Abstract
Plumage colours bestowed by carotenoid pigments can be important for visual communication and likely have a long evolutionary history within Aves. Discovering plumage carotenoids in fossil feathers could provide insight into the ecology of ancient birds and non-avian dinosaurs. With reference to a modern feather, we sought chemical evidence of carotenoids in six feathers preserved in amber (Miocene to mid-Cretaceous) and in a feather preserved as a compression fossil (Eocene). Evidence of melanin pigmentation and microstructure preservation was evaluated with scanning electron and light microscopies. We observed fine microstructural details including evidence for melanin pigmentation in the amber and compression fossils, but Raman spectral bands did not confirm the presence of carotenoids in them. Carotenoids may have been originally absent from these feathers or the pigments may have degraded during burial; the preservation of microstructure may suggest the former. Significantly, we show that carotenoid plumage pigments can be detected without sample destruction through an amber matrix using confocal Raman spectroscopy.
Animal colours can be richly informative about aspects of behaviour such as foraging ecology and mate preference. Birds in particular display many striking hues and complex patterns of pigmentation. Examples of plumage adaptations include brightly coloured feathers for enticing potential mates, as well as cryptic patterns that allow a bird to hide in plain sight1,2,3. By analogy with modern birds, the behaviours and habitats of ancient birds and other feathered dinosaurs may be inferred from pigments in fossil plumage. Methods for describing one class of plumage pigment (melanins) have recently been developed for ancient feathers4,5. Modern birds display six chemically-distinct classes of feather pigment, of which carotenoids are the most common after melanins; carotenoids mostly confer yellow, orange and red colours to feathers6,7. The two key challenges for describing carotenoids in ancient plumage are 1) to find an environment that preserves molecular evidence for carotenoids, and 2) to ascertain a technique that provides unequivocal evidence for carotenoids. We focused on feathers in amber because encapsulation within amber may insulate carotenoid molecules against diagenetic alteration, and such preserved pigments could be detected with Raman spectroscopy.
Carotenoids have an unequivocal Raman spectral signal that is greatly enhanced by a resonance effect, allowing trace amounts to be detected8. Furthermore, Raman spectroscopy can be performed without sample preparation or destruction. Edwards and colleagues9 showed that inclusions in amber can be studied with minimal or no interference from the amber matrix when Raman spectra are collected with confocal optics and 1064 nm excitation.
With conventional Raman microscopy, laser light is channeled through a microscope objective towards a sample, and then scattered light is channeled back through the same objective towards a detector. Light scatters from the entire sample volume that is penetrated by incident light, and the greatest concentration of scattered photons are typically returned from the focal plane of the incident light. Accordingly, light is scattered from both the surface and internal volume of a translucent sample. Confocal Raman microscopy differs from conventional Raman microscopy by the inclusion of a confocal pinhole above the microscope objective. The pinhole allows only the photons scattered from the focal plane to reach the detector. For a translucent sample with an inclusion (e.g. amber containing a fossil feather), the inclusion can be positioned in the focal plane of the incident light and only the light scattered from the inclusion will reach the detector. Hence, confocal Raman microscopy usefully provides chemical information about an inclusion with minimal or no interference from the surface or surrounding matrix.
Near infrared excitation wavelengths are typically less sensitive than visible wavelengths when studying pigments with Raman spectroscopy. However, visible excitation wavelengths tend to induce fluorescence when interacting with amber9. Thus, from a signal vs. noise perspective, a Raman spectrum of an inclusion in amber collected with a near infrared wavelength can be substantially more informative than a Raman spectrum of the same inclusion collected with a visible wavelength. A confocal Raman microscope with a near infrared (NIR) laser is thus an ideal tool for seeking carotenoids preserved in amber.
We first conducted a pilot study to determine if the carotenoid pigments of a modern feather could be detected through an amber matrix. A yellow and black wing feather from a greenfinch (Carduelis chloris) was placed underneath a polished piece of amber and analysed with Raman spectroscopy. In a second series of experiments, we analysed six fossil feathers preserved in amber using Raman spectroscopy and light microscopy. Evidence for pigmentation in the fossil feathers was sought with Raman spectroscopy, and the preservation of fine-scale morphology was studied with light microscopy. Finally, we collected Raman spectra and scanning electron microscopy images from an ancient feather preserved as a carbonised compression fossil. Compression fossils have previously provided evidence for melanin pigmentation4,5, and here we compared Raman spectral evidence from two types of fossil feather (1: preserved in amber; 2: preserved in lake sediment) with spectra from a modern feather.
Results
Raman spectroscopy
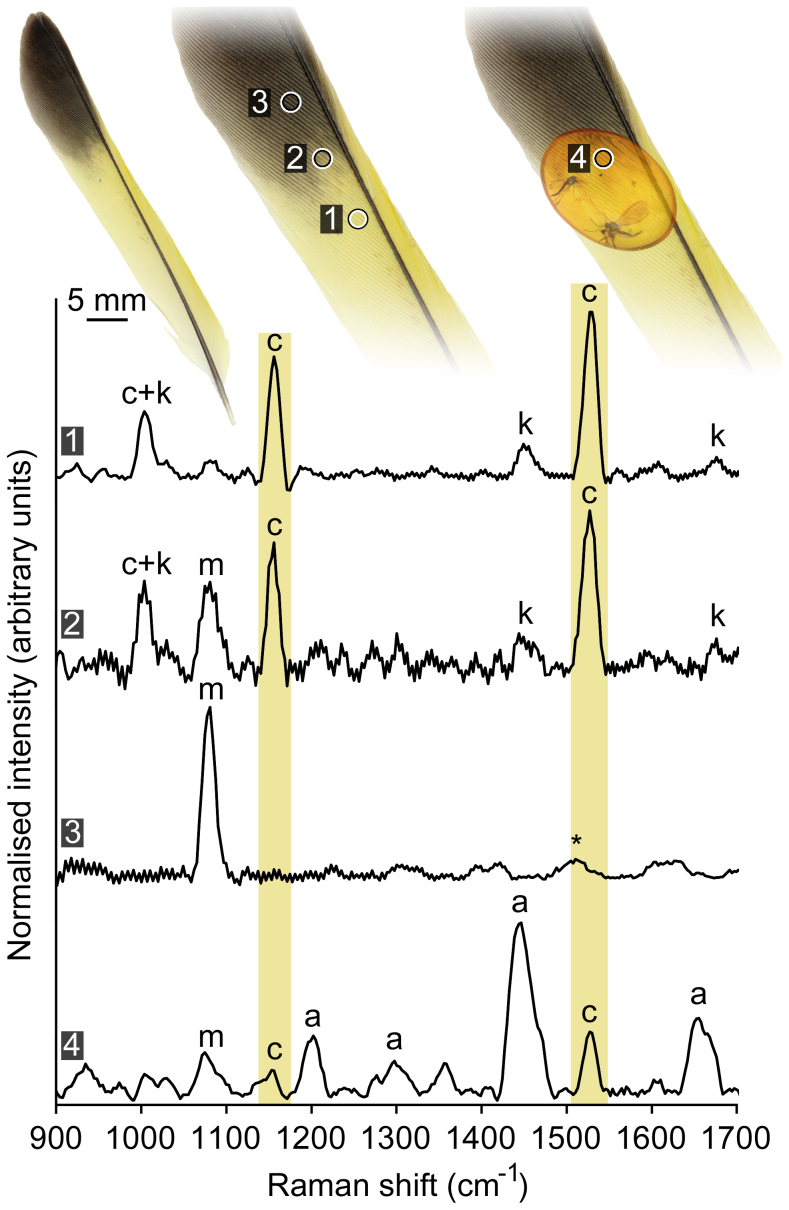
Raman spectra from the yellow barbs of a European greenfinch (Carduelis chloris i.e. modern bird) feather had distinctive carotenoid-informative bands at 1530 and 1153 cm−1 6,8,10,11 (Fig. 1). A third prominent band at 1001 cm−1 identified both the carotenoid pigment and the feather keratin. Bands at 1444 cm−1 and 1657 cm−1 also identified keratin. The spectrum from the black barbs of the same European greenfinch feather had a single band at 1078 cm−1 as the only discernible feature; assignments for the 1078 cm−1 band have not been found in published sources. The 1078 cm−1 band was evident alongside the 1530 cm−1, 1153 cm−1 and 1001 cm−1 bands in the yellow-black barb from the European greenfinch feather. The 1078 cm−1 band was not observed in spectra collected from the yellow feather barbs or the three instrument standards (acetaminophen, calcite, cyclohexane). Spectra from the black and yellow-black feather barbs were collected at low laser powers and consequently had high levels of spectral noise, which manifested as spectral artifacts after baseline correction and spectral smoothing (Fig. 1).
Figure 1. Carotenoid feather pigments can be detected through an amber matrix with Raman spectroscopy.
Band assignment symbols: a – amber; c – carotenoid; k – keratin; m – ‘melanin'; * – spectral noise. Although the spectrum from the melanin-pigmented black feather barbs contained a single spectral band (m), this band is not unequivocal evidence for the presence of melanin (see Fig. 2). Spectra have been baseline-corrected and smoothed; feather from a European greenfinch Carduelis chloris.
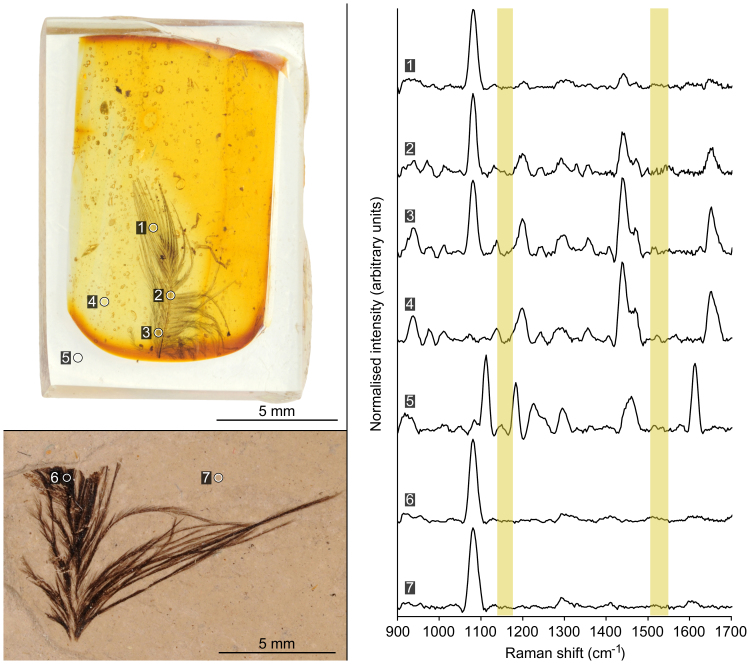
Five fossil feathers preserved in amber had been embedded in an epoxy resin. The epoxy resin had a distinct Raman spectrum with bands at 1110, 1181, 1220, 1289, 1450 and 1610 cm−1 (Fig. 2). Raman spectra from the amber matrices had bands at 1193, 1436, 1452 and 1647 cm−1. Note that carotenoid-identifying Raman bands were not observed in spectra collected from amber matrices (electronic supplementary information). All spectra from all analyses of the feathers in amber contained the 1078 cm−1 band, and many spectra contained amber spectral bands (Fig. 2). The 1078 cm−1 band was also the only feature in spectra from the Green River Formation fossil feather, and in spectra from the Green River Formation sedimentary matrix. Raman spectral evidence for carotenoid pigmentation was not recovered from the fossil feathers.
Figure 2. Raman spectra from a fossil feather preserved in amber and from a carbonised compression fossil.
Amber matrix, epoxy resin and fossil feather each had a distinct spectral signature. Near-identical spectra were collected from the fossil feathers and from the Green River Formation sediment. Yellow bars indicate locations where peaks are expected in spectra from carotenoids (see Fig. 1), showing that evidence for carotenoid pigmentation was not recovered from AMNH DR-14-32 (feather in amber) or USNM 584927 (compression fossil). Spectra have been baseline-corrected and smoothed. Spectral intensities have been normalized against the minimum and maximum values. Epoxy was only around the outside of the amber matrix, as oriented in the image, and not above and below the amber.
Microscopy
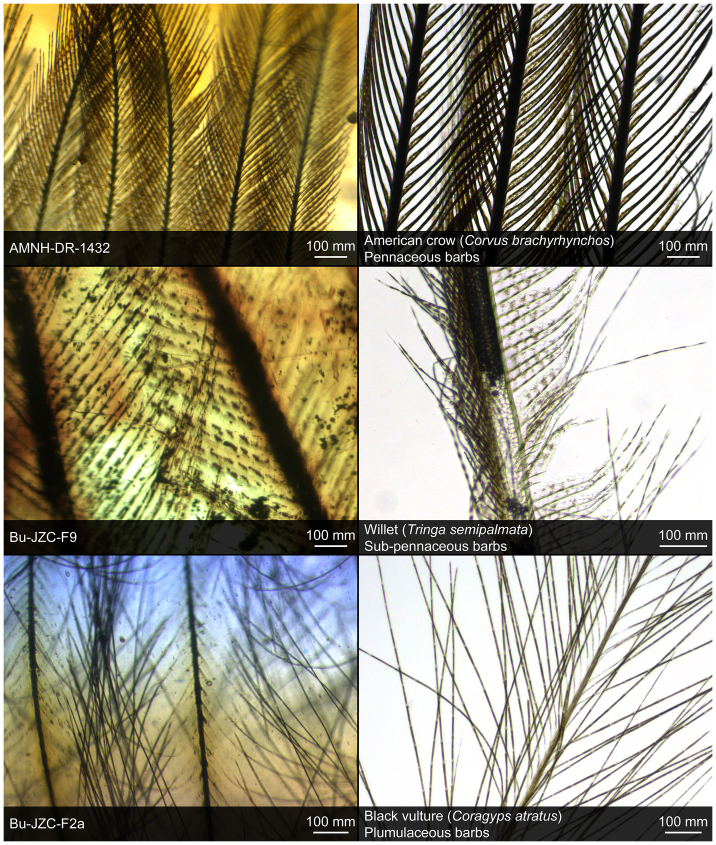
Feather microstructure was preserved with high fidelity in each of the fossil feathers in amber (Fig. 3). Morphological evidence for the order-level taxonomy of the fossil feathers was not observed, although morphological characteristics in three amber-preserved feathers were analogous to microstructures in modern feathers. The filamentous barbs of Bu-JZC-F2a were morphologically analogous to the plumulaceous barbs of a contour feather from a black vulture (Coragyps atratus) (Fig. 3). Feather barbs in Bu-JZC-F9 were morphologically similar to the sub-pennaceous barbs of a modern willet (Tringa semipalmata); dark bodies spaced along barbules in Bu-JZC-F9 were similar to pigment structures in a willet feather. The darkened barbs of AMNH-DR-1432 preserved pennaceous hooklets, and were morphologically analogous to the melanin-rich barbs of an American crow (Corvus brachyrhynchos).
Figure 3. Microstructures in modern and fossil feathers.
Fossil feathers in amber preserved microstructural details that were analogous to structures in an American crow (Corvus brachyrhynchos), a black vulture (Coragyps atratus) and a willet (Tringa semipalmata) feather. Morphological evidence for the order-level taxonomy of the fossil feathers was not observed. Feathers were studied with a Leica FS CB microscope (Leica Microsystems Inc., IL USA).
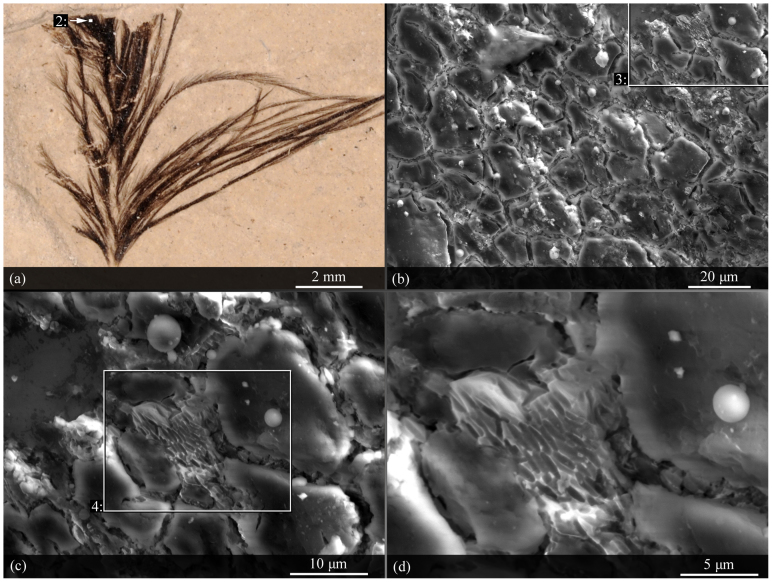
A compression fossil feather from the Green River Formation was imaged with a scanning electron microscope (Fig. 4). The fossil feather had a fractured surface, and the fracture edges and valleys contained elongate structures with long axis lengths of 2–3 μm (Fig. 4). The elongate textures were visually-consistent with negative casts of melanosomes described elsewhere5.
Figure 4. Trace evidence for melanin pigmentation in an Eocene fossil feather (USNM 584927).
(a). The dark material towards the distal tip of the feather was examined under a scanning electron microscope. (b–d). Small clusters of elongate, rounded structures within the dark region are morphologically consistent with fossil melanosome casts5. Parameters for scanning electron micrographs: (b). 12 kv accelerating voltage, 5.5 spot size, 8.4 mm working distance, 1.4 torr water vapor pressure; (c). 13 kv accelerating voltage, 5.3 spot size, 8.5 mm working distance, 1.4 torr water vapor pressure; (d). 13 kv accelerating voltage, 5.3 spot size, 8.4 mm working distance, 1.4 torr water vapor pressure. Contrast and brightness have been altered for image clarity.
Discussion
Raman spectroscopy is a viable technique for seeking carotenoid pigments in amber without sample destruction. Our analyses of the modern greenfinch feather showed that distinct evidence for carotenoids could be collected through an amber matrix. We studied yellow, yellow-black and black barbs on the greenfinch feather; black pigments in previous studies had been shown to be melanin12. Raman spectra from the yellow-black barbs contained carotenoid-informative bands, indicating that the co-deposited melanin did not obscure the Raman spectral evidence for carotenoids. Using our observations of modern feather barbs as a reference, we sought evidence for carotenoids in fossil feathers with Raman spectroscopy. The distinct Raman spectral bands attributed to carotenoid pigments were not observed in spectra from the fossil feathers in amber.
Raman spectroscopy can detect very low concentrations of carotenoids in feathers (see electronic supplementary information)13, and hence the absence of carotenoid spectral bands is not likely to be an issue of instrument sensitivity, but may instead indicate an original absence of carotenoid pigments or the diagenetic loss of carotenoids (e.g. by oxidation)14. Experimental degradation of modern bird feathers performed elsewhere to mimic the effects of fossilisation altered both the feather colour and the feather keratin12. In the present study, we did not recover chemical information about feather keratin, although we did observe micron-scale pennaceous and plumulaceous structures preserved inside the amber with fine detail. For diagenetic alteration to explain the absence of carotenoids, carotenoid degradation must have occurred in the absence of micron-scale physical alteration (cf. ref. 9). Alternatively, the pigments may have been originally absent from the feathers we studied. Based on our observations of modern birds, carotenoid pigments appear to be more common in pennaceous feathers than in plumulaceous feathers, and melanin pigments are substantially more common in both feather types compared with carotenoid pigments (unpublished data). Hence, the probability of collecting a carotenoid-pigmented fossil feather preserved in amber is likely smaller than the probability of finding a melanin-pigmented fossil feather.
Raman spectra from the melanin-pigmented greenfinch barbs were identical to the Raman spectra from the feathers in amber. Furthermore, the feathers in amber appear to preserve the same pigment microstructures that are observed in modern feathers with melanin colours (see Bu-JZC-F9 in Fig. 3). Unfortunately, our Raman spectral evidence does not provide an unequivocal diagnosis of melanin pigmentation. While a ‘melanin-type' spectrum was collected from a compression fossil with SEM evidence for melanin, a near-identical spectrum was collected from the surrounding Green River Formation sediment. The 1078 cm−1 Raman spectral band that we measured in each of these substrates has not previously been reported for a melanin-pigmented structure, and hence we are cautious to interpret the spectral feature as an analytical signal. The 1078 cm−1 is likely a collection artifact caused by sample fluorescence. In summary, our Raman analyses provided strong evidence for the absence of carotenoid pigments in the amber-preserved feathers, and an equivocal chemical signal for melanin pigmentation.
Raman spectroscopy is a good supplement to the tools currently used to describe pigments in well-preserved ancient feathers. Scanning electron microscopy and x-ray fluorescence provide evidence for melanin pigmentation in compression fossils, and Raman spectroscopy could provide evidence for carotenoid pigmentation in amber-preserved feathers. Raman could also be useful for identifying other feather pigments preserved in amber (e.g. psittacofulvins, porphyrins)6, and for studying other inclusions that may be pigmented (e.g. flowers)15. Although we did not find carotenoids in the feathers we studied, we have shown that confocal NIR Raman spectroscopy is an informative and non-destructive method for surveying the chemical composition of fossil feathers.
Methods
Modern and fossil feathers
Raman spectra were collected from the yellow and black wing feather of a European greenfinch (Carduelis chloris; male; USNM 637389). Yellow barbs provided reference spectra for carotenoid pigmentation, while black barbs provided reference spectra for melanin pigmentation12,16. Feathers from an American crow (Corvus brachyrhynchos), a black vulture (Coragyps atratus) and a willet (Tringa semipalmata) provided microstructural comparisons for the feathers in amber (feathers chosen because they had analogous characteristics to the feathers in amber). The modern feather samples were from the Division of Birds, National Museum of Natural History, Smithsonian Institution (NMNH), and were not collected for this study.
Six specimens of amber with feather inclusions were studied. Specimens were from the American Museum of Natural History (AMNH) collections, or were on loan to AMNH from private collectors. The amber specimens were transported from AMNH to NMNH for analysis and included one Miocene feather from the Dominican Republic (AMNH DR-14-32, pennaceous); two early Late Cretaceous specimens from New Jersey, USA (AMNH NJ JK 21, pennaceous; AMNH NJ 636, plumulaceous); as well as three mid-Cretaceous specimens from Myanmar (Bu-JZC F1-A, pennaceous; Bu-JZC F2-A, plumulaceous; Bu-JZC F9, sub-pennaceous)17,18,19. We compared the feathers in amber to an Eocene feather preserved as a compression fossil (USNM 584927; Colorado, USA; Green River Formation). Taxonomic affinities and body positions for any of the fossil feathers are unknown, and vane asymmetry was not observed in any of the pennaceous feathers.
Raman spectroscopy
Modern and fossil feathers were analysed with a Nomadic Raman microscope (BaySpec, San Jose CA, USA), using a 1064 nm excitation Nd:YAG laser and a 512 pixel InGaAs array detector with a spectral range of 277 to 1886 cm−1. Spectra were collected through an LMPlan N 10× IR microscope objective (Olympus, Melville, NY, USA) or an EPlan 40× microscope objective (Nikon Instruments, Tokyo, Japan); minimum spot size diameters were 4.3 and 2 μm, respectively. The Raman microscope used a 25 μm confocal pinhole.
Specimens were analysed without additional sample preparation, although amber specimens had previously been trimmed and polished, and five of the amber specimens had been embedded in EpoTek 301-2, a high-grade epoxy resin. Spectra were collected from three standards at the beginning of each analytical session: an acetaminophen tablet, a piece of optical calcite, and a vial of cyclohexane. Spectra from feathers were compared to pigment and keratin spectra published elsewhere6,13.
A total of 70 spectra were collected from all fossil feathers in amber, from rachises and barbs that could be positioned within the focal range of the microscope objectives (AMNH DR-14-32 n = 3 spectra; AMNH NJ JK 21 n = 3; AMNH NJ 636 n = 26; Bu-JZC F1-A n = 5; Bu-JZC F2-A n = 10; Bu-JZC F9 n = 15). Spectra were also collected from epoxy and amber matrices (see electronic supplementary information). The focal length of each objective was not quantified; instead, feather inclusions were aligned (visually) with the focal plane of each microscope objective, i.e. the feathers were brought into focus under the microscope. Analysis duration and laser power were optimised to maintain good signal without damaging specimens. Collection durations were between 10 and 50 seconds for each spectrum, and the laser power was 30, 50 or 100 mW. Data were smoothed and baseline corrected in R 2.15.2 (R Core Team 2012; http://www.R-project.org/) using the ‘sgolay' function in the ‘signal' package (Liland & Mevik 2012, http://CRAN.R-project.org/package=baseline), and the ‘baseline' function in the ‘baseline' package (Signal developers 2013, http://r-forge.r-project.org/projects/signal/), respectively.
Scanning electron microscopy
The compression fossil feather was imaged with a Philips XL30 environmental scanning electron microscope (FEI, Hillsborough, OR, USA) using a wide angle gaseous secondary electron detector. The specimen was not coated with a conductive medium. Images were collected under a range of operating conditions, in response to spatial differences in conductivity: 6 to 15 kv accelerating voltage, 4.5 to 5.4 spot size, 8 to 9.6 mm working distance, 0.7 to 1.4 torr water vapor pressure.
Author Contributions
D.B.T. planned the study, collected data and wrote the manuscript. P.C.N. provided specimens and edited the manuscript. C.J.D. collected data and edited the manuscript. D.A.G. provided specimens. H.F.J. secured research funding, planned the study and edited the manuscript. All authors read and approved the manuscript.
Supplementary Material
Dataset 1
Dataset 2
Acknowledgments
We thank James Zigras and Jim Kane for loaning amber specimens to AMNH, Matthew Carrano (NMNH) for helpful comments, Scott Whittaker (NMNH) for SEM training, Finnegan Marsh and Mark Florence (NMNH) for access to fossil feathers. DBT was funded by a Peter Buck Postdoctoral Fellowship, administered by NMNH.
References
- Hill G. E. Female house finches prefer colourful males: sexual selection for a condition-dependent trait. Anim. Behav. 40, 563–572 (1990). [Google Scholar]
- Rohwer S. & Ewald P. W. The cost of dominance and advantage of subordination in a badge signaling system. Evolution 35, 441–454, 10.2307/2408193 (1981). [DOI] [PubMed] [Google Scholar]
- Gluckman T. L. & Cardoso G. C. The dual function of barred plumage in birds: camouflage and communication. J. Evolutionary Biol. 23, 2501–2506, 10.1111/j.1420-9101.2010.02109.x (2010). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Plumage color patterns of an extinct dinosaur. Science 1369, 1369–1372 (2010). [DOI] [PubMed] [Google Scholar]
- Wogelius R. A. et al. Trace Metals as Biomarkers for Eumelanin Pigment in the Fossil Record. Science 333, 1622–1626, 10.1126/science.1205748 (2011). [DOI] [PubMed] [Google Scholar]
- Thomas D. B., McGoverin C. M., McGraw K. J., James H. F. & Madden O. Vibrational spectroscopic analyses of unique yellow feather pigments (spheniscins) in penguins. J. Roy. Soc. Interface 10, 10.1098/rsif.2012.1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw K. J. in Bird Coloration volume 1. Mechanisms and Measurements (eds Hill, G. E. & McGraw, K. J.) 177–242 (Harvard University Press, 2006). [Google Scholar]
- Schulz H., Baranska M. & Baranski R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 77, 212–221, 10.1002/bip.20215 (2005). [DOI] [PubMed] [Google Scholar]
- Edwards H. G. M., Farwell D. W. & Villar S. E. J. Raman microspectroscopic studies of amber resins with insect inclusions. Spectrochim. Acta A 68, 1089–1095, 10.1016/j.saa.2006.11.037 (2007). [DOI] [PubMed] [Google Scholar]
- Hsu S. L., Moore W. H. & Krimm S. Vibrational spectrum of unordered polypeptide chain - Raman study of feather keratin. Biopolymers 15, 1513–1528, 10.1002/bip.1976.360150807 (1976). [DOI] [PubMed] [Google Scholar]
- Veronelli M., Zerbi G. & Stradi R. In situ resonance Raman spectra of carotenoids in bird's feathers. J. Raman Spectrosc. 26, 683–692 (1995). [Google Scholar]
- McNamara M. E., Briggs D. E. G., Orr P. J., Field D. J. & Wang Z. Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biol. Lett. 9, 10.1098/rsbl.2013.0184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. B., McGraw K. J., James H. F. & Madden O. Non-destructive descriptions of carotenoids in feathers using Raman Spectroscopy. Anal. Methods 6, 1301–1308 (2014). [Google Scholar]
- Briggs D. E. G. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. Roy. Soc. B 354, 7–16, 10.1098/rstb.1999.0356 (1999). [Google Scholar]
- Poinar G. Jr Fossil palm flowers in Dominican and Baltic amber. Botan. J. Linn. Soc. 139, 361–367 (2002). [Google Scholar]
- Stradi R., Celentano G., Rossi E., Rovati G. & Pastore M. Carotenoids in bird plumage—I. The carotenoid pattern in a series of Paleartic Cardulinae. Comp. Biochem. Physiol. B 110, 131–143 (1995). [Google Scholar]
- Grimaldi D. A. in Amber, Resinite, and Fossil Resins Vol. 617 ACS Symposium Series (eds Anderson, K. B. & Crelling, J. C.) 203–217 (1995). [Google Scholar]
- Grimaldi D. & Case G. R. A feather in amber from the Upper Cretaceous of New Jersey. Am. Mus. Novit. 3126, 1–6 (1995). [Google Scholar]
- Grimaldi D. A., Engel M. S. & Nascimbene P. C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 3361, 1–71 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset 1
Dataset 2