Abstract
Eosinophilia and its cellular activation are hallmark features of asthma, as well as other allergic/TH2 disorders, yet there are few, if any, reliable surface markers of eosinophil activation. We have employed a FACS-based genome-wide screening system to identify transcriptional alterations in murine lung eosinophils recruited and activated by pulmonary allergen exposure. Using a relatively stringent screen with false-positive correction, we identified 82 candidate genes that could serve as eosinophil activation markers and/or pathogenic effector markers in asthma. Carbonic anhydrase IV (Car4) was a top dysregulated gene with 36-fold induction in allergen-elicited pulmonary eosinophils, which was validated by quantitative PCR, IHC and by flow cytometry. Eosinophil CAR4 expression was kinetically regulated by IL-5 but not IL-13. IL-5 was both necessary and sufficient for induction of eosinophil CAR4. While CAR4-deficient mice did not have a defect in eosinophil recruitment to the lung nor a change in eosinophil pH-buffering capacity, allergen-challenged chimeric mice that contained Car4−/− hematopoietic cells aberrantly expressed a series of genes enriched in biological processes involved in epithelial differentiation, keratinization, and anion exchange. In conclusion, we have determined that eosinophils express CAR4 following IL-5 or allergen exposure, and that CAR4 is involved in regulating the lung transcriptome associated with allergic airway inflammation; as such, CAR4 has potential value for diagnosing and monitoring eosinophilic responses.
Keywords: lung eosinophils, asthma, eosinophil activation, bone marrow transfer
Introduction
Asthma is a chronic inflammatory process characterized by lung and airway inflammation accompanied by the infiltration of lymphocytes, basophils, mast cells, macrophages, and eosinophils (1, 2). Eosinophilia is a hallmark feature of asthma, and it is well recognized that eosinophil migration is dependent on the eotaxin chemokines (3, 4) and the critical eosinophilopoietic and activating cytokine – interleukin 5 (IL-5) (4, 5). Eosinophils elicit key effector functions in asthma pathogenesis by directing dendritic cell activation, promoting antigen presentation, activating mast cells, and releasing pleotropic cytokines (See (5) for review). Accordingly, eosinophils are now being pharmacologically targeted by a variety of new drugs (6, 7), such as anti-IL-5 therapeutics (8, 9). Molecular characterization of the eosinophil transcriptome by genome-wide array offers a potentially promising approach to comprehensively screen for molecules associated with eosinophil activation during allergic inflammation.
In order to molecularly characterize eosinophil activation in allergic lung inflammation, we performed a genome-wide mRNA microarray analysis of FACS-purified murine eosinophils from allergen (ovalbumin-OVA)- or control (saline)-exposed lung samples. Transcriptome analysis revealed a pronounced differential expression of 82 genes in eosinophils obtained from allergen-exposed compared to control mice. While multiple candidate genes could serve as eosinophil activation markers, carbonic anhydrase 4 (Car4) expression on activated eosinophils was both pronounced and unexpected. CAR4 belongs to a family of Zn2+-containing metalloenzymes that has numerous members (CAR1 to CAR15, sharing minimal homology) that interconvert HCO3− and CO2 (HCO3− + H+ → H2O + CO2, for recent review see (10) by Waheed and Sly). CARs maintain acid-base balance in multiple tissue compartments so that harmful metabolic by-products can be rapidly removed, critical anions readily re-absorbed and specific pH optimally maintained. CAR4 is expressed on CNS neurons, renal tubule epithelium, pulmonary capillary endothelium, and intestinal epithelium for metabolic acidosis buffering, HCO3− re-absorption, lung CO2 excretion, and anion exchanges, respectively (11–13).
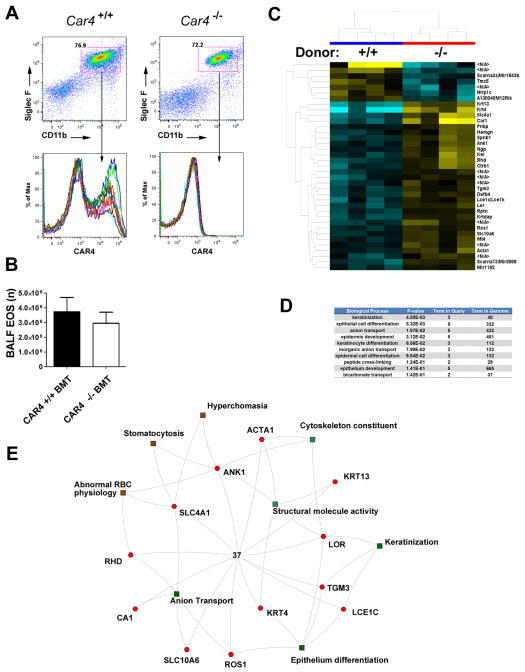
We validated the up-regulated eosinophil Car4 expression by quantitative PCR, protein staining, and by comparing Car4+/+ vs Car4−/− mice. IL-5 was necessary and sufficient to induce CAR4 expression, as demonstrated in allergen-challenged Il5−/− mice and following IL-5 administration in vivo. A genome-wide expression array analysis following transplantation with Car4+/+ and Car4−/− bone marrow identified differential expression of 37 transcripts in the asthmatic lung that was enriched in alterations in epithelial differentiation and keratinization processes. Collectively, we have determined that CAR4 is specifically upregulated in allergen-elicited pulmonary eosinophils, dependent on IL-5, and likely indirectly involved in a series of epithelial responses during allergic inflammation.
Materials and Methods
Mice
Car4−/− mice on C57BL/6J background were obtained from Jackson Laboratory (Bar Harbor, ME) from a recovery of cryo-preserved embryos. Due to the known breeding deficiency of Car4−/− homozygous mice (11), heterozygous matings were used in all experiments. In some initial validation experiments, BALB/c mice were subjected to CAR4 FACS staining, with no eosinophil CAR4 expression difference found between the two strains. All mice used in this study were housed in specific pathogen–free conditions at Cincinnati Children’s Hospital Medical Center (CCHMC) under IACUC-approved protocols. All mice were housed in a room with an ambient temperature of 22°C and a 12-hour light cycle.
Genome-wide microarray analysis on sorted eosinophils and qPCR validation
Tissue eosinophils were isolated from the perfused lung as previously described (14). In brief, eosinophils were sorted as DAPI−CCR3+Siglec-F+CD45+CD4−CD8a−CD19−B220−side scatterhigh (SSChigh) cells from 10 animals using FACS Aria (BD Biosciences). Total RNA from sorted eosinophils (purity 99%) was extracted by a standard TRIzol (Life Technologies) RNA isolation and subsequently with an RNeasy Mini Kit (QIAGEN). RNA integrity was validated by the Agilent 2100 bio-analyzer (Agilent Technologies). Eosinophil mRNA was amplified and labeled with the WT-Ovation Pico RNA Amplification System (NuGen) and subjected to the GeneChip Mouse Gene ST 1.0 Array chip (Affymetrix), which covers the whole mouse genome with 28,853 probe sets. The expression data were analyzed by the software of Genespring GX 11 (Agilent Technologies). Differential expression between activated lung eosinophils and saline controls was identified by upper 80th percentile expression, > 2.0-fold change, and a p value < 0.05 with a false discovery rate (FDR) correction. For the lung tissue microarray following bone marrow transplantation (BMT), Affymetrix Mouse Gene ST 2.0 Array chips and a filter of > 1.414 fold-change and Westfall-Young-corrected p value < 0.01 were used. For real-time PCR confirmation, the same mRNA sample used for microarray analysis was reverse transcribed with the iScript cDNA Synthesis Kit (Bio-Rad, 170-8891), and real-time PCR was performed with the iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using a pair of primers spanning exons 1 and 2 of the Car4 transcript (forward 5′-CATGCAGCTCCTTCTTGCTCT -3′; reverse 5′-TTTGAATCTCATAGCACCAGCC -3′), resulting in an 83-bp amplicon.
Flow cytometry analysis of eosinophil surface expression and signaling
Polychromatic flow cytometry compensation was preset in pilot studies for up to 5 channels on a FACS Canto II flow cytometer (BD Biosciences). Antibodies/dyes used in this study were purchased from Biolegend (CD11c-Pacific Blue N418, human Siglec 8 7C9, human CD11b ICRF44, human CD45 2D1), Invitrogen (LIVE/DEAD Violet dye), BD Biosciences (CD45-FITC 30-F11, Siglec F-PE E50-2440, CD11b-PECy7 M1/70, Gr-1 PerCP/Cy5.5 RB6-8C5), and R&D Systems (msCAR4, AF2414). Staining was performed on ice for 30 minutes in staining buffer (0.5% BSA, 0.01% NaN3 in 1× HBSS) with manufacturer-suggested titers. A secondary staining with the anti-goat AF488 was used for all CAR4 staining. Stained cells were resuspended and subjected to analysis with the BD FACS Canto II flow cytometer. In some experiments, stained cells were fixed before being subjected to FACS analysis in a controlled fashion. Raw flow cytometry data were analyzed by FlowJo software (Tree Star Inc.). For phosphor-FACS, eotaxin-1 (25 ng/mL) stimulation (2 minutes) was used for p44/42 MAPK activation assessment. Cells were immediately fixed by 2% formaldehyde at 37°C for 10 minutes, followed by ice-cold methanol permeabilization for 30 minutes. Specific Thr202/Tyr204 p44/42 antibody (E10, Cell Signaling Technology, #4375) was used together with previously mentioned eosinophil markers to assess eosinophil MAPK activation. For flow-imaging analysis of bronchoalveolar fluid (BALF) eosinophils, stained cells were fixed with 2% formaldehyde and run through the ImageStreamX system (Amnis Corp.), following the manufacturer’s instructions as previously reported (14).
CAR4 immunohistochemistry
A consistent lobe (lower-right) of mouse lung tissue was fixed in 10% formalin under negative pressure for 6 hours before regular paraffin embedding and sectioning procedures. After citric buffer antigen retrieval, the 5μm sections were incubated with either anti-CAR4 polyclonal antibody (R&D, AF2414) or IgG control (R&D, AB-108-C) in 1% rabbit serum overnight in 4 degree, followed by incubation with biotinylated rabbit-anti-goat secondary antibody for 1 hour at room temperature. The slides were developed by Vectastain Elite ABC kit (Vector lab) following the standard manufacturer’s protocol and counterstained by hematoxylin.
Bone marrow-derived eosinophils
Whole bone marrow was cultured using a modification of previously described methods (15). During the 14-day culture, the culture media was supplemented with FLT3 and SCF at 100 ng/mL (Peprotech, NJ) for the first 4 days and IL-5 at 10 ng/mL (Peprotech, NJ) for the following 10 days. Results from H&E staining and FACS analysis indicated that the purity of eosinophils was > 90% at day 14.
Experimental asthma induction
For the OVA model, mice were sensitized with 100 μg of OVA/Alum twice, 14 days apart. For OVA intranasal challenge, OVA (100 μg in 50 μL saline) was administered for 3 consecutive days. Mice were sacrificed 48 hours after the 3rd OVA challenge for eosinophilia assessment. For the Aspergillus model, 100 μg Aspergillus fumigatus (GREER, NC) was dissolved in sterile saline and administered intranasally every other day into wild-type or IL-5–transgenic mice (BALB/c or C57BL6); a total of 5 challenges were given unless otherwise noted in the results. For the CC10-IL-13 transgenic model, IL-13 expression is driven by the lung epithelium promoter CC10 under the control of doxycycline (16), and the asthma model was established after the mice were treated with doxycycline for 4 weeks.
Bone marrow transfer experiments
CD45.1 recipients received sequential doses of irradiation, 7 Gy and then 4.75 Gy, before a bolus transfer of 1.1×107 RBC-lysed donor CD45.2 Car4+/+ or Car4−/− whole bone marrow cells. CD45.1 recipient mice were fed with food containing doxycycline for 2 weeks thereafter and periodically checked for CD45.2 blood CD11b+ cell engraftment efficiency by FACS. Allergen challenge started when the CD11b+ engraftment was greater than 99% for animals around 3 weeks after the transfer.
Lactate induced acidification
Car4+/+ and Car4−/− bone marrow-derived eosinophils were loaded with 5 μM pH-sensitive dye SNARF-1AM and then incubated with 15 mM lactate for 20 minutes. Intracellular pH (pHi) was determined by flow cytometry comparing to eosinophils whose pHi was clamped with nigericin as previously described (17).
Statistical analysis
Statistical significance was analyzed using a two-tailed student t-test in all instances unless otherwise noted. Data are graphed as mean ± standard error of the mean (SEM). A p < 0.05 was deemed as significant in experiments not involving a microarray, whose criteria are specifically mentioned in the Methods and Results sections.
Results
Eosinophil RNA microarray identifies a unique transcriptome with Car4 as a lead transcript
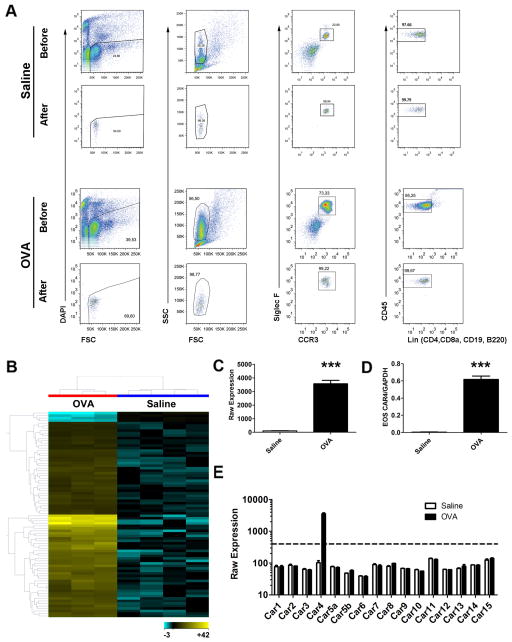
After lung perfusion and collagenase digestion, lung tissue eosinophils were FACS-sorted for DAPI−CCR3+Siglec-F+CD45+CD4−CD8a−CD19−B220− and SSChigh events, from saline-challenged lung (Fig. 1A upper panel) and OVA-challenged lung (Fig. 1A lower panel). Eosinophil sorting purity (> 95–99%) in saline and OVA samples was confirmed by back-running the sorted samples with an identical gating strategy (Fig. 1A). Microarray analysis of these isolated cells led to the identification of a total of 82 genes that were differentially expressed, 78 upregulated and 4 downregulated, in eosinophils from OVA-challenged vs. saline-challenged lungs (Fig. 1B and Table S1). Among the most-induced genes, Car4 mRNA was robustly upregulated by 36-fold (p < 0.0001, FDR-corrected p = 0.01) in eosinophils following OVA challenge (Fig. 1C). We next confirmed the Car4 mRNA upregulation by qPCR from the same sorted RNA sample, verifying a dramatic 165-fold increase after normalizing to Gapdh (p < 0.0001, Fig. 1D). To assess the potential involvement of other CARs, we also collectively analyzed the differential expression level of all 15 CAR family member transcripts embedded on the array and found that eosinophil Car4 upregulation was unique among all of the CAR members screened (Fig. 1E).
Figure 1. Unique transcriptome of activated lung eosinophils and Car4 validation.
A, After allergen (OVA, n = 3) and saline (n = 4) challenge, lung tissue eosinophils were isolated by FACS following enzymatic digestion to harvest eosinophil RNA. Live eosinophil events were identified as DAPI−CCR3+Siglec-F+CD45+CD4−CD8a−CD19−B220−SSChigh with a purity > 95–99% confirmed by sample back-run (Saline and OVA, before and after FACS sorting graphs shown). B, Eosinophil mRNA was subjected to genome-wide mRNA microarray (Affymetrix mouse ST 1.0 chip), resulting in 78 upregulated and 4 downregulated genes following activation (upper 80th percentile expression, fold change > 2.0, FDR-corrected p < 0.05). On the basis of these significant genes, a heat-map was established double-clustered on entity (gene, y axis) and condition (treatment, x axis), with yellow indicating high expression and blue indicating low expression (Table S1). C, The Affymetrix raw expression value of Car4 probes, saline (n = 4) vs. OVA (n = 3)-challenged eosinophils. D, The microarray finding of Car4 overexpression was validated by quantitative PCR (with Gapdh normalization). E, The Affymetrix raw expression values of all 16 Car family members embedded on the microarray were displayed, saline vs. OVA challenged, with a value of 400 units deemed as significantly expressed (*** p < 0.001, all data presented as mean ± SEM). EOS, eosinophils.
Lung eosinophil CAR4 surface expression is driven by TH2 inflammation
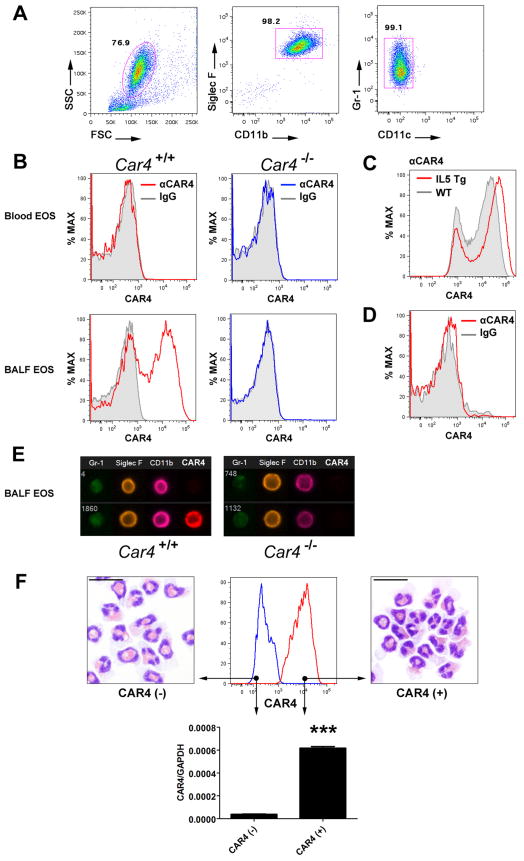
We aimed to validate the CAR4 expression at the protein level on both BALF and lung tissue eosinophils following allergen (Aspergillus fumigatus) challenge (18), in which eosinophils are a major population in BALF and lung tissue after 5–6 allergen challenges. Car4+/+ and Car4−/− mice were used to test the specificity of the antibody staining, while polychromatic FACS readily identified a pronounced eosinophil population (typically up to 80% of BALF cells) by SSChighSiglec-F+CD11b+Gr-1lowCD11c− serial gating (Fig. 2A), resulting in > 99% purity from BALF (Fig. 1A and 2F). We therefore used a similar gating strategy to study eosinophil populations by FACS throughout this study. Gated on BALF eosinophils, CAR4 staining controlled by non-specific IgG staining revealed robust CAR4 surface staining only on Car4+/+ BALF eosinophils, but not on Car4−/− BALF eosinophils and not on blood eosinophils from the same animal of either genotype (Fig. 2B). We identified a comparable CAR4 expression pattern in the OVA-challenging model that was originally used in the sorting-based microarray (Fig. S1A). We also assessed CAR4 surface expression on major leukocytes in the lung, BALF and blood. Of note, unlike lung eosinophils, which expressed surface CAR4 only following allergen challenges; lung macrophages (under homeostasis or allergen challenge), but not lymphocytes and neutrophils, also expressed CAR4 on their surface independent of allergic inflammation (Fig. S2A).
Figure 2. CAR4 protein expression on lung eosinophils during allergic inflammation.
A, Eosinophils (~106) are present in the airway in the mold allergen (Aspergillus fumigatus)–induced experimental asthma murine model. A FACS serial gating strategy identifies bronchoalveolar (BALF) and lung tissue eosinophils (EOS) as SSChighSiglec-F+CD11b+GR-1+CD11c− events with > 99% purity confirmed by sorting. B, Following allergen challenge, CAR4 surface expression (αCAR4) on Car4+/+ (red histogram) and Car4−/− (blue histogram) blood and BALF eosinophils are illustrated after serial gating (solid grey, IgG control). Representative FACS histograms of at least 5 independent experiments are shown. C, Following allergen challenge, similar CAR4 expression pattern is shown on lung tissue eosinophils (gated) acquired after tissue digestion and anti-CAR4 staining (αCAR4, solid grey, wild-type [WT]; red, CD2-IL5 transgenic mice [IL5 Tg]). D, With experimental asthma induced in CC10-IL13 transgenic mice and driven by 4 weeks of doxycycline exposure, CAR4 surface expression on BALF eosinophils was assessed (red) in reference to IgG staining (solid grey). E, Flow-imaging (Imagestreams) micrographs of the surface expression of CAR4 together with eosinophil markers. F, With Aspergillus challenges, BALF cells were FACS sorted for eosinophils by the aforementioned gating strategy. CAR4+ (red histogram) and CAR4− (blue histogram) eosinophils were back-run by FACS to confirm sorting purity. Car4 quantitative PCR was performed with RNA extracted from sorted CAR4+ and CAR4− eosinophils, which were also used to prepare a cytospin slide for morphological evaluation under 400× microscopy (25-μm bar superimposed). (***, p < 0.001, two-tailed student t-test, mean ± SEM)
We next examined CAR4 surface expression on lung tissue eosinophils from wild-type and Il5 transgenic mice and identified a similar heterogeneous expression pattern, with the latter exhibiting more robust CAR4 expression (Fig. 2C), suggesting a role for IL-5 in CAR4 induction. As IL-13 is sufficient to elicit extensive lung eosinophilia (19), we utilized the CC10-IL-13 transgenic system (16) to assess the role of IL-13 in eosinophil CAR4 induction. Of note, IL-13 overexpression failed to induce eosinophil CAR4 expression (Fig. 2D) despite the presence of extensive eosinophilic lung inflammation.
Notably, heterogeneous expression of CAR4 was consistently observed after 5–6 allergen challenges, typically resulting in a double-peak histogram with ~50% of the eosinophils remaining CAR4 negative (CAR4−). FACS imaging micrographs identified surface labeling of CAR4 and the co-existence of CAR4+ and CAR4− eosinophils in the BALF from mice with allergic inflammation (Fig. 2E). FACS sorting of CAR4+ and CAR4− eosinophils demonstrated that the CAR4+ eosinophils contained 20-fold higher Car4 transcript than CAR4− eosinophils despite comparable morphology (Fig. 2F). In addition, heterogeneous CAR4 expression on eosinophils did not correlate with eosinophil physical parameters (forward and side scatter) nor a panel of known eosinophil/granulocyte surface markers related to key functions (CCR3, Siglec-F, CD11b, CD11c, Gr-1, α4, αE, β1, β2 and β7 integrin, and L-selectin/CD62L, IL5R/CD125, CD69, CD86, CD25 and intracellular IL4) (Fig. S1B). Additionally, an unaltered eotaxin-CCR3 signaling strength was found based on p44/42 MAPK activation capacity of CAR4+ and CAR4− eosinophils (Fig. S1C, Car4+/+ sample used).
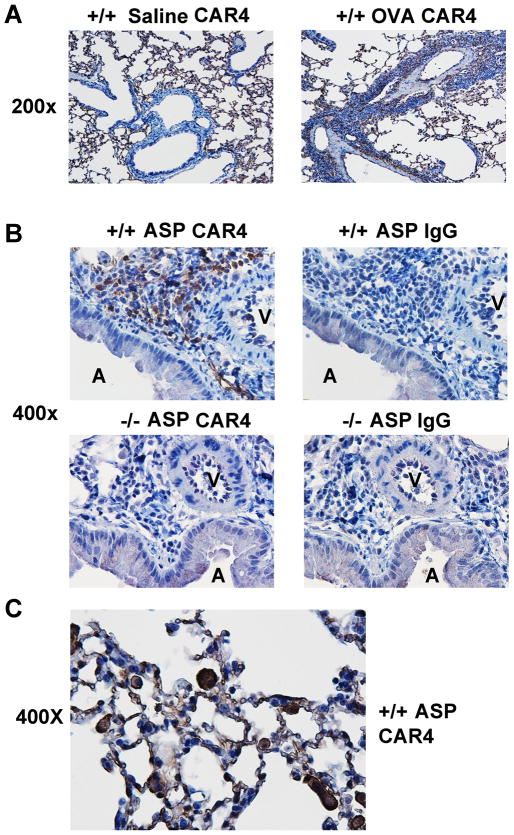
Additionally, we also examined CAR4 distribution in the normal and allergic lung by immunohistochemistry (IHC). In the OVA asthma model, while CAR4 was positive in the alveolar area regardless of treatment, CAR4 positive infiltrating inflammatory cells were only found in OVA lung in the anatomical regions traversing blood vessel and airway (the asthmatic pathogenic zone), an area clearly CAR4-negative in the saline control (Fig. 3A, 200×). At higher power magnification (400×), in a different asthma model (induced by Aspergillus fumigatus antigen), CAR4 positive events were only found in the CAR4 +/+ lung stained with CAR4, not in the −/− lung, and not in the IgG control staining in both cases (Fig. 3B). High power micrograph focusing on the alveolar region illustrated the CAR4 expression on lung macrophages and eosinophils (Fig. 3C)
Figure 3. CAR4 expression in the allergic lung by immunohistochemistry.
A, CAR4 IHC staining (at 200×) in saline and OVA challenged lung. B, Aspergillus-challenged CAR4 +/+ and −/− lungs stained with either anti-CAR4 or IgG antibody, with 400× micrographs shown. V, blood vessel; A, airway. C, CAR4 staining from the respiration/alveoli area of CAR4 +/+ Aspergillus-challenged lung (400× micrograph magnified to ~550× electronically).
IL-5 is necessary and sufficient for eosinophil CAR4 surface induction
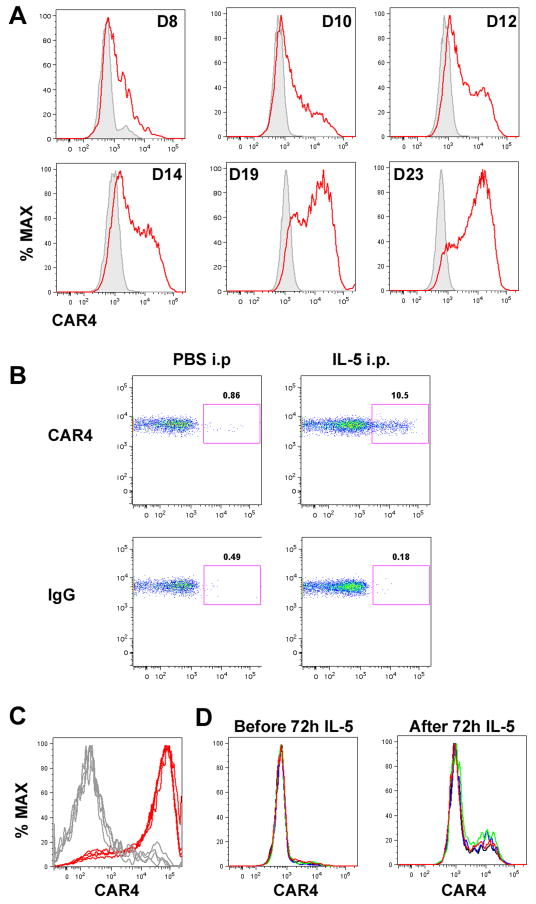
The enhanced CAR4 expression in eosinophils from IL-5 transgenic mice led us to hypothesize that IL-5 is a key driver of CAR4 induction during allergic airway inflammation. To test this hypothesis, the ability of IL-5 to modulate CAR4 expression during eosinophil development was assessed (15). Indeed, CAR4 on developing eosinophils was kinetically induced by IL-5 in vitro, reaching ~80% CAR4 positive around day 20 (Fig. 4A, gated eosinophils shown). Additionally, delivery of exogenous IL-5 (1 μg) into the peritoneal cavity of wild-type mice induced CAR4 upregulation on eosinophils (Fig. 4B, gated eosinophils shown). Lastly, we also established allergic inflammation in allergen-challenged Il5+/+ and Il5−/− mice and found that BALF eosinophils from Il5−/− mice were largely CAR4− (Fig. 4C); whereas, eosinophils from Il5+/+ mice were mostly CAR4+. Of note, CAR4 demonstrated more homogenous staining under these experimental conditions, likely due to administering seven allergen challenges. To further determine whether IL-5 was necessary and sufficient, we exposed BALF cells from Il5−/− allergen-challenged mice to exogenous IL-5 for 72 hours (20 ng/mL) and compared the eosinophil CAR4 expression before and after IL-5 exposure. IL-5 treatment indeed induced CAR4 expression (Fig. 4D). Compared to Car4+/+ eosinophils, Car4−/− bone marrow–derived eosinophils did not exhibit a difference in differentiation in terms of specific surface marker expression, yield, or morphology (Fig. S1D and data not shown).
Figure 4. Role of IL-5 for CAR4 induction.
A, Bone marrow–derived eosinophils CAR4 expression following IL-5 induction (10 ng/mL). Gated eosinophil CAR4+ expression histograms (red) (in reference to IgG staining (grey)) on cultured eosinophils were plotted on different days. B, IL-5 (1 μg) or saline (PBS) was administrated into the peritoneal cavity of naïve mice. After 24 hours, peritoneal eosinophil CAR4 expression was assessed by FACS, and CAR4 expression on gated eosinophils (CD45+SSChighSiglec-F+CD11b+GR-1+CD11c− ) is shown on the double-plot (CCR3-CAR4) controlled by IgG staining. C, Histograms of bronchoalveolar (BALF) eosinophil CAR4 expression from allergen-challenged Il5+/+ (red) and Il5−/− (grey) mice (n = 4 mice per group, superimposed). D, In the same experiment, the total BALF cells from Il5−/− asthmatic mice, largely consisting of CAR4− eosinophils, were cultured in IL-5-containing media (20 ng/mL) for 72 hours. Surface CAR4 expression was compared before and after the IL-5 treatment (n = 4 mice per group, superimposed, 2.8 ± 0.2% vs. 15.5 ± 3.5% CAR4+, mean ± SD).
Biochemical assessment of putative Car4 functions on eosinophils
Considering the reported functions of CAR4 (11, 12, 20), we examined the cellular buffering capacity by bone marrow–derived eosinophils. Cellular pH buffering capacity was readily observed for eosinophils, with Car4+/+ and Car4−/− eosinophils having similar activity (Fig. 5A). A well-established function of membrane-bound carbonic anhydrases is to enable lactate transport into cells by buffering extracellular pH following lactate-H+-co-transport (21, 22). Since readout of this process is intracellular acidification following lactate treatment, the intracellular pH in cultured eosinophils was monitored by a pH-sensitive dye following addition of lactate. While the intracellular acidification was readily observed, no difference in intracellular acidification was found between CAR4 +/+ and −/− eosinophils (Fig. 5B).
Figure 5. Cellular buffering capacity of Car4+/+ and Car4−/− eosinophils.
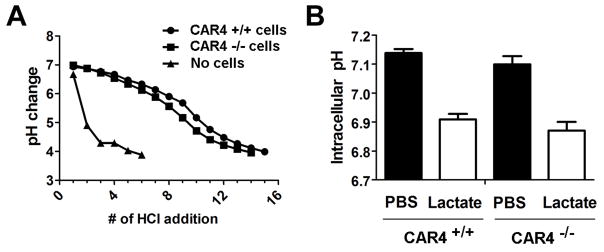
A, With > 90% purity, Car4+/+ and Car4−/− bone marrow–derived eosinophils (EOS, 2×106) were assessed for their cellular pH buffering capacity in saline without IL-5 following repetitive addition of 5 μL of 0.1 M HCl into 1 mL of cell suspension each time, with cell-free saline solution alone as negative control without cellular buffering. B, The intracellular pH changes in Car4+/+ and Car4−/− eosinophils were graphed before and after the exogenous lactate challenge (20 minutes), demonstrating no difference in buffering capacity which is required for lactate transport into cells. (mean ± SEM)
A novel epithelium-modulating function of eosinophil CAR4
We next investigated whether CAR4 expression on eosinophils could affect eosinophil migration and levels in the allergic airway. For this purpose, we allergen-challenged irradiated CD45.1 bone marrow recipient mice that have been adoptively transferred with either Car4+/+ or Car4−/− bone marrow from CD45.2 donor mice. CD45.2 donor engraftment (CD45.2) was > 99% as assessed by the presence of CD45.2+CD11b+ granulocytes, including eosinophils, in the blood of CD45.1 recipients (Fig. S2B); thus, the results can be attributed to hematopoietic cells rather than lung tissue cells (such as endothelial cells, which are known to express CAR4 (20)). FACS analysis of BALF eosinophil CAR4 expression confirmed the bone marrow transfer efficiency (Fig. 6A), while FACS enumeration revealed no difference in eosinophil levels between mice receiving Car4+/+ and Car4−/− cells (Fig. 6B). For direct evaluation of eosinophil trafficking, we applied a recently reported eosinophil adoptive transfer model (23) and found no migration difference between Car4+/+ and Car4−/− adoptively transferred eosinophils following recipient allergen challenges. Likewise, direct allergen challenge of Car4+/+ and Car4−/− mice (without bone marrow transplantation) revealed no difference in airway eosinophilia (Fig. S2C).
Figure 6. Allergic airway induction in Car4 bone marrow chimeric mice.
A, CD45.1 recipients were transplanted with CD45.2 Car4+/+ and Car4−/− bone marrow after irradiation and subsequently allergen challenged after ensuring CD11b+ engraftment > 99%. Eosinophilia was measured in Car4+/+ and Car4−/− recipients, and bronchoalveolar (BALF) eosinophil (EOS) Car4 donor genotype was verified. B, Total BALF eosinophil levels were quantified by FACS with serial gating (n = 7 per group, mean ± SEM). C, From the same bone marrow transplant (BMT) experiment, lung tissue microarray identified a cluster of 37 dysregulated genes (30 upregulated and 7 downregulated, Car4+/+ vs. Car4−/− donor), with a statistical criteria of corrected p < 0.01(Westfall-Young false correction) and fold change > 1.414. N/A, transcripts associated with unknown genes. D, On the basis of these 37 dysregulated genes, gene ontology analysis identified the top ten biological processes potentially associated with CAR4 functionality. The p values and number of entities in each category is shown. E, A schematic illustration of the interactive biological processes/pathways involving these 37 dysregulated genes.
The robust CAR4 induction on eosinophils during asthma suggests roles in lung tissue inflammatory processes; however, CAR4 did not directly affect eosinophil lung migration. In order to further understand the function of eosinophil CAR4 in asthma, we examined the transcriptional change of the whole-lung tissue from Car4+/+ and Car4−/− CD45.2 bone marrow recipient mice that had been subsequently allergen challenged. With asthma induction, we identified 37 genes differentially expressed in the lung of mice that were reconstituted with Car4+/+ compared with Car4−/− hematopoietic cells. The clustered heap-map identified 30 upregulated genes and 7 downregulated genes (Fig. 6C, see Table S2 for gene list) in Car4 +/+ recipients vs. Car4−/− recipients (n = 4 mice per group). Gene ontology analysis (http://toppgene.cchmc.org) (24) identified several pulmonary epithelium–related pathways, including keratinization, epithelial differentiation, anion exchange, and epidermal development (the top ten bio-processes listed in Fig. 6D). The genes and their functional interactions are displayed in a proposed interactive network (Fig. 6E).
Discussion
Recently, a key role of eosinophils in promoting asthma exacerbations has become increasingly recognized as agents that attenuate eosinophils (e.g. humanized anti–IL-5) are showing promising effects in eosinophilic asthma patients (6, 25). Responsiveness to biological interventions in asthma, such as anti–IL-5 and anti-IgE, appear to be particularly effective in patients with elevated levels of eosinophils, which are primarily identified by levels of sputum eosinophils (26). These findings, as well as decades of research correlating asthma severity with eosinophil levels and the release of eosinophilic granule constituents into the lung interstitium, highlight the importance of eosinophil activation in asthma. However, specific molecules associated with eosinophil activation have not been convincingly identified even though these would have great potential value in stratifying patients for therapeutic interventions.
We used genome-wide microarray analysis on sorted murine eosinophils to identify candidate markers of activated eosinophils. The aberrant and robust expression of CAR4 on lung eosinophils was quite surprising, with Car4 being among the most dysregulated transcripts. There have been no reports regarding hematopoietic expression of this CO2/bicarbonate catalyst gene other than in osteoclasts, which express CAR4 and CAR2 (27). In osteoclasts, CAR2 and CAR4 expression have been speculated to create an acidic milieu. Notably, the human asthmatic airway is an acidic microenvironment (28); therefore, infiltrating leukocytes could potentially modulate the local pH if they expressed surface CAR. While we did not observe a difference in cellular and intracellular buffering capacity (11, 12) in vitro, we did demonstrate the pH buffering capacity of eosinophils, consistent with prior findings (29). CAR4 buffering is also accompanied by a readily detectable intracellular activity (30). As a fine assessment of eosinophil CAR4 function, we also employed the lactate induced acidification assay, in which the subtle cellular change due to extracellular pH buffering is measured. Specifically, CAR4 buffering is mirrored by lactate-H+-cotransport, which depends on CAR4 buffering and results in a reduction in intracellular pH, a known function of CAR4 (21). Despite the negative phenotype, these results do not rule out the possibility that eosinophil-expressed CAR4 contributes to acid balance in the lung in vivo. Interestingly, CAR4 is expressed on the outer surface of the plasma membrane of IL-5-activated eosinophils, consistent with a function in trans rather than a role in cis. Nevertheless, CAR4+ and CAR4− eosinophils exhibited similar functional responses in terms of recruitment to the lung and eotaxin signaling.
On the basis of IL-5 inducing CAR4 in vitro and in vivo, we proposed that CAR4 is modulated by the TH2 cytokine IL-5 (4). It is conceivable that the novel function of CAR4 in asthma pathogenesis is related to the eosinophil effector function following cellular activation. Notably, eosinophilia in the asthmatic lung is not sufficient to induce eosinophil CAR4 expression because overexpression of IL-13 does not upregulate CAR4 in the absence of pathogenic level of IL-5. Recent research suggests that orally administrated CAR1 positively regulates regulatory T cells and negatively regulates TH17 cells with a negative impact on inflammatory bowel disease pathogenesis (31), CAR2 and CAR4 are associated with pathogenesis of cystic fibrosis (32) and infectious diarrhea (13), and CAR9 may be associated with gastrointestinal malignancies (33), suggesting a link between CARs and immune regulation.
CAR4 is known to be expressed on alveolar capillary endothelium (20), hindering precise phenotypic interpretation of the regular Car4−/− mice, as CAR4 is expressed on both hematopoietic and non-hematopoietic lung tissues. The bone marrow chimeric design overcomes this anatomical confounding factor in that a difference is solely attributable to CD45+ leukocytes. Therefore, the genes dysregulated within the resident lung tissue following bone marrow transfer are likely due to the robust CAR4 expression on infiltrating eosinophils, while the recipient’s basal lung CAR4 expression may explain why Car4 did not appear on the significant gene list due to dosage dilution and limited sensitivity. Accordingly, in the context of the induction of asthma and CAR4 expression, the gene ontology analysis of the lung tissue microarray data highlights a variety of potential functions for eosinophil CAR4, including the regulation of the keratinization, differentiation of the epithelium, and bicarbonate and anion transport. A recent microarray study from human asthmatic sputum identified a series of 57 genes characterizing airway eosinophil activation (34). Despite intriguing results and similar genome-wide approaches, this was not an eosinophil-specific approach, which may explain the absence of CAR4 on their screening list. Also, the contribution from CAR4 expressed by non-eosinophil leukocytes (such as macrophages) in our study cannot be entirely ruled out.
In summary, we have utilized a practical FACS/genome-wide screening–based discovery system to interrogate transcriptional changes of eosinophils during their recruitment to the asthmatic lung. The plasma membrane–associated metabolizing enzyme CAR4 was found to be aberrantly expressed on lung eosinophils after IL-5–induced activation in allergic airway inflammation. Although CAR4 does not directly regulate the intensity of eosinophilia, we have identified a series of lung transcripts that provide insight into the potential functions that eosinophil CAR4 may have in the context of allergic airway inflammation, namely in the processes of airway epithelial cell differentiation, anion exchange, and keratinization. Collectively, this study highlights the significance of using a cell-specific, genome-wide screening model in the context of a disease model and identifies CAR4 as an eosinophil surface molecule tightly upregulated in IL-5–elicited eosinophilia.
Supplementary Material
Acknowledgments
This work is supported by the NIH grants R37 AI045898 and R01 AI083450, the CURED (Campaign Urging Research for Eosinophilic Disease) Foundation, the Food Allergy Research & Education (FARE), and the Buckeye Foundation.
The authors thank Shawna Hottinger for editorial assistance.
References
- 1.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunological reviews. 2011;242:106–127. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. Innate and adaptive immune responses in asthma. Nature medicine. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nature medicine. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME. Eosinophilia. The New England journal of medicine. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ME, Hogan SP. The eosinophil. Annual review of immunology. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. New drugs for asthma. Nature reviews Drug discovery. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- 7.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nature reviews Drug discovery. 2013;12:117–129. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarogiannis S, Gourgoulianis KI, Kostikas K. Anti-interleukin-5 therapy and severe asthma. The New England journal of medicine. 2009;360:2576. doi: 10.1056/NEJMc090685. author reply 2577. [DOI] [PubMed] [Google Scholar]
- 9.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Waheed A, Sly WS. Membrane Associated Carbonic Anhydrase IV (CA IV): A Personal and Historical Perspective. Sub-cellular biochemistry. 2014;75:157–179. doi: 10.1007/978-94-007-7359-2_9. [DOI] [PubMed] [Google Scholar]
- 11.Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, Sly WS. Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16771–16776. doi: 10.1073/pnas.0508449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Alvarez BV, Chakarova C, Jiang L, Karan G, Frederick JM, Zhao Y, Sauve Y, Li X, Zrenner E, Wissinger B, Hollander AI, Katz B, Baehr W, Cremers FP, Casey JR, Bhattacharya SS, Zhang K. Mutant carbonic anhydrase 4 impairs pH regulation and causes retinal photoreceptor degeneration. Human molecular genetics. 2005;14:255–265. doi: 10.1093/hmg/ddi023. [DOI] [PubMed] [Google Scholar]
- 13.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infection and immunity. 2009;77:3639–3650. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. Journal of immunology. 2012;188:1075–1082. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. Journal of immunology. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. The Journal of clinical investigation. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Mose E, Zimmermann N. Proton channel HVCN1 is required for effector functions of mouse eosinophils. BMC immunology. 2013;14:24. doi: 10.1186/1471-2172-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra A, Weaver TE, Beck DC, Rothenberg ME. Interleukin-5-mediated allergic airway inflammation inhibits the human surfactant protein C promoter in transgenic mice. The Journal of biological chemistry. 2001;276:8453–8459. doi: 10.1074/jbc.M009481200. [DOI] [PubMed] [Google Scholar]
- 19.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. The American journal of pathology. 2006;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming RE, Crouch EC, Ruzicka CA, Sly WS. Pulmonary carbonic anhydrase IV: developmental regulation and cell-specific expression in the capillary endothelium. The American journal of physiology. 1993;265:L627–635. doi: 10.1152/ajplung.1993.265.6.L627. [DOI] [PubMed] [Google Scholar]
- 21.Hallerdei J, Scheibe RJ, Parkkila S, Waheed A, Sly WS, Gros G, Wetzel P, Endeward V. T tubules and surface membranes provide equally effective pathways of carbonic anhydrase-facilitated lactic acid transport in skeletal muscle. PloS one. 2010;5:e15137. doi: 10.1371/journal.pone.0015137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Klier M, Schuler C, Halestrap AP, Sly WS, Deitmer JW, Becker HM. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. The Journal of biological chemistry. 2011;286:27781–27791. doi: 10.1074/jbc.M111.255331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen T, Besse JA, Mingler MK, Fulkerson PC, Rothenberg ME. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6067–6072. doi: 10.1073/pnas.1220572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic acids research. 2010;38:W96–102. doi: 10.1093/nar/gkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corren J. Anti-interleukin-5 antibody therapy in asthma and allergies. Current opinion in allergy and clinical immunology. 2011;11:565–570. doi: 10.1097/ACI.0b013e32834c3d30. [DOI] [PubMed] [Google Scholar]
- 26.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. The New England journal of medicine. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 27.Riihonen R, Supuran CT, Parkkila S, Pastorekova S, Vaananen HK, Laitala-Leinonen T. Membrane-bound carbonic anhydrases in osteoclasts. Bone. 2007;40:1021–1031. doi: 10.1016/j.bone.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. American journal of respiratory and critical care medicine. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 29.Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME, Zimmermann N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood. 2009;114:2774–2782. doi: 10.1182/blood-2009-05-220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider HP, Alt MD, Klier M, Spiess A, Andes FT, Waheed A, Sly WS, Becker HM, Deitmer JW. GPI-anchored carbonic anhydrase IV displays both intra- and extracellular activity in cRNA-injected oocytes and in mouse neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1494–1499. doi: 10.1073/pnas.1221213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori K, Yamanishi H, Ikeda Y, Kumagi T, Hiasa Y, Matsuura B, Abe M, Onji M. Oral administration of carbonic anhydrase I ameliorates murine experimental colitis induced by Foxp3-CD4+CD25- T cells. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.1212612. [DOI] [PubMed] [Google Scholar]
- 32.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nature medicine. 2010;16:313–318. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidan E, Mentese A, Ozdemir F, Deger O, Kavgaci H, Caner Karahan S, Aydin F. Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Medical oncology. 2013;30:540. doi: 10.1007/s12032-013-0540-9. [DOI] [PubMed] [Google Scholar]
- 34.Esnault S, Kelly EA, Schwantes EA, Liu LY, Delain LP, Hauer JA, Bochkov YA, Denlinger LC, Malter JS, Mathur SK, Jarjour NN. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PloS one. 2013;8:e67560. doi: 10.1371/journal.pone.0067560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.