SUMMARY
World Health Organization-classified very severe pneumonia due to Pneumocystis jirovecii infection is recognized as a life-threatening condition in human immunodeficiency virus (HIV) infected infants. We recount the use of nasal bubble continuous positive airway pressure (BCPAP) in an HIV-infected African infant with very severe pneumonia and treatment failure due to suspected infection with P. jirovecii. We also examine the potential implications of BCPAP use in resource-poor settings with a high case index of acute respiratory failure due to HIV-related pneumonia, but limited access to mechanical ventilation.
Keywords: HIV, Pneumocystis jirovecii pneumonia, BCPAP, sub-Saharan Africa, early infant diagnosis
AMONG human immunodeficiency virus (HIV) infected African children, the World Health Organization (WHO) classification of very severe pneumonia due to Pneumocystis jirovecii infection is common,1 a major cause of mortality2 and highly predictive of death,3 despite treatment recommendations of high-dose cotrimoxazole, prednisolone, broad-spectrum antibiotics and supplemental oxygen in both confirmed and suspected P. jirovecii cases.4,5 We report the use of nasal bubble continuous positive airway pressure (BCPAP) in the management of an HIV-infected Malawian infant with very severe pneumonia and treatment failure due to probable P. jirovecii.5
CASE REPORT
A 3-month-old full-term male with unknown HIV status presented to a Malawian referral hospital with fever, cough and difficulty in breathing. Cough began 9 days prior to presentation, accompanied by progressively labored respiration and subjective fever for 2 days. The mother detailed the infant to be without rhinorrhea, breastfeeding well and with normal urine and stool patterns. She disclosed that she was HIV-infected but had not received prophylaxis for prevention of mother-to-child transmission of HIV or anti-retroviral therapy (ART). No further pertinent past medical history, including sick contacts suspicious for tuberculosis, was described.
On examination, the infant’s weight for height percentile was 100%, the axillary temperature was 38.1°C, pulse and respiratory rates were respectively 188 and 86/min, and transcutanous oxygen saturation (SpO2) was 74% in room air. The patient appeared well-hydrated but ill, with nasal flaring and severe lower chest wall indrawing. White plaques were observed on the oral mucosa, chest auscultation was clear bilaterally and no further cardiovascular abnormalities were reported, with the exception of tachycardia.
No malaria parasites were observed on blood film, the serum blood sugar was 6.4 mmol/dl and a chest radiograph demonstrated bilateral patchy infiltrates (Figure 1). The infant’s rapid HIV antibody test returned positive and, pending a definitive HIV DNA polymerase chain reaction (PCR) test, he was classified as presumptively HIV-infected.6 Blood culture, sputum induction, Mantoux testing and blood gas analysis were not routinely available, and the results from a complete blood count were lost. Admission diagnoses of thrush and very severe pneumonia were made,5 and the child’s SpO2 improved to 92% after commencing 21 of supplemental oxygen from an oxygen concentrator.
Figure 1.
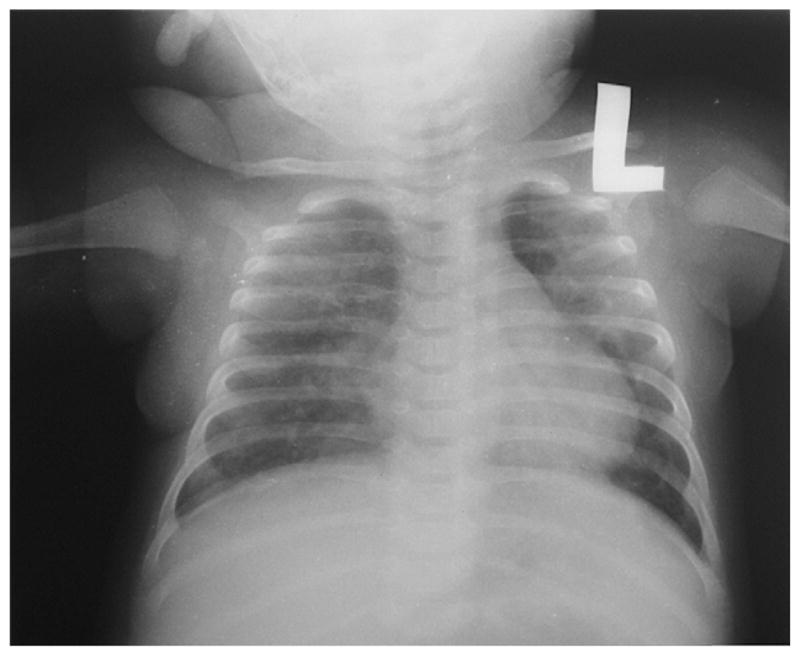
Anteroposterior radiograph of the chest demonstrating bilateral patchy infiltrates.
P. jirovecii was suspected due to the presumed presence of HIV infection, young age, lack of cotrimoxazole preventive therapy, as well as severe hypoxia, patchy bilateral infiltrates on chest imaging and respiratory distress in the context of clear chest auscultation.5 Laboratory confirmation of P. jirovecii infection was not available. High-dose oral cotrimoxazole, oral prednisolone, oral nystatin, intravenous benzylpenicillin and intravenous gentamycin treatment were commenced according to WHO-recommended dosing and frequency.5
On day 3 of hospitalization, the infant met WHO-defined treatment failure due to persistent low-grade fevers, more labored breathing pattern and an SpO2 of 78%, despite oxygen therapy.5 A repeat chest radiograph demonstrated worsening disease, and the patient was transferred to a five-bed pediatric high dependency unit staffed with BCPAP-trained nurses. Under pediatrician supervision, BCPAP was initiated, as mechanical ventilation was unavailable. Intravenous ceftriaxone 80 mg/kg/day was also substituted for benzylpenicillin and gentamycin, and an orogastric tube and intravenous fluids were started.
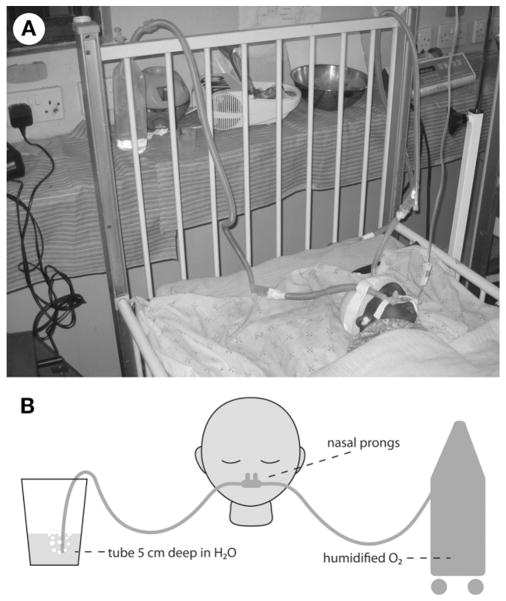
The BCPAP set-up utilized size 4 Hudson® nasal prongs (Hudson RCI, Research Triangle Park, NC, USA), affixed so there was no oral air-leak, and humidified oxygen from an Airsep® oxygen concentrator (Airsep Corp, Buffalo, NY, USA) to achieve a positive-end expiratory pressure (PEEP) of 5 cm of water (Figure 2). The infant’s SpO2 increased to 96%. After 7 days of continuous BCPAP without sedation or adjustment of the PEEP level, the infant was successfully weaned to room air. Following the completion of 10 days of ceftriaxone and 14 days of nystatin, the patient was discharged on hospital day 14 to finish 21 days of cotrimoxazole treatment accompanied by a prednisolone taper. The child was then initiated on ART at a local HIV clinic after the HIV DNA PCR returned positive and the CD4 cell count was noted to be 8%.
Figure 2.
Photograph (A) and diagram (B) of nasal bubble continuous positive airway pressure circuit.
DISCUSSION
We report the case of an infant with HIV-related pneumonia and progressive respiratory failure despite oxygen supplementation in which the administration of BCPAP appears to have provided benefit by increasing the infant’s SpO2 from 78% to above 90%. Although the diagnosis of P. jirovecii was not confirmed, this case illustrates BCPAP as a simple method of respiratory support that may be both feasible and beneficial for HIV-infected infants with acute respiratory failure, irrespective of etiology, in resource-poor settings without access to mechanical ventilation.
In industrialized countries, the optimal care for HIV-infected infants with respiratory failure due to P. jirovecii includes mechanical ventilation.7 Conversely, in developing countries where the burden of HIV-infected infants with respiratory failure due to P. jirovecii is highest, mechanical ventilation is not widely used due to prohibitive costs and insufficient technical expertise.
BCPAP is a respiratory support strategy that has been successfully utilized as an alternative to mechanical ventilation in preterm, low birth weight infants with respiratory failure in industrialized countries.8 The positive pressure in BCPAP is provided throughout the respiratory cycle to a spontaneously breathing infant. Within the closed BCPAP respiratory circuit, the expiratory end of ventilation tubing is inserted beneath a column of water to a depth equivalent to the desired pressure, which is usually 5–10 cm of water.8 BCPAP also creates bubbling in the water column that produces high frequency vibrations of the infant’s chest that may improve gas exchange.8 Compared to mechanical ventilation, BCPAP requires little technical expertise, can be successfully managed by nurses, is relatively inexpensive and has low complication rates in infants.9 Although Koyamaibole et al. demonstrated in Fiji that nurse-administered BCPAP in a neonatal cohort reduced the need for mechanical ventilation by more than 50%,9 no similar studies have been conducted in a high HIV-prevalent region or at the district hospital level.
While we have experienced promising results using BCPAP for subsequent cases of very severe pneumonia and progressive respiratory failure in HIV-infected infants, this case report highlights the urgent need for further, more rigorous evaluation of the feasibility and benefit of BCPAP in the case management of pediatric HIV-related pneumonia in resource-poor settings.
Acknowledgments
The authors offer their gratitude to the child and his family described in this case report, as well as to all of their patients that they respectfully serve. They also thank their colleagues and partners, including Columbia University Medical Center, New York, United States, and World Altering Medicine, New York, United States, for the donation of the BCPAP equipment and oxygen concentrators, respectively.
This work was sponsored in part by the National Institutes of Health (R24 TW007988) through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University. The funders had no role in the decision to publish or the preparation of the manuscript.
References
- 1.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necroscopy in African children dying from respiratory illnesses: a descriptive necroscopy study. Lancet. 2002;360:985–990. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 2.Graham SM, Mtitimila EI, Kamanga HS, Walsh AL, Hart CA, Molyneux ME. Clinical presentation and outcome of Pneumocystis carinii pneumonia in Malawian children. Lancet. 2000;355:369–373. doi: 10.1016/S0140-6736(98)11074-7. [DOI] [PubMed] [Google Scholar]
- 3.Zar HJ, Dechaboon A, Hanslo D, Apolles P, Magnus KG, Hussey G. Pneumocystis carinii pneumonia in South African children infected with human immunodeficiency virus. Pediatr Infect Dis J. 2000;19:603–607. doi: 10.1097/00006454-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 4.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva, Switzerland: WHO; 2005. [Accessed September 2010]. http://whqlibdoc.who.int/publications/2005/9241546700.pdf. [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Geneva, Switzerland: WHO; 2010. [Accessed September 2010]. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed] [Google Scholar]
- 7.Wong HR, Chundu KR. Improved outcome for young children with AIDS, Pneumocystis carinii pneumonia, and acute respiratory failure. Pediatr Pulmonol. 1994;18:114–118. doi: 10.1002/ppul.1950180211. [DOI] [PubMed] [Google Scholar]
- 8.Polin RA, Sahni R. Newer experience with CPAP. Semin Neonatol. 2002;7:379–389. doi: 10.1053/siny.2002.0132. [DOI] [PubMed] [Google Scholar]
- 9.Koyamaibole L, Kado J, Qovu JD, Colquhoun S, Duke T. An evaluation of bubble-CPAP in a neonatal unit in a developing country: effective respiratory support that can be applied by nurses. J Trop Pediatr. 2006;52:249–253. doi: 10.1093/tropej/fmi109. [DOI] [PubMed] [Google Scholar]