Abstract
Background
Quadrivalent human papillomavirus (HPV) vaccine, for protection against sexually transmitted HPV infection, is licensed for females and males 9–26 years on a 3-dose schedule (0, 2, and 6 months; Standard schedule). Vaccine uptake has been low and catch-up vaccination of older adolescents using an alternate dosing schedule may increase coverage. This study tested the non-inferiority of the immunogenicity of an alternate dosing schedule (0, 2, 12 months) among college age males.
Methods
220 18–25 year old males were randomly assigned to Standard or Alternate schedules. Blood samples were drawn immediately before Dose 1 and 2–6 weeks after Dose 3 and analyzed for antibody titers using a Luminex immunoassay. A value <1.5 for the upper 95% confidence interval (CI) bound of the Standard to Alternate schedule geometric mean titer (GMT) ratio was deemed non-inferior.
Results
Participants averaged 21.3 years old; 19.1% were non-white; completion rate was 93%. The anti-HPV titers for the Alternate schedule group were non-inferior to those of Standard schedule group for all four HPV vaccine virus types. Our results also demonstrated superiority of the Alternate schedule group for all four HPV vaccine virus types.
Conclusion
A delayed third dose at 12 months is immunologically non-inferior and superior for four HPV virus types. Using an alternate dosing schedule offers more flexibility to receive the 3-dose HPV vaccine and may result in higher vaccination rates among college-age males.
Keywords: Human papillomavirus, HPV vaccine, immunization, non-inferiority
Introduction
In 2006, the Advisory Committee on Immunization Practices (ACIP) licensed quadrivalent human papilloma virus (HPV) vaccine for use among females ages 9–26 years [1]. During the ensuing years, an enormous body of literature has been published regarding the acceptability of the vaccine among physicians, parents, adolescents, adult men and women, as well as barriers to and facilitators of HPV vaccine uptake. In addition, the manufacturer undertook a public relations/educational campaign aimed at informing the public about the protection from cervical cancer provided by the HPV vaccine. Yet, HPV vaccination rates among females are lower than national goals of 80% [2]. In 2011, based on a survey of the U.S. population, only one third of girls 13–17 years of age had received ≥3 doses of HPV vaccine [3]. Some of the reported barriers to full HPV vaccination are cost of vaccine, the three-dose schedule at 0, 2 and 6 months and its attendant difficulties, parental reluctance to vaccinate their young daughters against a sexually transmitted virus, and lack of physician recommendation, among others [4, 5].
Several facts indicate the need for another approach to prevention of HPV-related disease: 1) HPV infection represents more than 70% of all incident and prevalent cases of sexually transmitted infections in the US [6], translating to 17.9 million new infections annually among 15–24 year olds; 2) vaccination against HPV among females is not at sufficient levels to protect them from cervical cancer; and 3) an estimated 21,000 cancers in females and 12,000 cancers in males annually in the U.S are HPV-related [7].
To address the need to also protect men from HPV related cancers and genital warts, the ACIP permitted quadrivalent HPV vaccine for use among males ages 9–26 years in 2009 [8], with full recommendations published in 2011 [9]. This action was also intended to increase protection of women against cervical cancer by reducing male-to-female spread of the virus. Some of the same barriers to vaccination of males have been reported including, less supportive provider attitudes towards vaccinating males than females [10], lack of provider recommendation [11], lack of knowledge about the vaccine, not perceiving risk of HPV infection or benefit of vaccination, and preference for vaccinating at an older age [12]. Early estimates of HPV vaccination uptake among males are low with only 8.3% of adolescent males receiving at least one one dose in 2011 [3].
The three dose vaccination schedule has also been reported as a barrier to HPV vaccine uptake among males [5], as evidenced by the fact that only 28.1% of 13–17 year olds who have initiated the vaccination schedule have completed the series as of 2011 [13]. Adherence to the three dose vaccination schedule may be difficult for young men, because their frequency of contact with the health care system generally decreases at this age [14, 15]. Among those 18–26 years old, university health services can provide easy access to vaccination and other preventive health services, but an academic calendar may not be conducive to completing the standard schedule on time unless the first dose is administered early in the academic year. The purpose of this study was to test non-inferiority of an alternate administration schedule of 0, 2 and 12 months to the standard 0, 2 and 6 months schedule in a group of college-age men. We hypothesized that the immune response to the Alternate dosing schedule would be non-inferior to that of the Standard dosing schedule.
METHODS
This study was approved by the University of Pittsburgh Institutional Review Board (PRO10070407) and registered at ClinicalTrials.gov as NCT01184079.
Participants
From October 2010 through May 2011, men 18–25 years of age, were recruited using a variety of strategies including fliers, class announcements, recommendations by university health centers, emails to campus organizations, bus and campus newspaper advertisements, and targeted Facebook® advertisements. Potential participants were excluded if they had: more than four lifetime sexual partners, health problems that would interfere with the immune response or ability to complete the study, a hospitalization during the past year, hypersensitivity to yeast or HPV vaccine components, inability to complete the scheduled appointments, received HPV vaccine previously or if they were taking any immunosuppressive medications. Out of 311 men who were screened, 91 were excluded for not meeting inclusion criteria, leaving 220 enrollees of whom 204 completed the study.
Interventions
Participants read and signed informed consent forms prior to starting the study and completed eligibility screening forms before each dose of vaccine. Participants were randomized as they were scheduled for the initial visit using a simple random number sequence to determine the order of assignment into the Standard schedule or the Alternate schedule. Participants were aware of their group assignment. Following each vaccination visit, participants were screened for adverse events. Height and weight were measured at the final visit. Data collection and intervention schedules are shown in Table 1. Data collection was completed on May 29, 2012.
Table 1.
Participants, Intervals, per-protocol windows and activity by visit
| Visit | Activity | Intention to treat (n) | Day | Allowable per protocol window | Per protocol (n) |
|---|---|---|---|---|---|
| Standard Dosing Schedule | |||||
|
| |||||
| 1 | Blood draw, Dose 1 | 109 | 0 | - | 109 |
| 2 | Dose 2 | 108 | 60 days after Dose 1 | 51–70 days after Dose 1 | 103 |
| 3 | Dose 3 | 107 | 183 days after Dose 1 | 174–193 days after Dose 1 | 87 |
| 4 | Post Dose 3 blood draw | 107 | 30 days after Dose 3 | 14–49 days after Dose 3 | 87 |
|
| |||||
| Alternate Dosing Schedule | |||||
|
| |||||
| 1 | Blood draw, Dose 1 | 111 | 0 | - | 111 |
| 2 | Dose 2 | 110 | 60 days after Dose 1 | 51–70 days after Dose 1 | 110 |
| 3 | Dose 3 | 101 | 365 days after Dose 1 | 356–375 days after Dose 1 | 94 |
| 4 | Post Dose 3 blood draw | 97 | 30 days after Dose 3 | 14–49 days after Dose 3 | 88 |
Sample Processing and Immunogenicity Testing
Vaccine storage and delivery followed standard procedures. Blood samples were drawn immediately prior to the first dose and 2–6 weeks after the third dose into serum separator tubes. Samples were spun at 3200 rpm for 10–15 minutes and serum was transferred to labeled nunc cryovials. Cryovials were stored at −70°C. Frozen nunc tubes were shipped on dry ice to the laboratory by an express carrier. Serology testing for each of the four HPV types was performed at PPD Vaccines and Biologics Laboratory (Wayne, PA) using a competitive Luminex immunoassay (cLIA) that measures type-specific antibodies to neutralizing epitopes on the virus-like particles (VLPs) as described in Dias et al [16].
Objectives
In this randomized controlled trial, the primary goal was to determine whether the post Dose 3 geometric mean titers (GMTs) for men in the Alternate schedule group (N=111) were non-inferior to those in the Standard schedule group (N=109). Non-inferiority means that the difference in GMTs between the Standard and Alternate schedule groups was small enough to support the conclusion that Alternate schedule group also benefitted from HPV vaccination. That is, non-inferiority was demonstrated if the upper bound of the two-sided 95% confidence interval (CI) of the ratio of GMTs (Standard schedule GMT divided by the Alternate schedule GMT) was smaller than1.5 [17, 18]. Although our clinical trial was not intended to show superiority of the Alternate schedule to the Standard schedule, we also examined immunological response superiority, defined as, the lower bound of the two-sided 95% CI of the GMT ratio (Alternate schedule GMT divided by the Standard schedule GMT) larger than1.0 [18].
Sample Size
The formula used for calculating sample size was: (1 + 1/u) (Zα + Zβ)2 σ2/[log (RGMT) −δ0] [17] where u is the ratio of the size of the Standard schedule to Alternate schedule groups (u =1, for equal size groups); one-sided alpha (0.025), that is divided by 4 to account for multiplicity of 4 serotypes, a non-inferiority margin (δ0) equal to natural log(0.67), the expected ratio of geometric mean titers RGMT set at 0.8, and a standard deviation of 1.26 (personal communication, Alfred J. Saah, 2007). Sample size for a power of 80% was calculated to be 75 participants in each arm [17], but was increased to 110 to allow for the possibility of dropouts and baseline seropositive participants.
Statistical Analyses
Descriptive analyses of participants’ characteristics were performed for all randomized participants at baseline, overall and comparing those randomized to each of the two dosing schedules. Participants who had anti-HPV serum cLIA levels >20 milliMerck units/mL (mM/mL) for HPV types 6 and 16, >16 mM/mL for type 11, and >24 mM/mL for type 18 were considered to be seropositive at baseline [19] and were excluded from further analyses only for the type(s) for which they were seropositive. Other participants dropped out of the study because of failing to: 1) receive Dose 2 or Dose 3 at all; or 2) return for Dose 2, Dose 3 or the final blood draw within their respective study-designated windows (see Table 1). These individuals were excluded from subsequent “per protocol” analyses.
Because post-vaccination antibody titers were skewed, the data were natural log-transformed and then used to calculate HPV type-specific GMTs and 95% CIs for each group [20]. One-way analysis of variance (ANOVA) was used to compare continuous variables while the Pearson Chi-square test was used to compare categorical variables. In addition, reverse cumulative distribution curves for each virus type were plotted to visualize the difference in the log-transformed titers between participants randomized into the Standard and Alternate dosing schedules.
To examine the association between HPV type-specific titers and the time between receiving Dose 2 and Dose 3, linear regressions were conducted using log-transformed titers as the dependent variable and days between receipt of Dose 2 and Dose 3 as the independent variable, controlling for participants’ characteristics. Statistical significance for the analyses was set at P < 0.05.
RESULTS
Baseline Characteristics
The study groups did not differ in baseline demographics including age, race, smoking status, body mass index (BMI), and year in school (Table 2).
Table 2.
Baseline Characteristics of Participants Overall and by Randomized Group
| Characteristic | Overall (n=220) | Standard dosing schedule (n=109) | Alternate dosing schedule (n=111) | P-value |
|---|---|---|---|---|
| Age, mean years (SD) | 21.3 (2.2) | 21.4 (2.2) | 21.3 (2.3) | 0.671 |
| Nonwhite % | 19.1 | 20.2 | 18.0 | 0.683 |
| Smoker, % | 9.6 | 7.3 | 11.7 | 0.270 |
| BMI (SD) | 25.4 (7.5) | 26.0 (9.2) | 24.8 (5.2) | 0.958 |
| Year in school, % | ||||
| Freshman | 11.4 | 11.0 | 11.7 | 0.283 |
| Sophomore | 24.6 | 24.8 | 24.3 | |
| Junior | 15.5 | 10.1 | 20.7 | |
| Senior | 9.6 | 11.9 | 7.2 | |
| Graduate Student | 29.6 | 33.0 | 26.1 | |
| Non-student | 9.6 | 9.2 | 9.9 | |
BMI = body mass index; SD = standard deviation
Study Completion and Protocol Window Violation
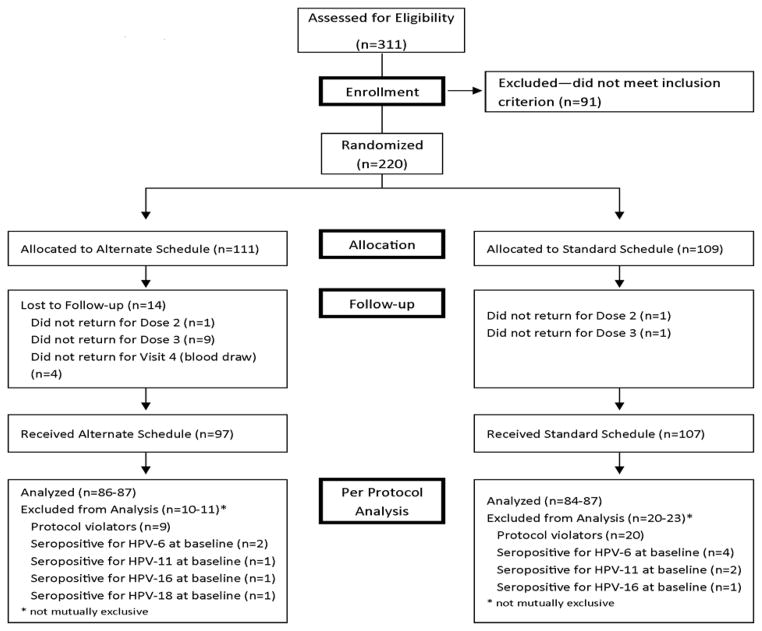
Figure 1 presents the enrollment, treatment allocation, follow-up and final disposition of the two study groups. Out of 220 participants enrolled, 204 completed the study. In the Standard schedule group two were lost to follow up; one did not return after Dose 1 and one did not return after Dose 2; while in the Alternate schedule group 14 were lost to follow up; one did not return after Dose 1, nine did not return after Dose 2, and four did not return for the post Dose 3 blood draw. Among the 204 participants who completed the study, there were 21 violations by 20 participants of the timing protocol in the Standard schedule group, five participants violated the Dose 2 window and 16 violated the Dose 3 window, compared with seven violations of the timing protocol in the Alternate schedule group; zero violated the Dose 2 window and seven violated the Dose 3 window. In addition, three individuals violated the second blood draw window in the Alternate schedule group.
Figure 1.
Recruitment, Enrollment, Treatment Allocation, Follow-up, Per Protocol Analysis
Antibody responses
Based on the cutoff values indicated above, eight participants (six in the Standard schedule group and two in the Alternate schedule group) were seropositive at baseline for one or more HPV types; six were seropositive for HPV type 6, three for HPV type 11, two for HPV type 16 and one for HPV type 18. One participant in each group did not respond to any of the HPV types; non-responders were included in the “intention-to-treat” and “per protocol including non-responders” analyses only.
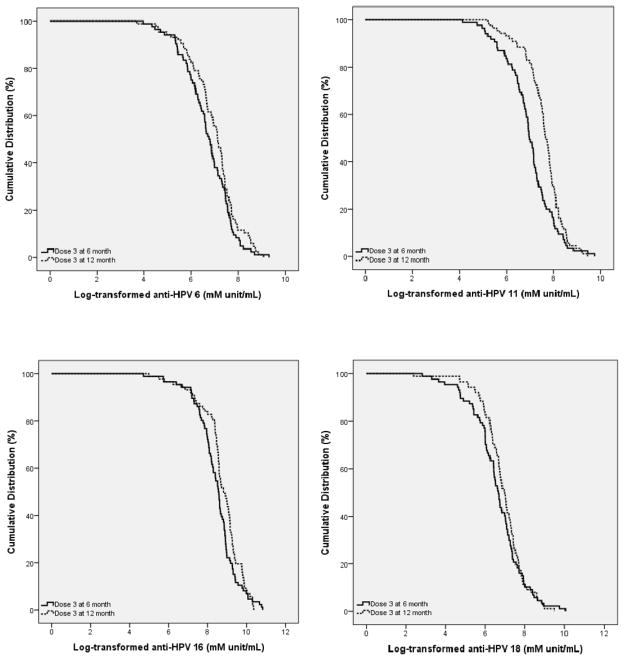
Reverse cumulative distribution curves are presented for all four HPV types in Figures 2a–d. GMTs are indicated in Table 3 for: 1) all participants who completed the study and were not seropositive at baseline (intention-to-treat); 2) those participants who completed all visits per protocol and were not seropositive at baseline including non-responders; and 3) those participants who completed all visits per protocol and were not seropositive at baseline, excluding non-responders. These analyses demonstrated that the immunological responses to HPV vaccine for the Alternate schedule group were non-inferior to those for the Standard schedule group for all four HPV virus types as indicated by the upper bounds of Standard to Alternate schedule GMT ratios that were all less than the accepted standard of 1.5. Our results also demonstrated superiority for all four vaccine virus types because the lower bound of the Alternate to Standard schedule GMT ratios were all greater than the accepted standard of 1.0.
Figure 2.
Reverse Cumulative Distribution Curves of Log Transformed Antibody Titers by HPV type
Solid line = anti-HPV for Dose 3 at 6 months (Standard schedule); dashed line = anti- HPV for Dose 3 at 12 months (Alternate schedule); mM unit/mL: milliMerck unit/mL.
Table 3.
Geometric Mean Titers of Post HPV Vaccine Dose 3 by HPV Type and Vaccination Schedule
| HPV Type |
Standard dosing schedule | Alternate dosing schedule | Upper Bound GMT ratio* (S)/(A) | Lower Bound GMT ratio** (A)/(S) | ||
|---|---|---|---|---|---|---|
| n | GMT (95% CI) mM units/mL (S) |
n | GMT (95% CI) mM units/mL (A) |
|||
| Intention-to-treat population# | ||||||
| 6 | 103 | 794 (565–1009) | 95 | 1063 (793–1312) | 0.77 | 1.40 |
| 11 | 105 | 1043 (689–1382) | 96 | 1961 (1480–2403) | 0.58 | 2.16 |
| 16 | 106 | 4555 (3243–5788) | 96 | 6186 (4947–7315) | 0.79 | 1.53 |
| 18 | 107 | 709 (338–1103) | 96 | 1049 (728–1350) | 0.82 | 2.15 |
| Per-protocol population including non-responders | ||||||
| 6 | 84 | 813 (558–1050) | 86 | 1066 (796–1313) | 0.80 | 1.43 |
| 11 | 85 | 1064 (654–1458) | 87 | 1946 (1583–2273) | 0.64 | 2.42 |
| 16 | 86 | 4713 (3186–6141) | 87 | 6219 (5018–7305) | 0.84 | 1.58 |
| 18 | 87 | 749 (328–1197) | 87 | 1008 (683–1312) | 0.91 | 2.08 |
| Per-protocol population excluding non-responders | ||||||
| 6 | 83 | 837 (576–1078) | 85 | 1081 (808–1330) | 0.81 | 1.40 |
| 11 | 84 | 1088 (670–1489) | 86 | 1996 (1628–2328) | 0.64 | 2.43 |
| 16 | 85 | 4926 (3341–6404) | 86 | 6496 (5258–7615) | 0.84 | 1.57 |
| 18 | 86 | 782 (344–1248) | 86 | 1062 (722–1380) | 0.90 | 2.10 |
HPV=human papillomavirus; CI=confidence interval; GMT=geometric mean titer; mM/mL=milliMerck units/mL; A=Alternate schedule; S=Standard schedule
Study completers, including those who violated protocol windows
Demonstrated non-inferiority for all four vaccine virus types because upper bound GMT ratio (S)/(A) < 1.5.
Demonstrated superiority for all four vaccine virus types because lower bound GMT ratio (A)/(S) > 1.0.
The average number of days between receipt of Dose 2 and Dose 3 was 131 days for the Standard schedule group and 317 days for the Alternate schedule group. Regression models that included participants from both groups, using HPV-type specific, log-transformed titers as the dependent variable and controlling for age, body mass index (BMI) and smoking status were conducted. Results (Table 4) demonstrate a significant, positive relationship between level of titers and the length of time between receipt of Dose 2 and Dose 3 for HPV types 11, 16 and 18 (P<0.05). This relationship indicates that antibody titer levels were more likely to be higher with increasing length of time between the second and third dose of the vaccine, supporting the use of the Alternate dosing schedule. For HPV type 11, age was significantly associated with lower GMTs; for HPV type 18, smoking was significantly associated with lower GMTs.
Table 4.
Association of Log-Transformed Post HPV Vaccination Titers with Days Between Dose 2 and Dose 3
| Variable | HPV-6 | HPV-11 | HPV-16 | HPV-18 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Coefficient (SE) | P-value | Coefficient (SE) | P-value | Coefficient (SE) | P-value | Coefficient (SE) | P-value | |
| Days between Doses 2 & 3 | 0.002 (0.001) | 0.074 | 0.003 (0.001) | <0.001 | 0.002 (0.001) | 0.049 | 0.002 (0.001) | 0.039 |
| Age | −0.067 (0.037) | 0.072 | −0.096 (0.032) | 0.003 | −0.059 (0.035) | 0.095 | −0.066 (0.039) | 0.087 |
| White (ref: Non-white) | 0.108 (0.209) | 0.605 | −0.155 (0.182) | 0.398 | 0.099 (0.199) | 0.621 | −0.367 (0.218) | 0.095 |
| Body mass index | −0.005 (0.014) | 0.741 | −0.016 (0.013) | 0.200 | −0.011 (0.014) | 0.445 | −0.018 (0.015) | 0.243 |
| Smoker (ref: non-smoker) | −0.205 (0.309) | 0.508 | −0.134 (0.270) | 0.620 | −0.411 (0.297) | 0.168 | −0.754 (0.327) | 0.022 |
| Intercept | 7.980 (0.919) | <0.001 | 9.123 (0.797) | <0.001 | 9.759 (0.878) | <0.001 | 8.619 (0.964) | <0.001 |
HPV=Human papillomavirus; SE=Standard error; R2= the proportion of variability in the data accounted for by the model. For HPV-6, R2=0.051, for HPV-11, R2=0.184, for HPV-16, R2=0.063, for HPV18, R2=0.102
Participants reported side effects following 646 separate vaccinations; 172 local and general reactions were reported, with no difference in proportion of side effects reported between Standard (24.4%) and Alternate (28.9%) schedule groups (P=0.26). The majority of side effects were pain and redness at the injection site (86%; n=148), with the remainder composed of fever (3.5%; n=6), and miscellaneous symptoms (10.5%; n=18). There were no reports of any serious side effects.
Discussion
There is compelling evidence of the personal and public health impact of HPV vaccination of both males and females. For example, quadrivalent HPV vaccine has been shown to be highly effective for preventing persistent HPV infection that can lead to orogenital cancers [7] and genital warts caused by HPV-6 and HPV-11 infections [21]. Almost all (≥99.5%) vaccinated persons develop an antibody response after 3 doses and current studies indicate that efficacy remains high for 2–5 years following vaccination [21–23]. Modeling studies have demonstrated the efficacy and cost effectiveness of vaccinating females and males against HPV [24–27]. Although HPV vaccine has been licensed and recommended for females since 2006 and for males since 2011, national self-reported HPV vaccination rates are low for both initiation (53% for females and 8.3% for males) and three-dose completion (35% for females and 1.3% for males)[13].
In an effort to overcome one barrier to vaccination, i.e., the need to visit to a health care provider three times within six months to receive the three-dose series, we compared an alternate HPV vaccine administration schedule (0, 2 and 12 months) with the standard administration schedule (0, 2 and 6 months). For all four HPV virus types contained in the quadrivalent HPV vaccine, the Alternative schedule was both non-inferior and superior to the Standard schedule. These findings are similar to a study among college-age females [28] in which the Alternate 0, 2 and 12 month dosing schedule was non-inferior for all four vaccine virus types. Furthermore, as in the previous study of females [28] and studies of hepatitis B vaccine [29], the longer the time period between the priming dose 1 and the boosting dose 3, the higher the antibody titers were for all four HPV virus types.
These findings of non-inferiority and superiority of the Alternate schedule are important for the following reasons. First, adolescent males typically reduce their contact with the health care system [14, 15] and a more flexible dosing schedule may encourage return visits for doses 2 and 3. Secondly, when individuals are not vaccinated during the early teen years, as recommended by Centers for Disease Control and Prevention, college is a good time for catchup vaccination. Most colleges and universities offer some student health services including prevention and treatment of sexually transmitted infections at little or no out-of-pocket expense. However, unless the series is started early in the academic year, it may be difficult to complete it within six months. A dosing schedule of 0, 2, and 12 months can increase the opportunities for young men to be completely vaccinated at their student health service.
Sixteen participants were out of window for their receiving Dose 3 in the Standard schedule group compared with seven in the Alternate group. This finding may be indicative of the difficulty of scheduling college-age men within a specific time frame and thus lends support to the need for flexibility in the dosing schedule.
One participant in each group did not respond to any of the vaccine HPV types. This antibody response rate of intention-to-treat participants (202/204 = 99%) was comparable to previously published data reporting that 99.5% of vaccinees have a positive antibody response to HPV virus-like particles [22, 30].
Strengths and Limitations
Because the competitive Luminex immunoassay tends to under represent antibody response, the values reported in this study may underestimate the actual antibody response to the vaccine [30]. Drop-out rates were higher in the Alternate schedule group; however, sample size requirements were met for all HPV types. Given the short follow-up of this study, the long-term effect of the Alternate dosing schedule on clinical outcomes is unknown. Antibody titers were somewhat higher in this study than among similarly aged males in the studies cited in the HPV vaccine package insert [31] and a related publication [23], although less than those for males vaccinated at 9–15 years of age [31]. This may reflect our advantaged population, consisting of mostly college students in which only 10% smoked tobacco, in contrast to a multinational population with presumably higher tobacco use. It may also reflect changes within the laboratory over time.
Conclusion
To date, non-inferiority of alternate administration schedules for HPV has not been tested for college-age men. For all HPV vaccine types, the GMT ratios indicated non-inferiority and superiority of the alternate vaccine administration schedule. The knowledge that the final dose of HPV vaccine is no less effective at 12 months than at 6 months may provide support for a more flexible dosing schedule. A broader time period for receiving the third HPV vaccine may help to ensure that busy young men who are working and/or going to school complete the three-dose series for full protection.
Acknowledgments
Funding
The authors and this work (NCT01184079) were supported in part by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc., manufacturer of Gardasil® quadrivalent human papillomavirus vaccine. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co., Inc. The project described was also supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
Footnotes
Conflict of Interest
The authors have no other conflicts to report regarding HPV vaccine.
References
- 1.US Food and Drug Administration. Vaccines, Blood & Biologics - Gardasil. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042 [Cited 2013, March 13]
- 2.US Department of Health and Human Services. Healthy People 2020. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23 [Cited 2013, May 30]
- 3.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years--United States. Morbidity and Mortality Weekly Report (MMWR) 2011;60:1117–23. [PubMed] [Google Scholar]
- 4.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30:3546–56. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 5.Walhart T. Parents, adolescents, children and the human papillomavirus vaccine: a review. International Nursing Review. 2012;59:305–11. doi: 10.1111/j.1466-7657.2012.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2008. Sexually Transmitted Diseases. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Human Papillomavirus--Associated Cancers--United States, 2004–2008. Morbidity and Mortality Weekly Report (MMWR) 2012;61:258–61. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2009. ACIP Provisional Recommendations for HPV Vaccine; pp. 1–2. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report (MMWR) 2011;60:1705–8. [PubMed] [Google Scholar]
- 10.Perkins RB, Clark JA. Providers’ attitudes toward human papillomavirus vaccination in young men: challenges for implementation of 2011 recommendations. American Journal of Men’s Health. 2012;6:320–3. doi: 10.1177/1557988312438911. [DOI] [PubMed] [Google Scholar]
- 11.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the national immunization survey-teen. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liddon N, Hood J, Wynn BA, Markowitz LE. Acceptability of human papillomavirus vaccine for males: a review of the literature. The Journal of Adolescent Health. 2010;46:113–23. doi: 10.1016/j.jadohealth.2009.11.199. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National and State Vaccination Coverage Among Adolescents Aged 13 through 17 Years--United States, 2010. Morbidity and Mortality Weekly Report (MMWR) 2011;60:1117–23. [PubMed] [Google Scholar]
- 14.Dempsey AF, Freed GL. Health Care Utilization by Adolescents on Medicaid: Implications for Delivering Vaccines. Pediatrics. 2010;125:43–9. doi: 10.1542/peds.2009-1044. [DOI] [PubMed] [Google Scholar]
- 15.Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, PGS National health care visit patterns of adolescents: Implications for delivery of new adolescent vaccines. Archives of Pediatrics & Adolescent Medicine. 2007;161:252–9. doi: 10.1001/archpedi.161.3.252. [DOI] [PubMed] [Google Scholar]
- 16.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clinical and Diagnostic Laboratory Immunology. 2005;12:959–69. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang WW, Mehrotra DV, Chan IS, Heyse JF. Statistical considerations for noninferiority/equivalence trials in vaccine development. Journal of Biopharmaceutical Statistics. 2006;16:429–41. doi: 10.1080/10543400600719251. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Guidance for Industry Non-Inferiority Clinical Trials. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf [Cited 2013, March 1]
- 19.Giuliano AR, Lazcano-Ponce E, Villa L, Nolan T, Marchant C, Radley D, et al. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. The Journal of Infectious Diseases. 2007;196:1153–62. doi: 10.1086/521679. [DOI] [PubMed] [Google Scholar]
- 20.Endo Y. Estimate of confidence intervals for geometric mean diameter and geometric standard deviation of lognormal size distribution. Powder Technology. 2009;193:154–61. [Google Scholar]
- 21.Barr E, Gause CK, Bautista OM, Railkar RA, Lupinacci LC, Insinga RP, et al. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. American Journal of Obstetrics and Gynecology. 2008;198:261, e1–11. doi: 10.1016/j.ajog.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45:609–7. doi: 10.1086/520654. [DOI] [PubMed] [Google Scholar]
- 23.Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Vardas E, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clinical and Vaccine Immunology : CVI. 2012;19:261–7. doi: 10.1128/CVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baussano I, Garnett G, Segnan N, Ronco G, Vineis P. Modelling patterns of clearance of HPV-16 infection and vaccination efficacy. Vaccine. 2011;29:1270–7. doi: 10.1016/j.vaccine.2010.11.082. [DOI] [PubMed] [Google Scholar]
- 25.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–67. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. The New England Journal of Medicine. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E, et al. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college-aged women. Journal of Womens Health (Larchmt) 2010;19:1441–7. doi: 10.1089/jwh.2009.1753. [DOI] [PubMed] [Google Scholar]
- 29.Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine. 1989;7:106–10. doi: 10.1016/0264-410x(89)90046-7. [DOI] [PubMed] [Google Scholar]
- 30.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. Journal of Infectious Diseases. 2009;200:166–71. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merck & Co I. Gardasil Package Insert. 2009. [Google Scholar]