Abstract
Serotonin (5-HT) has been recognized for decades as an important signaling molecule in the gut, but it is still revealing its secrets. We continue to discover novel gastrointestinal (GI) functions of 5-HT, as well as actions of gut-derived 5-HT outside of the gut, and we are learning how 5-HT signaling is altered in GI disorders. Furthermore, new therapeutic targets related to 5-HT signaling are being identified that can hopefully be exploited to alleviate the symptoms of functional GI disorders. Conventional functions of 5-HT in the gut involving intrinsic reflexes include stimulation of propulsive and segmentation motility patterns, epithelial secretion, and vasodilation. Activation of extrinsic vagal and spinal afferent fibers results in slowed gastric emptying, pancreatic secretion, satiation, pain and discomfort, as well as nausea and vomiting. Within the gut, 5-HT also exerts non-conventional actions that include serving as a pro-inflammatory signaling molecule and as a trophic factor to promote the development and maintenance of neurons and interstitial cells of Cajal. Platelet 5-HT, which comes from the gut, can promote hemostasis, influence bone development, and contribute to allergic airway inflammation. 5-HT3 receptor antagonists and 5-HT4 receptor agonists have been used to treat functional disorders with diarrhea or constipation, respectively. More recently, the synthetic enzyme, tryptophan hydroxylase has been targeted, and there are recent findings suggesting that epithelial 5-HT4 receptors could be targeted to provide a safe and effective treatment for constipation. Here we provide an overview of these serotonergic actions and treatment strategies.
Introductory remarks
One could argue that we should be using the term enteramine, rather than serotonin, when referring to 5-hydroxytryptamine (5-HT). After all, this substance was first extracted from rabbit gastric mucosa in 1937 by the Italian pharmacologist, Vittorio Erspamer, who named the bioactive amine that he discovered enteramine1. About a decade later, Maurice Rapport, Arda Green and Irvine Page of the Cleveland Clinic reported they had isolated a compound from bovine serum that they called serotonin for its vasoconstrictive properties2. Within a few years, it was determined that the structure of both of these compounds is 5-HT, and it appears that the second name stuck because it was made available to researchers by Upjohn Pharmaceuticals, who referred to the compound as serotonin3. Early on, Erspamer postulated that 5-HT must play an important role in gut function because he detected it in the gastrointestinal (GI) tracts of every vertebrate that he studied, from fish to frogs to pigeons to primates, and because he found that the vast majority of the serotonin in the body is synthesized and contained within the GI tract. Nevertheless, it is doubtful that Erspamer could have envisioned the variety and broad distribution of 5-HT receptors that are expressed in GI tissues, the array of functions that 5-HT serves in the gut, the actions of gut-derived serotonin outside of the GI tract, or the number of serotonergic targets that are in use or in development for the treatment of GI disorders. These are the areas of focus of this review.
Serotonin signaling in the gut
Mucosal 5-HT signaling in the gut
The majority of 5-HT in the body is synthesized, stored, and released from a subset of enteroendocrine cells called enterochromaffin (EC) cells in the intestinal mucosa4, 5 (Fig 1a,c). Because the intrinsic and extrinsic afferent nerves of the gut do not reach into the lumen where they could sample for nutrients, toxins or other cues to initiate reflex responses, EC cells function as sensory transducers that respond to chemical and mechanical stimuli6. Enterochromaffin cells use the rate limiting enzyme tryptophan hydroxylase 1 (TpH1) to synthesize serotonin7, 8. As is the case for other types of enteroendocrine cells, the secretory granules of EC cells are predominantly located at the basal surface, and this is thought to represent the major site of 5-HT release. However, there is morphological evidence for 5-HT release at the apical surface of EC cells9, and this is consistent with the ability to detect 5-HT release at the mucosal surface with electrochemistry techniques10–12. As described in greater detail below, 5-HT released from EC cells mediates many GI functions, including peristalsis, secretion, vasodilation and perception of pain or nausea, through activation of a diverse family of 5-HT receptors on intrinsic and extrinsic afferent nerve fibers that are located in the lamina propria.
Figure 1.
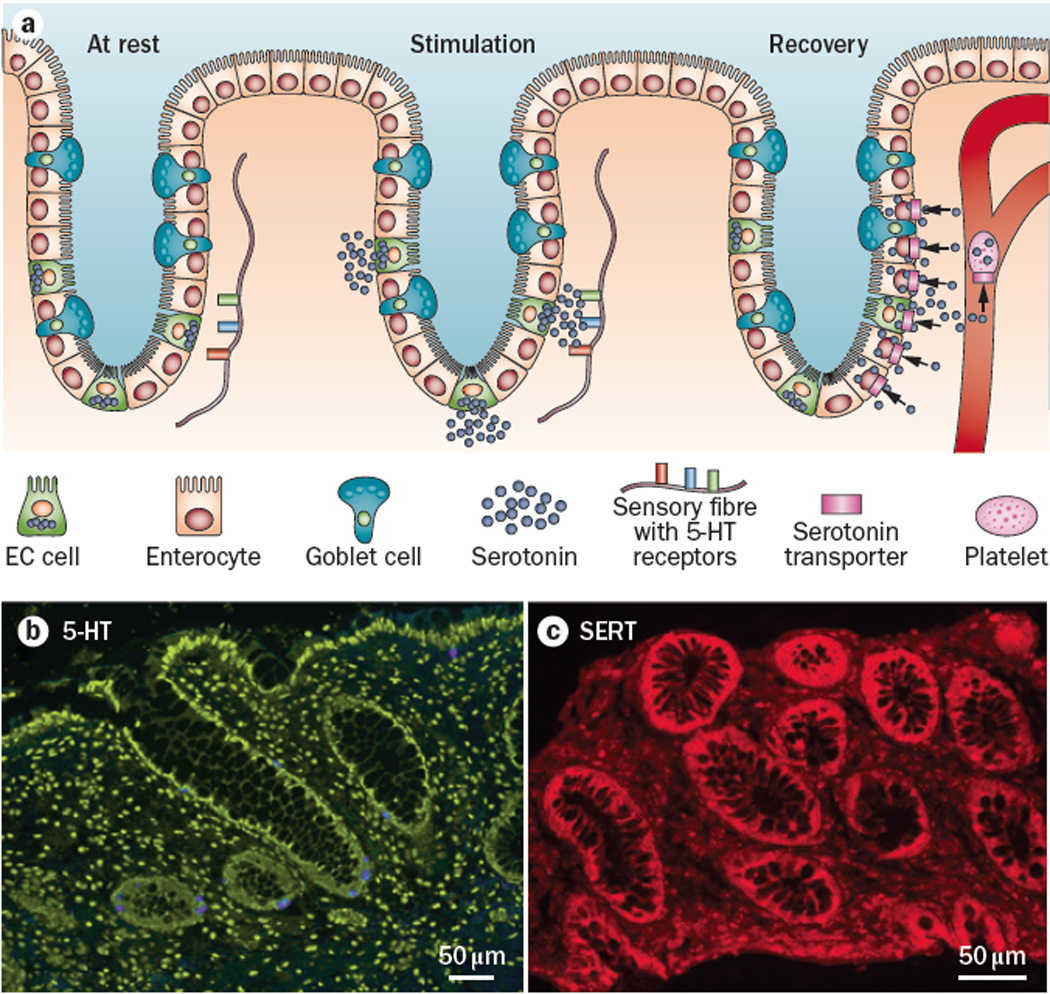
In the intestinal epithelium, 5-HT is synthesized and released by a small subset of cells called enterochromaffin (EC) cells, and the 5-HT is then removed from the interstitial space by the serotonin-selective reuptake transporter (SERT), which is expressed by essentially all epithelial cells. a. Immunstaining for 5-HT in a biopsy from the transverse colon of one of the authors (GMM). 5-HT is stained blue, and the section is counterstained with the nucleic acid stain, yoyo. Note that there are no mast cells stained for 5-HT in this micrograph; 5-HT-immunoreactive mast cells are common in mouse and rat, but not in human. b. Immunostaining for SERT in a human rectal biopsy specimen demonstrating that essentially all cells in the colonic glands are SERT-immunoreactive. c. Schematic diagram illustrating the sequence of events involved in 5-HT signaling in the gut. At rest, 5-HT is synthesized by EC cell. Upon mechanical or chemical stimulation, 5-HT is released into the interstitial space of the lamina propria and binds to receptors on nearby nerve fibers. The 5-HT signaling is terminated during the recovery phase. 5-HT is transported by SERT into epithelial cells where it is enzymatically degraded, or it enters the blood stream where it is transported into platelets where it is stored for future use.
An important property of intercellular signaling is the termination of the signal by local enzymatic degradation, or by uptake into nearby cells or nerve terminals for recycling or degradation. In the case of 5-HT, there are no intercellular degradative enzymes, and since it is a highly charged molecule at physiological pH, it requires a specialized transport mechanism to cross the plasma membrane where it can be degraded by intracellular enzymes such as monoamine oxidase13, 14. The serotonin-selective reuptake transporter (SERT), which is also responsible for 5-HT uptake in the brain, is expressed by essentially all epithelial cells of the intestinal mucosa13–16 (Fig. 1b,c). The serotonin transporter is a member of the neurotransmitter:sodium symporter (NSS) family, TC 2.A.22, which includes the monoamine transporters DAT (dopamine) and NAT (noradrenaline), and internalizes 5-HT via a sodium- and chloride-dependent mechanism17, 18. Since all of the epithelial cells in the lining of the intestines appear to express SERT, it serves as a selective sponge to remove 5-HT from the interstitial space following release by EC cells. By functioning to inactivate 5-HT through rapid reuptake into epithelial cells of the intestinal mucosa, SERT serves as a critical molecule in the local regulation of 5-HT availability and actions in the intestines. Studies of the intestine during early postnatal development, and in adult intestine, have demonstrated that decreased SERT function, via lower expression levels of SERT19, 20 or pharmacological blockade20, lead to higher levels of extracellular 5-HT. Serotonin that is not taken up into the cells of the epithelial lining enters the circulation via the dense capillary bed of the lamina propria, where it is taken up into platelets expressing SERT (Fig. 1c).
It is worth noting that regulation of SERT transcription in the intestinal epithelium differs from that for neuronal SERT. In intestinal epithelial cells, transcription starts downstream from the start site used in neurons, and there is a region of intron 1 located between these start sites that can regulate epithelial SERT expression, but does not affect neuronal SERT expression 21, 22. If the gut-specific signaling pathways were identified that could influence SERT expression and function, it would be possible to differentially regulate gut 5-HT signaling with drugs that target the gut epithelium.
The lamina propria is rich in mast cells, and murine mast cells are capable of synthesizing and releasing 5-HT. However, under basal conditions, human mast cells in the mucosal layer of the gut do not appear to synthesize 5-HT. Figure 1 illustrates 5-HT-immunoreactivity in the human colon, and while EC cells are obvious, mast cells are not observed. This is consistent with the findings of Buhner and Schemann, who also failed to detect 5-HT immunoreactivity in human mast cells23. On the other hand, there is evidence that human enteric mast cells express TpH24. Also, in some individuals with mastocytosis, 5-HT levels are increased in a manner that does not correlate with platelet numbers25. Interestingly, individuals with mastocytosis who have low levels of circulating 5-HT are more likely to exhibit GI and neurological complaints26. Regardless, the fact that mast cells in mice express 5-HT indicates that mast cells must be considered as a source of 5-HT in murine studies, and it is also possible that activated mast cells synthesize and release 5-HT under pathological conditions in humans, contributing to conditions such as hypersensitivity.
Neuronal 5-HT signaling in the gut
While the major depository of the body’s 5-HT is the mucosa of the GI tract, enteric neurons also express 5-HT. Like neurons in the brain, synthesis of 5-HT by enteric neurons is dependent on TpH27, 8. It is estimated that approximately 2% of the neurons in the myenteric plexus are serotonergic 27, and they synapse preferentially, but not exclusively, on other serotonergic neurons, forming a chain of serotonergic interneurons that project downstream along the gut tube 28, 29.
Studies of stimulus-evoked synaptic responses in the myenteric plexus demonstrate that 5-HT released from these neurons can contribute to both fast and slow excitatory postsynaptic potentials (EPSPs). Virtually all evoked fast EPSPs in the enteric nervous system (ENS) are mediated predominantly by acetylcholine acting at nicotinic receptors; however, roughly 10% of fast EPSPs also include a component sensitive to 5-HT3 receptor antagonists30. The small proportion of fast synaptic events including a serotonergic component corresponds with the small percentage of neurons in the myenteric plexus that express 5-HT, and the small proportion of neurons that appear to be surrounded by 5-HT-immunoreactive nerve terminals. It is reasonable to presume that these represent synaptic events involved in descending projections. This is consistent with the finding that descending reflex pathways that ultimately affect circular muscle activity are sensitive to 5-HT3 receptor inhibition31. On the other hand, the projection patterns of enteric 5-HT neurons are difficult to reconcile with data showing that inhibition of 5-HT3 receptors can interrupt ascending contractile reflexes in response to mucosal stimulation32. The physiological role of 5-HT-mediated slow EPSPs is less clear, but antagonists of 5-HT1P33, 34 and 5-HT735 receptors can inhibit them.
Alterations in mucosal 5-HT signaling in pathophysiological conditions
Because of its importance as a signaling molecule in the gut, numerous studies have investigated various elements of mucosal 5-HT signaling in inflamed intestinal mucosa from human biopsies and in animal models of intestinal inflammation. Most studies have reported changes in one aspect or another, and here we aim to highlight the common findings in the context of potential mechanisms underlying the relationship between altered mucosal 5-HT signaling and altered GI function and sensation.
Inflammation
Human studies have been conducted with mucosal biopsies from a number of inflammatory conditions, including Crohn’s disease36, 37, ulcerative colitis 16, 36, 37, diverticulitis38 and active celiac disease39, 40. In patients with ulcerative colitis, there is a decrease in 5-HT content and a trend towards fewer EC cells in the mucosa, although mucosal 5-HT release does not appear to be altered16. In celiac disease, elevations in 5-HT content, EC cell numbers, and circulating 5-HT have been reported, whereas in diverticulitis, no changes in 5-HT content or EC cell numbers have been detected. While changes at the upstream end of the mucosal 5-HT signaling pathway vary amongst these inflammatory disorders, all of the conditions described above are associated with decreased epithelial SERT expression levels. It is worth noting that a decrease in SERT has not been detected in inflamed pediatric samples41.
Serotonin signaling has also been investigated in a number of animal models of intestinal inflammation, including TNBS colitis19 and ileitis42 in guinea pig, and TNBS colitis21, DSS colitis43, Citrobacter rodentium enteritis44, and Trichinella spiralis enteritis45 in mouse. In all of these conditions, 5-HT levels and EC cell numbers are increased, and where studied, 5-HT release is increased as well. Another consistent feature amongst these models is a decrease in epithelial SERT expression, and it has been demonstrated that this decrease in SERT can lead to increased 5-HT availability under both basal and stimulated conditions19, supporting the concept that 5-HT signaling is affected by both release and reuptake events.
The findings that mucosal 5-HT content and EC cell numbers are elevated in the animal models and in celiac disease, whereas they are decreased in inflammatory bowel disease (IBD), may reflect the duration of the inflammatory condition, with animal models and active celiac disease representing shorter time points than chronic IBD. With regard to SERT, it is likely that expression is down-regulated by the inflammatory response. In the Caco-2 human epithelial cell line, SERT mRNA and protein levels, and 5-HT uptake are decreased in an additive manner by treatment with interferon gamma and tumor necrosis factor alpha, or by conditioned medium from activated lymphocytes46. Co-culture of Caco-2 cells with E. coli also down-regulates SERT expression47 and epithelial SERT expression and function in the intestine is decreased in mice with an enteropathogenic E. coli infection47. Thus, SERT expression and function are down regulated in inflammatory conditions leading to elevated 5-HT availability locally, and in the circulation.
Functional GI disorders
Many aspects of functional GI disorders are confusing and controversial, and the role of mucosal 5-HT signaling is no exception. Irritable Bowel Syndrome (IBS) is characterized by chronic abdominal pain or discomfort, associated with altered stool frequency or form, in the absence of organic disease that would be likely to explain the symptoms48. IBS patients also typically exhibit a reduced threshold for pain, or visceral hypersensitivity. Due to an absence of clear pathological markers, as in intestinal inflammation, the diagnosis is made based on presence of symptoms and further classified by the nature of the predominant stool pattern; IBS-C (constipation), IBS-D (diarrhea), or IBS-M (mixed or alternating) 48, 49. These subgroups also have physiological relevance as stool consistency (hard to watery) reflects the amount of water absorption as a function of intestinal transit time 49. Mucosal 5-HT signaling has been investigated in IBS-D and IBS-C, but not in IBS-M.
Possibly the first molecular alteration reported in the GI tracts of individuals with IBS was a decrease in SERT expression in specimens from individuals with IBS-D or IBS-C16. These findings led to much discussion and follow-up studies by several groups because it was intuitively difficult to fathom how a decrease in SERT could be associated with the divergent symptoms of diarrhea and constipation.
In IBS-D, no differences in basal or stimulated mucosal 5-HT release are detected relative to healthy controls, despite the finding that both mucosal 5-HT content and TpH1 mRNA levels are decreased16. While Camilleri and colleagues did not detect a change in mucosal SERT mRNA in their population of IBS-D patients50, decreased SERT expression in IBS-D has been reported by others in adults40, 51 as well as children41. In patients with IBS-C, no change was detected in the study by Camilleri50.
As described above, circulating 5-HT comes primarily from the gut, and represents the 5-HT that is not captured by SERT in the cells of the epithelial lining. Therefore, circulating 5-HT is often studied in GI disorders as a reflection of 5-HT availability in the mucosa. Postprandial 5-HT levels are elevated in platelet-poor plasma samples obtained from IBS-D52–54 or post-infectious IBS54 patients, but they have been reported to be reduced54 or unchanged53 in IBS-C. In contrast, platelet 5-HT levels are reduced in IBS-D55, but they are doubled as compared to healthy controls in IBS-C53. Collectively, these results are consistent with a decrease in 5-HT uptake by the intestinal epithelium in both of these forms of IBS since it appears that more 5-HT is ending up in the circulation. The ability to detect elevated postprandial 5-HT levels in platelet-poor blood in IBS-D, but not IBS-C, may reflect differences in platelet SERT function in these disorders. Uptake of 5-HT into platelets appears to be disrupted in individuals with IBS-D40, 55, 56. This could account for the elevated 5-HT in platelet poor plasma samples from patients with IBS-D, given that 5-HT is normally difficulty to detect in such samples, even postprandially. Further studies will be required to determine whether SERT function is altered in the mucosa and/or platelets of individuals with IBS-C.
The pattern of mucosal 5-HT signaling changes that have been detected in chronic constipation differ from those reported in IBS-C or IBS-D, but as in these forms of IBS, the net result appears to be an increase in 5-HT availability. In chronic constipation, SERT expression is not altered 57, but 5-HT content, EC cell numbers, and 5-HT release are increased 57, 58. The differences in 5-HT fingerprints of these disorders suggest that they represent different entities.
While mounting evidence strongly supports the concept that mucosal 5-HT signaling is altered in functional GI disorders, the cause and effect relationship has not been unequivocally resolved. It is clear that increasing mucosal 5-HT availability can alter intestinal function. Chronic treatment of mice with the selective serotonin reuptake inhibitor (SSRI) paroxetine decreased stool output and delayed upper GI transit59, and mice lacking SERT exhibit alternating bouts of diarrhea and constipation15, similar to individuals who suffer from IBS-M. In humans, chronic exposure to SSRIs enhances orocecal transit60, but also can induce constipation by reducing peristaltic activity 61. On the other hand, acute SSRI administration increases colonic motility and high-amplitude propagated contractions associated with abdominal cramping62. These findings suggest that altered mucosal 5-HT signaling could contribute to the symptoms of IBS. Furthermore, changes in gut function do not necessarily lead to changes in the elements of 5-HT signaling as demonstrated in a study of mucosal biopsies from individuals with opiate-induced constipation57. In this study, no differences were detected in 5-HT content or release, EC cell numbers, TpH1 mRNA or SERT mRNA levels in individuals with opiate-induced constipation as compared to healthy controls.
The cause of decreased SERT expression in IBS has not been determined. One possibility is that decreased SERT expression could involve increased permeability and a related low level of inflammation as is thought to exist in IBS63. Another possibility is that individuals with IBS have a genetic predisposition for decreased SERT expression. There is a polymorphic region in the human SERT gene (SERT-LPR), upstream of exon 1a, that comprises long (l) and short (s) variants depending on variable numbers of tandem repeats64. Interest in this polymorphism was heightened by the finding that, when expressed in lymphocytes, the s/s and l/s genotypes are associated with lower levels of SERT expression and function, as compared to the l/l genotype64. A possible relationship exists between SERT-LPR alleles and psychiatric disorders, anxiety-related personality traits, suicide, alcoholism, and responsiveness to antidepressants65. Studies of the SERT-LPR in humans with IBS have demonstrated trends in some populations, but a meta-analysis of these findings failed to detect a clear relationship between the SERT-LPR and IBS66, although SERT expression level in the gut does appear to be influenced by the SERT-LPR genotype 67. Furthermore, there appears to be a relationship between the SERT-LPR genotype and depressive symptoms with IBS, with IBS sufferers with the short allele being more likely to have a history of depression 68. A study of the single nucleotide polymorphism, rs25531, demonstrated the carriers of the rare G allele are three times as likely to have IBS than healthy controls 69.
Functions of 5-HT in the gastrointestinal tract
Conventional actions of 5-HT in the gut
One of the unique features of the ENS is the existence of intrinsic reflex circuitry that mediates the basic regulated activities of tissues in the GI tract necessary for digestion and the elimination of waste products. These regulated activities include (1) motility - generation of motor patterns via coordinated contractions and relaxations of the muscularis externa, which are required for mixing and propelling the luminal contents, (2) epithelial secretion, which is involved in diluting and dissolving luminal contents for digestion, and (3) vasodilation, which facilitates the active processes of digestion and absorption. The various intrinsic reflex circuits that control these fundamental activities can be mediated by the release of 5-HT from EC cells. In addition 5-HT released from EC cells can also activate receptors on extrinsic afferent fibers to send signals to the central nervous system.
Motility
The concept that 5-HT synthesized and released by EC cells in the intestinal mucosa can play a role in initiating peristalsis was originally proposed by Edith Bülbring and her colleagues in the late 1950’s70–73. Amongst their observations, they found that 5-HT is released by the EC cells in an intraluminal pressure-dependent manner72, and that intraluminal infusion of 5-HT restored peristalsis when it had been halted pharmacologically70. Since that time, it has been demonstrated in a number of experimental paradigms, including human preparations, that stroking of the mucosal surface results in 5-HT release19, 74–76. Serotonin release can also be induced by mechanical stimulation of BON cells, a human cell line model of EC cells77. It is clear from the elegant studies of groups led by Jack Grider78, Terry Smith79, and Joel Bornstein31, 80 that 5-HT released in response to mucosal stimulation can activate the ascending contractile and descending relaxant limbs of the peristaltic reflex. Additional support for a role of 5-HT release in propulsive motility comes from ex vivo19, 78, 81 and in vivo 82, 83 studies demonstrating that antagonists of 5-HT3 and/or 5-HT4 receptors slow intestinal motility, as well as a study demonstrating that corticotropin-releasing hormone-induced defecation is reversed by peripheral actions of the 5-HT3 receptor antagonist, ramosetron84. Furthermore, in isolated guinea pig colon preparations, propulsive motility is disrupted by desensitization of 5-HT4 receptors85, or high concentrations of the SERT inhibitor fluoxetine13, which presumably leads to 5-HT receptor desensitization by increasing 5-HT concentrations in the interstitial space.
In summarizing one of her papers, Edith Bülbring concluded, “Whether 5-HT is of primary importance for the initiation of the peristaltic reflex could not be decided as the amount of 5-HT was never reduced to zero…”71. New studies once again raise the question of the primary importance of 5-HT in the initiation of peristalsis. Recently, Gershon and colleagues demonstrated that intestinal transit and colonic bead expulsion, which presumably involve peristalsis, are unchanged in TpH1 deficient mice86. Furthermore, Spencer and colleagues removed the mucosa from mouse and guinea pig colonic preparations and were still able to demonstrate peristalsis activated by stretch of the tissue87, 88. Based on our current knowledge, it is probably best to conclude that 5-HT released from EC cells is sufficient, but not necessary, to initiate the peristaltic reflex.
Another motor pattern that is generated by enteric nerves is segmentation, which consists of alternating contractions of the muscularis in a given region without forward propulsion of the luminal contents. It serves to mix the contents of the small intestine and maximize their exposure to digestive enzymes during the digestive process. Experimentally, segmentation can be activated by luminal infusion of fatty acids, and a recent study demonstrates that mucosal 5-HT is likely involved in the production of this motor pattern. In an ex vivo guinea pig small intestine assay, segmentation was induced by addition of the SERT inhibitor, fluoxetine, and segmentation patterns initiated by fatty acids or fluoxetine are inhibited by antagonists of the 5-HT3 or 5-HT4 receptor, as well as CCK receptor antagonists89. The authors therefore concluded that both 5-HT and CCK are involved in the regulation of segmentation motor patterns.
Secretion
In the intestinal epithelium, active secretion can be initiated by intrinsic enteric reflexes that are comprised of primary afferent (sensory) and secretomotor neurons with cell bodies located in nearby submucosal ganglia 90. The afferent neurons receive signals from projections to the lamina propria, and when stimulated, they synaptically activate secretomotor neurons, which in turn, release neurotransmitters such as acetylcholine and vasoactive intestinal peptide (VIP) to activate secretion from the epithelial cells. Serotonin released from EC cells is an important activator of these reflex-mediated secretory responses77, 91, 92. Mechanical stimulation of the mucosal surface leads to 5-HT release, which activates neurally mediated Cl- and bicarbonate secretion. The neurogenic secretory responses appear to be mediated primarily by 5-HT3 and 5-HT4 receptors, but 5-HT1P receptors may also be involved90, 91, 93–96 97. Serotonin released from EC cells can also stimulate secretion through a direct paracrine action on nearby enterocytes, and this response is mediated by the activation of 5-HT2 receptors94. Serotonin-initiated secretion contributes to the dilution and neutralization of luminal contents, but it is also involved in the protective response to eliminate luminal pathogens98–102.
Vasodilation
Vascular tone is regulated solely by the sympathetic division of the autonomic nervous system throughout most of the body. A unique feature of the gut is the ability of intrinsic reflex circuitry to locally regulate blood vessel diameter. The vasodilatory motor neurons are located in submucosal ganglia, but two types of reflex circuits appear to exist: local circuits (≤ 2mm) consisting of afferent/sensory and motor neurons in the submucosal plexus, and a farther reaching circuit (up to 18 mm) that includes submucosal afferent neurons, projections to the myenteric plexus where signals are passed up and down stream along the gut tube, and submucosal vasodilator motor neurons103–107. These reflexes likely involve the same afferent neurons that activate secretomotor responses, and vasodilatory responses that are activated by mucosal stimulation are blocked by inhibition of 5-HT3 and 5-HT4 receptors96.
Activation of extrinsic afferent nerves
Early efforts to record from extrinsic afferent fibers innervating the GI tract were hampered by the fact that animals were fasted prior to the experiments, and the afferents were quiescent in the absence of the chemical and mechanical stimuli that normally lead to their activation108. The first electrophysiological recordings of vagal afferent fibers innervating the gut were accomplished by Autar Singh Paintal109, 110. He demonstrated in the 1950’s that quiescent sensory fibers innervating the stomach and intestine could be awakened by vascular infusion of phenyldiguanide. Phenyldiguanide, also known as phenylbiguanide, was subsequently shown to be a potent 5-HT3 receptor agonist.
Vagal afferent fibers arising in the upper GI tract send messages that result in a variety of responses upon activation by 5-HT. Pharmacological and immunohistochemical studies have demonstrated that vagal afferent nerve endings in the mucosal layer of the gut express 5-HT3 receptors111–114. Furthermore, infusion of hyperosmotic solutions or carbohydrate breakdown products into the lumen of the duodenum evokes release of 5-HT from EC cells which acts on 5-HT3 receptors to stimulate vagal afferent neurons115. Glucose in the lumen also causes 5-HT release from EC cells, and activates a vago-vagal reflex that inhibits gastric emptying116. These stimuli also result in pancreatic secretion117, and both the gastric inhibitory response and the pancreatic secretory response are sensitive to 5-HT3 receptor antagonists117. Satiety signals arising from the gut also involve 5-HT release since lipid-induced suppression of food intake is mediated entirely by simultaneous activation of 5-HT3 and CCK1 receptors118. In humans, inhibition of 5-HT3 receptors increases the volume of a liquid meal that is ingested 119, supporting a role for 5-HT receptors and vagal afferent signaling in the regulation of satiety and hunger in humans.
In addition to activation of digestive and homeostatic reflexes described above, 5-HT released from EC cells in the intestine can lead to feelings of intense discomfort. Cytotoxic chemotherapeutic agents such as cisplatin cause a robust release of 5-HT and activation of 5-HT3 receptors on vagal afferent fibers resulting in nausea and emesis 120–122. It is hard to imagine how a simple signaling molecule such as 5-HT, acting on one class of receptor, present on the same set of afferent fibers, can lead to such a multitude of reflex responses. One possible factor in sorting and processing these signals could involve simultaneous activation of vagal afferent nerves that express CCK1 receptors, which are distinct from those that express the 5-HT3 receptor123. Another contributing factor may be that the amount of 5-HT released in response to chemotherapeutic agents is far greater than that released in response to nutrients and osmotic signals.
While less is known about 5-HT-induced activation of spinal afferent fibers innervating the gut, as compared to 5-HT and vagal afferents, it is clear from in vivo124 and ex vivo125 studies of colonic afferent nerves that spinal afferents express 5-HT3 receptors on their peripheral endings. In studies of noxious colorectal distension in the rat, the 5-HT3 antagonist, alosetron inhibited reflex responses and c-fos immunoreativity in the dorsal horn124. Furthermore, the sensitivity of spinal afferents to 5-HT is enhanced during inflammation, both in terms of the proportion of afferent fibers responding to 5-HT and the intensity of the response126. This is consistent with the recent finding that the number of nerve fibers expressing 5-HT3 receptors is increased in the mucosal layer of the DSS-inflamed colon127. Interestingly, the hypersensitivity to 5-HT persists following recovery from inflammation126, suggesting that the analgesic effect of 5-HT3 agonists in IBS could involve attenuation of 5-HT’s ability to stimulate of spinal afferent terminals in the gut.
Non-conventional actions of 5-HT in the gut
In addition to the 5-HT-mediated activities described above, recent studies have revealed that this simple in structure, but complex in actions, signaling molecule can also serve as a trophic factor as well as a pro-inflammatory mediator in the GI tract.
Neuroprotective and trophic factor actions of 5-HT
Since enteric serotonergic neurons depend on TpH2 for the synthesis of 5-HT, TpH2 deficient mice have been used to investigate the roles of neuronal 5-HT in the bowel. An interesting finding of these studies is that 5-HT appears to be an important factor in the development and survival of enteric neurons. Mice lacking the TpH2 gene exhibit a lower density of myenteric neurons, particularly dopaminergic and GABAergic nerve cells128. These actions of 5-HT may involve the 5-HT2B receptor, which has been shown to influence enteric neuronal expansion, particularly in the developing gut. Stimulation of 5-HT2B receptors enhances the differentiation of enteric neurons in cultures from both dissociated cultures of mixed fetal gut cells and in cultures of neural crest-derived cells isolated from the gut129, indicating that 5-HT may influence the fate of developing enteric neurons through actions on 5-HT2B receptors.
The 5-HT2B receptor is also expressed by interstitial cells of Cajal (ICC) and is important for the integrity of the ICC network. 5-HT promotes the survival of ICC cells in culture via actions on 5-HT2B receptors130, and the density of ICC is decreased in 5-HT2B receptor deficient mice. Modeling of these changes suggests that alterations of the ICC network would impair slow wave propagation and ultimately motility and transit in the intestines131.
The 5-HT4 receptor also enhances the development and survival of enteric neurons. Studies from 5-HT4 knockout mice indicate that the density of neurons is not altered at birth, but 5-HT4 receptor deficiency leads to a postnatal reduction in enteric neurons, and treatment of adult mice with a 5-HT4 agonists promotes the generation of new enteric neurons132, 133. Furthermore, studies of cultured enteric neurons show that stimulation of 5-HT4 receptors increases the proliferation and/or survival of enteric neurons and enhances neurite outgrowth132, 133. The results of these investigations are supported by the studies of Miyako Takaki and colleagues demonstrating that 5-HT4 receptor stimulation enhances the formation of neural networks in an embryoid body culture system134. They also show significant improvement in regeneration of enteric reflexes and recovery of a defecation reflex that are disrupted by colonic resection135, 136.
TpH2 deficient mice exhibit disrupted intestinal transit86. Given these trophic/protective influences of 5-HT on the integrity of the myenteric plexus it is not clear whether the dysmotility findings from the TpH2 knockout mouse experiments reflect an alteration in serotonergic neurotransmission and/or changes in the circuitry due to loss of neurons.
Pro-inflammatory actions of 5-HT in the gut
As described above, 5-HT availability is increased in various animal models of intestinal inflammation, as well as human inflammatory conditions. Recent evidence strongly suggests that 5-HT can act as a pro-inflammatory signaling molecule in the mucosal layer of the gut. On one hand, inflammation is intensified in the IL-10 knockout and TNBS models of colitis when 5-HT availability is increased by lack of the 5-HT transporter in SERT deficient mice 137, 138. On the other hand, the extent of inflammation in DSS and DNBS colitis models is reduced when mucosal 5-HT availability is reduced by deletion of the gene that encodes TpH1 or by reduction of 5-HT synthesis with parachlorophenylalanine139. Bypassing TpH1 in TpH1-deficient mice by treatment with the 5-HT precursor, 5-hydroxytryptophan increased the severity of colitis in these animals. The pro-inflammatory actions of 5-HT in the mucosa involves a chain of events that are likely initiated by activation of 5-HT receptors on dendritic cells in the lamina propria140.
Functions of gut-derived 5-HT outside of the GI tract
It has long been generally accepted that “virtually all” peripheral 5-HT arises from the gut. This notion is based on the early studies of Erspamer and Bertaccini who demonstrated that total gastroenterectomy in rats or dogs caused a disappearance of the metabolite 5-Hydroxyindoleacetic acid (5-HIAA) from the urine, and dramatically lowered 5-HT levels in the blood, spleen and lungs141, 142. They also demonstrated that 5-HT levels in portal venous blood are much higher than those of vena cava blood. Based on these findings, and the ability of SERT-expressing platelets to distribute 5-HT throughout the body, it has been presumed that non-cerebrospinal actions of 5-HT outside of the gut involve 5-HT arising from enteric EC cells.
Many tissues express 5-HT receptors, and actions of 5-HT outside of the gut include vasoconstriction or vasodilation (depending on the vascular bed), platelet aggregation, hematopoiesis, regulation of bone density, bronchoconstriction, itching sensation and nociception143. Furthermore, 5-HT appears to be involved in heart and mammary development, as well as oocyte maturation. The roles of 5-HT in decreased bone density and allergic airway inflammation (AAI) are of particular interest in the context of this review because TpH inhibitors have been tested as a therapeutic strategy for these conditions.
Low Density Lipoprotein Receptor-Related Protein-5 (Lrp5) is a Wnt coreceptor that is important in the control of bone density, and there is evidence that it affects bone indirectly through regulation of 5-HT synthesis by EC cells 144. In Lrp5 deficient mice, TpH1 expression and mucosal 5-HT concentrations are elevated. Increased serotonin levels decrease bone formation by interacting with 5-HT1B receptors to inhibit osteoblast proliferation. Inhibition of TpH1 has been shown to have protective action in an ovariectomy model of osteoporosis, further supporting a role for 5-HT as a regulator of bone growth and density145.
A role for 5-HT in the manifestation of allergic airway inflammation has been well established, yet the source of the 5-HT was not clearly defined until recently, and it was not known whether TpH1 was involved in synthesis of that 5-HT. A recent study by Idzko and colleagues demonstrated that platelets provide the 5-HT that contributes to asthma pathogenesis, and that symptoms in a mouse model of allergic airway inflammation are greatly reduced in TpH1 deficient mice or mice treated with a TpH1 inhibitor146. These findings indicate that the 5-HT that interacts with airway tissues during asthma attacks originates in the gut, and that TpH1 inhibitors acting in the gut could provide a novel therapeutic approach for the treatment of asthma.
While it is likely that most, if not all, platelet serum arises in the gut, recent evidence indicates that TpH1 expression can be activated locally in a variety of tissues147. For example, there is considerable controversy as to whether it is gut-derived 5-HT, and/or 5-HT from local TpH1-expressing osteoclasts, that regulates bone remodeling by affecting both oseaclast and osteoblast development148. Furthermore, mouse mammary epithelium expresses mRNA for TpH1 as well as and aromatic amine decarboxylase when stimulated by prolactin149, and cells expressing TpH1 in the placenta give rise to 5-HT that is critical for brain development150. Regardless of the source of 5-HT regulating these systems under normal circumstances, pharmacological interventions and pathological conditions that affect gut 5-HT signaling, and ultimately circulating 5-HT levels, have the potential to impact many other tissues.
Serotonergic targets for the treatment of GI disorders
5-HT receptors in the GI tract
Serotonin receptors are widely expressed within the GI tract, and five of the seven known families, 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors, are expressed in the gut and can affect gut functions151 (Fig. 3). The 5-HT3 and 5-HT4 receptor subtypes have been most extensively studied in the gut, and have been targeted for the treatment of diarrhea and constipation, respectively.
Figure 3.
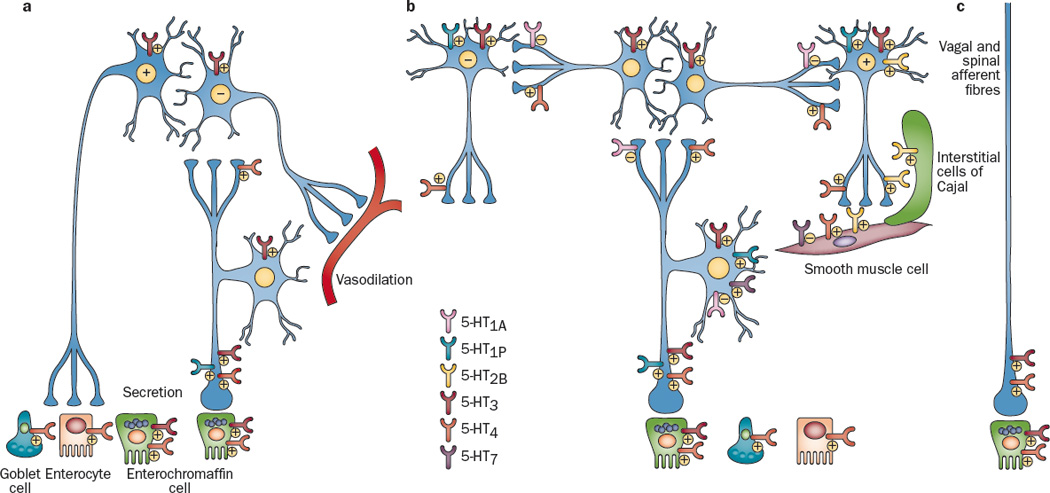
Distribution of 5-HT receptors on enteric neurons, extrinsic nerve fibers and other excitable cells in the gut. At least 6 subtypes of 5-HT receptors are expressed in the wall of the gut, and they can exert excitatory and/or inhibitory influences depending on their location and on the target cell type. a. intrinsic circuits for epithelial secretion and vasodilation. The motor neuron may be the same neuron, but it promotes epithelial secretion and relaxes vascular smooth muscle. b. Intrinsic circuit for propulsive motility. c. extrinsic vagal and spinal afferent fibers. The “+” and “-“ symbols indicate excitatory and inhibitory actions, respectively. References: 5-HT1A171, 186–188; 5-HT1P33, 74, 92, 97, 186, 189–191; 5-HT2129, 130, 192–194; 5-HT333, 74, 81, 106, 112, 116, 117, 119, 123, 124, 152, 186; 5-HT411, 74, 133, 168, 170, 171, 173, 186, 195; 5-HT735, 196.
5-HT3 receptors
Because of their value as a therapeutic target in the gut, 5-HT3 receptors have been extensively investigated. They are expressed on the excitable cells of the gut, including intrinsic and extrinsic afferent nerves that extend into the mucosa, interneurons, inhibitory and excitatory motor neurons, ICCs, smooth muscle and enterocytes (Fig. 3). As described above, 5-HT3 receptors are involved in the activation of intrinsic and extrinsic afferent nerves by 5-HT released from EC cells, and they contribute to a small subset of EPSPs. In an elegant and technically difficult investigation, Bertrand and colleagues demonstrated that AH neurons in the myenteric plexus, which serve as primary afferent neurons and project to the mucosa, are directly activated by 5-HT applied to the mucosa, and that these responses are exclusively mediated by 5-HT3 receptors152.
Initial therapeutic use of 5-HT3 receptor antagonists arose from the landmark discovery in 1986 that specific 5-HT3 receptor antagonists could inhibit cisplatin-induced emesis153, 154. 5-HT3 receptor antagonists likely exert their anti-nausea effects via at least two sites of action, vagal afferents in the gut and in the area postrema, and they are particularly effective in treating the acute phases of chemotherapy- and radiation therapy-induced nausea and emesis121, 122. A common adverse side effect of 5-HT3 receptor antagonist being used for anti-nausea and anti-emetic purposes is constipation, and this action has been exploited for the treatment of IBS-D83, 155.
5-HT3 antagonists have been shown to be effective in treating both the diarrhea and abdominal discomfort symptoms of IBS-D156, 157. While the precise mechanisms of action of these compounds have not been definitively determined, it is likely that they inhibit 5-HT3 receptors located on intrinsic and extrinsic afferent nerve fibers in the mucosa that are activated by 5-HT released from EC cells. They also inhibit the stimulation of 5-HT3 receptors on interneurons and motor neurons that contribute to fast EPSPs. Collectively, this would decrease propulsive motility and secretion locally within the gut, thus alleviating diarrhea, as well as decreased signaling from spinal afferent nerves that deliver signals of pain and discomfort to the CNS. As described above, the 5-HT3 antagonist, alosetron, inhibits activation of dorsal horn neurons in response to noxious distention of the colon124, indicating that 5-HT3 receptors are involved in the activation of pain signals from the gut. However, the viscero-analgesic benefits of 5-HT3 antagonists may also involve central mechanisms of action158.
With the knowledge that 5-HT3 receptors can participate in the activation of propulsive motility and secretory responses in the gut, 5-HT3 agonists have been developed and tested for the treatment of constipation. Emphasis has been concentrated on partial agonists because 5-HT3 receptors desensitize rapidly, and as outlined above, stimulation of 5-HT3 receptors on vagal and spinal afferent fibers can lead to nausea and abdominal discomfort, respectively. One such compound is pumosetrag (DDP733/MKC733), which is being developed for the treatment of IBS-C and has also been shown to reduce reflux events in individuals with gastroesophageal reflux disease159, 160. Another, somewhat counterintuitive strategy, is to use a high affinity, low intrinsic activity 5-HT3 receptor partial agonists for the treatment of IBS-D160. The concept being proposed is that these compounds would interrupt activation of 5-HT3 receptors by excessive background levels of 5-HT, such as those that are thought to exist in IBS-D, while allowing the receptors to be activated during reflex responses, thus reducing the chance of developing constipation.
5-HT4 receptors
Agonists of the 5-HT4 receptor alleviate constipation in IBS-C and in chronic constipation, they provide pain relief in IBS-C, and they can accelerate the rate of gastric emptying161–164. It is interesting to note that benzamides, such as metoclopramide, cisapride and renzapride, were recognized as prokinetic agents in the gut even before the existence of 5-HT4 receptors was discovered, and because they enhanced acetylcholine release from myenteric neurons165, 166, they were initially considered cholinergic compounds. Soon after the discovery of 5-HT4 receptors, it was determined that these compounds are 5-HT4 receptor agonists, and efforts were made to determine the mechanisms by which they promote motility. Considerable physiological data suggest that 5-HT4 receptor stimulation accelerates the peristaltic reflex11, 167–169. Furthermore, transgenic mice that lack 5-HT4 receptors exhibit slowed colonic motility133, suggesting that 5-HT4 receptors are physiologically activated and contribute to the regulation of propulsive motility, and/or they promote normal motility through their trophic actions mentioned above. Within the muscularis externa, 5-HT4 agonists act presynaptically on nerve terminals to enhance the release of acetylcholine169–173. By acting in this manner, they are thought to enhance naturally occurring reflex activity rather than to generate neurotransmission. This is an important distinction because efficient propulsive motility is a complex and carefully orchestrated process, and simply activating transmitter release by neurons throughout the gut tube actually inhibits propulsive motility174.
Unfortunately, while shown to be effective, the 5-HT4 agonists that were initially made available for the treatment of constipation, cisapride and tegaserod, were ultimately removed or restricted because of potential cardiovascular side effects. It is thought that the adverse effects of the early 5-HT4 compounds are related to their relative lack of selectivity, and resultant actions on other targets, including hERG potassium channels, dopamine receptors, 5-HT1 and 5-HT2 receptors 175, 176. Therefore, more selective agonists such as prucalopride, naranopride (ATI-7505), mosapride, and velusetrag (TD-5108) are becoming available or are being developed to fill this gap.
We have recently published the results of a study that reveals a novel target for 5-HT4 agonists that could provide clinical benefits while minimizing the risk of adverse side effects11. In ex vivo motility and peristaltic reflex assays, we and others found that 5-HT4 agonists accelerate propulsive motility when they are infused into the lumen or applied to the mucosal surface11, 78, 168, 177; however addition of agonists to the bathing solution where they would be in position to access the nerve terminals of the peristaltic reflex circuitry did not enhance motility11. These results suggested the existence of 5-HT4 receptors in the mucosa. Evaluation of fluorescence in a strain of mice in which the promoter of the 5-HT4 gene drives the expression of enhanced green fluorescent protein revealed that essentially all of the cells of the colonic mucosa express the 5-HT4 receptor (Fig. 4). Additional studies demonstrated the presence of transcript for this receptor in the colonic mucosa of mouse, rat and human . Furthermore, mucosal application of 5-HT4 agonists activated 5-HT release from EC cells, mucus discharge by goblet cells and Cl- secretion by enterocytes, and 5-HT4 receptor antagonists blocked these actions. Any or all of these actions could alleviate constipation, and formulating 5-HT4 agonists such that they target the colon without being absorbed could result in a safe and effective treatment of IBS-C and chronic constipation.
Figure 4.
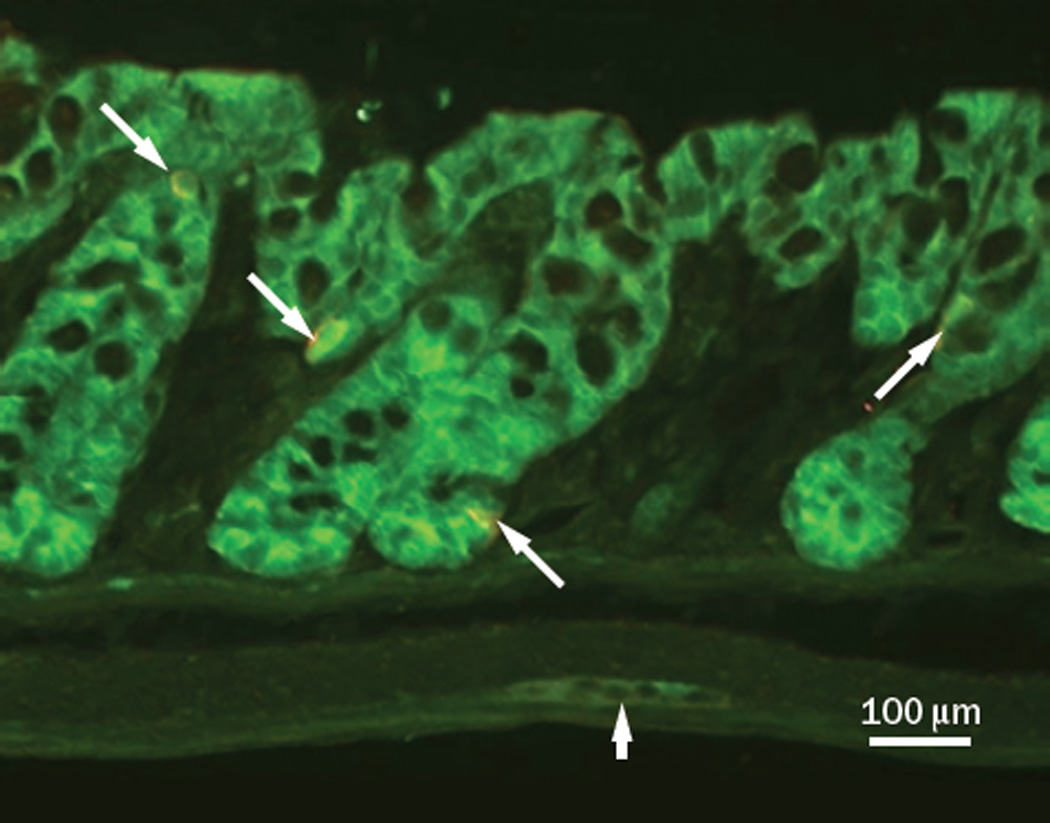
The 5-HT4 receptor is expressed by virtually all epithelial cells, including EC cells, in the murine colon11. This is a photomicrograph from a section of the distal colon of a 5-HT4R(BAC)-eGFP mouse in which cells that express the 5-HT4 receptor fluoresce green. Green fluorescence is present in all of the epithelial cells, as well as a layer of cells in the muscularis mucosa, and in a myenteric ganglion (block arrow). This section was also processed for 5-HT-immunoreactivity to label EC cells, shown in red (arrows).
A welcome, but puzzling, action of 5-HT4 receptor agonists is that they attenuate visceral pain arising from the colon. In humans, oral administration of the 5-HT4 receptor agonist tegaserod reduced rectal sensitivity to distension in individuals with IBS178 Furthermore, intraperitoneal or intraluminally administered tegaserod attenuated nociceptive responses, in a visceral hypersensitivity model involving awake, freely moving rats, 11, 179. The reason this is puzzling is that 5-HT4 receptor stimulation leads to activation of the cyclic AMP-protein kinase A pathway, which has an excitatory effect on neuronal activity, as is seen with the presynaptic facilitory actions of 5-HT4 agonists. It has been proposed that the anti-nociceptive actions of these compounds involve their non-selective inhibitory action on 5-HT2B receptors, but the actions of tegaserod in these studies are blocked by 5-HT4 receptor antagonists11, 180. Furthermore, intraluminal administration of the selective 5-HT4 receptor antagonist ATI-7505 inhibits visceral hypersensitivity11. One possible mechanism by which 5-HT4 receptors could attenuate visceral nociception is through an inhibitory action on intramural mechanoreceptor activation. In a rectal distension study in cats, tegaserod decreased afferent discharge at nociceptive intraluminal pressures without altering compliance181. However, even if mechanoreception is inhibited by 5-HT4 agonists, the precise mechanism of this inhibition has not been clearly determined.
Tryptophan hydroxylase
There is substantial evidence that 5-HT released from EC cells can activate enteric secretory and motor reflexes, as well as send signals to the CNS. Therefore, it stands to reason that suppressing this signaling apparatus by decreasing 5-HT release could possibly attenuate the symptoms of IBS-D. Accordingly, efforts have been made to develop TpH inhibitors that decrease GI 5-HT levels without affecting serotonergic signaling in the brain by limiting their ability to cross the blood-brain barrier182–184. Results of a double-blind, placebo-controlled study of one of these compounds, LX-1031, indicate that this approach shows promise for treating the symptoms of non-constipating IBS183. In this 4 week trial, LX-1031 resulted in significant relief of pain and discomfort, and in improvement of stool consistency. Treatment with the TpH inhibitor decreased peripheral 5-HT levels, as reflected by dose-dependent decreases in urine levels of the 5-HT metabolite, 5-HIAA, and interestingly, there was a correlation between symptom improvement and reduction in 5-HIAA.
Concluding remarks
Despite our best efforts over the past 6 decades to understand 5-HT signaling in the gut, many unanswered questions remain, and new questions are still arising. It is generally accepted that 5-HT plays a role in many GI functions and in sending signals from the gut to the CNS. Also, it is clear that drugs that target 5-HT signaling molecules are effective at alleviating the symptoms of functional GI disorders. Figuring out how to identify subpopulations of individuals with a given type of IBS that respond best to these treatments is an important goal. One aspect of 5-HT biology not extensively addressed in this review is the interrelationship between genetic polymorphisms in the genes of 5-HT signaling molecules and various forms of IBS. This is because no clear answers have yet emerged, but it is important to keep trying. Another important goal is to understand more precisely the distributions of 5-HT receptors and to determine which receptors in what locations are physiologically relevant. Much of our information is from whole organ or in vivo assays with which it is impossible to resolve the precise locations of receptors involved in a given reflex response. Gaining the resolution to answer these questions will require returning to the labor intensive, low throughput approaches such as those used by Bertrand and colleagues to demonstrate that 5-HT3 receptors are located on mucosal projections from myenteric primary afferent neurons. Another emerging feature of 5-HT signaling is that most of the targets of therapies for FGIDs are expressed by epithelial cells or within 20 µm of the epithelial surface. These include Tph1, as well as 5-HT3 and 5-HT4 receptors. Attempting to reach these receptors directly, rather than via the systemic circulation, could greatly improve the benefit to risk ratio.
Figure 2.
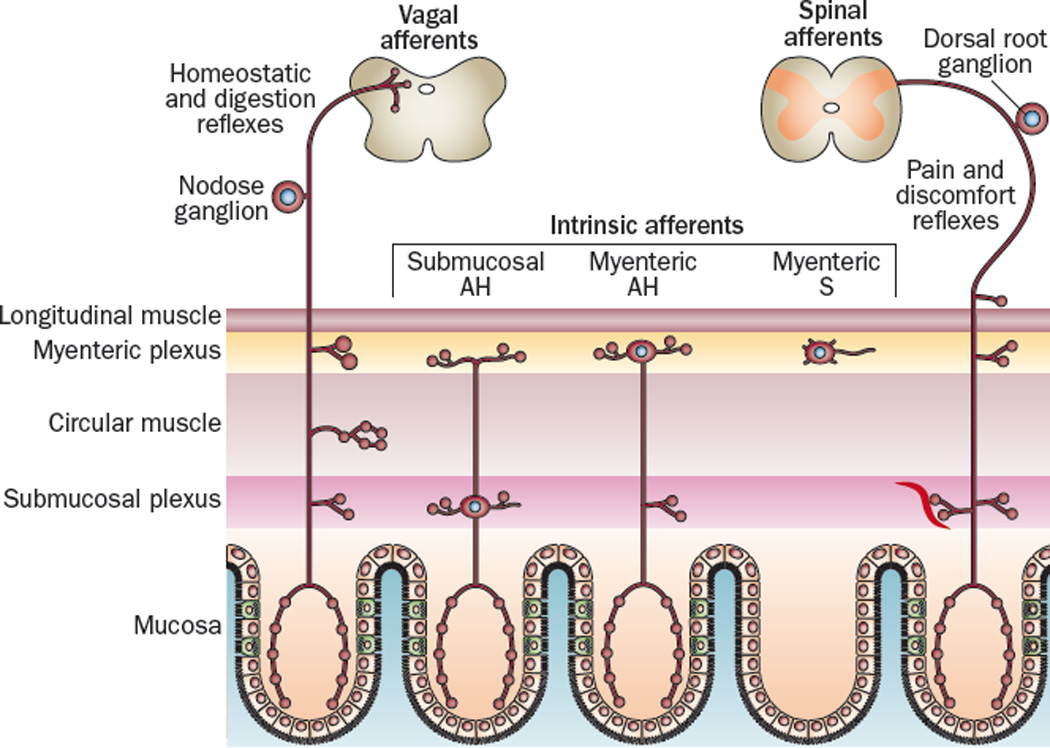
Most of the intrinsic and extrinsic primary afferent neurons that innervate the gut extend processes into the lamina propria of the mucosal layer where they can become exposed to 5-HT released by EC cells185. These include vagal afferent fibers arising from the nodose ganglion, spinal afferent fibers arising from dorsal root ganglia, and intrinsic AH neurons located in submucosal and myenteric ganglia. There is also a class of mechanosensative S neurons in the myenteric ganglia that do not project to the mucosal layer.
Key Points.
Serotonin is an important gastrointestinal signaling molecule that conveys signals from the lumen of the gut to intrinsic and extrinsic sensory neurons, and contributes to synaptic signals in the enteric nervous system.
Fundamental properties of mucosal serotonin signaling are altered in response to inflammation and in functional GI disorders.
Actions of serotonin released from mucosal enterochromaffin cells include activation of intrinsic reflexes such as peristalsis, segmentation, secretion and vasodilation. Serotonin also can activate signals sent to the CNS that stimulate digestive reflexes and can cause abdominal pain and discomfort, satiety, or nausea.
Recent investigations have demonstrated that mucosal serotonin can promote intestinal inflammation, and serotonin in the muscularis propria can promote neuron and interstitial cell of Cajal survival, and promote neural regeneration. Furthermore, gut-derived serotonin can have extra-alimentary actions such as influencing bone densitity and contributing to allergic airway inflammation.
Because of its importance as a signaling molecule in the gut, compounds that affect serotonin signaling in the gut have been developed, including drugs that target 5-HT3 receptors, 5-HT4 receptors, and tryptophan hydroxylase.
Because most targets related to serotonin signaling in the gut are within or close to the epithelial surface, emphasis should be placed on compounds that can mediated their actions without enteric the circulation, thus minimizing adverse actions while maximizing efficacy.
Review criteria.
In the process of preparing this Review we used our collective 35 years of experience studying serotonin signaling and serotonin receptors in the gastrointestinal tract, and we conducted a number of literature searches. PubMed database searches involved combinations of terms that included serotonin, SERT, tryptophan hydroxylase, receptor, inflammation, IBS, motility, secretion, vagal afferent, spinal afferent, intrinsic afferent, AH neuron, gastrointestinal, intestine, stomach, colon, and enteric nervous system.
Acknowledgements
This work was supported by NIH grants DK62267 (to GMM). The authors thank Dr. Brigitte Lavoie for editorial assistance.
Biographies
Gary Mawe is a Professor of Neurological Sciences, and Adjunct Professor of Pharmacology and of Gastroenterology and Hepatology at the University of Vermont College of Medicine. He has been studying 5-HT signaling in the gastrointestinal tract since he began his postdoctoral training in 1984 under the direction of Dr. Michael Gershon. His research activities currently focus on pathophysiological changes in mucosal serotonin signaling, enteric neuronal excitability, and neuromuscular transmission that likely contribute to altered gut function in inflammatory and functional GI disorders.
Jill Hoffman is a postdoctoral research fellow in the Division of Digestive Diseases at the University of California, Los Angeles. She completed her Ph.D. dissertation with Gary Mawe at the University of Vermont in 2011 on serotonin signaling and altered neurotransmission during colitis. Her current research is focused on the involvement of abdominal adipose tissue in the pathophysiology of Inflammatory Bowel Disease.
Footnotes
Conflicts of interest The authors have no conflicts to disclose.
Author Contributions GMM and JMH both contributed to formulating the outline, conducting literature searches, writing, and editing the manuscript
References
- 1.Erspamer V, Vialli M. Ricerche sul secreto delle cellule enterocromaffini. Boll d Soc Med-chir Pavia. 1937;51:357–363. [Google Scholar]
- 2.Rapport MM, Green AA, Page IH. Partial purification of the vasoconstrictor in beef serum. J Biol Chem. 1948;174:735–741. [PubMed] [Google Scholar]
- 3.Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology. 1999;21:2S–8S. doi: 10.1016/S0893-133X(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 4.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 6.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 7.Cote F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 9.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–113. doi: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand PP, Hu X, Mach J, Bertrand RL. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1228–G1236. doi: 10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JM, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel BA, Bian X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 13.Wade PR, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 15.Chen JJ, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coates MD, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Blakely RD, et al. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 18.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 19.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bian X, Patel B, Dai X, Galligan JJ, Swain G. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenterology. 2007;132:2438–2447. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linden DR, et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 22.Ozsarac N, Santha E, Hoffman BJ. Alternative non-coding exons support serotonin transporter mRNA expression in the brain and gut. J Neurochem. 2002;82:336–344. doi: 10.1046/j.1471-4159.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- 23.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012;1822:85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Yu PL, et al. Immunohistochemical localization of tryptophan hydroxylase in the human and rat gastrointestinal tracts. J Comp Neurol. 1999;411:654–665. doi: 10.1002/(sici)1096-9861(19990906)411:4<654::aid-cne9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Kushnir-Sukhov NM, Brittain E, Scott L, Metcalfe DD. Clinical correlates of blood serotonin levels in patients with mastocytosis. Eur J Clin Invest. 2008;38:953–958. doi: 10.1111/j.1365-2362.2008.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa M, et al. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 28.Young HM, Furness JB. Ultrastructural examination of the targets of serotonin-immunoreactive descending interneurons in the guinea pig small intestine. J Comp Neurol. 1995;356:101–114. doi: 10.1002/cne.903560107. [DOI] [PubMed] [Google Scholar]
- 29.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 30.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 31.Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil. 2002;14:255–264. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- 32.Yuan SY, Bornstein JC, Furness JB. Investigation of the role of 5-HT3 and 5-HT4 receptors in ascending and descending reflexes to the circular muscle of guinea-pig small intestine. Br J Pharmacol. 1994;112:1095–1100. doi: 10.1111/j.1476-5381.1994.tb13196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci U S A. 1986;83:9799–9803. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaki M, Branchek T, Tamir H, Gershon MD. Specific antagonism of enteric neural serotonin receptors by dipeptides of 5-hydroxytryptophan: evidence that serotonin is a mediator of slow synaptic excitation in the myenteric plexus. J Neurosci. 1985;5:1769–1780. doi: 10.1523/JNEUROSCI.05-07-01769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monro RL, Bornstein JC, Bertrand PP. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience. 2005;134:975–986. doi: 10.1016/j.neuroscience.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Magro F, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–224. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- 37.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 38.Costedio MM, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman NS, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Foley S, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–1443. doi: 10.1053/j.gastro.2011.01.052. e1. [DOI] [PubMed] [Google Scholar]
- 41.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut. 2007;56:186–194. doi: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL, Lomax AE. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol. 2010;298:G446–G455. doi: 10.1152/ajpgi.00318.2009. [DOI] [PubMed] [Google Scholar]
- 44.O'Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol. 2006;8:646–660. doi: 10.1111/j.1462-5822.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 45.Wheatcroft J, et al. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 46.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 47.Esmaili A, et al. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology. 2009;137:2074–2083. doi: 10.1053/j.gastro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longstreth GF, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 49.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55–60. [PubMed] [Google Scholar]
- 50.Camilleri M, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053–G1060. doi: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 52.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 54.Dunlop SP, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 55.Franke L, et al. Serotonin transporter activity and serotonin concentration in platelets of patients with irritable bowel syndrome: effect of gender. J Gastroenterol. 2010;45:389–398. doi: 10.1007/s00535-009-0167-y. [DOI] [PubMed] [Google Scholar]
- 56.Bellini M, et al. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–2711. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 57.Costedio MM, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am J Gastroenterol. 2010;105:1173–1180. doi: 10.1038/ajg.2009.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lincoln J, Crowe R, Kamm MA, Burnstock G, Lennard-Jones JE. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology. 1990;98:1219–1225. doi: 10.1016/0016-5085(90)90336-y. [DOI] [PubMed] [Google Scholar]
- 59.Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil. 2006;18:464–471. doi: 10.1111/j.1365-2982.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 60.Chial HJ, et al. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol. 2003;284:G130–G137. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
- 61.Leroi AM, et al. Prolonged stationary colonic motility recording in seven patients with severe constipation secondary to antidepressants. Neurogastroenterol Motil. 2000;12:149–154. doi: 10.1046/j.1365-2982.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- 62.Tack J, Broekaert D, Corsetti M, Fischler B, Janssens J. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther. 2006;23:265–274. doi: 10.1111/j.1365-2036.2006.02724.x. [DOI] [PubMed] [Google Scholar]
- 63.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 64.Heils A, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 65.Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 66.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang YM, et al. Serotonin transporter gene promoter region polymorphisms and serotonin transporter expression in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol Motil. 2012;24:560–565. doi: 10.1111/j.1365-2982.2012.01902.x. e254-5. [DOI] [PubMed] [Google Scholar]
- 68.Jarrett ME, et al. Relationship of SERT polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol Res Nurs. 2007;9:161–169. doi: 10.1177/1099800407307822. [DOI] [PubMed] [Google Scholar]
- 69.Kohen R, et al. The serotonin transporter polymorphism rs25531 is associated with irritable bowel syndrome. Dig Dis Sci. 2009;54:2663–2670. doi: 10.1007/s10620-008-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol Chemother. 1958;13:444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bulbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol. 1959;146:29–53. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bulbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- 74.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 75.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- 76.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil. 2004;16:511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 77.Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001;108:1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- 79.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bian XC, Bornstein JC, Bertrand PP. Nicotinic transmission at functionally distinct synapses in descending reflex pathways of the rat colon. Neurogastroenterol Motil. 2003;15:161–171. doi: 10.1046/j.1365-2982.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- 81.Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 1996;271:G849–G857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- 82.Haga K, Asano K, Fukuda T, Kobayakawa T. The function of 5-HT3 receptors on colonic transit in rats. Obes Res. 1995;3(Suppl 5):801S–810S. doi: 10.1002/j.1550-8528.1995.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 83.Talley NJ, et al. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci. 1990;35:477–480. doi: 10.1007/BF01536922. [DOI] [PubMed] [Google Scholar]
- 84.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827–G831. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- 85.Grider JR. Desensitization of the peristaltic reflex induced by mucosal stimulation with the selective 5-HT4 agonist tegaserod. Am J Physiol Gastrointest Liver Physiol. 2006;290:G319–G327. doi: 10.1152/ajpgi.00326.2005. [DOI] [PubMed] [Google Scholar]
- 86.Li Z, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659, 670. doi: 10.1053/j.gastro.2009.09.020. 670 e1-2. [DOI] [PubMed] [Google Scholar]
- 88.Spencer NJ, et al. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol. 2011;301:G519–G527. doi: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- 89.Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin (5-HT) and cholecystokinin (CCK) mediate nutrient induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- 90.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 91.Cooke HJ, Sidhu M, Wang YZ. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Motil. 1997;9:181–186. doi: 10.1046/j.1365-2982.1997.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 92.Sidhu M, Cooke HJ. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. Am J Physiol. 1995;269:G346–G351. doi: 10.1152/ajpgi.1995.269.3.G346. [DOI] [PubMed] [Google Scholar]
- 93.Townsend Dt, Casey MA, Brown DR. Mediation of neurogenic ion transport by acetylcholine, prostanoids and 5-hydroxytryptamine in porcine ileum. Eur J Pharmacol. 2005;519:285–289. doi: 10.1016/j.ejphar.2005.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown DR. Mucosal protection through active intestinal secretion: neural and paracrine modulation by 5-hydroxytryptamine. Behav Brain Res. 1996;73:193–197. doi: 10.1016/0166-4328(96)00095-2. [DOI] [PubMed] [Google Scholar]
- 95.Safsten B, Sjoblom M, Flemstrom G. Serotonin increases protective duodenal bicarbonate secretion via enteric ganglia and a 5-HT4-dependent pathway. Scand J Gastroenterol. 2006;41:1279–1289. doi: 10.1080/00365520600641480. [DOI] [PubMed] [Google Scholar]
- 96.Vanner S, Macnaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):39–43. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 97.Cooke HJ, Sidhu M, Wang YZ. Activation of 5-HT1P receptors on submucosal afferents subsequently triggers VIP neurons and chloride secretion in the guinea-pig colon. J Auton Nerv Syst. 1997;66:105–110. doi: 10.1016/s0165-1838(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 98.Sjoqvist A, Cassuto J, Jodal M, Lundgren O. Actions of serotonin antagonists on cholera-toxin-induced intestinal fluid secretion. Acta Physiol Scand. 1992;145:229–237. doi: 10.1111/j.1748-1716.1992.tb09360.x. [DOI] [PubMed] [Google Scholar]
- 99.Lundgren O. 5-Hydroxytryptamine, enterotoxins, and intestinal fluid secretion. Gastroenterology. 1998;115:1009–1012. doi: 10.1016/s0016-5085(98)70275-6. [DOI] [PubMed] [Google Scholar]
- 100.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sorensson J, Jodal M, Lundgren O. Involvement of nerves and calcium channels in the intestinal response to Clostridium difficile toxin A: an experimental study in rats in vivo. Gut. 2001;49:56–65. doi: 10.1136/gut.49.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kordasti S, Sjovall H, Lundgren O, Svensson L. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut. 2004;53:952–957. doi: 10.1136/gut.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brookes SJ, Steele PA, Costa M. Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res. 1991;263:471–481. doi: 10.1007/BF00327280. [DOI] [PubMed] [Google Scholar]
- 104.Galligan JJ, Costa M, Furness JB. Changes in surviving nerve fibers associated with submucosal arteries following extrinsic denervation of the small intestine. Cell Tissue Res. 1988;253:647–656. doi: 10.1007/BF00219756. [DOI] [PubMed] [Google Scholar]
- 105.Vanner S. Myenteric neurons activate submucosal vasodilator neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G380–G387. doi: 10.1152/ajpgi.2000.279.2.G380. [DOI] [PubMed] [Google Scholar]
- 106.Vanner S, Jiang MM, Surprenant A. Mucosal stimulation evokes vasodilation in submucosal arterioles by neuronal and nonneuronal mechanisms. Am J Physiol. 1993;264:G202–G212. doi: 10.1152/ajpgi.1993.264.2.G202. [DOI] [PubMed] [Google Scholar]
- 107.Reed DE, Vanner SJ. Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol. 2003;553:911–924. doi: 10.1113/jphysiol.2003.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- 109.Paintal AS. A method of locating the receptors of visceral afferent fibres. J Physiol. 1954;124:166–172. doi: 10.1113/jphysiol.1954.sp005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paintal AS. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature. 1953;172:1194–1195. doi: 10.1038/1721194a0. [DOI] [PubMed] [Google Scholar]
- 111.Glatzle J, et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 112.Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol. 1998;509(Pt 3):717–727. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Round A, Wallis DI. Further studies on the blockade of 5-HT depolarizations of rabbit vagal afferent and sympathetic ganglion cells by MDL 72222 and other antagonists. Neuropharmacology. 1987;26:39–48. doi: 10.1016/0028-3908(87)90042-6. [DOI] [PubMed] [Google Scholar]
- 114.Ireland SJ, Tyers MB. Pharmacological characterization of 5-hydroxytryptamine-induced depolarization of the rat isolated vagus nerve. Br J Pharmacol. 1987;90:229–238. doi: 10.1111/j.1476-5381.1987.tb16844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raybould HE, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- 118.Savastano DM, Covasa M. Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors. Physiol Behav. 2007;92:434–442. doi: 10.1016/j.physbeh.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 119.Janssen P, Vos R, Van Oudenhove L, Tack J. Influence of the 5-HT3 receptor antagonist ondansetron on gastric sensorimotor function and nutrient tolerance in healthy volunteers. Neurogastroenterol Motil. 2011;23:444–449. doi: 10.1111/j.1365-2982.2010.01655.x. e175. [DOI] [PubMed] [Google Scholar]
- 120.Hillsley K, Grundy D. Plasticity in the mesenteric afferent response to cisplatin following vagotomy in the rat. J Auton Nerv Syst. 1999;76:93–98. doi: 10.1016/s0165-1838(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 121.Gale JD. Serotonergic mediation of vomiting. J Pediatr Gastroenterol Nutr. 1995;21(Suppl 1):S22–S28. doi: 10.1097/00005176-199501001-00008. [DOI] [PubMed] [Google Scholar]
- 122.Tyers MB, Freeman AJ. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology. 1992;49:263–268. doi: 10.1159/000227054. [DOI] [PubMed] [Google Scholar]
- 123.Hillsley K, Grundy D. Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci Lett. 1998;255:63–66. doi: 10.1016/s0304-3940(98)00690-9. [DOI] [PubMed] [Google Scholar]
- 124.Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hicks GA, et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002;544:861–869. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coldwell JR, Phillis BD, Sutherland K, Howarth GS, Blackshaw LA. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol. 2007;579:203–213. doi: 10.1113/jphysiol.2006.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsumoto K, et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab Invest. 2012;92:769–782. doi: 10.1038/labinvest.2012.14. [DOI] [PubMed] [Google Scholar]
- 128.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417. doi: 10.1053/j.gastro.2012.05.007. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wouters MM, et al. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 131.Du P, et al. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT(2B) knockout mice with altered interstitial cells of Cajal networks. Biophys J. 2010;98:1772–1781. doi: 10.1016/j.bpj.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil. 2007;19(Suppl 2):19–24. doi: 10.1111/j.1365-2982.2007.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takaki M, et al. In vitro enhanced differentiation of neural networks in ES gut-like organ from mouse ES cells by a 5-HT4-receptor activation. Biochem Biophys Res Commun. 2011;406:529–533. doi: 10.1016/j.bbrc.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 135.Katsui R, et al. A new possibility for repairing the anal dysfunction by promoting regeneration of the reflex pathways in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1084–G1093. doi: 10.1152/ajpgi.00345.2007. [DOI] [PubMed] [Google Scholar]
- 136.Matsuyoshi H, et al. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil. 2010;22:806–813. doi: 10.1111/j.1365-2982.2010.01474.x. e226. [DOI] [PubMed] [Google Scholar]
- 137.Haub S, et al. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil. 2010;22:826–834. doi: 10.1111/j.1365-2982.2010.01479.x. e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bischoff SC, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–G695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 139.Ghia JE, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 140.Li N, et al. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bertaccini G. Tissue 5-hydroxytryptamine and urinary 5-hydroxyindoleacetic acid after partial or total removal of the gastro-intestinal tract in the rat. J Physiol. 1960;153:239–249. doi: 10.1113/jphysiol.1960.sp006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Erspamer V, Testini A. Observations on the release and turnover rate of 5-hydroxytryptamine in the gastrointestinal tract. J Pharm Pharmacol. 1959;11:618–623. doi: 10.1111/j.2042-7158.1959.tb12603.x. [DOI] [PubMed] [Google Scholar]
- 143.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yadav VK, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Durk T, et al. Production of Serotonin by Tryptophan Hydroxylase 1 and Release via Platelets Contribute to Allergic Airway Inflammation. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201208-1440OC. [DOI] [PubMed] [Google Scholar]
- 147.Amireault P, Sibon D, Cote F. Life without Peripheral Serotonin: Insights from Tryptophan Hydroxylase 1 Knockout Mice Reveal the Existence of Paracrine/Autocrine Serotonergic Networks. ACS Chem Neurosci. 2013;4:64–71. doi: 10.1021/cn300154j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chabbi-Achengli Y, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2012;109:2567–2572. doi: 10.1073/pnas.1117792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsuda M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 150.Bonnin A, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 152.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 153.Costall B, Domeney AM, Naylor RJ, Tattersall FD. 5-Hydroxytryptamine M-receptor antagonism to prevent cisplatin-induced emesis. Neuropharmacology. 1986;25:959–961. doi: 10.1016/0028-3908(86)90030-4. [DOI] [PubMed] [Google Scholar]
- 154.Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol. 1986;88:497–9. doi: 10.1111/j.1476-5381.1986.tb10228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Talley NJ. Review article, 5-hydroxytryptamine agonists and antagonists in the modulation of gastrointestinal motility and sensation: clinical implications. Aliment Pharmacol Ther. 1992;6:273–289. doi: 10.1111/j.1365-2036.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 156.Andresen V, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert Opin Pharmacother. 2003;4:2089–2098. doi: 10.1517/14656566.4.11.2089. [DOI] [PubMed] [Google Scholar]
- 159.Choung RS, et al. A novel partial 5HT3 agonist DDP733 after a standard refluxogenic meal reduces reflux events: a randomized, double-blind, placebo-controlled pharmacodynamic study. Aliment Pharmacol Ther. 2008;27:404–411. doi: 10.1111/j.1365-2036.2007.03591.x. [DOI] [PubMed] [Google Scholar]
- 160.Evangelista S. Drug evaluation: Pumosetrag for the treatment of irritable bowel syndrome and gastroesophageal reflux disease. Curr Opin Investig Drugs. 2007;8:416–422. [PubMed] [Google Scholar]
- 161.Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007:CD003960. doi: 10.1002/14651858.CD003960.pub3. [DOI] [PubMed] [Google Scholar]
- 162.McCallum RW, Prakash C, Campoli-Richards DM, Goa KL. Cisapride. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1988;36:652–681. doi: 10.2165/00003495-198836060-00002. [DOI] [PubMed] [Google Scholar]
- 163.Degen L, Petrig C, Studer D, Schroller S, Beglinger C. Effect of tegaserod on gut transit in male and female subjects. Neurogastroenterol Motil. 2005;17:821–826. doi: 10.1111/j.1365-2982.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 164.Baeyens R, Reyntjens A, Verlinden M. Cisapride accelerates gastric emptying and mouth-to-caecum transit of a barium meal. Eur J Clin Pharmacol. 1984;27:315–318. doi: 10.1007/BF00542167. [DOI] [PubMed] [Google Scholar]
- 165.Fernandez AG, Massingham R. Peripheral receptor populations involved in the regulation of gastrointestinal motility and the pharmacological actions of metoclopramide-like drugs. Life Sci. 1985;36:1–14. doi: 10.1016/0024-3205(85)90280-2. [DOI] [PubMed] [Google Scholar]
- 166.Pfeuffer-Friederich I, Kilbinger H. In: Proceedings of the Ninth International Symposium of GI Motility. Roman C, editor. Lancaster, UK: MTP Press; 1984. pp. 527–534. [Google Scholar]
- 167.Craig DA, Clarke DE. Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine receptor. J Pharmacol Exp Ther. 1990;252:1378–1386. [PubMed] [Google Scholar]
- 168.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 169.Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989;96:1257–1264. doi: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 170.Galligan JJ, Pan H, Messori E. Signalling mechanism coupled to 5-hydroxytryptamine4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus. Neurogastroenterol Motil. 2003;15:523–529. doi: 10.1046/j.1365-2982.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 171.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266:G230–G238. doi: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- 172.Fang X, et al. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil. 2008;20:80–93. doi: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 173.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–G1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 174.Hoffman JM, McKnight ND, Sharkey KA, Mawe GM. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil. 2011;23:673–e279. doi: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- 175.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 176.Tack J, et al. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745–767. doi: 10.1111/j.1365-2036.2012.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Foxx-Orenstein AE, Jin JG, Grider JR. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am J Physiol. 1998;275:G979–G983. doi: 10.1152/ajpgi.1998.275.5.G979. [DOI] [PubMed] [Google Scholar]
- 178.Sabate JM, et al. Sensory signalling effects of tegaserod in patients with irritable bowel syndrome with constipation. Neurogastroenterol Motil. 2008;20:134–141. doi: 10.1111/j.1365-2982.2007.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Greenwood-Van Meerveld B, Venkova K, Hicks G, Dennis E, Crowell MD. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol Motil. 2006;18:76–86. doi: 10.1111/j.1365-2982.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 180.Greenwood-Van Meerveld B, Campbell-Dittmeyer K, Johnson AC, Hicks GA. 5-HT2B receptors do not modulate sensitivity to colonic distension in rats with acute colorectal hypersensitivity. Neurogastroenterol Motil. 2006;18:343–345. doi: 10.1111/j.1365-2982.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 181.Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil. 2002;14:221–227. doi: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 182.Camilleri M. LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil. 2011;23:193–200. doi: 10.1111/j.1365-2982.2010.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brown PM, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Q, et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 185.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 186.Galligan JJ. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurons. Behav Brain Res. 1996;73:199–201. doi: 10.1016/0166-4328(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 187.Galligan JJ, North RA. Opioid, 5-HT1A and alpha 2 receptors localized to subsets of guinea-pig myenteric neurons. J Auton Nerv Syst. 1991;32:1–11. doi: 10.1016/0165-1838(91)90229-v. [DOI] [PubMed] [Google Scholar]
- 188.Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol. 1996;364:439–455. doi: 10.1002/(SICI)1096-9861(19960115)364:3<439::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 189.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tack JF, Janssens J, Vantrappen G, Wood JD. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol. 1992;263:G838–G846. doi: 10.1152/ajpgi.1992.263.6.G838. [DOI] [PubMed] [Google Scholar]
- 191.Tack J. The physiology and the pathophysiology of the gastric accommodation reflex in man. Verh K Acad Geneeskd Belg. 2000;62:183–207. discussion 207-10. [PubMed] [Google Scholar]
- 192.Borman RA, et al. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol. 2002;135:1144–1151. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. 5-HT(2A) receptors: location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877–G893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 194.Tharayil VS, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–469. doi: 10.1111/j.1365-2982.2009.01435.x. e109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tonini M, et al. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology. 2005;129:1557–1566. doi: 10.1053/j.gastro.2005.08.005. [DOI] [PubMed] [Google Scholar]


