Abstract
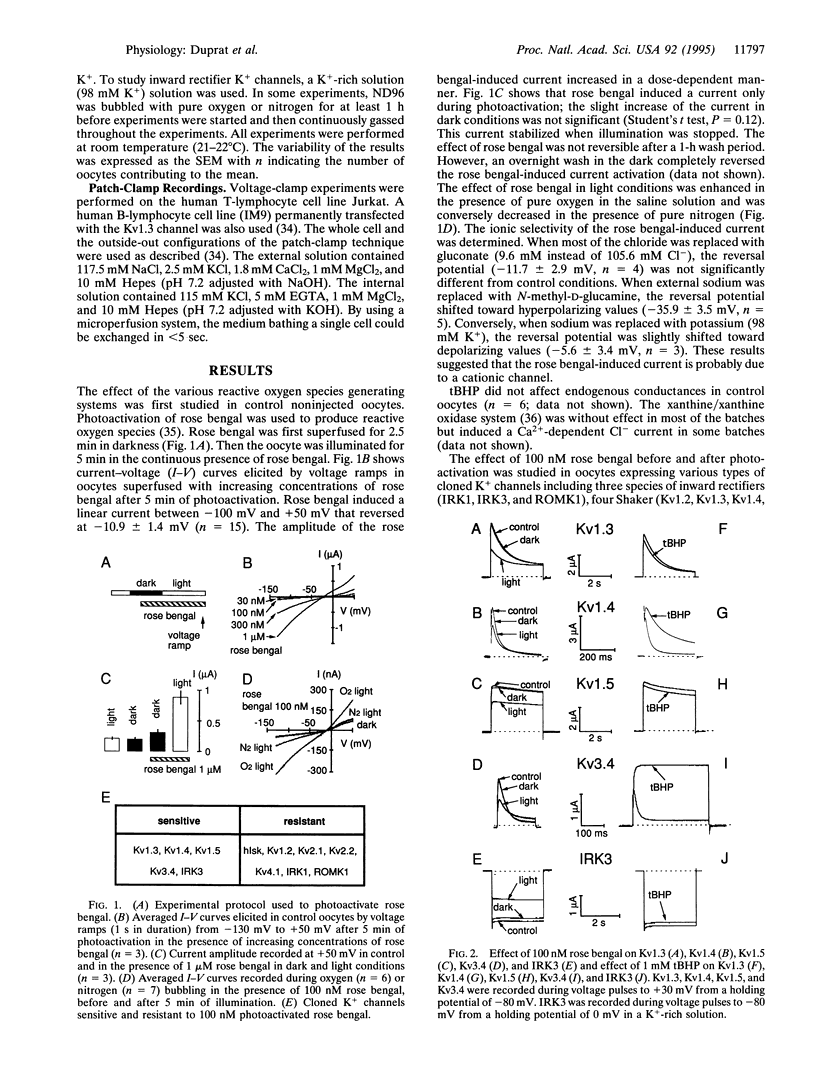
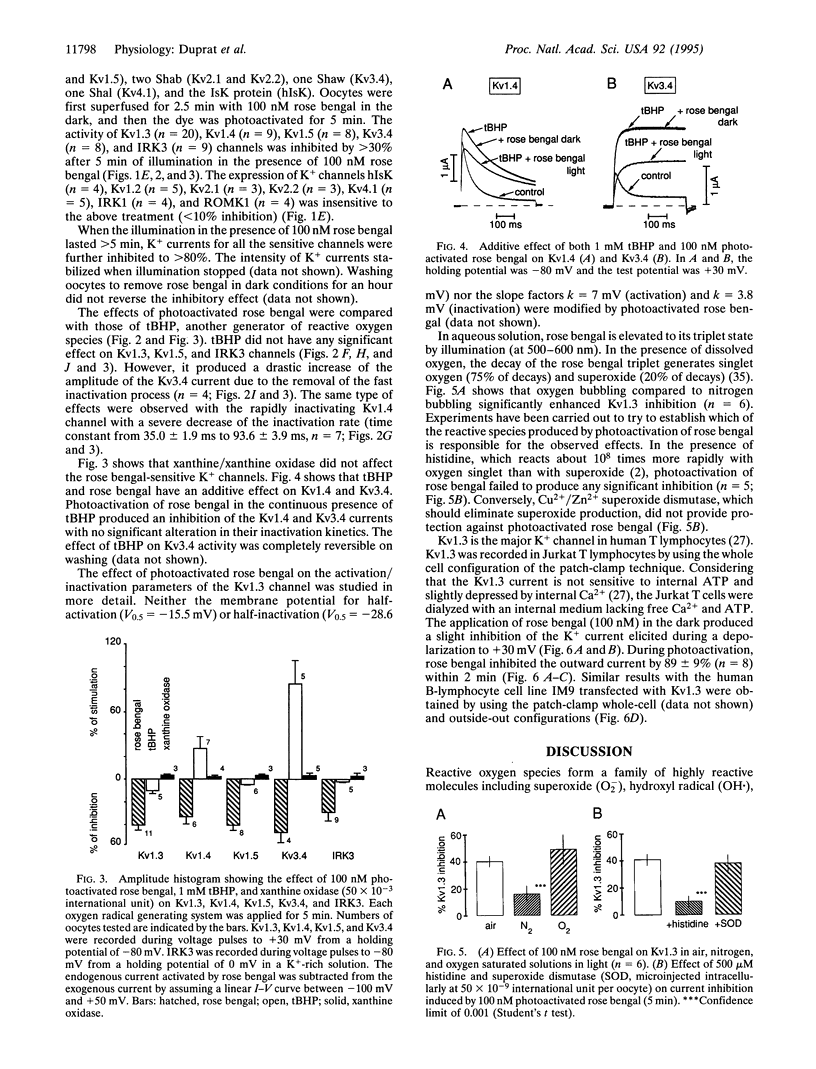
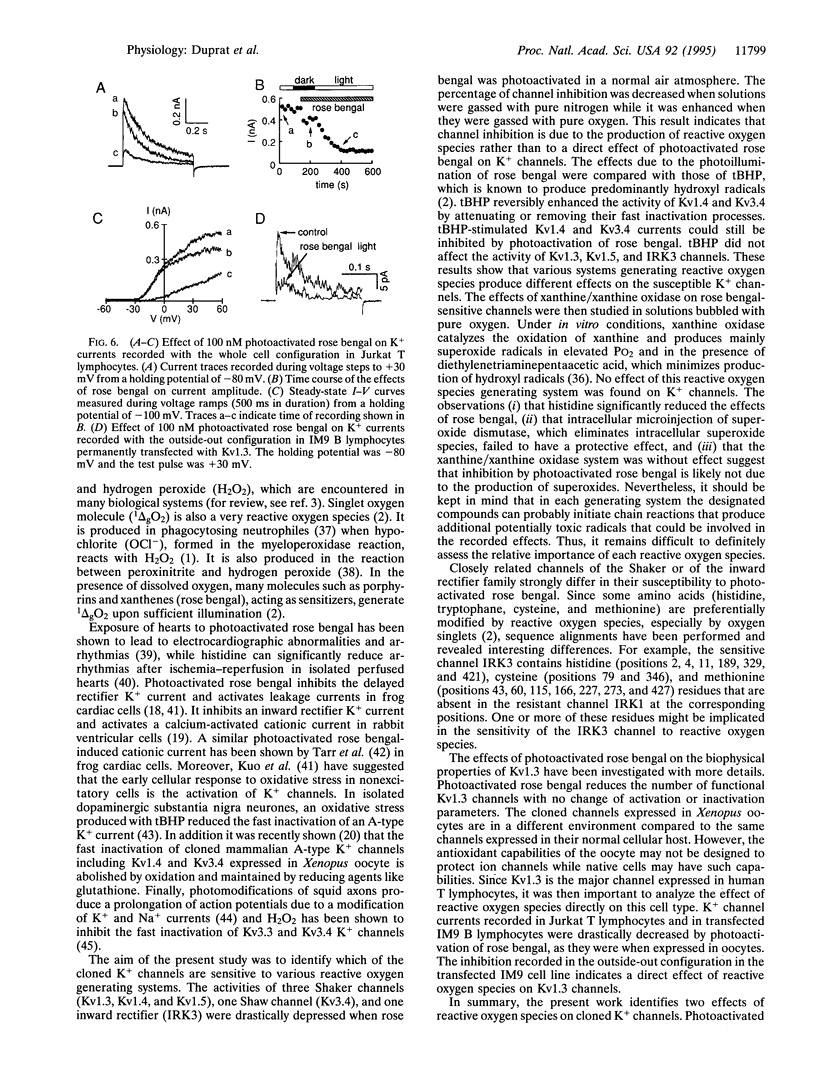
Free radical-induced oxidant stress has been implicated in a number of physiological and pathophysiological states including ischemia and reperfusion-induced dysrhythmia in the heart, apoptosis of T lymphocytes, phagocytosis, and neurodegeneration. We have studied the effects of oxidant stress on the native K+ channel from T lymphocytes and on K+ channels cloned from cardiac, brain, and T-lymphocyte cells and expressed in Xenopus oocytes. The activity of three Shaker K+ channels (Kv1.3, Kv1.4, and Kv1.5), one Shaw channel (Kv3.4), and one inward rectifier K+ channel (IRK3) was drastically inhibited by photoactivation of rose bengal, a classical generator of reactive oxygen species. Other channel types (such as Shaker K+ channel Kv1.2, Shab channels Kv2.1 and Kv2.2, Shal channel Kv4.1, inward rectifiers IRK1 and ROMK1, and hIsK) were completely resistant to this treatment. On the other hand tert-butyl hydroperoxide, another generator of reactive oxygen species, removed the fast inactivation processes of Kv1.4 and Kv3.4 but did not alter other channels. Xanthine/xanthine oxidase system had no effect on all channels studied. Thus, we show that different types of K+ channels are differently modified by reactive oxygen species, an observation that might be of importance in disease states.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attali B., Guillemare E., Lesage F., Honoré E., Romey G., Lazdunski M., Barhanin J. The protein IsK is a dual activator of K+ and Cl- channels. Nature. 1993 Oct 28;365(6449):850–852. doi: 10.1038/365850a0. [DOI] [PubMed] [Google Scholar]
- Attali B., Lesage F., Ziliani P., Guillemare E., Honoré E., Waldmann R., Hugnot J. P., Mattéi M. G., Lazdunski M., Barhanin J. Multiple mRNA isoforms encoding the mouse cardiac Kv1-5 delayed rectifier K+ channel. J Biol Chem. 1993 Nov 15;268(32):24283–24289. [PubMed] [Google Scholar]
- Attali B., Romey G., Honoré E., Schmid-Alliana A., Mattéi M. G., Lesage F., Ricard P., Barhanin J., Lazdunski M. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J Biol Chem. 1992 Apr 25;267(12):8650–8657. [PubMed] [Google Scholar]
- Baldwin T. J., Tsaur M. L., Lopez G. A., Jan Y. N., Jan L. Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991 Sep;7(3):471–483. doi: 10.1016/0896-6273(91)90299-f. [DOI] [PubMed] [Google Scholar]
- Bing O. H. Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol. 1994 Aug;26(8):943–948. doi: 10.1006/jmcc.1994.1115. [DOI] [PubMed] [Google Scholar]
- Bright J., Khar A. Apoptosis: programmed cell death in health and disease. Biosci Rep. 1994 Apr;14(2):67–81. doi: 10.1007/BF01210302. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Bechara E. J., Medeiros M. H., Briviba K., Sies H. Singlet molecular oxygen production in the reaction of peroxynitrite with hydrogen peroxide. FEBS Lett. 1994 Dec 5;355(3):287–289. doi: 10.1016/0014-5793(94)01224-5. [DOI] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dixon S. J. Ion transport, membrane potential, and cytoplasmic pH in lymphocytes: changes during activation. Physiol Rev. 1989 Apr;69(2):417–481. doi: 10.1152/physrev.1989.69.2.417. [DOI] [PubMed] [Google Scholar]
- Guillemare E., Honoré E., Pradier L., Lesage F., Schweitz H., Attali B., Barhanin J., Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992 Dec 15;31(49):12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Lesage F., Barhanin J., Lazdunski M. Different types of K+ channel current are generated by different levels of a single mRNA. EMBO J. 1992 Jul;11(7):2465–2471. doi: 10.1002/j.1460-2075.1992.tb05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P. M., Glatt C. E., Bredt D. S., Yellen G., Snyder S. H. A novel K+ channel with unique localizations in mammalian brain: molecular cloning and characterization. Neuron. 1992 Mar;8(3):473–481. doi: 10.1016/0896-6273(92)90275-i. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Khan A. U., Kasha M. Singlet molecular oxygen evolution upon simple acidification of aqueous hypochlorite: application to studies on the deleterious health effects of chlorinated drinking water. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12362–12364. doi: 10.1073/pnas.91.26.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kukreja R. C., Loesser K. E., Kearns A. A., Naseem S. A., Hess M. L. Protective effects of histidine during ischemia-reperfusion in isolated perfused rat hearts. Am J Physiol. 1993 May;264(5 Pt 2):H1370–H1381. doi: 10.1152/ajpheart.1993.264.5.H1370. [DOI] [PubMed] [Google Scholar]
- Kuo S. S., Saad A. H., Koong A. C., Hahn G. M., Giaccia A. J. Potassium-channel activation in response to low doses of gamma-irradiation involves reactive oxygen intermediates in nonexcitatory cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):908–912. doi: 10.1073/pnas.90.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama Y., Bernier M., Hearse D. J. Singlet oxygen-induced arrhythmias. Dose- and light-response studies for photoactivation of rose bengal in the rat heart. Circulation. 1989 Nov;80(5):1432–1448. doi: 10.1161/01.cir.80.5.1432. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Rodgers M. A. Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and DNA. Photochem Photobiol. 1987 Jan;45(1):79–86. doi: 10.1111/j.1751-1097.1987.tb08407.x. [DOI] [PubMed] [Google Scholar]
- Lesage F., Duprat F., Fink M., Guillemare E., Coppola T., Lazdunski M., Hugnot J. P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994 Oct 10;353(1):37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- Lingueglia E., Renard S., Waldmann R., Voilley N., Champigny G., Plass H., Lazdunski M., Barbry P. Different homologous subunits of the amiloride-sensitive Na+ channel are differently regulated by aldosterone. J Biol Chem. 1994 May 13;269(19):13736–13739. [PubMed] [Google Scholar]
- Matsuura H., Shattock M. J. Effects of oxidant stress on steady-state background currents in isolated ventricular myocytes. Am J Physiol. 1991 Nov;261(5 Pt 2):H1358–H1365. doi: 10.1152/ajpheart.1991.261.5.H1358. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993 Nov;16(11):439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P., Narahashi T. Internal and external application of photodynamic sensitizers on squid giant axons. J Membr Biol. 1977 Sep 14;36(2-3):159–173. doi: 10.1007/BF01868149. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Schröter K. H., Ruppersberg J. P., Wunder F., Rettig J., Stocker M., Pongs O. Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain. FEBS Lett. 1991 Jan 28;278(2):211–216. doi: 10.1016/0014-5793(91)80119-n. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Agardh C. D., Bengtsson F. Free radicals and brain damage. Cerebrovasc Brain Metab Rev. 1989 Fall;1(3):165–211. [PubMed] [Google Scholar]
- Steinbeck M. J., Khan A. U., Karnovsky M. J. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J Biol Chem. 1992 Jul 5;267(19):13425–13433. [PubMed] [Google Scholar]
- Tarr M., Arriaga E., Goertz K. K., Valenzeno D. P. Properties of cardiac I(leak) induced by photosensitizer-generated reactive oxygen. Free Radic Biol Med. 1994 Apr;16(4):477–484. doi: 10.1016/0891-5849(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J. C., Tseng G. N., Schwartz A., Tanouye M. A. Molecular cloning and functional expression of a potassium channel cDNA isolated from a rat cardiac library. FEBS Lett. 1990 Jul 30;268(1):63–68. doi: 10.1016/0014-5793(90)80973-m. [DOI] [PubMed] [Google Scholar]
- Valenzeno D. P., Tarr M. Membrane photomodification of cardiac myocytes: potassium and leakage currents. Photochem Photobiol. 1991 Feb;53(2):195–201. doi: 10.1111/j.1751-1097.1991.tb03923.x. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E., Rudy B. Modulation of K+ channels by hydrogen peroxide. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- Wang W., Sackin H., Giebisch G. Renal potassium channels and their regulation. Annu Rev Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- Yu B. P. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994 Jan;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]



