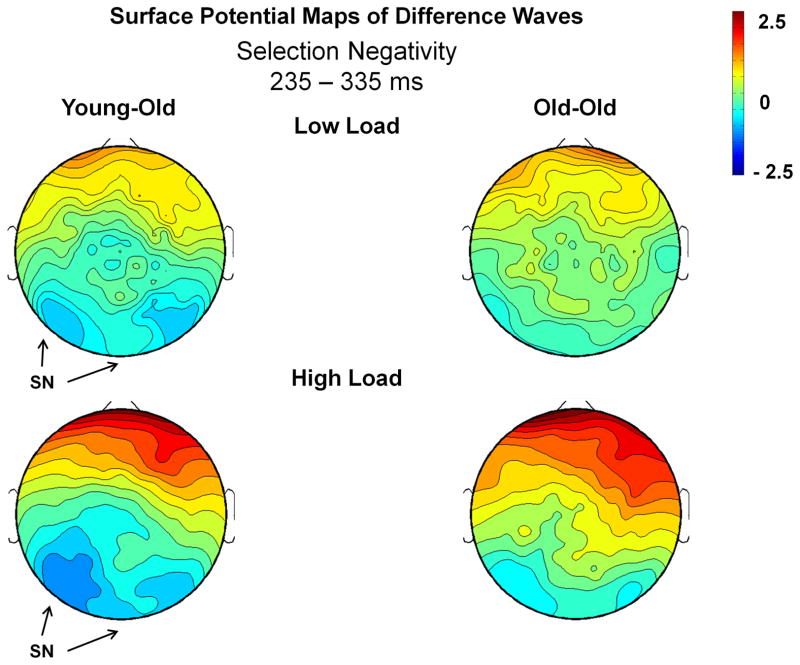
Figure 6 presents the surface potential maps of the Attend – Ignore difference waves during the temporal interval of the SN, illustrating that the most prominent differences between conditions occurred at lateral posterior scalp regions. The local negative peak was measured at 282 ms (SD = 26 ms). There was no effect of age group (F(1,36) = 1.30, p = .26). There was a marginal effect of memory load (F(1,36) = 3.12, p = .09), due to the SN peaking slightly later (~13 ms) in the high load task.
Figure 6.
Surface potential maps of the Attend – Ignore differences waves for the SN interval (235–335 ms). Arrows illustrate the presence of the SN (the difference in electrophysiological activity between Attend and Ignore conditions) in only the young-old group.
Amplitude
The posterior SN was measured as the mean amplitude between 235–335 ms in response to standard stimuli at the posterior ROIs LOT and ROT. An ANOVA of the mean amplitude revealed an effect of condition (F(1,36) = 4.55, p = .04), and a condition x ROI interaction (F(1,36) = 6.82, p = .01). The effect of condition was due to more negative-going ERPs under the Attend condition than under the Ignore condition (Figure 3). This resulted in a negativity (SN) for the Attend – Ignore difference waves (Figure 6). The interaction between condition and ROI was present because the largest difference between conditions was found at the left occipito-temporal ROI, LOT. There was no memory load x condition interaction (F(1,36) = .01, p = .92) and no overall effect of age (F(1,36) = .005, p = .94). Most relevant to the aims of this study, there was an age x condition interaction (F(1,36) = 4.64, p = .04), which was not modulated by memory load (F(1,36) = .13, p = .72). To better understand this result, the young-old and old-old groups were analyzed separately. A within-group comparison revealed an effect of condition, consistent with an SN, for the young-old group (F(1,14) = 11.86, p = .004), but no difference between conditions for the old-old group (F(1,22) < .001, p = .99).
To help delineate whether differences between groups in the size of the SN were dependent on the age-related decline in the amplitude of the N1 component, the ANOVA was run again using the mean amplitude of N1 (averaged across Attend and Ignore conditions) as a covariate. After controlling for the size of the N1, the interaction between age and condition became marginal (F(1,35) = 3.83, p = .06).
Correlations
The common cause hypothesis was evaluated by determining if the magnitude of age-related differences was similar across ERP components. It is noteworthy that we found no correlations between the size of either the P1 or N1 and the size of the SP or SN.2 To explore the possibility that augmented anterior selective attention operations may serve to compensate for impairment in posterior visual processing and selection, the relationship between the SP and SN was examined. We observed a strong inverse correlation between SN amplitude and SP amplitude (r(36) = .45, p = .005), which remained robust even when controlling for age and executive capacity (r(34) = .45, p = .005); as the size of the SN decreased, the size of the SP increased. To further test the hypothesis that a decline in executive capacity and top-down control functions was the source of diminished SN amplitude, we examined the relationship between raw scores on Digit Symbol Coding, Letter-Number Sequencing, and Trail-Making Part A (which were worse for old-old subjects) and the size of the SN component. No correlations were observed between these variables (with or without controlling for age).
Discussion
This study utilized temporally precise ERPs to examine age-related differences in selective attention among older adults. Our use of color as the defining feature of early selection, and the sequential presentation of stimuli in attend (relevant) vs. ignore (irrelevant) feature channels were consistent with a large body of research on selective attention (Czigler, 1996; Eimer, 1997; van der Stelt et al., 1998; Martin-Loeches et al., 1999; Kopp et al., 2007; Gazzaley et al., 2008; Daffner et al., 2012a; Daffner et al., 2012b). The P1 and N1 components served as indices of bottom-up sensory-perceptual processing. Consistent with this formulation, neither the amplitude nor latency of these early components was modulated by the attentional condition. The anterior SP was used as a marker of frontally mediated top-down processing, and the posterior SN served as an index of the influence of top-down operations on bottom-up processing.
As reviewed in the Introduction, different theories of cognitive aging would generate different expectations about the potential patterns of age-related changes in ERP responses among older adults. For example, the frontal deficit hypothesis would lead to the expectation of finding a reduction in the size of the SP and SN, but not the P1 and N1. This hypothesis also would predict larger age-associated declines in the SP and SN under the high load than low load task. The hypothesis about cognitive aging that emphasizes bottom-up deficits would lead to the prediction of a decline in the amplitude of the P1, N1, and SN, but not the SP, and the common cause hypothesis would generate the expectation of finding that the magnitude of age-related decline was similar across all ERP components.
The major results of the study of older adults can be summarized as follows: salient age-associated reductions were found in the size of the posterior N1 and the posterior SN. There were no age-associated differences in the P1 amplitude. The anterior SP was very well preserved in old-old age. Its size inversely correlated with that of the posterior SN. Lastly, none of the components measured were modulated by task load.
The marked decline in the size of the N1 component indicates an age-related disruption in bottom up processing among old-old subjects. Because there were no reliable differences between groups in visual acuity, it is unlikely that the N1 finding was driven by peripheral visual factors. The posterior N1 has been most commonly associated with preliminary visual discrimination and categorization (Hillyard et al., 1998a; Vogel & Luck, 2000; Martinovic et al., 2011), which may facilitate subsequent selection mechanisms. The age-associated reduction in the N1 response suggests that older individuals may have to rely on degraded visual information when carrying out subsequent attention operations, thus supporting hypotheses that emphasize the role of bottom-up deficits. A problem with this interpretation is that our other marker of bottom-up processing, the P1 component, did not show a diminished response in the old-old group. For reasons that remain to be established, considerable variability exists in the literature regarding whether there are age-related decreases (Ceponiene et al., 2008; (Czigler & Balazs, 2005), increases (Diaz and Amenedo, 1998; Falkenstein et al., 2006; Kolev et al., 2006), or no changes (Celesia and Daly, 1977; Curran et al., 2001; De Sanctis et al., 2007) in the P1 amplitude. Moreover, it is not uncommon for studies to report that the pattern of age-related changes differs between the N1 and P1 components (Talsma et al., 2006); De Sanctis et al., 2007; Falkenstein et al., 2006; Kolev et al., 2006).
Interestingly, research has suggested that the P1 and N1 components have different neural generators and functional significance (Mangun & Hillyard, 1991; Schechter et al., 2005; Rossion & Caharel, 2011). They are differentially influenced by magno- and parvocellular visual systems, with the magnocellular pathway playing a greater role in the P1 component and the parvocellular pathway playing a greater role in the N1 component (Schechter et al., 2005). Moreover, there is evidence of greater age-related decline in the parvocellular pathway (N1) (Elliott & Werner, 2010), which would be consistent with the findings of the current study.
In agreement with other reports in the literature (Kenemans et al., 1995; Zanto et al., 2010), our study revealed a marked age-related reduction in the size of the posterior SN. The ventral occipital cortex appears to be a critical source of SN activity (Hillyard et al., 1998a; Hillyard et al., 1998b), which may represent the enhanced perceptual processing of relevant stimulus dimensions within visual feature selection areas (Hillyard & Anllo-Vento, 1998; Kopp et al., 2007). The operations of the SN component reflect neural activity at the intersection between bottom-up and top-down processing. The SN depends on the delivery of sensory-perceptual data that are subjected to anterior control mechanisms (Hillyard & Anllo-Vento, 1998; Kopp et al., 2007). As such, the age-related impairment in processing indexed by the reduced SN could be due to suboptimal top-down control operations, degraded sensory-perceptual information, or both.
The frontal deficit hypothesis would attribute the diminished SN in old-old adults to impairments in top-down activity. Surprisingly, we obtained limited support for this explanation. The frontal deficit hypothesis leads to the expectation that subjects with lower executive capacity would have smaller SN amplitudes. We found that the raw scores on three of the tests of executive capacity, Letter-Number Sequencing, Digit-Symbol Coding, and Trail Making were lower for old-old than young-old subjects. However, worse performance on these tests did not correlate with a reduction in the size of the SN component. Moreover, according to load theory (de Fockert et al., 2001), increases in task demands siphon off limited resources (hypothesized to be reduced with cognitive aging), and thus interfere with the top-down control of selective attention (Lavie, 2005). This leads to the prediction of larger age-related decline in SN and SP amplitude under the high load than low load task, which was not observed.
However, it is important to point out that because the posterior SN was not measurable in old-old subjects, even under the low load task, inferences about the failure to find further decline in this component under the high load task are constrained (i.e., floor effect). There are several reasons why we do not believe that the absence of an SN under the low load task should automatically be interpreted as a reflection of impaired attentional control. It would suggest that for the old-old subjects, the requirements of the low load task were so demanding that they did not have sufficient resources to carry out top-down control mechanisms, which seems unlikely. It is noteworthy that old-old subjects performed the low load task very well, exhibiting no differences in accuracy or RT compared to young-old subjects. Additionally, one might expect that if the old-old group were at the limits of their capacity under the low load task, an increase in load would impact other operations that likely share in this pool of resources. However, we did not observe a reduction in the size of the anterior SP under the high load task.
A bottom-up explanation for the age-associated changes in SN was supported by finding that the difference between young-old and old-old groups in the size of the SN did not remain significant after accounting for the size of the N1. This result raises the possibility that the process indexed by the N1 component serves as a mediating variable in the age-related decline in the SN component. However, we need to acknowledge the weaknesses of this argument. First, the change in significance after controlling for N1 amplitude was very small. Second, the size of the N1 and SN was not correlated, which would be expected if the N1 were the principal source of an age-related decline in SN. That said, the marked reduction of N1 amplitude provides evidence of disrupted early sensory-perceptual functioning, leaving open the possibility that impairment of another bottom-up process, not measured in the current study, is more directly linked to the SN.
The classic version of the common cause hypothesis posits a deterioration in a common underlying neurophysiological variable that impacts all phases of information processing. This theory leads to the expectation of age-related declines across markers of all stages of information processing. However, we found that age-associated declines were specifically associated with the N1 and SN components, with no evidence of deterioration in either the P1 or the SP. Moreover, the theory predicts that age-related differences in early stages of visual processing would account for much of the variance in age-related differences at later stages. However, there were no correlations between the markers of sensory-perceptual processing and indices of selective attention.
One of the most striking findings of the study was the preservation of the anterior SP among old-old subjects. This component reflects frontal activity and top-down operations. It is sensitive to task goals and indexes operations involved in evaluating features according to their relevance (Luck & Hillyard, 1994; Potts & Tucker, 2001; Potts, 2004). According to EEG source localization analyses, the SP activity is at least partially generated by regions in the prefrontal cortex (van der Stelt et al., 2001; Jonkman et al., 2004; Potts, 2004), which is in keeping with fMRI data highlighting the critical role of prefrontal regions in attentional control (Rissman et al., 2009; Rossi et al., 2009; Zanto et al., 2011). Our finding of no age-related decline in the size of the anterior SP is consistent with most reports in the literature (Looren de Jong et al., 1988; Kenemans et al., 1995; Czigler, 1996). In fact, Talsma et al. (2006) demonstrated an age-related increase in the size of the SP in young-old adults relative to young adults.
We found a robust inverse relationship between the changes in the SN and SP: the more attenuated the SN, the larger the SP. This pattern would be consistent with the posterior-anterior shift in aging (PASA) theory, which suggests that the relationship between reduced occipitotemporal activity and increased frontal activity is the reflection of a compensatory mechanism (Davis et al., 2008). It would also support the scaffolding theory of aging and cognition (STAC), which suggests that age-related increases in frontal activity may represent adaptive activation of complementary neural circuits to achieve cognitive goals in response to declining neural structure and function (Park & Reuter-Lorenz, 2009). One way to interpret our results within the framework of these recently proposed theories is that impairments of bottom-up processing disrupt the selective attention operations indexed by the posterior SN, which are compensated by augmentation of frontal top-down control activity, indexed by the anterior SP. The alternative is to argue that the age-related reduction of the SN is a manifestation of frontal and not bottom-up deficits. However, this framework leads to the awkward suggestion that top-down control systems are impaired, leading to a disruption of the posterior SN, but at the same time are well-preserved, allowing for compensatory anterior SP activity.
In summary, compared to their young-old counterparts, old-old adults exhibit diminished posterior N1 and SN components, but a well-preserved anterior SP component. The results of this investigation do not completely match any of the anticipated ideal-typical patterns derived from three major theories of cognitive aging, however they seem to come closest to fitting the bottom-up hypothesis. A key issue involves determining the most appropriate interpretation of the age-related decline in the SN component. The sustained SP among old-old subjects suggests the relative preservation of anterior, top-down functioning compared to young-old adults, while their markedly attenuated posterior N1 highlights the potential role of degraded early bottom-up visual processing in selective attention deficits. More research is needed to substantiate this hypothesis. It remains to be determined whether our findings apply to earlier stages in the aging process. It is plausible that age-associated impairment in selective attention among young-old adults mainly reflects deterioration in top-down functioning. However, it seems unlikely that our results only apply to old-old individuals, given studies in the literature on young-old adults that report age-related declines in the size of the N1 (Czigler & Balazs, 2005; Ceponiene et al., 2008; Zanto et al., 2010) and SN (Kenemans et al., 1995; Zanto et al., 2010), but stability or even augmentation of the size of the SP (Looren de Jong et al., 1988; Kenemans et al., 1995; Czigler, 1996; Talsma et al., 2006).
Future investigation should include not only young-old and old-old adults, but also young and middle-aged subjects in order to gain a more comprehensive view of changes in information processing across the life span. The current study was limited to the investigation of age-related differences in individuals with high executive capacity relative to age-appropriate norms. This approach allowed us to argue that the observed differences between groups were due to the aging process and not executive capacity or task performance. It also reduced the likelihood that subjects in our old-old group were suffering from early dementia or mild cognitive impairment, which would have confounded normal with diseased cognitive aging. However, to determine the generalizability of our findings, future investigations using this paradigm should incorporate cognitively average-performing individuals, being mindful of excluding those with evidence of mild cognitive impairment or other neurological disease.
Acknowledgments
Source of Funding: This research was funded in part by NIA grant R01 AGO17935 and by generous support from the Wimberly family, the Muss family, and the Mortimer/Grubman family. The authors would like to thank Marissa Keppley and Christine Dunant for their excellent administrative assistance.
Footnotes
In one old-old subject visual acuity was inadvertently not obtained, and in another old-old subject visual acuity was 20/80. To ensure that differences between the young-old and old-old groups in the N1 and SN components were not driven by these two old-old subjects, statistical analyses were re-run after excluding them. No changes were found in the pattern or reliability of the results.
The amplitude of the P1 and N1 components was collapsed across condition, memory load, and posterior ROIs (LOT and ROT). The amplitude of the SP and SN was collapsed across memory load and across the anterior ROIs (CF, LAL, RAL, LAM, RAM) for the SP and lateral posterior ROIs (LOT and ROT) for the SN.
Conflicts of Interest
There are no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychological Association; 1994. [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Mayer KU. The Berlin aging study: Aging from 70 to 100. New York: Cambridge University Press; 1999. [Google Scholar]
- Ceponiene R, Westerfield M, Torki M, Townsend J. Modality-specificity of sensory aging in vision and audition: Evidence from event-related potentials. Brain Res. 2008;1215:53–68. doi: 10.1016/j.brainres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–99. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: Results from the 90+ study. Neurology. 2008;71:337–43. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: An ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Czigler I. Age, color processing and meaningfulness: An event-related potential study. Int J Psychophysiol. 1996;22:25–34. doi: 10.1016/0167-8760(96)00010-4. [DOI] [PubMed] [Google Scholar]
- Czigler I, Balazs L. Age-related effects of novel visual stimuli in a letter-matching task: An event-related potential study. Biol Psychol. 2005;69:229–42. doi: 10.1016/j.biopsycho.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Riis J, et al. Cognitive status impacts age-related changes in attention to novel and target events in normal adults. Neuropsychology. 2007;21:291–300. doi: 10.1037/0894-4105.21.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, et al. Increased responsiveness to novelty is associated with successful cognitive aging. J Cogn Neurosci. 2006;18:1759–73. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Sun X, Tarbi E, Rentz DM, Holcomb PJ, Riis JL. Does compensatory neural activity survive old-old age? Neuroimage. 2011;54:427–38. doi: 10.1016/j.neuroimage.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Tarbi EC, Haring AE, et al. The influence of executive capacity on selective attention and subsequent processing. Front Hum Neurosci. 2012a;6:167. doi: 10.3389/fnhum.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Zhuravleva TY, Sun X, et al. Does modulation of selective attention to features reflect enhancement or suppression of neural activity? Biol Psychol. 2012b;89:398–407. doi: 10.1016/j.biopsycho.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Cabeza R. Age-related changes in hemispheric organization. In: Cabeza R, Nyberg L, DP, editors. Cognitive neuroscience of aging. New York: Oxford University Press; 2005. pp. 325–53. [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que pasa? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Ramchurn A, van Velzen J, Bergstrom Z, Bunce D. Behavioral and ERP evidence of greater distractor processing in old age. Brain Res. 2009;1282:67–73. doi: 10.1016/j.brainres.2009.05.060. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–6. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Eimer M. An event-related potential (ERP) study of transient and sustained visual attention to color and form. Biol Psychol. 1997;44:143–60. doi: 10.1016/s0301-0511(96)05217-9. [DOI] [PubMed] [Google Scholar]
- Elliott SL, Werner JS. Age-related changes in contrast gain related to the M and P pathways. J Vis. 2010;10(4):1–15. doi: 10.1167/10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–6. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Goh JO. Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–62. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter RM, Aine CJ. Brain mechanisms of visual selective attention. In: Parasuraman R, Davies DR, editors. Varieties of attention. Orlando: Academic Press Inc; 1984. pp. 293–321. [Google Scholar]
- Hartley AA, Kieley JM. Adult age differences in the inhibition of return of visual attention. Psychol Aging. 1995;10:670–83. doi: 10.1037//0882-7974.10.4.670. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower G, editor. Psychology of learning & motivation. Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95:781–7. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Munte TF. Selective attention to color and location: An analysis with event-related brain potentials. Percept Psychophys. 1984;36:185–98. doi: 10.3758/bf03202679. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Teder-Salejarvi WA, Munte TF. Temporal dynamics of early perceptual processing. Curr Opin Neurobiol. 1998a;8:202–10. doi: 10.1016/s0959-4388(98)80141-4. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Phil Trans R Soc B. 1998b;353:1257–70. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, stroop, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–78. [Google Scholar]
- Johannes S, Munte TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Brain Res Cogn Brain Res. 1995;2:189–205. doi: 10.1016/0926-6410(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Kenemans JL, Kemner C, Verbaten MN, van Engeland H. Dipole source localization of event-related brain activity indicative of an early visual selective attention deficit in ADHD children. Clin Neurophysiol. 2004;115:1537–49. doi: 10.1016/j.clinph.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Kemps E, Newson R. Comparison of adult age differences in verbal and visuo-spatial memory: The importance of ‘pure’, parallel and validated measures. J Clin Exp Neuropsychol. 2006;28:341–56. doi: 10.1080/13803390490918228. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Smulders FT, Kok A. Selective processing of two-dimensional visual stimuli in young and old subjects: Electrophysiological analysis. Psychophysiology. 1995;32:108–20. doi: 10.1111/j.1469-8986.1995.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Kinsella K, He W. International Population Report. Washington: US Government Printing Office; 2009. An aging world: 2008 US Census Bureau. [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54:107–43. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kopp B, Tabeling S, Moschner C, Wessel K. Temporal dynamics of selective attention and conflict resolution during cross-dimensional go-nogo decisions. BMC Neurosci. 2007;8:68. doi: 10.1186/1471-2202-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133:339–54. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging. 1994;9:339–55. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging. 2001;16:196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- Looren de Jong H, Kok A, van Rooy JC. Early and late selection in young and old adults: An event-related potential study. Psychophysiology. 1988;25:657–71. doi: 10.1111/j.1469-8986.1988.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, MacLeod CM, editors. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 145–62. [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17:1057–74. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Martin-Loeches M, Hinojosa JA, Rubia FJ. Insights from event-related potentials into the temporal and hierarchical organization of the ventral and dorsal streams of the visual system in selective attention. Psychophysiology. 1999;36:721–36. [PubMed] [Google Scholar]
- Martinovic J, Mordal J, Wuerger SM. Event-related potentials reveal an early advantage for luminance contours in the processing of objects. J Vis. 2011:11. doi: 10.1167/11.7.1. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Filion DL. Aging, selective attention, and inhibitory processes: A psychophysiological approach. Psychol Aging. 1992;7:65–71. doi: 10.1037//0882-7974.7.1.65. [DOI] [PubMed] [Google Scholar]
- McLaughlin PM, Szostak C, Binns MA, Craik FI, Tipper SP, Stuss DT. The effects of age and task demands on visual selective attention. Can J Exp Psychol. 2010;64:197–207. doi: 10.1037/a0020650. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, et al. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain Cogn. 2002;49:277–96. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Newson RS, Kemps EB, Luszez MA. Cognitive mechanisms underlying decrements in mental synthesis in older adults. Aging Neuropsychology and Cognition. 2003;10:28–43. [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–5. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Potts GF, Tucker DM. Frontal evaluation and posterior representation in target detection. Brain Res Cogn Brain Res. 2001;11:147–56. doi: 10.1016/s0926-6410(00)00075-6. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–82. [Google Scholar]
- Riis JL, Chong H, McGinnnis S, et al. Age-related changes in early novelty processing as measured by ERPs. Biol Psychol. 2009;82:33–44. doi: 10.1016/j.biopsycho.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, et al. Compensatory neural activity distinguishes different patterns of normal cognitive aging. Neuroimage. 2008;39:441–54. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. The effect of non-visual working memory load on top-down modulation of visual processing. Neuropsychologia. 2009;47:1637–46. doi: 10.1016/j.neuropsychologia.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009;192:489–97. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception. Vision Res. 2011;51:1297–311. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. J Cogn Neurosci. 2010;22:1224–34. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. Clin Neuropsychol. 1992;6:53–62. [PubMed] [Google Scholar]
- Sawaki R, Katayama J. Top-down directed attention to stimulus features and attentional allocation to bottom-up deviations. J Vis. 2008;8(4):1–8. doi: 10.1167/8.15.4. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–15. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3:384–90. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin aging study (BASE) Psychol Aging. 2003;18:318–31. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Smid HG, Jakob A, Heinze HJ. An event-related brain potential study of visual selective attention to conjunctions of color and shape. Psychophysiology. 1999;36:264–79. doi: 10.1017/s0048577299971135. [DOI] [PubMed] [Google Scholar]
- Talsma D, Kok A, Ridderinkhof KR. Selective attention to spatial and non-spatial visual stimuli is affected differentially by age: Effects on event-related brain potentials and performance data. Int J Psychophysiol. 2006;62:249–61. doi: 10.1016/j.ijpsycho.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: Findings from an older australian population. Gerontology. 2006;52:386–94. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Sujuan G, Lane KA, et al. Mild cognitive dysfunction: An epidemiological perspective with an emphasis on African americans. J Geriatr Psychiatry Neurol. 2007;20:215–26. doi: 10.1177/0891988707308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt O, Kok A, Smulders FT, Snel J, Boudewijn Gunning W. Cerebral event-related potentials associated with selective attention to color: Developmental changes from childhood to adulthood. Psychophysiology. 1998;35:227–39. doi: 10.1017/s0048577298961303. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, van der Molen M, Boudewijn Gunning W, Kok A. Neuroelectrical signs of selective attention to color in boys with attention-deficit hyperactivity disorder. Brain Res Cogn Brain Res. 2001;12:245–64. doi: 10.1016/s0926-6410(01)00055-6. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Wang TH, Kruggel F, Rugg MD. Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia. 2009;47:1352–61. doi: 10.1016/j.neuropsychologia.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 4. San Antonio, TX: Pearson; 2008. [Google Scholar]
- West R. Visual distraction, working memory, and aging. Mem Cognit. 1999;27:1064–72. doi: 10.3758/bf03201235. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–9. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–61. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]