Abstract
This study aimed to further elucidate the function of Roundabout proteins in endothelium. We show that both Robo1 and Robo4 are present in human umbilical vein endothelial cells (HUVECs) and have knocked expression down using small interfering RNA (siRNA) technology. Roundabout knockout endothelial cells were then studied in a variety of in vitro assays. We also performed a yeast 2-hybrid analysis using the intracellular domain of Robo4 as bait to identify interacting proteins and downstream signaling. Both Robo1 and Robo4 siRNA knockdown and transfection of Robo4-green fluorescent protein inhibited endothelial cell movement and disrupted tube formation on Matrigel. Consistent with a role in regulating cell movement, yeast 2-hybrid and glutathione-S-transferase pulldown analyses show Robo4 binding to a Wiskott-Aldrich syndrome protein (WASP), neural Wiskott-Aldrich syndrome protein, and WASP-interacting protein actin-nucleating complex. We have further shown that Robo1 forms a heterodimeric complex with Robo4, and that transfection of Robo4GFP into HUVECs induces filopodia formation. We finally show using Robo1 knockdown cells that Robo1 is essential for Robo4-mediated filopodia induction. Our results favor a model whereby Slit2 binding to a Robo1/Robo4 heterodimer activates actin nucleation-promoting factors to promote endothelial cell migration.—Sheldon, H., Andre, M., Legg, J. A., Heal, P., Herbert, J. M., Sainson, R., Sharma, A. S., Kitajewski, J. K., Heath, V. L., Bicknell, R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors.
Keywords: roundabouts, angiogenesis, cell signaling
Originally identified inDrosophila neurones, the Slit/Roundabout family of neuronal guidance genes are also found in the endothelium of the vertebrate vasculature (1, 2). The family comprises 3 predominantly neuronal Roundabouts (Robo1, 2, and 3) together with the more sequence divergent, endothelial-restricted Roundabout, Robo4 (3,4,5). Robo1 is also present in vertebrate endothelium (6). There are 3 Slit ligands, each of which binds to the 3 neuronal Roundabouts (Robo1, 2, and 3). Although most studies have described a repulsive guidance role for Robos in the developing axonal growth cone, the function of Roundabouts in endothelium and angiogenesis remains controversial (4, 6, 7). Thus, although Robo1 and 4 are thought to be involved in endothelial guidance and migration, their precise role has been difficult to pinpoint because of the lack of suitable assays in endothelial systems. In vivo knockdown and overexpression of Robo4 in the zebrafish point to a vascular guidance role, and similar studies in cultured endothelium have shown that overexpression of Robo4 impairs cell migration (8, 9).
Previous downstream signal transduction studies have implicated mammalian enabled (Mena), ERK (extracellular signal related kinase), FAK (focal adhesion kinase), and more recently Cdc42 and Rac1 RhoGTPase in Robo4 signaling (9,10,11). The most detailed study of Robo4 signaling identified Cdc42 and Rac1 as downstream players, leading these authors to propose that Robo4 mediates attractive guidance mechanisms (9). Although an early study suggested that Robo4 bound Slit2, this has failed to be substantiated by others (7, 12). Indeed, knowledge of the X-ray crystal structure of the Robo1/Slit2 complex has shown that Robo4 lacks many of the critical Slit2 binding residues, and it is considered highly unlikely that Slits are ligands for Robo4 (13). The study by Kaur et al.(9) concluded that a minimal region of 54 amino acids in the extracellular region is sufficient to bind a non-Slit ligand and activate intracellular signaling pathways; however, no ligand was identified. To date, there is no convincing evidence for the existence of a Robo4 ligand.
In the absence of a ligand, we have used small interfering (siRNA) knockdown as a probe of Robo4 function in HUVECs. Following Robo4 knockdown, endothelial cells were studied in a variety of in vitro endothelial behavioral assays. A yeast 2-hybrid analysis was performed using the intracellular domain of Robo4 as bait to characterize the downstream signaling pathway of Robo4. Last, interactions between Robo4 and Robo1/Slit2 in the endothelial cell have also been explored.
MATERIALS AND METHODS
Plasmids and adenovirus production
Robo4-green fluorescent protein (GFP) -tagged plasmids were constructed using pEGFP-N1 (Invitrogen, Carlsbad, CA, USA). Robo4FL-GFP was cloned into the pAdlox shuttle vector and then underwent Cre-lox recombination with donor virus to create Ad/Robo4FL-GFP. Robo1Fc was created using the pIG vector. Full-length WASP was cloned into pcDNA 3.1/myc/His (Invitrogen). pRK5-HA-GST was generated by cloning glutathione-S-transferase (GST) into the BamH1 sites of pRK5-HA (N-terminal HA tag; ref. 14). The intracellular domain of Robo4 (amino acids 495–1007) was then cloned into the NotI/HindIII sites. To generate WASP-GST, full-length WASP was cloned into BglII/EcoRI sites.
Cell culture and immunoblotting
All cell lines were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS). The cell lines used were SHSY5Y (human neuroblastoma), U87 MG (human glioma), HEK293Ts (human kidney), MRC5 (human lung), MCF7 (human breast adenocarcinoma), HDMEC (human dermal microvascular endothelial cell), PC3 (human prostate cancer), HeLa (human cervical cancer), and COS7 (monkey fibroblast). Human umbilical vein endothelial cells (HUVECs) were purchased from TCS Cellworks and cultured in HUVEC medium [M199 supplemented with 10% FCS, large vessel endothelial growth supplement (TCS Cellworks Ltd, Claydon, Buckingham, UK) and 2 mM l-glutamine]. Antibodies used include anti-Robo1 (Abcam, Cambridge, MA, USA; ab7279), anti-WASP (Millipore, Billerica, MA, USA; 07–066), 3F10 anti-HA (Roche, Basel, Switzerland), anti-Robo4 (Abcam; ab10547), and anti-β tubulin (Sigma, Gillingham, Dorset, UK; TUB 2.1). MR1 and MR7 anti-Robo4 and 9E10 antimyc were all obtained from Cancer Research UK (Lincoln’s Inn Fields, London, UK).
Cell lysates
Cell lysates were generated by incubating on ice for 15 min with lysis buffer (0.4% Nonidet P-40, 150 mM NaCl, 50 mM Tris, 1 mM EDTA, 10% glycerol, 1 mM PMSF, and 1 μg/ml each of chymostatin, antipain, leupeptin, and pepstatin). Lysates were clarified by centrifugation at 15,000 g for 10 min at 4°C, and the protein concentration was determined by Bio-Rad protein assay (Bio-Rad, Hemel Hempstead, UK). We probed 50 μg total protein with relevant antibodies. The relative intensities of the immunoblot bands were measured using a plug-in for Image J (U.S. National Institutes of Health, Bethesda, MD, USA).
RNA interference and adenoviral protein expression
The siRNA oligos against human Robo1 and Robo4 were designed using the Dharmacon siDesign Center program (Dharmacon, Lafayette, CO, USA) and selected according to the Reynold’s criteria (15). The oligos used were the following: Robo1(1), GCAGGUACUUGGAGGAUAU; Robo1(2), GCAACAAGAUGAAUUAGAA; Robo4(1), GCCAAGACUACGAGUUCAA; Robo4(2), CUACGAGUUCAAAGUGAGA; and negative control duplex (Eurogentec, Southampton, UK; OR-0030-neg05).
The siRNA duplexes and negative control duplex were chemically synthesized by Eurogentec. Early passage HUVECs (<p5) were plated at 1 × 106 cells per 10 cm/0.1% gelatin-coated dish. The following day, the siRNA oligos were transfected using Lipofectamine RNAiMAX (Invitrogen) as per the manufacturer’s instructions, and the optimal concentrations of duplexes for knockdown were determined by titration analysis (see figures). For adenoviral protein expression, HUVECs were infected at multiplicity of infection 50 in serum-free medium for 1 h with agitation, and assays were performed 24 h later.
Immunofluorescence microscopy, live cell imaging, and filopodia counting
Subconfluent layers of HUVECs expressing full-length Robo4 GFP or GFP alone were fixed in 4% formaldehyde for 10 min, neutralized in 50 mM NH4Cl in PBS for 10 min, and permeabilized for 4 min in 0.1% Triton-X-100 in PBS. Coverslips were incubated in primary and secondary antibodies for 30 min with 3 washes in PBS between every step. To visualize F-actin, coverslips were incubated in TRITC phalloidin (Invitrogen). The coverslips were mounted in Mowiol containing p-phenyldiamine antifade. Slides were viewed using a Bio-Rad MRC100 confocal laser scanning microscope or using a Zeiss Axioskop2 microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Hamamatsu digital camera C4742–95 (Hamamatsu Photonics, Hamamatsu, Japan) and ×63 oil-immersion objective. The numbers of filopodia were counted, and a Kruskal-Wallis nonparametric test was performed to determine whether there was a statistically significant difference. The probability P values are shown above their corresponding peaks in the figures, with significance set at 5% (P<0.05). For live cell imaging, HUVECs were plated on 0.1% gelatin-coated 25-mm glass coverslips. Cells were imaged using an ×63 oil objective 24 h postinfection with Robo4-GFP adenovirus. Robo4-GFP expression was confirmed using fluorescent microscopy, before movies were taken under differential interference contrast for 100 frames at 3 s/frame.
GST pulldowns
HEK293T cells were transfected for 48 h with epitope-tagged constructs. Lysates were generated as described above and boiled for 5 min in 4× SDS-PAGE loading buffer. The lysates were probed with 3F10 anti-HA and relevant antibody to confirm transfection of both constructs. The remaining lysates were incubated for 2 h at 4°C on a rotator with 25 μl total bed volume of glutathione beads. The beads were pelleted by centrifugation for 30 s at 1000 g and washed 4 times in 1 ml ice-cold lysis buffer. The beads were resuspended in 20 μl 1× SDS-PAGE loading buffer and boiled for 5 min before analysis by SDS-PAGE and immunoblot.
Coimmunoprecipitations
HUVECs were lysed and clarified, and lysate samples were taken. The Protein G beads (Cancer Research UK) were washed 3 times in lysis buffer, and a 25 μl bed volume of beads was incubated with the cell lysates on a rotator at 4°C. The beads were pelleted at 1000 g for 30 s, and the supernatant was removed. The beads were washed 3 times in lysis buffer and termed the no-antibody, beads-only negative control in the figures. After 5 μg of Abcam anti-Robo4 was added, the lysates were rotated at 4°C for 1 h; 25 μl bed volume of Protein G beads was then added and incubated for a further 1 h at 4°C. The beads were pelleted at 1000 g for 30 s, the supernatant was removed, and the beads were washed 3 times in 1 ml lysis buffer. The beads were analyzed by immunoblot and termed the IP lane.
FACS analysis
FACS was performed on unpermeablized HUVECs with a monoclonal antibody to the extracellular domain of Robo4 (MR7) and goat anti-mouse fluorescein isothiocyanate conjugated secondary. Isotyped IgG2a was included as a negative control.
Yeast 2-hybrid screen
The yeast 2-hybrid analysis was carried out using the C terminus of Robo4 (residues 495-1007) and a human placental cDNA library according to the Matchmaker™ Library Construction and Screening Kit instructions (Clontech, Mountain View, CA, USA). Yeast 2-hybrid positives were identified using a combined basic local alignment search tool (BLAST) and genome position searches. Each sequence was translated in 6 frames and BLAST searched against the Refseq human peptide database. In conjunction, the human genome position for each sequence was determined to see which gene it overlapped with.
RNA isolation, reverse transcriptase-polymerase chain reaction (RT-PCR), and quantitative real-time PCR (qPCR)
RNA was extracted from cells using the RNeasy Micro Kit (Qiagen, Crawley, UK) and cDNA produced from 2 μg total RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). qPCR was performed using the Exiqon System (Roche, Basel, Switzerland). Probes were purchased from Exiqon A/S (Vedbaek, Copenhagen, Denmark). The qPCR oligo sequences are as follows: Actin-forward, GCACCCAGCACAATGAAGA; Actin-reverse, CGATCCACACGGAGTACTTG; Flotilin2-forward, CTCAGCTTCACCATCAAGGAC; Flotilin2-reverse, TCAGCATCTCTCTGCACCAC; Robo1-forward, AAAGTAGCACGACGGCAAAT; Robo1-reverse, GGCACTGAGACGCATGAAA; Robo4-forward, CTGCAGAGGCTTGGAAGG; Robo4-reverse, GCAGTGGGGAACTGTTGG; OAS1-forward, GGTGGAGTTCGATGTGCTG; OAS1-reverse, AGGTTTATAGCCGCCAGTCA; ISG20-forward, CACCCCTCAGCACATGGT; ISG20-reverse, TGGAAGTCGTGCTTCAGGT.
Matrigel assay
We plated 1.4 × 105 cells on polymerized Matrigel (BD Biosciences, Erembodegem, Belgium) in a 6-well plate. Wells were photographed after 8 h, and the number of nodes was quantified from 5 random fields of view. The average number of nodes per field of view (normalized to 100 for the mock control) is plotted in the figures.
Scratch wound assay
We seeded 1 × 105 cells into 24-well plates and allowed them to adhere for 24 h. A scratch was performed using a 20-μl tip, and the cells were photographed at 0 and 8 h. Image J software was used to determine the difference between the surface area of the scratch at 0 and 8 h.
Modified Boyden chamber
Chemotaxis of HUVECs toward Slit and vascular endothelial growth factor (VEGF) was measured in a 48-well chemotaxis chamber using 8 μM Nucleopore polycarbonate filters (Neuro Probe Inc., Gaithersburg, MD, USA) coated in 0.1% gelatin. HUVECs were serum starved for 1 h in DMEM alone. After starvation, cells were passaged in minimal medium (DMEM with 1% FCS) and seeded into a Boyden chamber at 20,000 cells/well. We placed 30 μl of test factors (10 ng/ml VEGF, 40 pM Slit2N, 400 pM Robo1Fc and Robo4Fc in minimal medium) or minimal medium alone (basal migration) in the lower wells. Slit2-N was purified on a heparin column as described previously (16). The apparatus was left for 5 h at 37°C/5% CO2; the membrane was fixed in 100% methanol for 10 min and stained in 0.5% crystal violet in PBS for 10 min. Nonmigrated cells were wiped away with a cotton bud, and the membrane was imaged. The number of cells from 5 random fields of view were counted and plotted as fold increase migration relative to minimal medium control.
RESULTS
Robo4 knockdown or overexpression decreases endothelial motility and alters the pattern of tube formation
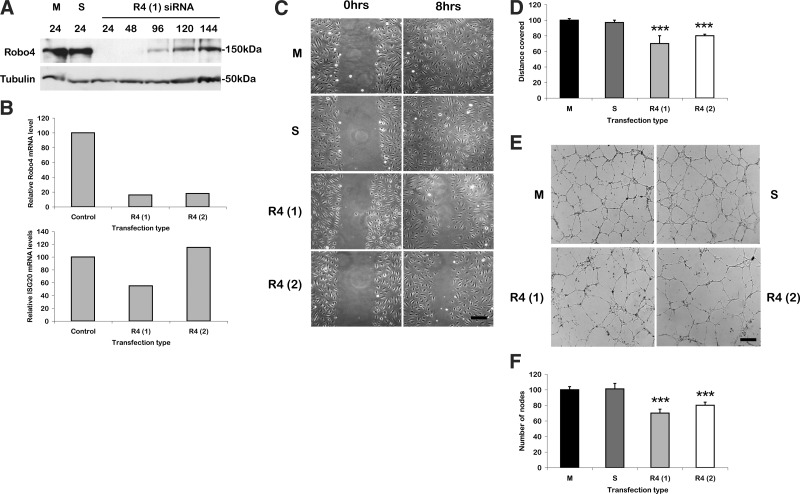
To examine Robo4’s role in endothelial cell migration and angiogenesis, Robo4 was transiently knocked down in HUVECs using 2 siRNA duplexes, termed R4(1) and R4(2). Inhibition of protein expression after 1 nM siRNA transfection was verified by immunoblot with a specific Robo4 antibody (Fig. 1A). At the mRNA level, qPCR analyses demonstrated that 1 nM transfection of both oligos depleted Robo4 mRNA levels by 90 and 85% respectively, consistent with the strong depletion of Robo4 protein expression (Fig. 1A, B). It is essential when using siRNA to show that the duplexes do not activate an interferon response, which can give rise to nonspecific phenotypes (17). qPCR analysis of ISG20 (interferon stimulated gene 20 kDa) expression showed no significant increase in its mRNA levels, indicating no interferon response with either of the Robo4 siRNA oligos (Fig. 1B). Therefore, both oligos efficiently depleted Robo4 mRNA and protein levels with no detectable off-target effects.
Figure 1.
Robo4 siRNA inhibits wound healing and Matrigel tube formation. A) Immunoblot of 1 nM R4(1) siRNA in HUVECs at different time points. B) qPCR analysis of Robo4 and ISG20 mRNA levels after transfection of 1 nM Robo4 siRNA oligos. Results normalized to flotilin2 loading control. C) Wound healing assay with Robo4 siRNA-transfected HUVECs. D) Measurements of the distance covered by each wound relative to the mock control. ***P < 0.001 vs. scrambled control. E) Matrigel with Robo4 siRNA-transfected HUVECs. Scale bars = 200 μm. F) Measurement of the average number of nodes present in Matrigel assay under the different conditions. ***P < 0.001 vs. mock control. Error bars ± sd. M, mock transfected; S, scrambled negative control duplex; R4(1), Robo4 duplex 1 siRNA; R4(2), Robo4 duplex 2 siRNA.
To examine the role of Robo4 in endothelial cell motility and angiogenesis, the siRNA oligos were used to deplete Robo4 expression, and the resultant phenotype was examined in scratch wound and Matrigel assays. In the scratch wound assay, Robo4 knockdown resulted in a significantly slower wound closure when compared to the mock and scrambled controls (Fig. 1C, D; P<0.001). In a Matrigel assay, Robo4 knockdown HUVECs showed an impaired ability to form a regular network, as fewer branches were formed and the network was uneven (Fig. 1E, F; P<0.001). These results confirm a role for Robo4 in endothelial cell movement and in vitro angiogenesis.
To complement the Robo4 siRNA studies, we investigated whether endothelial cell motility and guidance may also be affected by Robo4 overexpression. To test this hypothesis, a scratch wound assay was performed on HUVECs infected with either full-length FL-Robo4-GFP or GFP alone. HUVECs expressing FL-Robo4-GFP were less motile, and it took twice as long for the wound to close when compared to GFP expressing cells (Supplemental Fig. 1A, B; P<0.001). The ability of Robo4-overexpressing cells to form organized tube networks on Matrigel was also examined. Although FL-Robo4-GFP cells could elongate to form tubes, there were consistently fewer branches when compared to the GFP control (Supplemental Fig. 1C, D; P<0.001). The overall tube networks were irregular and contained large empty spaces, whereas the GFP control cells resulted in networks that were more uniform and dense. Therefore, overexpression of Robo4 resulted in a similar phenotype as Robo4 siRNA, implying that too little or too much Robo4 has the same detrimental effect on cell migration and tube formation.
Robo4 resides primarily in intracellular vesicles
In addition to studying the effects of Robo4-GFP expression on cell motility and tube formation, we studied its subcellular localization in HUVECs. FL-Robo4-GFP or GFP alone was infected using adenovirus into HUVECs, and protein expression was visualized using fluorescent microscopy. In contrast to the nuclear and diffuse cytoplasmic distribution of GFP alone, FL-Robo4-GFP localized to the perinuclear region of the cell and punctate, vesicle structures in the cytoplasm (Fig. 2A). The same distribution was seen in COS-7 cells when Robo4 was tagged with GFP, His, Flag, or Myc, suggesting that it is not aberrant localization due to GFP tagging (data not shown). HUVECs immunostained using MR1 anti-Robo4 also showed endogenous Robo4 in a similar, punctate and perinuclear distribution (Supplemental Fig. 2A). When expressed in COS-7 cells, the extracellular domain alone did not form vesicles. However, a Robo4 deletion construct containing the extracellular, transmembrane, and CC0 domains did localize to vesicles, thus indicating that the sequence responsible for vesicle localization is contained within this intracellular region (Supplemental Fig. 2B). However, despite the vesicular localization, FACS analysis with an antibody that recognizes the extracellular domain of Robo4 demonstrated that Robo4 was also present on the cell surface (Fig. 2B).
Figure 2.
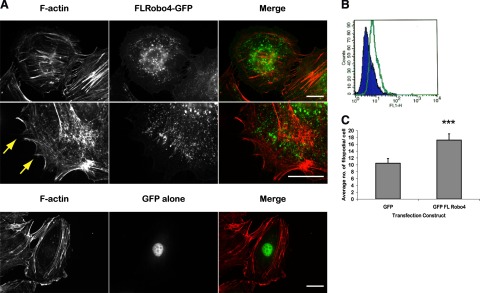
Robo4-GFP induces filopodia formation in HUVECs. A) FL-Robo4-GFP and GFP alone expressed in HUVECs and stained with TRITC phalloidin. Arrows indicate filopodia. Scale bars = 10 μm. B) FACS scan with a monoclonal antibody that recognizes the extracellular domain of Robo4 (MR7). Green, MR7; blue, isotyped IgG2a negative control. C) Quantitative analysis of filopodia numbers after expression of GFP alone or FL-Robo4-GFP (***P<0.001). Error bars ± se.
Live imaging of FL-Robo4-GFP expressing cells showed that they were very ruffled and spiky, with constant remodelling and movement at their edges (see Supplemental Fig. 2C for fluorescent images and Supplemental Movies 1 and 2). The movements involved both forward projection and subsequent retractions of the membrane, resulting in no overall net movement. Therefore, although Robo4-GFP-expressing cells can generate the filopodia and lamellipodia necessary for cell movement, they also show concomitant cell retraction and so cannot promote their migration. Following the vesicles using GFP imaging showed that subpopulations of the vesicles moved in sudden, linear movements toward the plasma membrane (Supplemental Movie 3). This was consistent with observations in fixed cells, because the FL-Robo4-GFP vesicles were often seen in parallel, linear arrays that colocalized with actin filaments (Supplemental Fig. 2D).
Overexpression of Robo4FL-GFP in HUVECs resulted in a spiky cellular morphology, with an increase in the number of filopodia compared to the GFP-infected cells (Fig. 2A, arrows). To quantify the phenotype, FL-Robo4-GFP and GFP-infected HUVECs were fixed and stained for F-actin, and the average number of filopodia was determined. Robo4FL-GFP expression resulted in a statistically significant increase in the number of filopodia (Fig. 2C; P<0.001). We conclude that Robo4 overexpression induces changes in cell shape and cytoskeletal rearrangement.
Robo4 interacts with WASP, NWASP, WIP, and Mena in endothelial cells
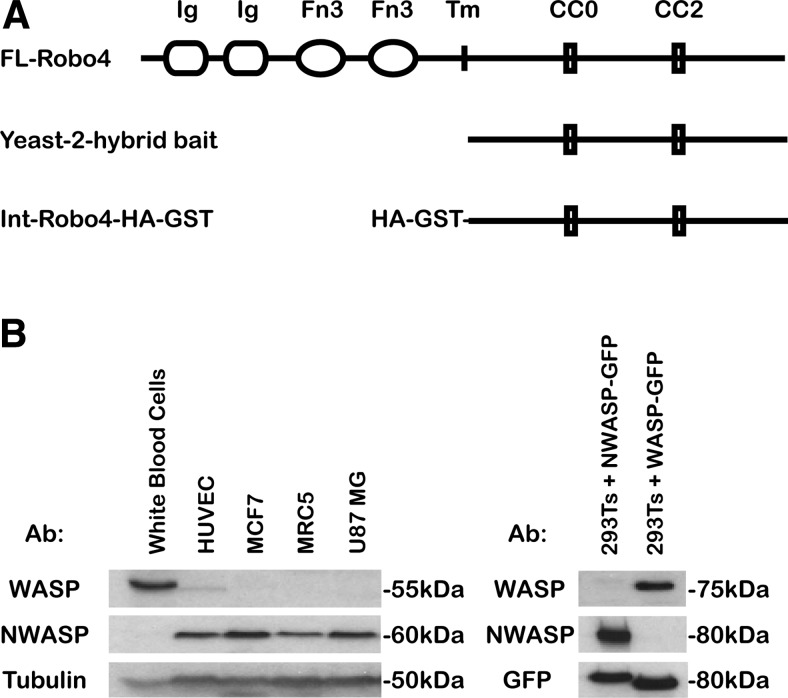
To begin to characterize the intracellular signaling pathways downstream of Robo4, the C-terminus of Robo4 was employed as bait in a yeast 2-hybrid analysis using a human placental library (Fig. 3A). Remarkably, the greatest numbers of hits were all to proteins involved in the regulation of the actin cytoskeleton, including WASP, neural WASP (NWASP), WASP-interacting protein actin-nucleating complex (WIP), and syndapin. In particular, the proline-rich domains of WASP and NWASP were identified several times as potential interacting proteins. In contrast to the ubiquitously expressed NWASP, WASP is exclusively expressed in cells of hematopoietic lineage (18). Although endothelial cells share this lineage, WASP expression has not previously been shown in HUVECs. To investigate the expression of WASP and NWASP in HUVECs, a panel of cell lysates was probed by immunoblot with anti-WASP, NWASP, and tubulin antibodies (Fig. 3B). WASP expression was high in white blood cells, with an ∼10-fold weaker expression in HUVECs. As expected, WASP was absent in MCF7, MRC5, and U87 MG cell lines. Thus, although the expression was relatively low, WASP protein is present in HUVECs and available for interaction with Robo4. NWASP was expressed in all lysates except white blood cells (Fig. 3B). Both the NWASP and WASP antibodies were specific to their respective proteins because they did not cross-react with the opposing GFP-tagged NWASP/WASP constructs overexpressed in HEK293T lysates (Fig. 3B).
Figure 3.
WASP and NWASP are expressed in HUVECs. A) Domain structure of full-length Robo4, the Robo4 yeast 2-hybrid bait, and intracellular Robo4-HA-GST constructs. Ig, immunoglobulin-like domain; Fn3, fibronectin type 3 domain; Tm, transmembrane domain; CC0/2, cytoplasmic conserved domains 0 and 2. B) Immunoblot analysis of WASP and NWASP expression in a variety of cell lines and HUVECs.
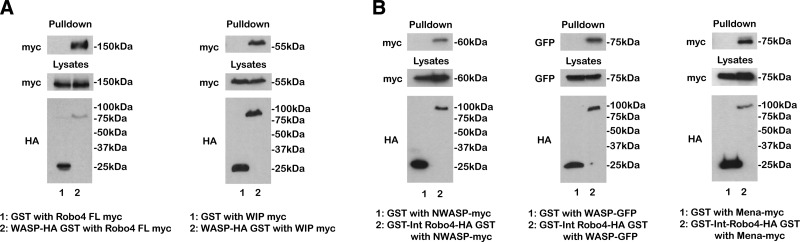
To investigate the binding of WASP to Robo4, we performed GST pulldown analyses. Because of degradation problems experienced using bacterially expressed Robo4 and WASP constructs, we adopted a cell-line approach, using a mammalian HA-GST expression vector. HA-GST vector alone or FL-WASP-HA-GST were cotransfected along with full-length FL-Robo4-myc into HEK293Ts and the lysates incubated with glutathione beads. Immunoblot of the lysates demonstrated that all constructs were successfully transfected, but Robo4-myc bound only to WASP-HA-GST and not the HA-GST alone control (Fig. 4A). As a positive control for the WASP construct, FL-WIP-myc was used, and this construct also bound to WASP-HA-GST (Fig. 4A). Therefore, GST pulldown analysis confirms WASP as a binding partner for Robo4.
Figure 4.
Robo4 binds WASP, NWASP, and Mena in GST pulldown analyses. A) Immunoblots of HA-GST alone and FL-WASP-HA-GST pulldowns with full-length Robo4-myc and a WIP-myc positive control. B) Immunoblots of HA-GST alone and Int-Robo4-HA-GST pulldowns with NWASP-myc, WASP-GFP, and a Mena-myc positive control.
To extend our analysis to include NWASP, the reciprocal experiment was performed by cloning the intracellular domain of Robo4 into pRK5-HA-GST (termed Int-Robo4-HA-GST; Fig. 3A). Unlike the equivalent bacterial GST construct, human Int-Robo4-HA-GST expressed well in HEK293Ts with minimal degradation (Fig. 4B). Mena, a previously identified binding partner for Robo4 and identified in our yeast 2-hybrid screen, bound to Int-Robo4-HA-GST as expected (Fig. 4B) (7). Interestingly, when cotransfected with epitope-tagged FL-WASP and NWASP, both WASP and NWASP bound to Int-Robo4-HA-GST (Fig. 4B). To further characterize the domain in WASP/NWASP responsible for binding to Robo4, we took advantage of a range of NWASP deletion constructs. When cotransfected with Int-Robo4-HA-GST, only constructs containing the proline-rich central domain were capable of binding to Robo4 (Supplemental Fig. 3A, B). Therefore, these GST pulldowns confirm the yeast 2-hybrid result, and because of the conserved domain structures of WASP and NWASP, it is likely that both proteins are binding through their proline-rich domains to the intracellular domain of Robo4.
Robo4 heterodimerizes with Robo1 and requires Robo1 to signal
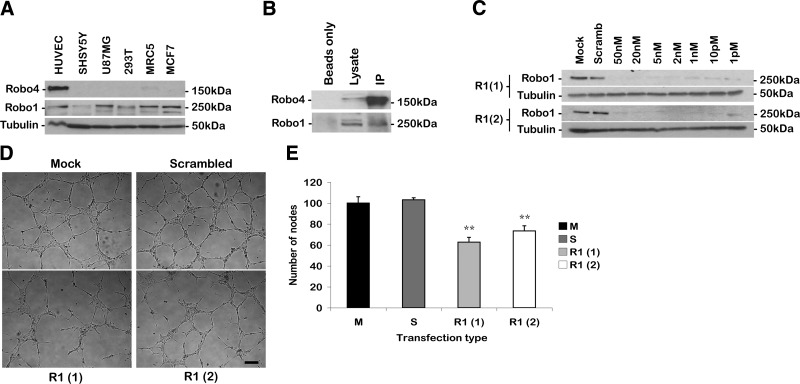
The siRNA and overexpression studies demonstrate that Robo4 is important in mediating cell movement in HUVECs. However, we wanted to probe further the mechanism by which Robo4 was functioning. The Roundabout receptors are known to homo- and heterodimerize, and this activity is important for their function (19, 20). To determine if other Roundabouts are expressed in HUVECs, we used qPCR and immunoblotting to probe a cell panel for Robo1 expression (Fig. 5A; Supplemental Fig. 4A). Robo1 was ubiquitously expressed, with high mRNA levels detected in SHSY5Y and U87 MG neuronal positive controls, but with significant transcript expression also in HUVECs (Supplemental Fig. 4A). Immunoblot of cell lysates with a polyclonal anti-Robo1 antibody detected a Robo1 band migrating near the 250-kDa marker. This is higher than the predicted 180-kDa molecular mass for Robo1, and, similar to Robo4, this is consistent with its N-terminal glycosylation. Robo1 protein was detected across the cell panel, with good expression in HUVECs (Fig. 6A). In contrast, Robo4 demonstrated endothelial-specific expression in both the qPCR and immunoblot analyses, as previously reported (Supplemental Fig. 4A; Fig. 5A) (3, 5). Therefore, both Robo1 and Robo4 are expressed in HUVECs.
Figure 5.
Robo1 siRNA inhibits in vitro angiogenesis. A) Immunoblot analysis of Robo4 and Robo1 protein expression across a panel of lysates. B) Coimmunoprecipitation analysis of Robo1-Robo4 binding. C) Titration analysis determining the optimal concentration of siRNA duplexes [R1(1) and R1(2)] required for maximum Robo1 knockdown. D) Matrigel assay with R1(1) and R1(2) siRNA-transfected HUVECs. Scale bar = 200 μm. E) Measurement of the number of nodes present in Matrigel assay under the different conditions. **P < 0.05 vs. mock control. Error bars ± sd.
Figure 6.
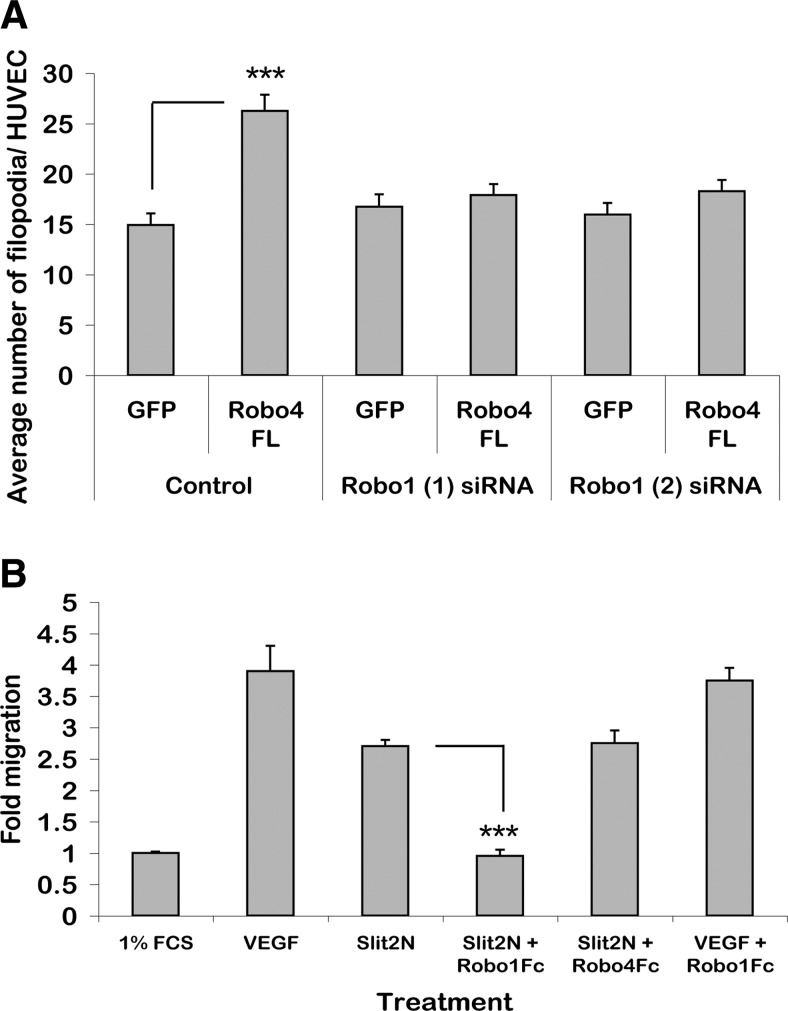
Robo1 and Robo4 cooperate in filopodia formation. A) Quantification of the number of filopodia in mock transfected or Robo1 siRNA-treated HUVECs expressing GFP alone or FL-Robo4-GFP. B) Modified Boyden chamber assay analyzing the migration of HUVECs to different stimuli. ***P < 0.001. Error bars ± se.
To examine heterodimerization, endogenous Robo4 was immunoprecipitated onto protein-G beads from HUVEC lysate. Robo1 and Robo4 immunoblots showed that endogenous Robo1 coimmunoprecipitated with Robo4, indicating that these proteins interact in HUVECs (Fig. 5B). Further evidence for Robo1-Robo4 heterodimerization was seen in COS-7 localization studies. When transfected alone, FL-Robo1-GFP localized to the membrane (Supplemental Fig. 4B). However, when cotransfected with FL-Robo4-myc, Robo1 relocalizes into Robo4-labeled vesicles (Supplemental Fig. 4C). This relocalization of Robo1 is consistent with Robo1 and Robo4 forming a complex.
To enable the analysis of Robo1 function in HUVECs, 2 siRNA oligos were designed specific to Robo1, termed R1(1) and R1(2). Initial experiments demonstrated that 50 nM concentration of both oligos efficiently silenced Robo1 expressed at 24, 48, and 72 h posttransfection (Supplemental Fig. 5A). At the mRNA level, qPCR analysis showed that both oligos significantly reduced Robo1 mRNA levels (90 and 80%, respectively) and did not induce the expression of interferon genes (Supplemental Fig. 5B). At the protein level, titration of the siRNA oligos down to 50 pM resulted in Robo1 expression returning, but still at a level significantly lower than the mock transfection (Fig. 6C). Here 2 nM was the optimum concentration required for each oligo to ensure maximum knockdown of Robo1 with minimum amount of oligo. Therefore, Robo1 expression in HUVECs can be potently and specifically depleted with these siRNA oligos.
To examine Robo1’s function in angiogenesis, the siRNA oligos were used to silence Robo1, and its effects were examined in a Matrigel assay. Similar to Robo4, the loss of Robo1 resulted in a more open meshwork of tubes on Matrigel (Fig. 5D, E; P<0.05). This result is consistent with Robo1 and Robo4 cooperating in regulating signaling pathways downstream of Slit.
As shown previously here and by Kaur et al.(9), Robo4 overexpression induces filopodia formation in endothelial cells (Fig. 2C). To probe the functional significance of Robo1-Robo4 interaction, we employed this filopodia phenotype as a functional output of Robo4 activation. When FL-Robo4-GFP is overexpressed in HUVECs, the Robo4-GFP expressing cells have a statistically significant increase in their numbers of filopodia relative to GFP expressing negative control cells (Fig. 6A; P<0.001). However, when overexpressed in Robo1 siRNA-treated cells, there is no induction of filopodia relative to the GFP-alone negative controls. This result demonstrates that Robo1 and Robo4 cooperate in regulating the actin cytoskeleton in HUVECs.
To determine whether Slit2 is functioning through binding to Robo1 or Robo4 in HUVECs, we used recombinant Slit2 in a modified Boyden chamber assay. The N-terminal portion of Slit2 (Slit2-N) has full biological activity and was released by salt washes of transfected 293 cells (21). At concentrations similar to those showing activity in neuronal migration assays, Slit2-N increased the migration of HUVECs above that of 1% FCS-alone basal migration control (Fig. 6B) (16). This increase in migration was suppressed by preincubation of Slit2-N with Robo1Fc but not with Robo4Fc (Fig. 6B; P<0.001). This confirms that migration is due to Slit2-N binding to Robo1 and not Robo4.
DISCUSSION
In recent years, the Roundabout family of single-pass, transmembrane receptors has been implicated in numerous cellular functions, including myogenesis, leukocyte chemotaxis, neuronal migration, and angiogenesis. In this study, we have used siRNA technology to investigate the role of Robo1 and Robo4 in endothelial biology. Our results suggest that Robo1 is involved in some manner with Robo4 in filopodia formation through remodeling of the actin cytoskeleton.
Robo4 localization in HUVECs
The subcellular localization and activity of Robo4 in HUVECs was probed using an adenoviral Robo4-GFP construct. When expressed in HUVECs, Robo4-GFP localized to intracellular vesicles. However, consistent with its role as a cell surface receptor, FACS analysis with antibody to the extracellular domain of Robo4 confirmed its cell surface expression. A concern here is that overexpression of the GFP-tagged protein in HUVECs leads to aggregates and the appearance in vesicles is artifactual. However, endogenous Robo4 also had a punctate, perinuclear distribution. These results are consistent with neuronal Roundabouts that are also sequestered intracellularly, with their appearance on the cell surface being highly regulated (22). Our preliminary analysis of the localizations of truncation mutants has identified the residues encompassing the transmembrane and CC0 intracellular domains as the sequence regulating this localization. However, further studies with site-directed mutants are required to isolate the precise domain required for vesicle formation. Interestingly, a subpopulation of the Robo4-GFP vesicles arranged into linear arrays that migrated rapidly from the perinuclear region of the cell to the plasma membrane. This may represent an active pool of Robo4 that can be trafficked to the cell surface to mediate migration.
Robo4 and Robo1 in angiogenesis
Robo4 is the most divergent member of the Roundabout protein family and is unique in that its expression has been detected only in endothelial cells (3, 5). Consistent with a role in angiogenesis, knockdown of Robo4 expression by siRNA results in inhibitory phenotypes in both woundhealing and Matrigel assays, similar to the effect of morpholino silencing of Robo4 in zebrafish (8).
The Roundabout receptors are known to heterodimerize, and therefore we were interested in determining whether Roundabout receptors, other than Robo4, are expressed in HUVECs. Robo1 expression in HUVECs is controversial, with conflicting reports (6, 7, 11). However, using qPCR and immunoblot analyses, we have demonstrated that Robo1 is clearly expressed in HUVECs. In culture, Robo1-Robo4 interactions were demonstrated in endogenous coimmunoprecipitation assays and the relocalization of Robo1 to Robo4-GFP labeled intracellular vesicles. Consistent with these molecules functioning together, Robo1 siRNA leads to poor tube formation on Matrigel.
To complement our siRNA studies, we investigated the effects of Robo4 overexpression in HUVECs. Morpholino knockdown in zebrafish embryos lead to defects in spatial and temporal vascular sprouting phenotype and angioblasts from the knockdown zebrafish actively seek guidance cues (8, 9). This phenotype can be explained by either positive or negative effects on cell migration. First, removal of an attractive guidance signal results in the collapse of a vessel. Or, in the absence of negative signals to direct their outgrowth, the endothelial cells cannot migrate correctly, resulting in the final decision to regress. Our studies clearly show that overexpression of Robo4 led to an impairment of the migratory ability of HUVECs and their ability to form a tubular mesh when seeded onto Matrigel. To generate directional movement, cells must produce a polarized leading front to mediate movement toward the signal. However, the unpolarized production of filopodia on all fronts that results from Robo4-GFP expression could inhibit directional movement, leading to a decrease in overall motility. Conversely, loss of coordinated actin rearrangements following Robo4 siRNA explains the subsequent loss of cell motility. This would also account for the knockdown effects on vascular development in zebrafish (8).
Robo4 binding to WASP proteins
To further delineate the signaling pathways downstream of Robo4, we used the intracellular domain as a “bait” in a yeast 2-hybrid screen. The results were striking in the number of proteins that it identified as binding to the intracellular domain of Robo4 that are involved in actin regulatory machinery. Mena has been previously identified as a binding partner for Robo4, and it generated several hits in the screen. The identification of a previously described binding partner for Robo4 corroborates the accuracy of the yeast 2-hybrid screen. Mena functions downstream of Cdc42 and IRSp53 to initiate filopodia formation and may represent one mechanism by which Robo4 can regulate the actin cytoskeleton (23). Novel hits included the WASP family proteins WASP/NWASP and their regulatory proteins, WIP and syndapin (24). It is interesting that WASP has previously been implicated in Robo4-mediated migration of 293 cells (9).
Whereas NWASP has a ubiquitous expression, WASP was originally found to have restricted expression to hematopoietic cells (18, 25). Although expressed at low levels relative to white blood cells, WASP is expressed in HUVECs and hence is available for binding to Robo4. GST pulldown experiments confirmed the binding of Mena, WASP, and NWASP to the intracellular domain of Robo4 and identified the proline-rich domain as the site in NWASP required for binding the intracellular domain of Robo4. The identification of the WASP regulatory protein WIP as a binding partner is particularly interesting because Robo4 may activate WASP-dependent actin polymerization through interactions with WIP. Alternatively, NWASP-syndapin binding may be required for the endocytosis and generation of Robo4-labeled vesicles (26, 27). The remarkable number of hits for this complex suggests that Robo4 acts as a molecular scaffold recruiting these proteins to enable actin nucleation, and the identification of these signaling molecules as interactors with Robo4 is entirely consistent with a role in actin assembly and filopodia formation. It will be interesting to determine the relative contributions of WASP and NWASP to Robo1/Robo4 signaling downstream of Slit.
The effect of Slit2 on endothelial cells is controversial. Slit2 has been reported both to attract migrating HUVECs by binding Robo1 and inhibit HUVECs (and microvascular endothelial cell) migration by binding to Robo4 (6, 11). In addition, Robo4 binding directly to Slit2 is debateable (4, 7, 28). When we used a physiological dose (40 pM) of the biologically active highly purified N-terminal protein (Slit2-N), we observed a stimulation of migration. Stimulation was blocked by preincubation with the extracellular domain of Robo1 (Robo1Fc) but not Robo4 (Robo4Fc), indicating that this effect is mediated through binding to Robo1. However, with Robo1 in a complex with Robo4, Slit2 may still mediate effects through Robo4 despite no direct interaction (4). Indeed, our data demonstrate that removal of Robo1 by siRNA blocks the induction of filopodia by overexpressed FL-Robo4-GFP. Therefore, Robo1 is necessary for at least partial activity of Robo4 in HUVECs.
This demonstration of Robo1-Robo4 cooperation is significant for understanding the mechanism behind Robo4 function in endothelial cells. In addition, it could explain how Robo1 can have apparent negative signaling activity in neurons but be promigratory in endothelium. When expressed alone, Robo1 may have a preset effect on migration, which, depending on the cell type, can subsequently be controlled by the expression of other Roundabout members. In neurons, where Robo4 is absent, Slit2 binding to Robo1 induces the binding of Rho GTPase activating proteins (srGAPs) to its CC3 domain. The srGAPs increase the intrinsic GTPase activity of Cdc42, leading to its inactivation. The localized deactivation of Cdc42 at the cell surface closest to the Slit source results in asymmetric actin polymerization and induces the cells to migrate away from the source of Slit (29,30,31). However, in HUVECs, Cdc42 activity is increased on Robo4 overexpression, and therefore Robo4-Robo1 dimerization may prevent srGAPs’ recruitment to the Robo1 CC3 domain, allowing Cdc42 to remain activated (9). Cdc42 activation and recruitment of WASP, NWASP, and WIP to Robo4 can lead to local actin polymerization at the site of Slit2 binding, which in turn will give rise to filopodia and the attraction of migrating cells to the source of Slit2.
In conclusion, work by our laboratory and others have provided evidence for a role for Robo4 in filopodia formation, endothelial migration, and guidance. These data begin to address the mechanisms by which these occur, but there is still much to determine. For example, how is WASP activity regulated by Slit binding to Robo1/Robo4, and how do the different downstream actin regulatory proteins orchestrate the physiological response to Robo4 activation? Future studies are required to further characterize these signaling pathways critical to endothelial cell migration.
Supplementary Material
Acknowledgments
This work was supported by Cancer Research UK grant A6766 and European Union FP6 LSHC-CT-2003-503233 grant “STROMA.” We thank Laura M. Machesky and Guillaume Bompard for the WASP and NWASP constructs. H.S., M.A., and J.A.L. contributed equally to the work. They designed and performed research and helped write the paper. P.H. designed and performed the modified Boyden chamber assay. J.M.H. carried out the bioinformatics analysis of the yeast 2-hybrid experiments. A.S.N. made recombinant adenoviruses (vital new reagents). J.K.K. advised on the design of experiments. V.L.H. assisted with some experiments and helped write the paper. R.B. conceived and supervised the entire project, including the writing of the paper.
References
- Seeger M., Tear G., Ferres-Marco D., Goodman C. S. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Kidd T., Brose K., Mitchell K. J., Fetter R. D., Tessier-Lavigne M., Goodman C. S., Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Huminiecki L., Gorn M., Suchting S., Poulsom R., Bicknell R. Magic Roundabout is a new member of the Roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- Legg J. A., Herbert J. M. J., Clissold P., Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Stekel D., Sanderson S., Heath V. L., Bicknell R. A novel method of differential gene expression analysis using multiple cDNA libraries applied to the identification of tumour endothelial genes. BMC Genomics. 2008;7:153–174. doi: 10.1186/1471-2164-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Xiao Y., Ding B. B., Zhang N., Yuan X., Gui L., Qian K. X., Duan S., Chen Z., Rao Y., Geng J. G. Induction of tumour angiogenesis by Slit-Robo signalling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Park K. W., Morrison C. M., Sorensen L. K., Jones C. A., Rao Y., Chien C. B., Wu J. Y., Urness L. D., Li D. Y. Robo4 is a vascular-specific receptor that inhibits endothelial cell migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Bedell V. M., Yeo S. Y., Park K. W., Chung J., Seth P., Shivalingappa V., Zhao J., Obara T., Sukhatme V. P., Drummond I. A., Li D. Y., Ramchandran R. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Castellone M. D., Bedell V. M., Konar M., Gutkind J. S., Ramchandran R. Robo4 signalling in endothelial cells implies attractive guidance mechanisms. J Biol Chem. 2006;281:11347–11356. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- Bashaw G. J., Kidd T., Murray D., Pawson T., Goodman C. S. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the Roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Seth P., Lin Y., Hanai J., Shivalingappa V., Duyao M. P., Sukhatme V. P. Magic Roundabout, a tumour endothelial marker: expression and signalling. Biochem Biophys Res Commun. 2005;332:533–541. doi: 10.1016/j.bbrc.2005.03.250. [DOI] [PubMed] [Google Scholar]
- Hohenester E., Hussain S., Howitt J. A. Interaction of the guidance molecule Slit with cellular receptors. Biochem Soc Trans. 2006;34:418–421. doi: 10.1042/BST0340418. [DOI] [PubMed] [Google Scholar]
- Morlot C., Thielens N. M., Ravelli R. B., Hemrika W., Romijn R. A., Gros P., Cusack S., McCarthy A. A. Structural insights into the Slit-Robo complex. Proc Natl Acad Sci U S A. 2007;104:14923–14928. doi: 10.1073/pnas.0705310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard T. H., Behrendt B., Launay S., Fütterer K., Machesky L. M. Identification and charaterisation of a novel human isoform of Arp2/3 complex subunit p16-ARC/ARPC5. Cell Motil Cytoskelet. 2003;54:81–90. doi: 10.1002/cm.10087. [DOI] [PubMed] [Google Scholar]
- Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet K. T., Brose K., Ma L., Wang K. H., Marillat V., Sotelo C., Tessier-Lavigne M., Chédotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledz C. A., Holko M., de Veer M. J., Silverman R. H., Williams B. R. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Derry J. M., Ochs H. D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Hivert B., Liu Z., Chuang C. Y., Doherty P., Sundaresan V. Robo1 and Robo2 are homophilic binding molecules that promote axonal growth. Mol Cell Neurosci. 2002;21:534–545. doi: 10.1006/mcne.2002.1193. [DOI] [PubMed] [Google Scholar]
- Sabatier C., Plump A. S., Le Ma Brose K., Tamada A., Murakami F., Lee E. Y., Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of Slit responsiveness required for midline crossing by commisural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Howitt J. A., Clout N. J., Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E., Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attaraction by Slit through a robo/DCC receptor complex. Science. 2001;291:1928–1939. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Krugmann S., Jordens I., Gevaert K., Driessens M., Vandekerckhove J., Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53: Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles N. M., Rhotagi R., Anton I. M., Medina M., Saville S. P., Miki H., Yamaguchi H., Takenawa T., Hartwig J. H., Geha R. S., Ramesh N. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat Cell Biol. 2001;3:484–491. doi: 10.1038/35074551. [DOI] [PubMed] [Google Scholar]
- Miki H., Miura K., Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearragement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- Kessels M. M., Qualmann B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002;21:6083–6094. doi: 10.1093/emboj/cdf604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels M. M., Qualmann B. Syndapin oligomers interconnect the machineries for endocytic vesicle formation and actin polymerization. J Biol Chem. 2006;281:13285–13299. doi: 10.1074/jbc.M510226200. [DOI] [PubMed] [Google Scholar]
- Suchting S., Heal P., Tahtis K., Stewart L. M., Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005;19:121–123. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- Lundström A., Gallio M., Englund C., Steneberg P., Hemphälä J., Aspenström P., Keleman K., Falileeva L., Dickson B. J., Samakovlis C. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18:2161–2171. doi: 10.1101/gad.310204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Li M., Labrador J. P., McEwen J., Lai E. C., Goodman C. S., Bashaw G. J. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci U S A. 2005;102:4613–4618. doi: 10.1073/pnas.0409325102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Ren X. R., Huang Y. Z., Xie Y., Liu G., Saito H., Tang H., Wen L., Brady-Kalnay S. M., Mei L., Wu J. Y., Xiong W. C., Rao Y. Signal transduction in neuronal migration: roles of the GTPase activating proteins and small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.