Abstract
Background
Data are available indicating an independent inverse relationship of dietary vegetable protein to the blood pressure (BP) of individuals. Here we assess whether BP is associated with glutamic acid intake (the predominant dietary amino acid, especially in vegetable protein) and with each of four other amino acids higher relatively in vegetable than animal protein (proline, phenylalanine, serine, cystine).
Methods and Results
Cross-sectional epidemiological study; 4,680 persons ages 40–59 -- 17 random population samples in China, Japan, U.K., U.S.A.; BP measurement 8 times at 4 visits; dietary data (83 nutrients, 18 amino acids) from 4 standardized multi-pass 24-hour dietary recalls and 2 timed 24-hour urine collections. Dietary glutamic acid (percent of total protein intake) was inversely related to BP. Across multivariate regression models (Model 1 controlled for age, gender, sample, through Model 5 controlled for 16 non-nutrient and nutrient possible confounders) estimated average BP differences associated with glutamic acid intake higher by 4.72% total dietary protein (2 s.d.) were −1.5 to −3.0 mm Hg systolic and −1.0 to −1.6 mm Hg diastolic (Z-values −2.15 to −5.11). Results were similar for the glutamic acid-BP relationship with each other amino acid also in the model, e.g., with control for 15 variables plus proline, systolic/diastolic pressure differences −2.7/−2.0 (Z −2.51, −2.82). In these 2-amino acid models, higher intake (2 s.d.) of each other amino acid was associated with small BP differences and Z-values.
Conclusions
Dietary glutamic acid may have independent BP lowering effects, possibly contributing to the inverse relation of vegetable protein to BP.
Keywords: dietary amino acids, glutamic acid, blood pressure, population study
INTRODUCTION
The population-based International Study on Macro/Micronutrients and Blood Pressure (INTERMAP) found a significant inverse relation of vegetable protein intake to blood pressure (BP) for individuals [1]. Among predominantly vegetable compared to animal protein consumers, intake of glutamic acid -- the most common dietary amino acid -- made up a higher percent of total protein, as did (to a lesser degree) cystine, proline, phenylalanine, and serine. We therefore hypothesized that the higher the intake of these five amino acids – and in particular glutamic acid – the lower the BP. Results are presented here.
METHODS
Basic Premises, Population Samples, Field Methods (1996–1999)
Basic INTERMAP premises are: multiple nutrients have small independent influences on BP of individuals that in combination summate as sizable -- clinically relevant -- effects. To detect impact of single nutrients on BP of individuals, standardized high-quality data are needed on large population samples. Accordingly, INTERMAP surveyed 4,680 men and women ages 40–59 from 17 population random samples in Japan (four samples), People’s Republic of China (PRC, three), United Kingdom (UK, two), United States (USA, eight) [2]. Participants were selected randomly from community or workplace population lists, arrayed into four age/gender strata. Staff were trained and certified by senior colleagues based on a common protocol. Each participant attended four times, visits 1 and 2 on consecutive days, visits 3 and 4 on consecutive days on average 3 weeks later. BP was measured twice/visit with a random zero sphygmomanometer and averaged. Measurements of height, weight, and data on daily alcohol consumption over the previous seven days were obtained at two visits. Dietary data were collected at each visit by multi-pass 24-hr recall [2,3]. All foods, drinks, supplements consumed in the previous 24 hours were recorded. For PRC and USA participants, monosodium glutamate (MSG, 66% and 46% glutamic acid respectively) was quantitated [4]; for Japan and UK participants MSG use was negligible and was not quantitated. Questionnaire data were obtained on demographic, biomedical, and other possible confounders. Each participant provided two 24-hour urine collections, start and end timed at the research center (visits 1–2 and 3–4); measurements included urinary volume, sodium, potassium, creatinine, and urea nitrogen (biomarker of total protein intake) [3,5]; 8% of specimens were split locally and sent blind to the Central Laboratory to estimate technical error [2].
Individuals were excluded because: did not attend all four visits; diet data considered unreliable; energy intake from any 24-hour dietary recall below 2,092 or greater than 20,920 kJ/day for women, 33,472 kJ/day for men; two urine collections not available; other data incomplete or indicated protocol violation (total 215 people). For each exclusion an alternative participant was recruited. The study received institutional ethics committee approval for each site; all participants gave written informed consent.
Statistical Methods
Food data of individuals were converted into nutrients (83 nutrients including 18 amino acids) with use of country-specific tables on nutrient composition of foods, updated and standardized across countries by the Nutrition Coordinating Center, University of Minnesota [2,6]. For nutrients supplying energy, intake was calculated as percent total energy; for others, as intake/1,000 kJ; also as amounts/24 hours; for amino acids, also as percent of total protein intake. Main food groups supplying each amino acid were assessed. Urinary values/24 hours were calculated as products of urinary concentrations and volumes standardized to 24 hours. Measurements/person were averaged, for BP and nutrients, across the four visits; for urinary excretions, across the two collections. For descriptive statistics, means and standard deviations (s.d.), numbers and percentages were calculated by country and study-wide. Reliability of BP and amino acid intakes (mean of four visits) was estimated from the formula 1/[1+(ratio/4)]×100, where the ratio is intra-individual variance/inter-individual variance, estimated separately for 8 gender/country strata and pooled by weighting each stratum-specific estimate by (sample size minus one). This gives a first approximation of effect of random error (day-to-day variability) on reliability of amino acid associations with BP; the statistic is estimated size of an observed coefficient as percent of theoretical coefficient in univariate regression analysis [7–10].
Associations among nutrients were explored by partial correlation, adjusted for sample, age, gender; pooled across countries, weighted by sample size. Multiple regression analyses were used to examine relationships of each of the five dietary amino acids (grams/day, % kJ, % total protein) to systolic and diastolic BP (SBP, DBP). Adjustment for confounders was done sequentially with use of 9 models (3 to 15 covariates) (Table 1), without and with height and weight [1,2]. Regression models were fit by country and coefficients pooled across countries, weighted by inverse of variance, to estimate overall association; cross-country heterogeneity of regression coefficients was tested by chi-square; interactions were assessed for age and gender; departures from linearity tested with quadratic terms. Regression coefficients were expressed as mm Hg for two s.d. difference in amino acid intake, from pooled within-country s.d. weighted by sample size. Statistical significance is presented as Z-values (Z-value=regression coefficient/standard error); equivalent p-values are in table footnotes. Sensitivity analyses were also done (Tables 1, S.6. – S.8.); including censored normal regression to adjust for potential antihypertensive treatment bias [11]. Adjusted mean SBP and DBP by country-specific quartiles of glutamic acid (% total protein), were calculated by ANOVA and plotted.
Table 1.
Estimated Mean Difference in Blood Pressure, Glutamic Acid Intake (% Total Protein) from Foods Higher by 2 s.d., Multiple Regression Analyses
| Model | Systolic BP | Diastolic BP | ||
|---|---|---|---|---|
| Difference, mm Hg | (Z-value) | Difference, mm Hg | (Z-value) | |
| Main Analyses – All 4,680 Participants | ||||
| 1 | −3.03* | (−5.11) | −1.44 | (−3.67) |
| 2 | −2.78* | (−4.73) | −1.39 | (−3.58) |
| 3 | −2.08* | (−3.51) | −1.02 | (−2.60) |
| 4 | −1.94 | (−2.95) | −1.19 | (−2.73) |
| 5a - P | −2.43 | (−3.59) | −1.48 | (−3.28) |
| 5b - Mg | −2.50 | (−3.66) | −1.63 | (−3.57) |
| 5c - Ca | −1.48 | (−2.21) | −0.96 | (−2.15) |
| 5d - Fe | −1.81 | (−2.73) | −1.15 | (−2.59) |
| 5e - Fiber | −1.95 | (−2.93) | −1.21 | (−2.72) |
| Sensitivity Analyses | ||||
| Adjusted also for Education (years), and Current Smoking (yes/no) – N=4,680 | ||||
| 4 | −1.88 | (−2.86) | −1.23 | (−2.81) |
| 5b - Mg | −2.46 | (−3.59) | −1.68 | (−3.67) |
| Adjusted also for Month of Field Survey – N=4,680 | ||||
| 4 | −1.95 | (−2.96) | −1.21 | (−2.77) |
| 5b - Mg | −2.52 | (−3.67) | −1.64 | (−3.56) |
| Adjusted also for Season of Field Survey – N=4,680 | ||||
| 4 | −1.93 | (−2.93) | −1.19 | (−2.74) |
| 5b - Mg | −2.49 | (−3.64) | −1.63 | (−3.56) |
| Adjusted also for Total Energy (kJ/day) – (N=4,680) | ||||
| 4 | −1.96 | (−2.98) | −1.19 | (−2.72) |
| 5b - Mg | −2.53 | (−3.70) | −1.64 | (−3.58) |
| Adjusted for Urinary Na/Creatinine and K/Creatinine Ratio (mmol/mmol) instead of Urinary Na and Urinary K (mmol/24-h) – (N=4,680) | ||||
| 4 | −1.99 | (−3.05) | −1.20 | (−2.76) |
| 5b - Mg | −2.54 | (−3.72) | −1.60 | (−3.49) |
| Adjusted also for Total Available Carbohydrate (% kJ) – (N=4,680) | ||||
| 4 | −1.74 | (−2.56) | −1.19* | (−2.64) |
| 5b - Mg | −2.07 | (−2.91) | −1.57 | (−3.30) |
| Adjusted also for Starch (% kJ) – (N=4,680) | ||||
| 4 | −1.50 | (−2.12) | −0.81 | (−1.73) |
| 5b - Mg | −2.27 | (−3.07) | −1.35 | (−2.71) |
| Censored normal regression adjusting for antihypertensive treatment – (N=4,680) | ||||
| 4 | −2.29 | (−3.10) | −1.40 | (−2.89) |
| 5b - Mg | −2.82 | (−3.66) | −1.81 | (−3.55) |
| Nonhypertensive Persons – (N=3,671) | ||||
| 4 | −1.20 | (−2.20) | −0.76* | (−1.88) |
| 5b - Mg | −1.65 | (−2.86) | −1.17 | (−2.75) |
| Excluding Persons with High Day-to-day Variability in Nutrient Intake and/or BP – (N=3,473) | ||||
| 4 | −2.45 | (−3.06) | −1.28 | (−2.42) |
| 5b - Mg | −2.83 | (−3.43) | −1.65 | (−2.99) |
| Glutamic Acid Expressed as grams/day (instead of % Total Protein) – (N=4,680) | ||||
| 4 + Total Energy (kJ/day) | −1.62 | (−1.96) | −1.05 | (−1.86) |
| 5b - Mg + Total Energy (kJ/day) | −0.18 | (−0.19) | −0.45 | (−0.74) |
| 4 + Total Energy + Height + Weight | −1.87 | (−2.36) | −1.21 | (−2.23) |
| 5b - Mg + Total Energy + Height + Weight | −1.48 | (−1.70) | −1.24 | (−2.10) |
| Glutamic Acid Expressed as % kJ (instead of % Total Protein) – (N=4,680) | ||||
| 4 | −1.06 | (−2.33) | −0.66 | (−2.15) |
| 5b - Mg | −0.22 | (−0.44) | −0.35 | (−1.02) |
| 4 + Height + Weight | −1.08 | (−2.48) | −0.66 | (−2.22) |
| 5b - Mg + Height + Weight | −0.86 | (−1.76) | −0.72 | (−2.17) |
Model 1: Controlled for Sample, Age, Gender
Model 2: Model 1 Variables + Special Diet (Yes/No), Supplement Intake (Yes/No), CVD-DM Diagnosis (Yes/No), Physical Activity (Medium + Heavy, hours/day), Family History of High BP (Yes, No or Unknown)
Model 3: Model 2 Variables + Urinary Na and Urinary K (mmol/24-h), 14-day Alcohol (grams/day)
Model 4: Model 3 Variables + Cholesterol (mg/1,000 kJ), Total SFA and Total PFA (% kJ)
Model 5a–5e, Main Analyses: Controlled for Model 4 variables + each stipulated nutrient (expressed per 1,000 kJ)
Sensitivity Analyses: controlled for Model 4 variables + each stipulated variable, or variables in Model 5b - Mg + each stipulated variable Month of field survey: mid-point between first and fourth clinic visit. Season of field survey: Winter = December/January/February; Spring = March/April/May; Summer = June/July/August; Fall = September/October/November
Z-value=regression coefficient/standard error; Z-value ≥ 1.96: uncorrected p ≤0.05; ≥ 2.58: uncorrected p ≤ 0.01; ≥3.29: uncorrected p ≤ 0.001
2 s.d. higher glutamic acid intake for % Total Protein – 4.72%; for grams/day – 9.60; for % Total Kilocalories – 1.00%
Test for cross-country heterogeneity significant, p <0.05.
Analyses were with SAS version 9.1 by Ian J. Brown and Queenie Chan. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
RESULTS
Descriptive Statistics
Multiple characteristics of the study population samples are provided in supplemental online Table S.1. Mean SBP ranged from 117.2 (Japan) to 121.3 mm Hg (PRC), mean DBP from 73.2 (PRC) to 77.3 mm Hg (UK). Consistently, glutamic acid was the predominant dietary amino acid, averaging for all 4,680 INTERMAP participants 15.7 grams/day, 3.0% kJ, 20.1% total protein. As grams/day and % kJ, these values were higher for persons from UK/USA than for those from Japan/PRC; as % total protein, glutamic acid intake was highest for PRC participants (24.1%), lowest for Japanese (17.8%) (for UK/USA, 20.5%/19.8%). Only 2% of women and men reported use of dietary supplements containing glutamic acid; intake from supplements and from foods plus supplements among supplement users averaged 0.5 grams/day and 16.4 grams/day.
Univariate estimates of reliability of glutamic acid intake, based on mean values from the four 24-hour recalls/participant, were: 68.1% of theoretical coefficient (grams/day), 60.7% (% kJ), 60.6% (% total protein) (Table S.2.), similar for men and women; across the four countries; and for the four other amino acids. BP reliability estimates were 94.3% (SBP) and 93.0% (DBP), high across all eight gender/country subgroups.
Partial Correlations
Expressed as grams/day or % kJ, partial correlations (adjusted for sample, age, gender) were high order (+0.83 to +0.90) for glutamic acid with proline, phenylalanine, serine, cystine (Tables S.3., S.4.), smaller for amino acids expressed as % total dietary protein-- +0.37 to +0.47 except for glutamic acid with proline (+0.80) (Table S.5.). Glutamic acid expressed as grams/day and as % kJ was positively correlated with dietary calcium, copper, iron, magnesium, phosphorus, selenium (partial r +0.21 copper to +0.62 phosphorus) (Tables S.3., S.4.); with glutamic acid expressed as % total protein, partial r values with these micronutrients (expressed as caloric density) were small, range +0.09 (Ca) to −0.10 (Mg and Se) except for phosphorus (−0.18) (Table S.5.). Glutamic acid as % total protein was positively correlated with total carbohydrate (+0.33) and starch (+0.39); inversely with alcohol (−0.17 to −0.19). Partial r data were similar for the four other amino acids.
Multiple Regression Analyses -- Glutamic Acid and BP
The glutamic acid-BP relation was stronger expressed as % total protein than as grams/day or % kJ. With glutamic acid intake (% total protein) from food higher by 2 s.d. (+4.72% total protein), in multivariate controlled models (Models 4–5e, Table 1), average SBP was lower by 1.5 to 2.5 mm Hg (Z-value −2.21 to −3.66); average DBP, by 1.0 to 1.6 mm Hg (Z −2.15 to −3.57). Results were qualitatively similar, with BP differences smaller, in corresponding analyses including height and weight) -- e.g., Model 5b-Mg, SBP lower by 1.8 mm Hg (Z −2.73); DBP, by 1.2 mm Hg (Z −2.70) (data not tabulated). Compared to Model 4 coefficients, those adjusted also for phosphorus or magnesium (5a-P, 5b-Mg) were larger for both SBP and DBP; those for iron or fiber (5d-Fe, 5e-fiber) were similar to Model 4; those for calcium (5c-Ca) were lower, particularly for SBP. With adjustment for vegetable protein, associations of glutamic acid and BP remained qualitatively similar; BP differences and Z-values quantitatively weaker (data not tabulated).
Sensitivity analyses yielded results similar to the foregoing, including for nonhypertensive persons, and adjusted for antihypertensive treatment, seasonality, and additional socio-demographic characteristics (smoking and education) (Table 1). BP differences and Z-values were largest with exclusion of persons with high day-to-day variability in nutrient intake and/or BP. Tests for age/gender interaction and quadratic nonlinearity constantly yielded non-significant results; most cross-country heterogeneity tests were non-significant. Despite no significant interaction terms, the inverse relation of glutamic acid to SBP was stronger for women than men; also, in Models 4, 5a–e, stronger in those ages 50–59 than 40–49 years (Tables S.10., S.11.).
All analyses yielded almost identical results with the independent variable glutamic acid from foods plus supplements (data not tabulated).
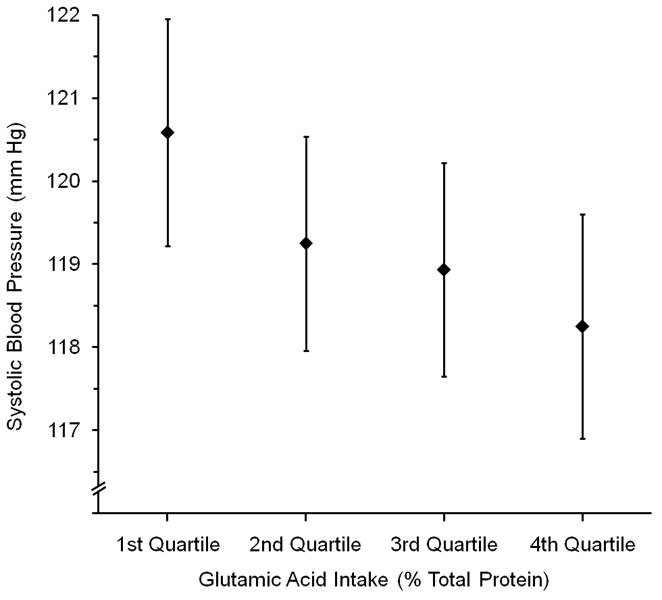
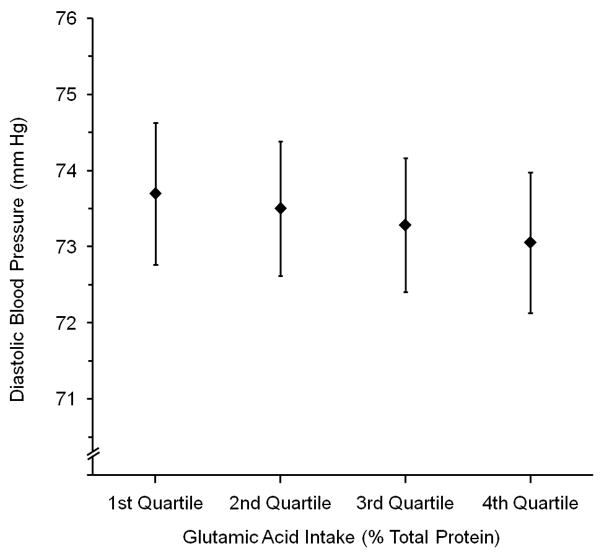
Figure 1 demonstrates successively lower mean SBP and DBP across quartiles 1 to 4 of country-specific glutamic acid intake, controlled for Model 5b-Mg covariates (P for Trend = <0.001 for SBP, 0.12 for DBP).
Figure 1.
Mean (a) Systolic and (b) Diastolic Blood Pressure (mm Hg) by Country-specific Quartiles of Glutamic Acid Intake (% Total Protein)*, Adjusted for Model 5b - Mg Covariates†, For All 4,680 Participants. Whiskers are 99% Confidence Intervals. P for Trend: for (a), <0.001; for (b), P =0.12
* Country-specific quartile cut-offs for glutamic acid intake (% Total Protein) were: for Japan, 16.8 (25th percentile), 17.6 (50th percentile), 18.6 (75th percentile); for PRC, 19.4, 25.1, 27.4); for UK, 19.4, 20.3, 21.5; for USA, 18.6, 19.7, 20.9
† Estimated by analysis of variance, overall (coefficients not pooled by country). Adjusted for Country (not sample), Age, Gender, Special Diet, Supplement Intake, CVD-DM Diagnosis, Physical Activity, Family History of High BP, Urinary Na, Urinary K, 14-Day Alcohol, Cholesterol, Total SFA, Total PFA, Magnesium (see Table 1 footnote for units)
Proline, phenylalanine, and serine (but not cystine) related to BP in a qualitatively similar way, with BP differences and Z-values smaller (Tables S.6. – S.8).
In multivariate models including glutamic acid and one other of the four amino acids (2 s.d. higher, % total protein), glutamic acid intake was associated with SBP 2.0 to 2.9 mm Hg lower, DBP 1.2 to 2.0 mm Hg lower (Z −2.32 to −3.63) (Table 2); For each other amino acid in these analyses, BP differences and Z-values were low order. With height and weight also in these regressions, BP differences and Z-values for the glutamic acid-BP relation were −1.4 to −2.2 mm Hg SBP and −0.8 to −1.7 mm Hg DBP (Z −1.65 to −2.44) (data not tabulated). Sensitivity analyses for these 2-amino acid assessments yielded findings generally similar to those in Table 2 (Table S.9.). The relation was generally less strong with amino acids expressed as grams/day or % kJ.
Table 2.
Estimated Mean Difference in Blood Pressure, Amino Acid Intake (% Total Protein) from Foods Higher by 2 s.d., Two Amino Acids in Same Model, Model 5b - Mg, All Participants (N=4,680)
| Variable (2 s.d.) | Systolic Blood Pressure | Diastolic Blood Pressure | ||
|---|---|---|---|---|
| Difference mm Hg | (Z-value) | Difference mm Hg | (Z-value) | |
| Glutamic Acid (4.72) | −2.72 | (−2.51) | −2.00 | (−2.82) |
| Proline (2.54) | +0.30 | (+0.26) | +0.55* | (+0.72) |
| Glutamic Acid (4.72) | −1.99 | (−2.48) | −1.24 | (−2.32) |
| Phenylalanine (0.35) | −0.72 | (−1.32) | −0.63 | (−1.71) |
| Glutamic Acid (4.72) | −2.27 | (−2.84) | −1.37 | (−2.58) |
| Serine (0.45) | −0.51 | (−0.96) | −0.40 | (−1.12) |
| Glutamic Acid (4.72) | −2.88 | (−3.63) | −1.56 | (−2.95) |
| Cystine (0.27) | +0.57 | (+1.00) | −0.09* | (−0.25) |
Model 5b - Mg: Controlled for Sample, Age, Gender, Special Diet, Supplement Intake, CVD-DM Diagnosis, Physical Activity, Family History of High BP, Urinary Na, Urinary K, 14-Day Alcohol, Cholesterol, Total SFA, Total PFA, Magnesium (see Table 1 footnote for units).
Z-value=regression coefficient/standard error; Z-value ≥ 1.96: uncorrected p ≤ 0.05; ≥2.58: uncorrected p ≤ 0.01; ≥ 3.29: uncorrected p ≤ 0.001.
Cross-country heterogeneity significant, p <0.05.
Results were nonsignificant for tests of age/gender interaction and quadratic nonlinearity with these 2-amino acid models, as were most tests for cross-country heterogeneity for the glutamic acid-BP relation (data not tabulated).
Food Sources of Glutamic Acid
Seven food groups -- four vegetable, three animal -- supplied most (83.6%; vegetable 41.9%, animal 41.7%) of the glutamic acid (Table 3, rows 2–5 and rows 9–11).
Table 3.
Food Groups Supplying Most Dietary Glutamic Acid, by Country
| Food Group | Japan (N=1,145) | PRC (N=839) | UK (N=501) | USA (N=2,195) | All (N=4,680) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | 14.19 g/d | 100.0% | 14.97 g/d | 100.0% | 16.83 g/d | 100.0% | 16.54 g/d | 100.0% | 15.72 g/d | 100.0% |
| Pasta, Rice, Noodles* | 3.03 | 21.3 | 3.01 | 20.2 | 0.31 | 1.8 | 0.85 | 5.1 | 1.71 | 10.9 |
| Bread, Rolls, Biscuits* | 1.06 | 7.5 | 0.15 | 1.0 | 4.19 | 24.9 | 2.22 | 13.4 | 1.77 | 11.3 |
| Vegetables, Beans | 0.96 | 6.8 | 1.47 | 9.8 | 1.21 | 7.2 | 1.10 | 6.6 | 1.14 | 7.3 |
| Grains, Flour, Cereals | 0.13 | 0.9 | 6.30 | 42.1 | 0.66 | 3.9 | 1.50 | 9.0 | 1.94 | 12.4 |
| Vegetarian Meat Substitutes | 1.07 | 7.5 | 0.33 | 2.2 | 0.04 | 0.2 | 0.11 | 0.6 | 0.37 | 2.4 |
| Cakes, Puddings, Cookies, Sweet Snacks* | 0.37 | 2.6 | 0.99 | 6.6 | 1.07 | 6.4 | 0.39 | 2.4 | 0.56 | 3.6 |
| Nuts, Seeds | 0.12 | 0.8 | 0.27 | 1.8 | 0.13 | 0.8 | 0.33 | 2.0 | 0.25 | 1.6 |
| Fish, Shellfish | 3.20 | 22.6 | 0.36 | 2.4 | 0.64 | 3.8 | 0.69 | 4.2 | 1.24 | 7.9 |
| Meat | 1.91 | 13.5 | 1.25 | 8.4 | 4.59 | 27.3 | 5.09 | 30.8 | 3.57 | 22.7 |
| Milk, Cheese, Dairy† | 0.87 | 6.2 | 0.03 | 0.2 | 2.76 | 16.4 | 2.63 | 15.9 | 1.75 | 11.1 |
| Eggs | 0.61 | 4.3 | 0.31 | 2.1 | 0.24 | 1.4 | 0.43 | 2.6 | 0.44 | 2.8 |
| Sum – Vegetable (Rows 2–8) | 6.73 | 47.4 | 11.53 | 77.1 | 7.60 | 45.20 | 6.48 | 39.2 | 7.76 | 49.4 |
| Sum – Animal (Rows 9–12) | 6.60 | 46.5 | 1.95 | 13.0 | 8.22 | 48.9 | 8.85 | 53.5 | 6.99 | 44.5 |
May include small quantities of glutamic acid of animal origin, e.g., from egg white.
Does not include ice cream.
DISCUSSION
Our main finding was a consistent inverse relationship of glutamic acid intake (% total protein) to BP, prevailing in repeated regression models with control for multiple confounders, non-dietary and dietary (including variables previously demonstrated to relate significantly and independently to BP (Na, K, alcohol intake, weight adjusted for height) [12]; also for intake of each of four other amino acids more common in vegetable than animal protein. It prevailed for women and men; for those ages 40–49 and 50–59; across four countries; for nonhypertensive persons; with control for month or season of dietary survey, socio-demographic characteristics, and was strongest with exclusion of individuals manifesting marked intra-individual variability in nutrient intake or BP -- results concordant with the tentative inference that dietary glutamic acid may have an etiologically significant favorable effect on BP of individuals. This novel finding novel needs replication in other populations and in trials.
Of 18 dietary amino acids quantitated, glutamic acid intake was consistently by far the most common. For predominantly vegetable versus predominantly animal protein consumers, glutamic acid constituted 23% versus 18% of total protein intake. Thus, given the previous INTERMAP finding of an independent inverse relation of vegetable protein intake to BP [1], it was an expected result that this most common amino acid (especially in vegetable protein) would be inversely related to BP.
As far as we know, this is the first paper on the relation of glutamic acid (or proline, phenylalanine, serine, cystine) intake to BP. Thus, earlier literature reporting lower BP in vegetarian than omnivorous populations did not deal with specific nutrients [13], and more recent papers -- from observational studies or controlled trials -- did not report on glutamic acid or the other four amino acids predominant in vegetable protein [1]. In the two DASH and the OMNIHEART feeding trials, dietary protein – particularly vegetable protein – was increased, hence also glutamic acid [14–16], but this modification was an overall one, so that no inference is possible as to individual nutrients producing BP reduction with the DASH/OMNIHEART eating pattern. Correspondingly, there are no data on total glutamic acid intake and BP in the 39 papers from a recent international symposium on glutamic acid [17]. The only related information is from small (N 11 to 52) short-term randomized controlled trials dealing with MSG [18–22], amount ranging from 1.5g tablet given with breakfast to 12g given after overnight fast, without effects on BP. In three east Asian studies [23–25], inverse relations were reported to SBP of urinary ratio of sulphate to urea (index of intake of sulfur-containing amino acids from animal protein); also of serum phenylalanine and serine; also overnight urinary cysteine; also 24-h urinary 3-methylhistidine (marker for animal protein intake). These papers reported no dietary-BP data, nor data on the five amino acids considered here.
Glutamate has been characterized as “an amino acid of particular distinction… an abundant biomolecule [with] involvement in multiple metabolic processes that play major roles…” [26]. Therefore, multiple mechanisms can be invoked as possibly accounting for a favorable effect of dietary glutamic acid on BP, e.g.: Oxidized in the intestinal tissues, it serves as an energy yielding or glutathione substitute [26]. Glutathione in its redox state can counteract oxidative injury from free radicals [27], and can enhance hypotensive effects of nitric oxide [28]. Dietary glutamate may also be a substrate for arginine [29], a precursor of nitric oxide and potent vasodilator [30]. Glutamate is an excitatory neurotransmitter; areas of the brain most sensitive to increased plasma glutamate -- potentially from dietary intake -- are those relatively unprotected by the blood-brain barrier, notably the hypothalamus, linking the nervous system to the endocrine system via the pituitary gland [26,31]. Glutamate excitation of hypothalamus neurons could affect vasoactive hormone production, though findings in human studies to date are negative [32]. Another possible pathway for favorable BP influence of higher glutamate intake is enhanced kidney size and function [33–35].
The inverse relation between dietary glutamic acid and BP is one of several independent associations of nutrients with BP found by the INTERMAP Study (as expected) [1,36–39]. The relation between glutamic acid and BP is stronger with glutamic acid expressed as % total protein, compared to its expression as grams/day or % kJ. This may be because glutamic acid expressed as % total protein correlates much less strongly with other variables possibly confounding than glutamic acid expressed as grams/day or % kJ. Compared to Model 4, glutamic acid-BP associations were larger in models adjusted also for phosphorus or magnesium, similar in models for iron or fiber, and smaller in models for calcium. This may reflect the different sign of the correlation between these variables and glutamic acid, but all partial r values are low order, hence any inference is conjectural. Another possibility is chance variation. For all models the glutamic acid-BP relationship remains qualitatively the same, i.e., inverse with all Z-values greater than 2.
Bias towards the null of exposure-BP associations induced by reduced BPs of treated hypertensive participants is a concern for all studies including such individuals [11]. Glutamic acid-BP associations were quantitatively similar in models adjusted for antihypertensive treatment effect compared to main analyses, indicating that bias of this kind is not substantial.
Limitations of our findings include: their cross-sectional nature, but they are the only population-based data available; effect size underestimation due to limited reliability in nutrient measurement (regression-dilution bias), despite multiple standardized state-of-the-art measurements; ability to control only partially (albeit considerably) for high-order collinearity among dietary variables of concern, less of a problem in analyses with amino acids expressed as percent of total dietary protein than as grams/day or percent total kilocalories; limited generalizability to persons younger than 40 and older than 59 years; apparent small effect size. This last limitation, anticipated by INTERMAP [2], must be kept in perspective: with “small” independent influences of multiple nutrients [1,36–39], combined effects become substantial, i.e., improved nutrition is capable of preventing/reducing unfavorable BP levels for most people, as DASH and OMNIHEART feeding trial findings demonstrate [14–16]. Also, long-term BP effects of habitual eating patterns, from childhood into middle age, may be greater, as data on salt intake and BP indicate [12]. Moreover, reduction of population average SBP by small amounts, e.g., 2 mm Hg, is estimated to result in mortality rates lower by 6% for stroke and 4% for coronary heart disease [12,40]. Finally, eating patterns based mainly on foods with predominantly vegetable protein -- high in glutamic acid, ω-3/ω-6 PFA, Ca, Mg, P, Fe, and other micronutrients, low/moderate in fats/saturated fats/cholesterol/refined sugars/caloric density, and in salt/alcohol, have multiple favorable influences -- on BP, serum lipids, cardiovascular disease risk, and general health.
In conclusion, we recorded an independent inverse relation of dietary glutamic acid to BP with control for multiple possible confounders. Glutamic acid -- the most common dietary amino acid, especially in vegetable protein -- may be a key component accounting for the previously reported inverse relation of vegetable protein intake to BP.
Supplementary Material
Acknowledgments
We thank all INTERMAP staff at local, national, and international centres for their invaluable efforts; a partial listing of these colleagues is given in Reference 2 of this article. We dedicate this paper to the memory of Vernon R. Young, world renowned amino acid investigator, whose exchange with the senior author years ago was seminal in setting the stage for the research reported here.
FUNDING SOURCES
Supported by grant 2-ROI-HL50490 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and by the National Institutes of Health Office on Dietary Supplements (Bethesda, Md); and by national agencies in China, Japan (the Ministry of Education, Science, Sports, and Culture, Grant-in-Aid for Scientific Research [A]. No. 090357003), and the United Kingdom.
Footnotes
DISCLOSURES
None.
References
- 1.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B for the INTERMAP Cooperative Research Group. Association between protein intake and blood pressure. The INTERMAP Study. Arch Intern Med. 2006;166:79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou B for the INTERMAP Research Group. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary) J Hum Hypertens. 2003;17:591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, Okayama A, Okuda N, Robertson C, Robinson F, Schakel S, Stevens M, Van Heel N, Zhao LC, Zhou BF for the INTERMAP Research Group. INTERMAP: the dietary data--process and quality control. J Hum Hypertens. 2003;17:609–622. doi: 10.1038/sj.jhh.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He K, Zhao L, Daviglus ML, Dyer AR, Van Horn L, Garside D, Zhu L, Guo D, Wu Y, Zhou B, Stamler J for the INTERMAP Cooperative Research Group. Association of monosodium glutamate intake with overweight in Chinese adults: The INTERMAP Study. Obesity. 2008;16:1875–1880. doi: 10.1038/oby.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer A, Elliott P, Chee D, Stamler J. Urinary biochemical markers of dietary intake in the INTERSALT Study. Am J Clin Nutr. 1997;65(suppl):1246S–1253S. doi: 10.1093/ajcn/65.4.1246S. [DOI] [PubMed] [Google Scholar]
- 6.Schakel SF, Dennis B, Wold AC, Conway R, Zhao L, Okuda N, Okayama A, Moag-Stahlberg A, Robertson C, Van Heel N, Buzzard IM, Stamler J. Enhancing data on nutrient composition of foods eaten by participants in the INTERMAP study in China, Japan, the United Kingdom, and the United States. J Food Comp Anal. 2003;16:395–408. doi: 10.1016/S0889-1575(03)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandits GA, Bartsch GE, Stamler J. Chapter 4. Method issues in dietary data analyses in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65(suppl):211S–227S. doi: 10.1093/ajcn/65.1.211S. [DOI] [PubMed] [Google Scholar]
- 8.Dyer AR, Shipley M, Elliott P for the INTERSALT Cooperative Research Group. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. I. Estimates of reliability. Am J Epidemiol. 1994;139:927–939. doi: 10.1093/oxfordjournals.aje.a117099. [DOI] [PubMed] [Google Scholar]
- 9.Dyer AR, Elliott P, Shipley M for the INTERSALT Cooperative Research Group. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. Am J Epidemiol. 1994;139:940–951. doi: 10.1093/oxfordjournals.aje.a117100. [DOI] [PubMed] [Google Scholar]
- 10.Dyer AR, Liu K, Sempos CT. 5. Nutrient data analysis techniques and strategies. In: Berdanier CD, Dwyer J, Feldman EB, editors. Handbook of Nutrition and Food. 2. Boca Raton, F.L; CRC Press LLC: 2005. pp. 93–103. [Google Scholar]
- 11.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 12.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65(suppl):626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol. 1974;100:390–398. doi: 10.1093/oxfordjournals.aje.a112050. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N for the DASH Collaborative Research Group. A clinical trial on the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 15.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH for the DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM for the OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. Results of the OmniHeart Randomized Trial. J Am Med Assoc. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 17.Fernstrom JD, Garattini S. International Symposium on Glutamate. J Nutr. 2000;130(suppl):891S–1079S. doi: 10.1093/jn/130.4.891S. [DOI] [PubMed] [Google Scholar]
- 18.Morselli PL, Garattini S. Monosodium glutamate and the Chinese restaurant syndrome. Nature. 1970;227:611–612. doi: 10.1038/227611a0. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblum I, Bradley JD, Coulston F. Single and double blind studies with oral monosodium glutamate in man. Toxicol Appl Pharmacol. 1971;18:367–373. doi: 10.1016/0041-008x(71)90129-3. [DOI] [PubMed] [Google Scholar]
- 20.Zanda G, Franciosi P, Tognoni G, Rizzo M, Standen SM, Morselli PL, Garattini S. A double blind study on the effects of monosodium glutamate in man. Biomedicine. 1973;19:202–204. [PubMed] [Google Scholar]
- 21.Yang WH, Drouin MA, Herbert M, Mao Y, Karsh J. The monosodium glutamate symptom complex: Assessment in a double-blind, placebo-controlled, randomized study. J Allergy Clin Immunol. 1997;99:757–762. doi: 10.1016/s0091-6749(97)80008-5. [DOI] [PubMed] [Google Scholar]
- 22.Prawirohardjono W, Dwiprahasto I, Astuti I, Hadiwandowo S, Kristin E, Muhammad M, Kelly MF. The administration to Indonesians of monosodium L-glutamate in Indonesian foods: An assessment of adverse reactions in a randomized double-blind, crossover, placebo-controlled study. J Nutr. 2000;130(suppl):1074S–1076S. doi: 10.1093/jn/130.4.1074S. [DOI] [PubMed] [Google Scholar]
- 23.Yamori Y, Kihara M, Nara Y, Ohtaka M, Horie R, Tsunematsu T, Note S. Hypertension and diet: multiple regression analysis in a Japanese farming community. Lancet. 1981;1(8231):1204–1205. doi: 10.1016/s0140-6736(81)92363-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Zhang X, Zhu A, Zhao L, Zhu S, Ruan L, Zhu L, Liang S. The relationship of dietary animal protein and electrolytes to blood pressure: a study on three Chinese populations. Int J Epidemiol. 1994;23:716–722. doi: 10.1093/ije/23.4.716. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Ikeda K, Yamori Y for the WHO-CARDIAC Study Group. Inverse relationship between urinary markers of animal protein intake and blood pressure in Chinese: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Int J Epidemiol. 2002;31:227–233. doi: 10.1093/ije/31.1.227. [DOI] [PubMed] [Google Scholar]
- 26.Young VR, Ajami AM. Glutamate: An amino acid of particular distinction. J Nutr. 2000;130(suppl):892S–900S. doi: 10.1093/jn/130.4.892S. [DOI] [PubMed] [Google Scholar]
- 27.Beutler E. Nutritional and metabolic aspects of glutathione. Ann Rev Nutr. 1989;9:287–302. doi: 10.1146/annurev.nu.09.070189.001443. [DOI] [PubMed] [Google Scholar]
- 28.Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999;34:507–514. doi: 10.1016/s0735-1097(99)00216-8. [DOI] [PubMed] [Google Scholar]
- 29.Reeds PJ, Burrin DG, Stoll B, Jahoor F. Intestinal glutamate metabolism. J Nutr. 2000;130:978S–782S. doi: 10.1093/jn/130.4.978S. [DOI] [PubMed] [Google Scholar]
- 30.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic–pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46:489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board, National Academy of Sciences. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C: National Academies Press; 2005. 10. Protein and Amino Acids; pp. 589–768. [Google Scholar]
- 32.Fernstrom JD. Pituitary hormone secretion in normal male humans: Acute responses to a large, oral dose of monosodium glutamate. J Nutr. 2000;130:1053S–1057S. doi: 10.1093/jn/130.4.1053S. [DOI] [PubMed] [Google Scholar]
- 33.Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press; 1951. [Google Scholar]
- 34.Wilson HEC. An investigation of the cause of renal hypertrophy in rats fed on a high protein diet. Biochem J. 1933;27:1348–1356. doi: 10.1042/bj0271348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S. Renal functional reserve in humans: Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75:943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 36.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon M, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, Steffen LM, Van Horn L, Yarnell J, Zhou B for the INTERMAP Research Group. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP Study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J for the INERMAP Cooperative Research Group. Dietary phosphorus and blood pressure. International population study on macronutrients and blood pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, Daviglus ML, Kesteloot H, Okayama A, Curb JD, Rodriguez BL, Elmer PJ, Steffen LM, Robertson C, Zhao L for the International Study of Macro-Micronutrients and Blood Pressure Research Group. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure. Hypertension. 2008;52:408–414. doi: 10.1161/HYPERTENSIONAHA.108.112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzoulaki I, Brown IJ, Chan Q, Van Horn L, Ueshima H, Zhao L, Stamler J, Elliott P, for the International Collaborative Research Group on Macro-/Micronutrients and Blood Pressure Relation of iron and red meat intake to blood pressure: Cross sectional epidemiological study. Brit Med J. 2008;337:a258. doi: 10.1136/bmj.a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT Study findings: public health and medical care implications. Hypertension. 1989;14:570–577. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.