Abstract
Systemic inflammatory response syndrome (SIRS) is a rare systemic inflammatory response associated with fever, tachycardia, profound hypotension, and respiratory distress, which has been reported in cancer patients receiving T cells genetically modified with chimeric antigen receptors to retarget their specificity to tumor-associated antigens. The syndrome usually occurs following significant in vivo expansion of the infused cells and has been associated with tumor destruction/lysis. Analysis of patient plasma has shown elevated cytokine levels, and resolution of symptoms has been reported after administration of steroids and/or antibodies (such as anti–tumor necrosis factor and anti-interleukin (IL)-6 receptor antibodies) that interfere with cytokine responses.To date, SIRS has not been reported in subjects receiving genetically unmodified T cells with native receptors directed against tumor antigens, in which greater physiological control of T-cell activation and expansion may occur. Here, however, we report a patient with bulky refractory Epstein-Barr virus (EBV)–associated lymphoma, who developed this syndrome 2 weeks after receiving T cells directed against EBV antigens through their native receptors. She was treated with steroids and etanercept, with rapid resolution of symptoms. SIRS may therefore occur even when T cells recognize antigens physiologically through their “wild-type” native receptors and should be acknowledged as a potential complication of this therapy.
Introduction
Several recent reports have described encouraging clinical responses in patients receiving T cells genetically modified to express a chimeric antigen receptor (CAR) targeting CD19, a B-cell lineage-specific antigen expressed on B-cell leukemia or lymphoma cells.1,2,3,4,5,6,7 In many of these patients, tumor response was associated with a marked expansion of CAR-modified T cells, often accompanied by a “cytokine storm” characterized by elevated levels of cytokines, including interleukin (IL)-6 and tumor necrosis factor-α (TNF-α), in the bloodstream. This has been attributed to the activation of tumor-directed T cells via their artificial receptors, which usually incorporate costimulatory moieties, followed by recruitment of other immune system effectors. Clinically, the presentation is similar to systemic inflammatory response syndrome (SIRS), a syndrome characterized by fever, tachycardia, and hypotension, which has also been observed after administration of cytokines or some monoclonal antibodies.8 The episodes occurring in recipients of CAR-modified T cells have been successfully treated with steroids or antibodies to tumor necrosis factor-α and IL6 receptor.9
By contrast, such complications have not previously been reported in subjects (numbering >150) who have received T cells expressing native receptors directed to tumor-associated Epstein-Barr virus (EBV) antigens, a difference that has been attributed to the more physiological antigen–receptor interactions that occur in this setting.10 Here, however, we now describe a SIRS-like syndrome that developed in a patient who received a T-cell product that was not genetically modified with a CAR but that recognized viral antigens through native receptors.
Results
Patient history
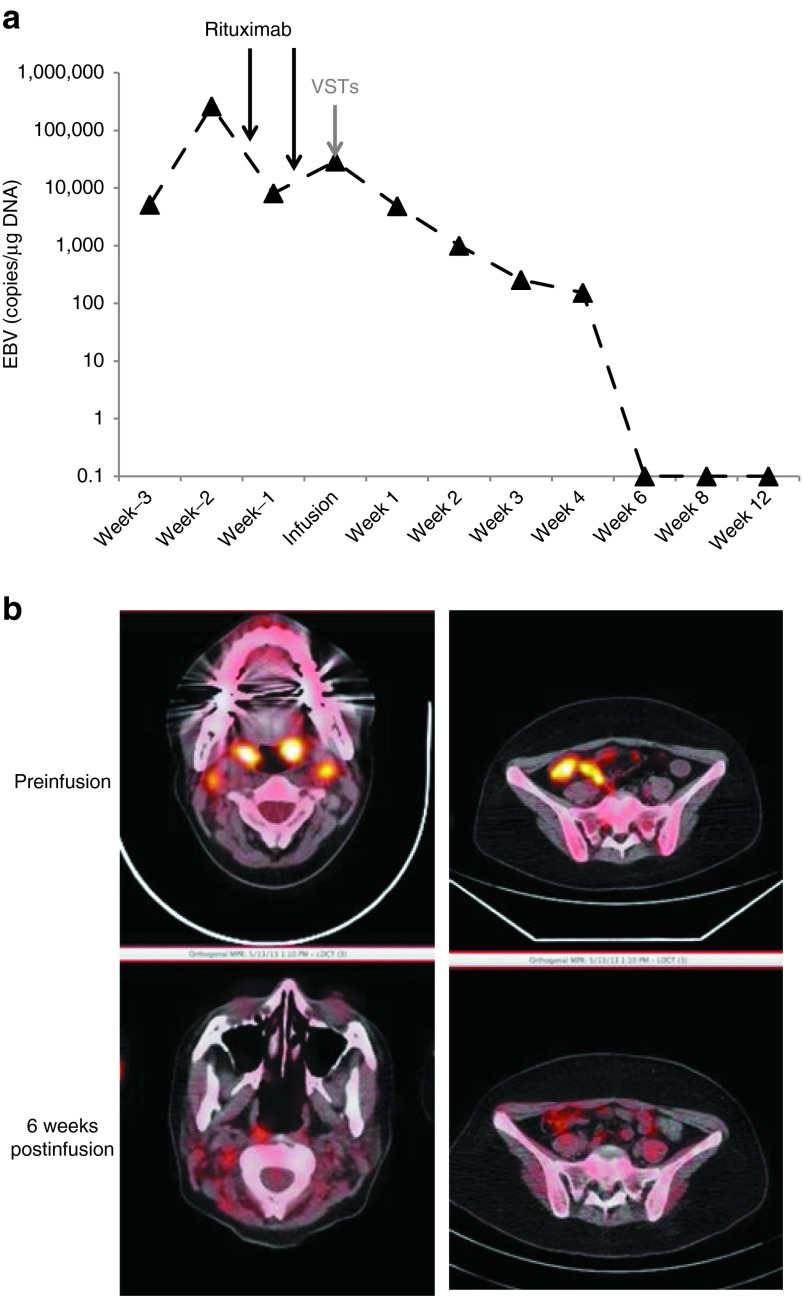
CAGT #3022 is a 19-year-old female with relapsed EBV-negative Hodgkin lymphoma who received a 9/10 human leukocyte antigen (HLA)–matched (DRB1 mismatch) transplant from an unrelated donor after conditioning with 600-cGy total body irradiation, fludarabine, and campath. Three months after transplant, she developed rapidly progressive EBV posttransplant lymphoproliferative disease with a rapid elevation in EBV DNA and positron emission tomography imaging showing extensive lymphadenopathy (Figure 1).
Figure 1.
Clinical response to VSTs. The patient was infused with donor-derived virus-specific T cells at a dose of 5 × 106 cells/m2 (dose level 1). Before receiving the cells, the patient had received rituximab twice. (a) The patient's EBV load over time, with arrows indicating the time points at which rituximab and T cells were administered. (b) Extensive disease on the PET–CT scan before SIRS and complete resolution on the follow-up scan 6 weeks later. EBV, Epstein-Barr virus; PET–CT, positron emission tomography–computed tomography; SIRS, systemic inflammatory response syndrome; VST, virus-specific T cells.
She received two doses of rituximab, but after an initial fall in EBV DNA, clinical examination and computed tomography scan showed progressive disease with development of lymph nodes in the neck, chest, abdomen, and elsewhere. Lactate dehydrogenase levels increased to 2,000 international units (IU), and bone marrow analysis showed an increased EBV viral load of >100,000 copies per microgram of DNA. Biopsy confirmed EBV-positive polymorphic posttransplant lymphoproliferative disorder. We obtained approval to treat her on a single-patient protocol on an established investigational new drug application with donor-derived ex vivo–expanded T cells that can be specific for up to five viruses including EBV11 (see Figure 2 for phenotype and specificity of the infused line), and she received a single dose of 5 × 106 cells/m2 with no immediate adverse effects. By 2 weeks postinfusion, her peripheral blood EBV DNA fell to the normal range and her clinical status improved, coincident with repeat imaging showing smaller nodes and adenoids and resolution of lung changes (Figure 1).
Figure 2.
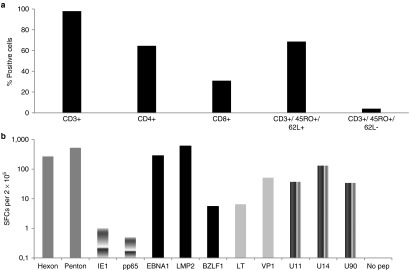
Immunophenotype and specificity of VST line. (a) The phenotype of the VST line on the day of cryopreservation. (b) The frequency of AdV- (Hexon and Penton), CMV- (IE1 and pp65), EBV- (LMP2, EBNA1, and BZLF1), BKV- (large T (LT) and VP1), and HHV6 (U11, U14, and U90)–reactive T cells in the VST line as measured by IFNγ enzyme-linked immunospot analysis per 2 × 105 input cells. Control was IFNγ release in response to stimulation with no pepmix. AdV, adenovirus; BKV, BK virus; CMV, cytomegalovirus; EBNA1, Epstein-Barr virus nuclear antigen 1; EBV, Epstein-Barr virus; HHV, human herpes virus; IE1, immediate-early 1; IFN, interferon; LMP2, latent membrane protein 2; pp65, phosphoprotein 65; VST, virus-specific T cells.
Two weeks after receiving virus-specific T cells (VSTs), she developed a new fever and was started on antibiotics. Over the next day, she developed skin rash, tachycardia, and hypotension and was transferred to the intensive care unit for inotropic support with dopamine and norepinephrine. She did not show any evidence of tumor lysis syndrome, and lactate dehydrogenase had already fallen to the normal range. After sepsis was excluded, she was given empirical etanercept and methylprednisolone (1 mg/kg daily, ×2). Her symptoms resolved within a few hours, and inotropic support was discontinued. Nine months after receiving VSTs, she remains well, and her EBV lymphoma remains in remission. However, her Hodgkin lymphoma progressed with R cervical node and mediastinal disease (biopsy confirmed as CD30-positive and EBV-negative), and she is receiving therapy with brentuximab.
T-cell persistence
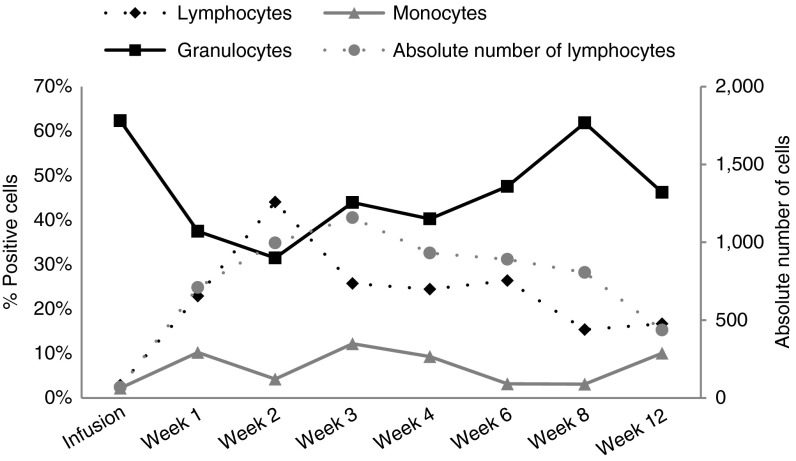
We tracked cellular immune reconstitution postinfusion. Figure 3 shows that there was a rapid rise in total lymphocyte count at week 2 when SIRS occurred. Subset analysis demonstrated that equivalent expansion occurred in both CD4+ and CD8+ T-cell compartments.
Figure 3.
Immune reconstitution. This figure shows the frequency of lymphocytes, monocytes, and granulocytes (% of total) before and at weeks 1, 2, 3, 4, 6, 8, and 12 postinfusion. Between the infusion and week 2 (period when SIRS occurred), the lymphocytes increased substantially (from 2.88% to 44% of total white blood cells). SIRS, systemic inflammatory response syndrome.
EBV-specific T-cell activity
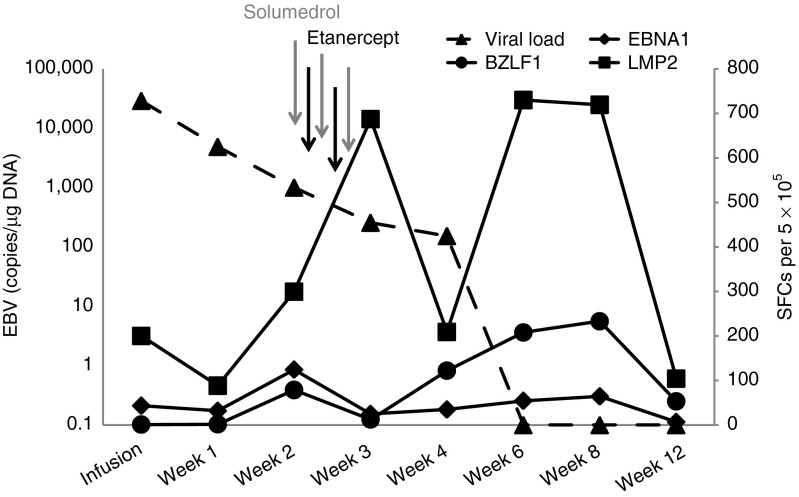
To determine whether the rise in total lymphocyte count was due to selective expansion of EBV-reactive cells, we tracked functional T-cell responses by interferon (IFN)-γ enzyme-linked immunospot (ELIspot) assay, stimulating the lymphocytes with the EBV antigens Epstein–Barr nuclear antigen 1 (EBNA1), latent membrane protein 2 (LMP2), and BZLF1 to which the infused T-cell line had responded (Figure 4). Preinfusion, the EBV load in peripheral blood was 22,364 copies/μg DNA and the patient had evidence of circulating EBV-specific T cells against EBNA1 and LMP2 (43 and 200 spot-forming cells (SFCs) per 5 × 105 peripheral blood mononuclear cells (PBMCs), respectively), with no activity against BZLF1 (0 SFC per 5 × 105 PBMCs). Two weeks postinfusion, during the episode of SIRS, the EBV load had decreased to 1,002 copies/μg DNA, whereas the frequency of EBV-reactive T cells had increased to 124, 300, and 79 SFCs per 5 × 105 PBMCs against EBNA1, LMP2, and BZLF1, respectively. The administration of solumedrol and etanercept between weeks 2 and 3 postinfusion reduced the numbers of T cells reacting to EBNA1 (decrease to 25 SFCs) and BZLF1 (decrease to 13 SFCs) at week 3, although the numbers of LMP2-reactive T-cells continued to increase (687 SFCs at week 3). However, 3 weeks later (at week 6 postinfusion), the frequency of T cells directed to EBNA1, LMP2, and BZLF1 had again increased to 54, 730, and 208 SFCs per 5 × 105 PBMCs, respectively. This secondary expansion, however, was not associated with the recurrence of SIRS. The patient also had increased T cells directed against the adenovirus antigens hexon and penton compared with the levels at week 4 postinfusion, although polymerase chain reaction studies did not detect any adenovirus in the peripheral blood. The subject also had transient reactivations of both BK virus (BKV) and human herpes virus 6 (HHV6) in the peripheral blood, both of which cleared following an increase in the frequency of T cells reactive against both viruses (Supplementary Figure S1).
Figure 4.
Immune response to EBV. This graph shows the EBV load (dotted line) on the day of infusion as well as the precursor frequency of T cells directed against the EBV antigens targeted in the infused T-cell line—EBNA1, LMP2, and BZLF1—before and after infusion. The time points at which etanercept and solumedrol were administered are also indicated. EBNA1, Epstein-Barr virus nuclear antigen 1; EBV, Epstein-Barr virus; LMP2, latent membrane protein 2.
Cytokine profile
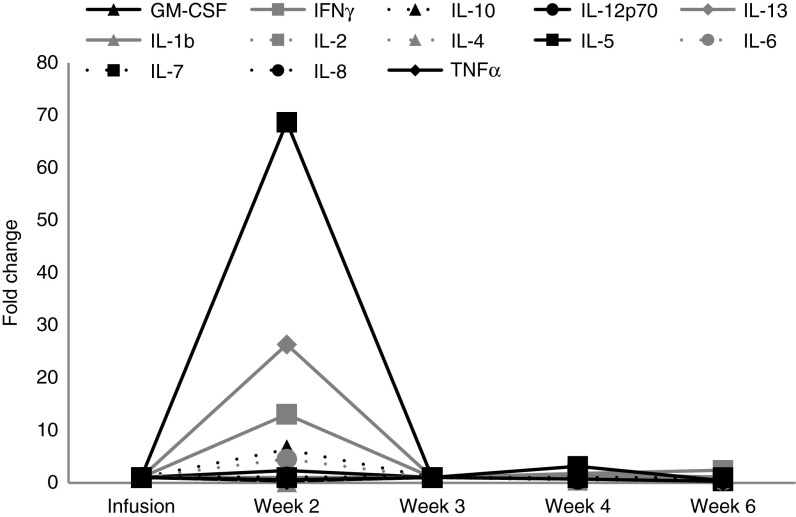
Figure 5 shows the plasma cytokine profile pre- and post-VST infusion. There was an elevation in IL5, IL13, IL10, IL6, and IFNγ levels (range: 5- to 69-fold change relative to baseline) at 2 weeks postinfusion, coincident with the onset of SIRS. Following administration of etanercept and solumedrol (with resolution of SIRS), cytokine levels returned to baseline at week 3 postinfusion.
Figure 5.
Cytokine levels. The figure shows the cytokine profile before and after T-cell infusion. There was an elevation in IL5, IL13, IL10, IL6, and IFNγ levels at 2 weeks postinfusion coincident with the onset of SIRS, which had returned to baseline levels by week 3 postinfusion, associated with the administration of etanercept and solumedrol. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; SIRS, systemic inflammatory response syndrome; TNF, tumor necrosis factor.
Discussion
This patient treated for a bulky EBV lymphoma with multivirus-specific T cells active against EBV, adenovirus, BKV, and HHV6 subsequently developed a clinical syndrome consistent with SIRS at 2 weeks postinfusion, but she responded to steroids and etanercept. A SIRS-like syndrome associated with elevated cytokine levels has been reported in several studies using T cells modified with CARs1,2,3,4,5,12,13 and in studies that evaluated administration of the CD19/CD3-bispecific T-cell receptor-engaging antibody blinatumomab;8 however, this study reports such a syndrome developing in a recipient of T cells recognizing tumor-expressed antigens via their native receptors.
CAR-modified T cells with costimulatory moieties included in the construct are subject to nonphysiological stimulation. Moreover, naive T cells may also be transduced, further predisposing recipients to this complication. We have completed several studies using virus-specific T cells with memory cell phenotype generated by exposure to viral antigens, and until now, we have not observed SIRS.14,15,16,17 However, we have recently modified our manufacturing methodology to rapidly generate virus-specific T cells using overlapping peptide pools as a stimulus followed by a 10-day expansion phase in the presence of the cytokines IL4 and IL7. These virus-specific T cells may be more predisposed to activation if they contain fewer memory cells and more naive cells than previous preparations. Contradicting this hypothesis, however, the phenotype of this and other lines generated with the shorter manufacturing process showed no difference in the proportions of memory and naive cells, although the rapidly generated VSTs contained greater numbers of cells expressing markers associated with a central memory phenotype (CD45RO+CD62L+).17,18
This episode of SIRS following the infusion of rapidly generated T cells with native antigen specificity prompted us to review our experience with 175 patients treated on other clinical studies with either donor-derived EBV or multivirus-specific T cells generated using longer ex vivo culture times.14,16,17,18 This review of >20 years of clinical data identified only one other patient who may have had a syndrome consistent with SIRS. Similar to patient #3022, this subject was treated for bulky EBV posttransplant lymphoproliferative disease with extensive pharyngeal involvement and had a vigorous inflammatory response with an immune infiltrate of genetically marked cells apparent on follow-up biopsy.19 This response produced progressive airway obstruction and mucosal sloughing, ultimately requiring mechanical ventilation. He also had reversible cardiac impairment and fevers during this response, but he subsequently made a full recovery and remains well >10 years later. Cytokine panels were not available when he was treated (in 1996), so we cannot definitively conclude that this was SIRS, but it seems likely.
Because this syndrome has clinical features similar to those seen in sepsis, it is important to exclude underlying bacterial, viral, or fungal infection. In our patient, there was no evidence of any infections other than EBV. In retrospect, some adverse events reported after T-cell infusions that were originally attributed to sepsis may in fact have been due to SIRS,12 and cytokine levels should be checked in patients presenting with this clinical picture. The cytokine levels we observed are somewhat different from those reported by other investigators whose patients developed SIRS after administration of CD19 CAR-modified T cells incorporating the 41BB costimulatory endodomain.2,9 These investigators did not report IL5 or IL13 levels in their studies, but they observed substantially elevated levels of IL6, IFNγ, and IL10. For example, Grupp et al. reported >100-fold increase relative to baseline for IL10 and IL6 and >1,000-fold increase for IFNγ.9 By contrast, our subject had <15-fold increase for IFNγ and <10-fold increase for IL6 and IL10. We obtained samples at the time of peak SIRS symptoms and snap-froze the material, so we believe that these differences are physiological and not the consequence of differences in sample handling.
Although our experience suggests that SIRS is rare in patients receiving donor-derived T cells to treat viral infections (2 of 175 patients), the risk of this complication may be elevated when treating patients with bulky disease that can induce marked expansion and activation of the infused T cells. Patients who develop symptoms consistent with SIRS in the absence of other causes should therefore have plasma cytokines measured and be treated with steroids or other immune suppression regimens. It may be helpful to measure plasma cytokines to follow responses to therapy or possibly to identify inflammatory pathways as potential therapeutic targets.
Materials and Methods
Patient. This patient was treated on a single-patient protocol on an established investigational new drug application approved by the US Food and Drug Administration, the Institutional Review Board, and the National Marrow Donor Program's Institutional Review Board. The parent protocol is open to recipients of allogeneic hematopoietic stem cell transplant who are at least in the 30th day posttransplant, have a life expectancy >30 days, with no severe kidney or liver disease, and without graft-versus-host disease of grade >2. Because the patient in this study was critically ill with progressive EBV lymphoma, we obtained approval from the US Food and Drug Administration to treat her on a single-patient protocol with donor multivirus-specific T cells.
VST generation. A VST line from the transplant donor was generated as previously described.11 Briefly, donor PBMCs were stimulated with overlapping peptide pools spanning target antigens for EBV, cytomegalovirus (CMV), adenovirus, BKV, and HHV6 viruses in the presence of the prosurvival cytokines IL4 and IL7. On days 9–11 of culture, VSTs were harvested, and samples were sent for quality assurance/quality control testing, as well as for phenotypic and functional studies. The remaining cells were cryopreserved for clinical use. Release criteria for VSTs included viability >70%, negative culture for bacteria and fungi after 7 days, endotoxin testing <5 EU (endotoxin units)/ml, negative result for mycoplasma, <10% killing of recipient/haploidentical phytohemagglutinin (Sigma-Aldrich, St Louis, MO)-activated lymphoblasts at a 20:1 ratio, and human leukocyte antigen identity.
Enzyme-linked immunospot assay. Enzyme-linked immunospot analysis was used to quantitate the frequency of T cells secreting IFNγ in response to EBV, HHV6, BKV, adenoviral, and CMV antigen exposure.11,20 SFCs and input cell numbers were plotted, and the frequency of T cells specific to each antigen was expressed as specific SFCs per input cells.
Detection of EBV, CMV, BKV, HHV6, and adenovirus DNA in PBMCs. To quantitate EBV load in patient blood, DNA was isolated from 3- to 5 × 106 PBMCs using an anion exchange column (Qiagen, Valencia, CA). The resulting DNA (500 ng) was analyzed by quantitative polymerase chain reaction, as previously described.21 Adenovirus, BKV, HHV6, and CMV DNAs were quantified by Viracor IBT Laboratories (Lee's Summit, MO).
Cytokine assay. The patient's blood was collected before infusion and at weeks 1, 2, 3, 4, and 6 postinfusion in heparin tubes and centrifuged for 5 minutes. Plasma was immediately collected, snap-frozen, and stored at −20 °C. The Milliplex Human Cytokine Immunoassay (Catalog #HSCYTO-60SKPMX13) (Millipore, Billerica, MA), which detects IL1β, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12p70, IL13, IFNγ, granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-α, was used to assess cytokine levels in the banked plasma samples. The assay was performed as per manufacturer's instructions, and samples were analyzed on a Luminex 200 (XMAP Technology, Austin, TX).
SUPPLEMENTARY MATERIAL Figure S1. In vivo expansion and clinical benefits of VSTs.
Acknowledgments
We are grateful to Amy Reyna—the study coordinator, Deborah Lyon for quality control testing, April Durett for phenotypic analyses, and Oumar Diouf for assistance with good manufacturing practice production. H.E.H. is supported by a Dan L Duncan Chair and M.K.B. is supported by a Fayez Sarofim Chair. The parent clinical trial was supported in part by the Production Assistance for Cellular Therapies (PACT) program [National Heart, Lung, and Blood Institute contract #HHSN268201000007C], Alex's Lemonade Stand Foundation, the Clinical Research Center at Texas Children's Hospital, and the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine. We also appreciate the support of resources shared by Dan L Duncan Cancer Center through support grant P30CA125123.
Supplementary Material
In vivo expansion and clinical benefits of VSTs.
References
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CR, Hanley PJ, Liu H, Torrano V, Lin YF, Arce JA, et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy. 2010;12:743–749. doi: 10.3109/14653241003709686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21:2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Gerdemann U, Keukens L, Keirnan JM, Katari UL, Nguyen CT, de Pagter AP, et al. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood. 2013;121:207–218. doi: 10.1182/blood-2012-05-430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–3981. doi: 10.1182/blood-2003-12-4287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo expansion and clinical benefits of VSTs.