Abstract
Gold nanoparticles provide an attractive and applicable scaffold for delivery of nucleic acids. In this review, we focus on the use of covalent and noncovalent gold nanoparticle conjugates for applications in gene delivery and RNA-interference technologies. We also discuss challenges in nucleic acid delivery, including endosomal entrapment/escape and active delivery/presentation of nucleic acids in the cell.
Introduction
Nucleic acids provide promising tools for therapeutic targets, including pathways and molecules involved in cancer and genetic disorders.1,2 Plasmid3 and minivector DNAs4 can be used to repair defective genes, and small interfering RNA (siRNA)5 can be used to regulate therapeutically relevant processes. In contrast with small-molecule therapeutics, however, nucleic acids require delivery vehicles for protection from nucleases and other environmental agents and to facilitate entry into the cell.6
Nucleic acid delivery vehicles are generally divided into two categories: biological and synthetic vectors. On the biological side, viral vectors provide efficient delivery; however, immunogenicity, carcinogenicity, and inflammation can become an issue for clinical applications.7,8,9 Traditional synthetic vectors—including cationic lipids,10 polymers,11,12 and dendrimers13—have been widely used for intracellular nucleic acid delivery. In practice, several lipid-based vectors (e.g., Transfectam14 and Lipofectamine15) have also been commercialized. However, in spite of their transfection efficacy and ease of large-scale production, nucleic acid delivery using these vectors still has limitations for clinical applications, e.g., low storage stability, lack of targeting efficacy, and limited in vivo tracking/monitoring.
Inorganic nanoparticles16,17,18,19,20,21 are emerging as synthetic vectors that feature several advantages relative to traditional lipid-based vectors, including tunable size and surface properties, multifunctional capabilities, and the ability to translate the physical properties of the metal core to the delivery vehicle. Gold nanoparticles (AuNPs),22,23,24,25,26 in particular, serve as attractive materials for nucleic acid delivery applications23,27,28,29,30,31,32,33,34 due to the following advantages. First, AuNPs can be fabricated in a scalable fashion with low size dispersity.35,36,37,38 Second, functional diversity can be readily achieved by the creation of multifunctional monolayers, allowing multiple functional moieties such as nucleic acids and targeting agents to be placed onto the particle surface.39,40 Finally, the cytotoxicity,26,41,42 biodistribution,43,44 and in vivo excretion properties45,46,47 can be modulated by regulating the particle size and surface functionality.
In this review, we will focus on the structural design of AuNPs for nucleic acid delivery, highlighting the unique chemical and biological properties of these particles.
Structure Design
There are two primary strategies for the design of nucleic acid carriers: covalent attachment and supramolecular assembly. Both of these approaches have been used with AuNPs, and each has their own advantages and challenges that we will discuss.
Covalent AuNP conjugates
Synthetic and biological compounds can be anchored onto the surface of AuNPs via the strong metal–ligand interaction between sulfur and gold (the S–Au binding).31 The S–Au interaction is partially covalent (~35%) and mostly electrostatic (~65%). Energy decomposition analysis indicates that gold has greater covalent character with sulfur ligands relative to Cu and Ag.48 In general, the “S–Au covalent bond” is a widely accepted nomenclature. In this section, we will discuss AuNPs functionalized with thiolated oligonucleotides for nucleic acid delivery.
Covalent attachment of nucleic acids to AuNPs is an effective means of transporting gene-silencing oligonucleotides, where the modification does not inhibit biological activity.49,50 Small silencing RNA reduces target gene expression in addition to inducing regulation by Argonaute proteins that are associated with target messenger RNA (mRNA) degradation.51 The application of RNA interference (RNAi) using AuNPs mainly involves delivery of microRNAs (miRNAs) and small interfering RNAs (siRNAs).52,53 Mirkin et al.54 synthesized a class of polyvalent nucleic acid AuNPs (pNA–AuNPs) by functionalizing AuNPs covalently with thiol-modified oligonucleotides and applied them to siRNA-based gene silencing. The dense shell of oligonucleotides on the surface of these NPs inhibits degradation by nucleases, protecting the payload. Surprisingly, cellular uptake of these pNA–AuNPs was quite rapid with >50 different cell lines, even though their strong negative charge would be expected to prevent uptake (see following text).55,56 Cellular uptake of pNA–AuNPs was strongly dependent on the density of the oligonucleotide on the particle surface, with higher density providing more efficient delivery.57 These “antisense particles” have higher affinity constants for their complementary nucleic acids than their linear counterparts, a key determinant of gene-silencing efficiency. This increased affinity is consistent with the cooperative binding theory that higher oligonucleotide packing densities lead to an increase in the association constant of DNA with AuNPs.58
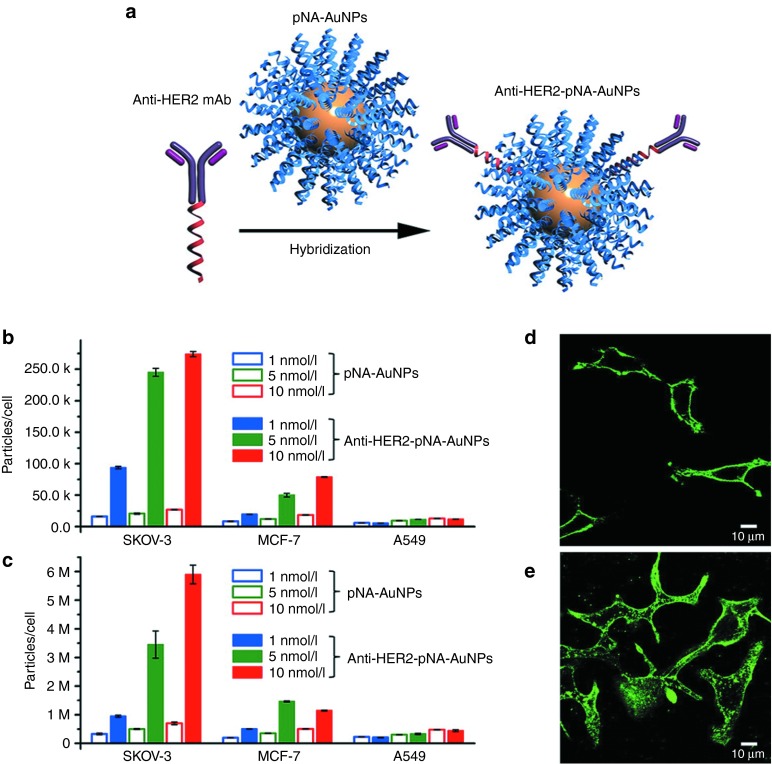
The mechanism of uptake of the strongly anionic pNA–AuNPs remained an open question for a number of years.55 A recent study by Mirkin59 demonstrated that spherical pNA–AuNPs, even at a low concentration, could bind strongly to class A scavenger receptors (SR-A), important receptors for mediating membrane transport. On binding to SR-A, endocytosis occurred via a lipid-raft-dependent, caveolae-mediated pathway. This mechanism provides “universal” delivery to diseased cells and healthy cells. Hybridizing monoclonal antibody–DNA conjugates with pNA–AuNPs imparts targeting capabilities to these constructs (Figure 1a), providing cell selectivity in uptake and greater gene knockdown in cells that overexpress the target antigen (Figure 1b–e).60
Figure 1.
Antibody-linked pNA-AuNPs for cellular targeting. Schematic illustration of (a) the synthesis of anti-HER2-pNA–AuNPs. The number of AuNPs per cell at (b) 4 °C and (c) 37 °C, after 4 hours of incubation. Significantly higher uptake was observed for HER2-overexpressing cells (SKOV-3) and moderate-expressing cells (MCF-7) with regard to targeted particles, whereas HER2-nonexpressing cells (A549) showed no selectivity. Confocal micrograph of SKOV-3 cells after incubating for 4 hours with 5 nmol/l anti-HER2-pNA–AuNPs at (d) 4 °C and (e) 37 °C. Antisense DNA strands were labeled with fluorescein at the 5′-end. mAb, monoclonal antibody. Reprinted with permission from ref. 60. Copyright 2012 American Chemical Society.
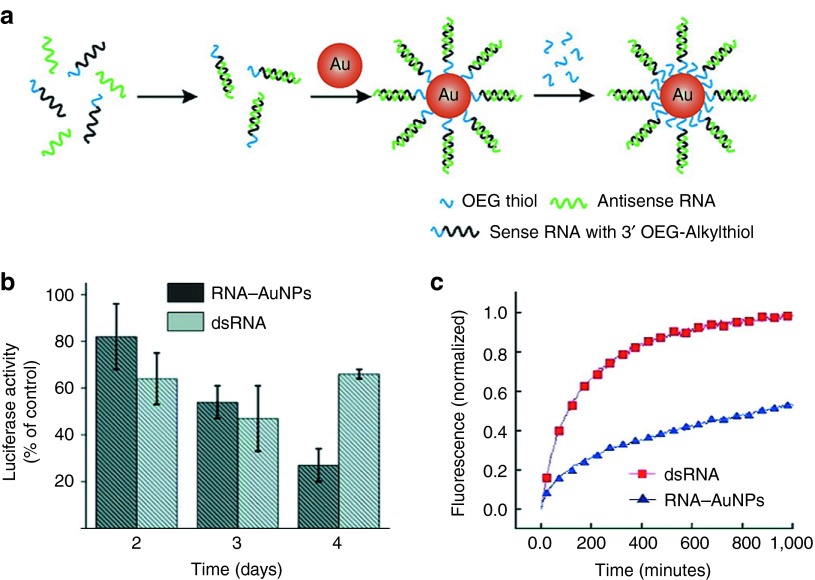
Mirkin et al.61 have used their “antisense particles” to functionalize AuNPs with mimics of synthetic tumor-suppressive miRNA—miR-205. On interaction with the 3′-untranslated region of target mRNA, these miRNA–AuNPs repressed the expression of miRNA target protein, achieving three times the efficiency of the co-carrier-supported molecular miRNAs. Unlike miRNAs, however, siRNAs are totally paired with target mRNA, resulting in transient silencing of the target gene.62 The instability of siRNAs caused by enzyme degradation, however, provides a challenge for siRNA delivery. Nagasaki et al.53,63 fabricated 15-nm AuNPs with SH-PEG-PAMA (thiol-bearing polyethylene glycol–poly(2-N,N-dimethylamino)ethylmethacrylate) and monothiolated siRNAs, where the amine-terminated copolymer enhances particle stability. Knockdown of ~60% of normalized luciferase expression was observed in HuH-7 cells. In later studies, RNase-free polyvalent siRNA–AuNPs were synthesized and delivered into HeLa cells. These densely siRNA-coated AuNPs had sixfold longer serum half-life than their molecular RNA duplex counterparts (Figure 2a–c).52
Figure 2.
Polyvalent siRNA–AuNPs for gene regulation. (a) Schematic depiction of the synthesis of polyvalent RNA–AuNP conjugates. (b) Knockdown of luciferase expression in HeLa cells over 4 days using polyvalent RNA–AuNP conjugates (3 nmol/l nanoparticle (NP) concentration, ∼100 nmol/l RNA duplex concentration) or double-stranded (ds) RNA (100 nmol/l). (c) Stability of RNA–AuNPs. Comparison of the stability of cyanine 5-labeled double-stranded (ds)RNA (red) and RNA–AuNPs (blue) in 10% serum. The increase in fluorescence intensity demonstrates the distance-dependent release of the fluorophore from the gold core, which is an efficient quencher of the fluorescence. Reprinted with permission from ref. 52. Copyright 2009 American Chemical Society. OEG thiol, oligoethylene glycol-thiol.
Topical transdermal delivery presents a particularly difficult challenge in therapeutic delivery.64,65 Recent studies have shown that through the use of appropriate skin conditioning agents, pNA-AuNPs can be used to transdermally deliver siRNA in an effective manner. In these studies, epidermal growth factor receptor was targeted, reducing epidermal thickness by ~40%. Moreover, no clinical or histological evidence of toxicity was found in treated skin.66
Although emphasis is often placed on the importance of cellular uptake of nucleic acid delivery systems, endosomal escape is perhaps the most challenging barrier for delivery.67,68 Recently, siRNA loaded in lipid NPs has shown low efficiency (1–2%) in escaping from the endosomes into the cytosol.69 The successful intracellular gene regulation by pNA–AuNPs,52,55,56 however, indicates effective endosomal escape of pNA–AuNPs. Mirkin et al.70 further demonstrated that cyanine 5-labeled pNA–AuNPs gather in endosomes 1 hour posttransfection; after 4 hours, they can be observed throughout the cytoplasm. However, the detailed mechanism regarding endosomal escape of pNA–AuNPs still remains elusive. In addition, cationic polymeric ligands (such as SH-PEG-PAMA) act as stabilizers for AuNPs against RNases and facilitate endosomal escape via the “proton sponge” effect. This will be discussed in detail in the next section. Nevertheless, the necessity for nucleic acids to be removed from the NP surface is still an open debate.
Noncovalent AuNP conjugates
Noncovalent nucleic acid delivery vehicles are an attractive alternative to covalent systems. Using supramolecular conjugates allows the use of unmodified nucleic acids, allowing delivery of DNA for gene therapy and of RNA for knockdown applications. With these systems, the synthetic versatility of the AuNP platform provides multiple options for vehicle design, such as mixed-monolayer-protected AuNPs (MM-AuNPs), amino acid–functionalized AuNPs (AA–AuNPs), and layer-by-layer-fabricated AuNPs (LbL-AuNPs).
The strong negative charge of nucleic acids makes cationic AuNPs obvious partners for self-assembly.71 In early studies, Rotello et al.72 created effective delivery vehicles for plasmid DNA using quaternary ammonium–functionalized AuNPs. These studies demonstrated that a number of parameters were important for successful transfection, including the AuNP-to-DNA ratio, surface charge coverage, and hydrophobicity (Figure 3a–c). In these AuNP–DNA complexes, the DNA is bent around the AuNPs into a “spaghetti and meatballs” motif, protecting the DNA from degradation by nucleases and other chemical agents.73 The changes in DNA conformation were further studied using circular dichroism and fluorescence experiments, demonstrating complex reversible structural changes that occurred on binding.74 In an analogous strategy, Klibanov and Thomas covalently attached ~2 kDa polyethyleneimine (PEI) chains to AuNPs and used these PEI–AuNP conjugates as delivery vectors of plasmid DNA into mammalian cells.75 The most potent conjugates were 12 times more efficient at plasmid DNA delivery than their unmodified PEI counterparts.
Figure 3.
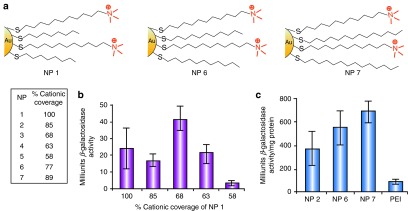
Mixed-monolayer-protected AuNPs for gene transfection. (a) Structures of cationic AuNPs with various amounts of cationic ligands (NP 1–5) and alkyl chain length (NP 6–7) used for transfection of DNA. (b) Transfection of β-galactosidase (β-gal) using NP–DNA complexes at 2,200:1 ratio (measured by β-gal activity). Levels of β-gal were determined by comparison with a standard curve using known protein concentrations. (c) Transfection efficiency of NPs 2, 6, and 7 at 2,200:1 NP/DNA ratio and commercially available PEI (60 kDa). NP, nanaoparticle; PEI, polyethyleneimine. Reprinted with permission from ref. 72. Copyright 2002 American Chemical Society.
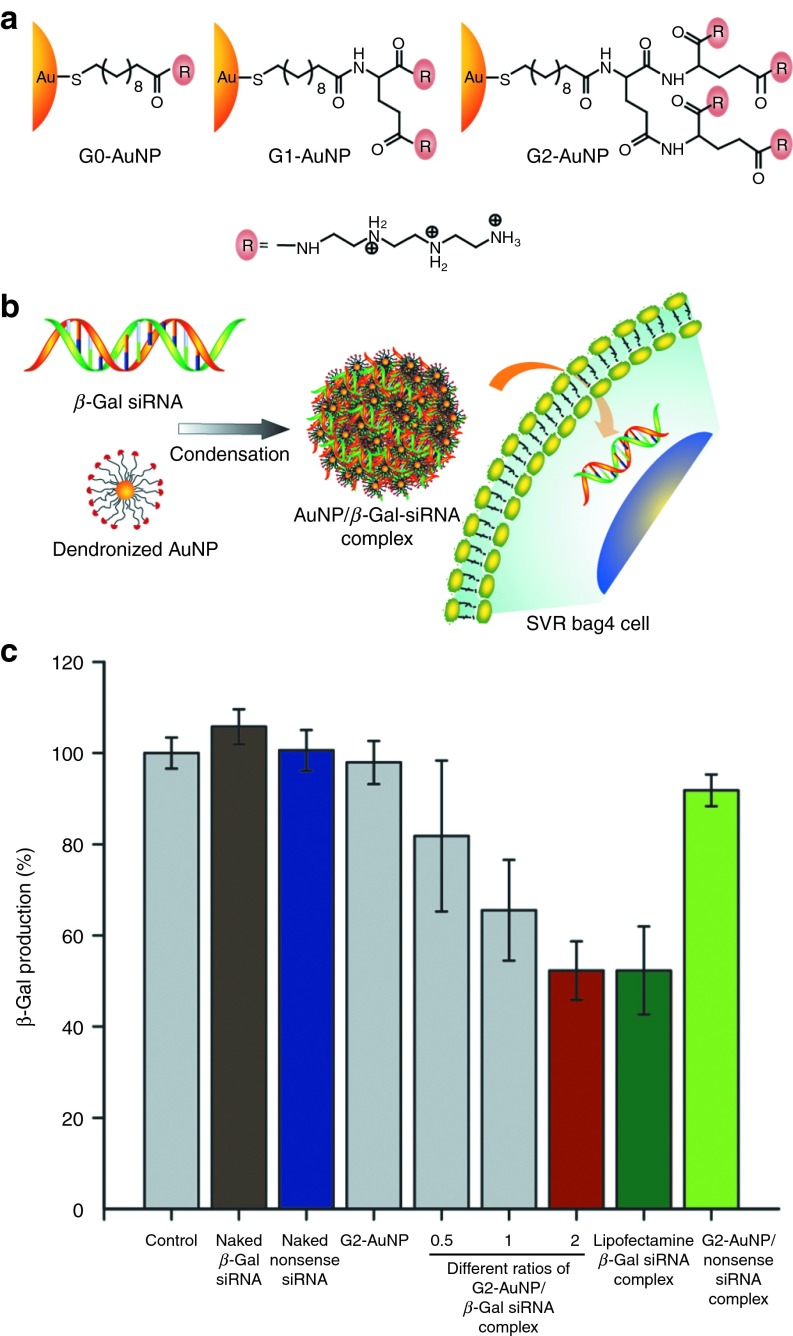
Amino acids provide versatile moieties for tuning AuNP functionality.76,77 Binding studies between cationic amino acid–functionalized AuNPs and double-stranded DNA (dsDNA) showed that side chains with increased cationic character were stronger binders than hydrophobic or neutral analogs.78 This concept was applied to gene delivery, where AuNPs featuring dendritic amino acid–based headgroups were shown to be effective gene delivery vehicles in vitro.79 As an example, first-generation lysine dendron (G1-Lys)–coated AuNPs were 28 times superior to polylysine in reporter gene expression. A similar dendron-based strategy was later used for siRNA delivery, where biodegradable glutamic acid scaffolds were functionalized with triethylenetetramine (Figure 4a).80 Second-generation dendronized AuNPs (G2-AuNPs) provided the most efficient knockdown, with β-galactosidase–siRNA (β-gal–siRNA) (Figure 4b) showing ~50% gene silencing with minimal cytotoxicity.
Figure 4.
siRNA delivery using dendronized AuNPs. (a) Chemical structure of G2-AuNP. (b) Schematic illustration of G2-AuNP/β-gal-siRNA complexation and transfection into SVR-bag4 cells. (c) Gene-silencing effect of naked β-gal-siRNA, naked nonsense siRNA, G2-AuNP, G2-AuNP/β-gal-siRNA complex, and G2-AuNP/nonsense-siRNA complex. β-gal-siRNA, β-galactosidase-siRNA; G2-AuNP, second-generation dendronized AuNPs; NP, nanoparticle; siRNA, small interfering RNA. Reprinted with permission from ref. 80. Copyright 2012 WILEY-VCH KGaA, Weinheim.
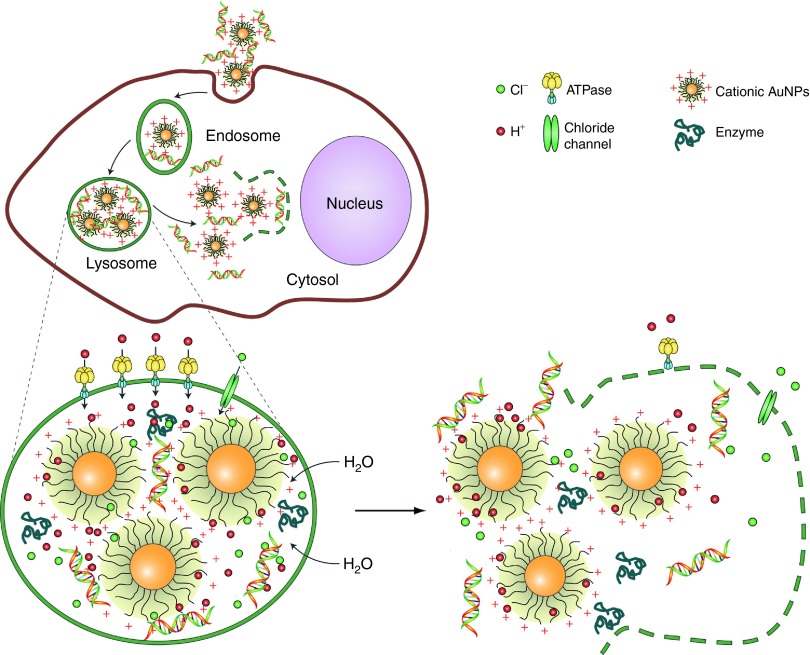
Although measuring the intracellular expression of β-galactosidase is an excellent way to investigate the transfection/knockdown efficiency, it does not shed much light on the uptake mechanism and the consequent endosomal release of noncovalent AuNP–NA conjugates. Noncovalent AuNP–nucleic acid (NA) conjugates are in general composed of cationic AuNPs that can facilitate endosomal escape via a “proton sponge” mechanism.67,81 The traditional “proton sponge” hypothesis explains that after fusion of the late endosome with a lysosome, materials (such as PEI) with amine groups are able to sequester protons once they enter the acidifying lysosomal compartment.82 The lysosomal “proton pump” process results in the retention of one Cl– ion and one water molecule for each proton entering the lysosome, maintaining electrical neutrality. This process results in lysosomal swelling and rupture, with materials released into the cytosol (Figure 5).67,83 However, a recent study did not observe any contribution from PEI in lysosomal pH change within a time frame of 0–24 hours, challenging the theory that the “proton sponge” is the dominant releasing mechanism.84
Figure 5.
Schematic illustration of the proton sponge effect leading to endosomal escape for cationic nanoparticles. ATPase, adenosine triphosphatase; NP, nanoparticle; PEI, polyethyleneimine. Adapted with permission from ref. 67. Copyright 2009 Macmillan Publishers.
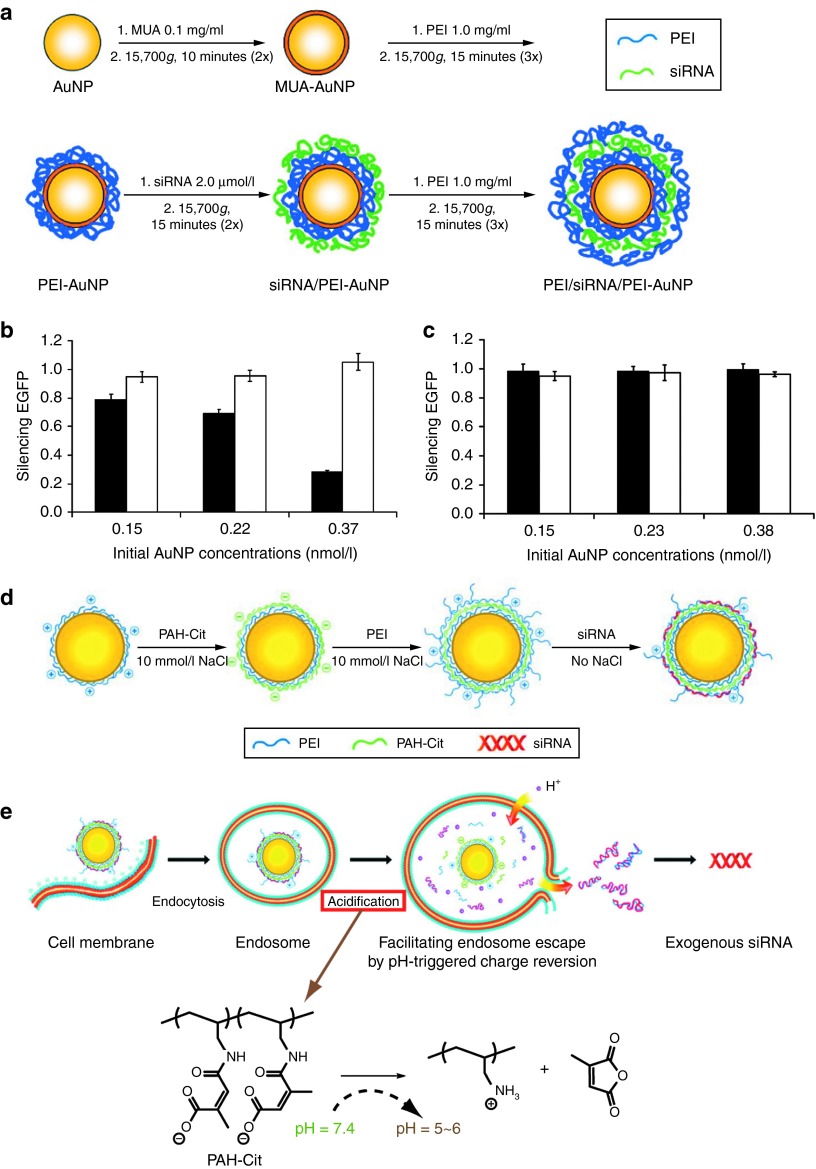
LbL fabrication using negatively charged nucleic acids and positively charged AuNPs provides a strategy for the controlled release of nucleic acids. In one example, mercaptoundecanoic acid–stabilized AuNPs were consecutively coated with layers of positively charged PEI and negatively charged siRNAs (Figure 6a).85 Both siRNA/PEI-AuNPs and PEI/siRNA/PEI-AuNPs showed much higher cellular uptake than citrate-stabilized AuNPs. PEI/siRNA/PEI-AuNPs showed nominal toxicity in cell culture despite containing high levels of PEI. For AuNP–NA conjugates constructed by the LbL method, the material on the top layer plays an important role in achieving endosomal escape.85 In the above example, when siRNAs were used as the outer layer, NPs were packed in the endosome but no enhanced green fluorescent protein knockdown occurred. By contrast, PEI/siRNA/PEI-AuNPs that have PEI on the outside provided 70% knockdown of enhanced green fluorescent protein (Figure 6b,c). This comparison indicates an endosomal escape mechanism of siRNA delivery by such LbL-AuNPs.85 Similarly, AuNPs with an incorporated anionic polyelectrolyte were prepared by an LbL method. Instead of embedding nucleic acids into two PEI layers, cis-aconitic anhydride–functionalized poly(allylamine) (PAH-Cit) was enclosed as a charge-reversal layer, with siRNA as the terminal layer (Figure 6d).86 By enhancing the capacity of PEI's “proton sponge” effect, the charge conversion occurring between pH 7.4 and pH 5.0 would facilitate the endosomal escape of these siRNA/PEI/PAH-Cit/PEI–AuNP complexes through membrane disruption. Gene silencing of ~80% was achieved by these complexes, whereas only 20% silencing was observed with controls lacking the charge-reversing element (Figure 6e).86
Figure 6.
siRNA delivery using LbL-AuNPs. Schematic representation of (a) layer-by-layer deposition applied to AuNPs. Gene silencing of enhanced green fluorescent protein (EGFP) in Chinese hamster ovary (CHO)-K1 cells stably expressing EGFP after addition of (b) PEI/siRNA/PEI-AuNPs and (c) siRNA/PEI-AuNPs at various initial concentrations. For LbL-AuNPs, either siRNA against EGFP (▪) was used or a nontargeted siRNA control (□) was used. Reprinted with permission from ref. 85. Copyright 2009 American Chemical Society. Schematic representation showing (d) the synthesis of LbL-AuNPs containing a charge-reversal layer (PAH-Cit). Schematic illustration of (e) enhanced intracellular payload release at endosome by a pH-dependent layer on AuNPs. LbL-AuNPs, layer-by-layer-fabricated AuNPs; MUA, 11-mercaptoundecanoic acid; NP, nanoparticles; PAH, cis-aconitic anhydride-functionalized poly(allylamine); PEI, polyethyleneimine; siRNA, small interfering RNA. Reprinted with permission from ref. 86. Copyright 2010 American Chemical Society.
Stimuli-Responsive Release of Nucleic Acid
Glutathione (GSH)-mediated release represents a promising approach for intracellular release of nucleic acids from AuNPs (Figure 7a).79 GSH is the most abundant thiol species in the cytoplasm, with intracellular GSH concentrations (1–10 mmol/l) dramatically higher than the extracellular levels (2 μmol/l in plasma).87 GSH features an overall negative charge; as a result, place exchange of GSH onto cationic particles diminishes and then reverses particle charge (Figure 7b,c). This strategy was hypothesized for the previously discussed dendron-based systems, an assertion validated by a dose-dependent increase in transfection efficiency79 on treatment of cells with glutathione monoester (GSH-OEt) (Figure 7d).88
Figure 7.
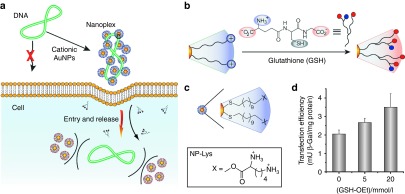
Glutathione-mediated intracellular gene release. (a) Schematic illustration of cationic gold nanoparticles (AuNPs) used as transfection vectors. (b) Schematic description of place exchange between native cationic ligands and cellular glutathione (GSH) on NP surface. (c) Chemical structure of lysine-based functionalized AuNPs. (d) Increase in transfection level depending on dose of glutathione monoester (GSH-OEt). Reprinted with permission from ref. 79. Copyright 2008 American Chemical Society.
Light-regulated release provides spatiotemporal control of nucleic acid release. In early studies, a photolabile AuNP was designed to photochemically uncage DNA in supramolecular AuNP complexes using near-ultraviolet (>350 nm) irradiation.89 By constructing a positively charged AuNP bearing a photocleavable o-nitrobenzyl ester linkage, the surface net charge of AuNPs could be switched from cationic to anionic under irradiation. The charge reversal yielded a negatively charged carboxylate group and consecutively released negatively charged DNA by electrostatic repulsion (Figure 8a). Efficient DNA delivery and release was also obtained in living cells, along with significant internalization of DNA into the nucleus.
Figure 8.
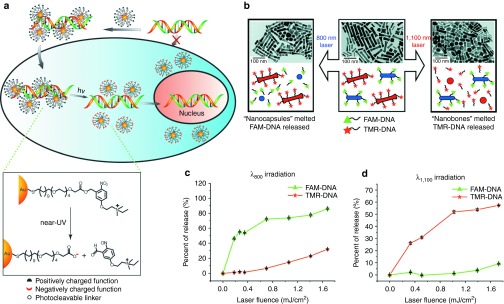
Light-regulated nucleic acid release in cells. Schematic illustration of (a) the release of DNA from the AuNP–DNA complexes on ultraviolet irradiation within the cell. Reprinted with permission from ref. 89. Copyright 2006 WILEY-VCH KGaA, Weinheim. Schematic illustration of (b) selective release of multiple DNA oligonucleotides from gold nanorods of different aspect ratios. Percentage release of 6-carboxyfluorescein–labeled DNA (FAM–DNA) and tetramethylrhodamine-labeled DNA (TMR-DNA) as a function of (c) λ800 laser fluence and (d) λ1100 laser fluence. Reprinted with permission from ref. 93. Copyright 2009 American Chemical Society.
Near-infrared irradiation can be used to broaden the application of light-regulated release, by increasing the depth of tissue penetration up to 10 cm.90 The strong surface plasmon absorption of gold nanorods (AuNRs) is tunable within the near-infrared range, making these particles excellent platforms for near-infrared applications.91 These AuNRs can be heated locally by using pulsed laser excitation, providing release of covalently bound nucleic acids on the rod surface of the AuNRs in a controlled fashion.92 The longitudinal surface plasmon resonance (SPRlong) of AuNRs can be tuned by altering the AuNR aspect ratio, providing a potential mechanism for wavelength-specific release. In one study, AuNRs with two different aspect ratios were functionalized with two different oligonucleotides—6-carboxyfluorescein–labeled DNA and tetramethylrhodamine-labeled DNA (Figure 8b). By applying this methodology, selective, externally controlled gene release was achieved by irradiating at 800 and 1100 nm, with a high level of payload delivery (Figure 8c,d).93 However, AuNRs demonstrated low endosomal release. Moreover, their cellular uptake was also lower than the uptake of spherical AuNPs due to their high aspect ratio and consequently lower number of available cell membrane receptor sites for binding.94 In addition, the difficulty associated with the endosomal and lysosomal escapes of AuNRs is a major concern for transfection efficiency.95 So far, there is no solid evidence for light-associated endosomal escape.
Conclusions and Outlook
Key properties of the AuNPs, such as biocompatibility, tunable size, and straightforward functionalization, make them attractive scaffolds for the creation of nucleic acid delivery vehicles. In particular, the design of AuNP–based covalent and noncovalent nucleic acid carriers significantly affects cellular uptake, endosomal escape, and nucleic acid release. To date, the potential of these systems has been mostly demonstrated using in vitro studies; however, there are issues to be resolved before AuNP–NA conjugates can be translated to clinical applications. First, minimizing short- and long-term cytotoxicity of AuNPs is essential. Numerous studies documented the biocompatibility of these therapeutic NPs using simple cytotoxicity experiments; however, detailed toxicological evaluation (cell membrane damage, oxidative stress, genotoxicity, and so on) needs to be properly addressed. Second, targeting of these vehicles to specific organs and tissues will be required to minimize side effects. This targeting can be achieved by (i) decorating the surface of delivery vehicles with specific antibodies targeted to the disease cells and (ii) grafting noninteracting functional groups (e.g., polyethylene glycol and zwitterionic entities) on the surface that eschew plasma protein adsorption, improving the pharmacokinetics and evading immune surveillance. Finally, immunological issues need to be fully explored before clinical use of any new material. Nonetheless, AuNPs provide a platform with all the attributes required to meet these challenges and should continue to provide important tools for in vitro applications, eventually yielding clinically important delivery vehicles.
Acknowledgments
The authors declare no conflict of interest. This work was supported by the National Institutes of Health (EB014277). Y.D. was supported by the China Scholarship Council (File No. 201208320185).
References
- Anderson WF. Human gene therapy. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Mulligan RC. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- Zhao N, Fogg JM, Zechiedrich L, Zu Y. Transfection of shRNA-encoding Minivector DNA of a few hundred base pairs to regulate gene expression in lymphoma cells. Gene Ther. 2011;18:220–224. doi: 10.1038/gt.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47:1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Takakura Y. Nonviral vector-mediated RNA interference: its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv Drug Deliv Rev. 2009;61:760–766. doi: 10.1016/j.addr.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Wu J, Yamanouchi D, Liu B, Chu CC. Biodegradable arginine-based poly(ether ester amide)s as a non-viral DNA delivery vector and their structure–function study. J Mater Chem. 2012;22:18983–18991. [Google Scholar]
- Yamanouchi D, Wu J, Lazar AN, Kent KC, Chu CC, Liu B. Biodegradable arginine-based poly(ester-amide)s as non-viral gene delivery reagents. Biomaterials. 2008;29:3269–3277. doi: 10.1016/j.biomaterials.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Kichler A, Zauner W, Ogris M, Wagner E. Influence of the DNA complexation medium on the transfection efficiency of lipospermine/DNA particles. Gene Ther. 1998;5:855–860. doi: 10.1038/sj.gt.3300658. [DOI] [PubMed] [Google Scholar]
- Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, Prato M, et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc. 2005;127:12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- Biju V, Anas A, Akita H, Shibu ES, Itoh T, Harashima H, et al. FRET from quantum dots to photodecompose undesired acceptors and report the condensation and decondensation of plasmid DNA. ACS Nano. 2012;6:3776–3788. doi: 10.1021/nn2048608. [DOI] [PubMed] [Google Scholar]
- Chen AA, Derfus AM, Khetani SR, Bhatia SN. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res. 2005;33:e190. doi: 10.1093/nar/gni188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, et al. PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: synthesis, complexation, and transfection. Adv Funct Mater. 2009;19:2244–2251. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsianti M, Lim M, Marquis CP, Amal R. Assembly of polyethylenimine-based magnetic iron oxide vectors: insights into gene delivery. Langmuir. 2010;26:7314–7326. doi: 10.1021/la9041919. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- Rana S, Bajaj A, Mout R, Rotello VM. Monolayer coated gold nanoparticles for delivery applications. Adv Drug Deliv Rev. 2012;64:200–216. doi: 10.1016/j.addr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell CM, Jung JM, Atukorale PU, Carney RP, Stellacci F, Irvine DJ. Oligonucleotide delivery by cell-penetrating “striped” nanoparticles. Angew Chem Int Ed Engl. 2011;50:12312–12315. doi: 10.1002/anie.201104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Saha K, Kim C, Rotello VM. The role of surface functionality in determining nanoparticle cytotoxicity. Acc Chem Res. 2013;46:681–691. doi: 10.1021/ar3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindy ME, Prud'homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- Panyala NR, Pena-Mendez EM, Josef H. Gold and nano-gold in medicine: overview, toxicology and perspectives. J Appl Biomed. 2009;7:75–91. [Google Scholar]
- Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater. 2009;19:1553–1566. [Google Scholar]
- Beaux MF, 2nd, McIlroy DN, Gustin KE. Utilization of solid nanomaterials for drug delivery. Expert Opin Drug Deliv. 2008;5:725–735. doi: 10.1517/17425247.5.7.725. [DOI] [PubMed] [Google Scholar]
- Roca M, Haes AJ. Probing cells with noble metal nanoparticle aggregates. Nanomedicine (Lond) 2008;3:555–565. doi: 10.2217/17435889.3.4.555. [DOI] [PubMed] [Google Scholar]
- Sanvicens N, Marco MP. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61:1027–1040. [Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Shenhar R, Rotello VM. Nanoparticles: scaffolds and building blocks. Acc Chem Res. 2003;36:549–561. doi: 10.1021/ar020083j. [DOI] [PubMed] [Google Scholar]
- Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. ). Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J Chem Soc, Chem Commun. 1994;7:801–802. [Google Scholar]
- Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JA, Overton KW, Speight ME, Oldenburg CN, Loo L, Robarge W, et al. Cellular uptake of gold nanoparticles passivated with BSA-SV40 large T antigen conjugates. Anal Chem. 2007;79:9150–9159. doi: 10.1021/ac0715524. [DOI] [PubMed] [Google Scholar]
- Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu ZJ, et al. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZJ, Carboni R, Quercio MJ, Jr, Yan B, Miranda OR, Anderton DL, et al. Surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish. Small. 2010;6:2261–2265. doi: 10.1002/smll.201000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 2013;46:761–769. doi: 10.1021/ar2003336. [DOI] [PubMed] [Google Scholar]
- Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Perrett S, Nie G. Understanding the particokinetics of engineered nanomaterials for safe and effective therapeutic applications. Small. 2013;9:1619–1634. doi: 10.1002/smll.201201630. [DOI] [PubMed] [Google Scholar]
- Pakiari AH, Jamshidi Z. Nature and strength of M-S bonds (m = Au, Ag, and Cu) in binary alloy gold clusters. J Phys Chem A. 2010;114:9212–9221. doi: 10.1021/jp100423b. [DOI] [PubMed] [Google Scholar]
- Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J Am Chem Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton-Jean AK, Langer R, Anderson DG. Five years of siRNA delivery: spotlight on gold nanoparticles. Small. 2011;7:1932–1937. doi: 10.1002/smll.201100761. [DOI] [PubMed] [Google Scholar]
- Seferos DS, Prigodich AE, Giljohann DA, Patel PC, Mirkin CA. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009;9:308–311. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–3821. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Wu G, Li Z, Mirkin CA, Schatz GC. What controls the melting properties of DNA-linked gold nanoparticle assemblies. J Am Chem Soc. 2003;125:1643–1654. doi: 10.1021/ja021096v. [DOI] [PubMed] [Google Scholar]
- Choi CH, Hao L, Narayan SP, Auyeung E, Mirkin CA. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc Natl Acad Sci U S A. 2013;110:7625–7630. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hao L, Hurst SJ, Mirkin CA. Antibody-linked spherical nucleic acids for cellular targeting. J Am Chem Soc. 2012;134:16488–16491. doi: 10.1021/ja306854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Patel PC, Alhasan AH, Giljohann DA, Mirkin CA. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small. 2011;7:3158–3162. doi: 10.1002/smll.201101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xie J. Progress on RNAi-based molecular medicines. Int J Nanomedicine. 2012;7:3971–3980. doi: 10.2147/IJN.S31897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Nakaogami J, Ishii T, Nagasaki Y. Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing. Chem Lett. 2006;35:1046–1047. [Google Scholar]
- Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci U S A. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Lévy R, Shaheen U, Cesbron Y, Sée V. Gold nanoparticles delivery in mammalian live cells: a critical review. Nano Rev. 2010;1:4889. doi: 10.3402/nano.v1i0.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates. Mol Pharm. 2009;6:1934–1940. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh CM, Esposito EA, 3rd, Boal AK, Simard JM, Martin CT, Rotello VM. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J Am Chem Soc. 2001;123:7626–7629. doi: 10.1021/ja015556g. [DOI] [PubMed] [Google Scholar]
- Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjug Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- Han G, Martin CT, Rotello VM. Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem Biol Drug Des. 2006;67:78–82. doi: 10.1111/j.1747-0285.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Goodman CM, Chari NS, Han G, Hong R, Ghosh P, Rotello VM. DNA-binding by functionalized gold nanoparticles: mechanism and structural requirements. Chem Biol Drug Des. 2006;67:297–304. doi: 10.1111/j.1747-0285.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2003;100:9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You CC, De M, Han G, Rotello VM. Tunable inhibition and denaturation of alpha-chymotrypsin with amino acid-functionalized gold nanoparticles. J Am Chem Soc. 2005;127:12873–12881. doi: 10.1021/ja0512881. [DOI] [PubMed] [Google Scholar]
- You CC, Agasti SS, Rotello VM. Isomeric control of protein recognition with amino acid- and dipeptide-functionalized gold nanoparticles. Chemistry. 2008;14:143–150. doi: 10.1002/chem.200701234. [DOI] [PubMed] [Google Scholar]
- Ghosh PS, Han G, Erdogan B, Rosado O, Krovi SA, Rotello VM. Nanoparticles featuring amino acid-functionalized side chains as DNA receptors. Chem Biol Drug Des. 2007;70:13–18. doi: 10.1111/j.1747-0285.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Chompoosor A, Yeh YC, Agasti SS, Solfiell DJ, Rotello VM. Dendronized gold nanoparticles for siRNA delivery. Small. 2012;8:3253–3256. doi: 10.1002/smll.201201141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, May S. Release of cationic polymer-DNA complexes from the endosome: a theoretical investigation of the proton sponge hypothesis. J Chem Phys. 2008;129:185105. doi: 10.1063/1.3009263. [DOI] [PubMed] [Google Scholar]
- Behr J. The proton sponge: a trick to enter cells the viruses did not exploit. Chimia. 1997;51:34–36. [Google Scholar]
- Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “proton sponge “ effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, Breunig M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9:2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z, et al. Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte. ACS Nano. 2010;4:5505–5511. doi: 10.1021/nn101638u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Hong R, Han G, Fernández JM, Kim BJ, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- Han G, You CC, Kim BJ, Turingan RS, Forbes NS, Martin CT, et al. Light-regulated release of DNA and its delivery to nuclei by means of photolabile gold nanoparticles. Angew Chem Int Ed Engl. 2006;45:3165–3169. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lin YP, Wang CW, Tzeng HC, Wu CH, Chen YC, et al. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J Am Chem Soc. 2006;128:3709–3715. doi: 10.1021/ja0570180. [DOI] [PubMed] [Google Scholar]
- Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2009;3:80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- Chen J, Irudayaraj J. Quantitative investigation of compartmentalized dynamics of ErbB2 targeting gold nanorods in live cells by single molecule spectroscopy. ACS Nano. 2009;3:4071–4079. doi: 10.1021/nn900743v. [DOI] [PubMed] [Google Scholar]