Abstract
Tobacco smoking is the leading preventable cause of death in the United States. A major negative health consequence of chronic smoking is hypertension. Untoward addictive and cardiovascular sequelae associated with chronic smoking are mediated by nicotine-induced activation of nicotinic receptors (nAChRs) within striatal dopaminergic and hypothalamic noradrenergic systems. Hypertension involves both brain and peripheral angiotensin systems. Activation of angiotensin type-1 receptors (AT1) release dopamine and norepinephrine. The current study determined the role of AT1 and angiotensin type-2 (AT2) receptors in mediating nicotine-evoked dopamine and norepinephrine release from striatal and hypothalamic slices, respectively. The potential involvement of nAChRs in mediating effects of AT1 antagonist losartan and AT2 antagonist, 1-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid (PD123319) was evaluated by determining their affinities for α4β2* and α7* nAChRs using [3H]nicotine and [3H]methyllycaconitine binding assays, respectively. Results show that losartan concentration-dependently inhibited nicotine-evoked [3H]dopamine and [3H]norepinephrine release (IC50: 3.9±1.2 and 2.2±0.7 μM; Imax: 82±3 and 89±6%, respectively). In contrast, PD123319 did not alter nicotine-evoked norepinephrine release, and potentiated nicotine-evoked dopamine release. These results indicate that AT1 receptors modulate nicotine-evoked striatal dopamine and hypothalamic norepinephrine release. Furthermore, AT1 receptor activation appears to be counteracted by AT2 receptor activation in striatum. Losartan and PD123319 did not inhibit [3H]nicotine or [3H]methyllycaconitine binding, indicating that these AT1 and AT2 antagonists do not interact with the agonist recognition sites on α4β2* and α7* nAChRs to mediate these effects of nicotine. Thus, angiotensin receptors contribute to the effects of nicotine on dopamine and norepinephrine release in brain regions involved in nicotine reward and hypertension.
Keywords: Angiotensin II receptors, dopamine, nicotinic acetylcholine receptors, norepinephrine, reward, smoking
1. INTRODUCTION
Tobacco smoking is the most preventable cause of death in the United States and is associated with increased cardiovascular disease, stroke, chronic lung disease, lung cancer and other cancers [1]. Nicotine, the most abundant alkaloid in tobacco, has intrinsic rewarding properties that contribute to tobacco dependence [2]. Activation of nicotinic acetylcholine receptors (nAChRs) by nicotine increases extracellular dopamine (DA) concentrations in brain reward circuits, including nucleus accumbens (NAc) and striatum [3, 4]. The NAc mediates primary reward, whereas the striatum is associated with habit formation and compulsivity [5-7]. Importantly, the transition from reward seeking to compulsive behavior associated with drug addiction appears to be mediated by a shift in activity from NAc to striatum [6, 7]. Consistent with this idea, smoking has been shown to be associated with decreased [11C]raclopride (DA D2 receptor antagonist) binding in striatum, but not in NAc in humans, using positron emission tomography [8]. Therefore, hedonic response to nicotine appears to involve DA release in striatum.
Tobacco dependence is a potent risk factor for cardiovascular diseases, including hypertension, atherosclerosis, myocardial infarction and aortic abdominal aneurysm [9, 10]. Specifically, the paraventricular nucleus of the hypothalamus is an important site of integration of neuroendocrine and autonomic responses controlling blood pressure [11]. Microinjection of norepinephrine (NE) into the paraventricular nucleus increases systolic and diastolic blood pressure in rats [12]. Importantly, intraperitoneal and intracerebroventricular injections of nicotine induces NE release in the paraventricular nucleus [13]. Taken together, nicotine-induced hypertension is mediated, at least in part, by hypothalamic NE.
nAChRs are ligand-gated ion channel receptors consisting of homo- or heteropentameric transmembrane proteins with a diverse subunit composition [14, 15]. nAChR subtype diversity in function and pharmacological response are attributed to the specific subunit compositions, including α2-α10 and β2-β4 subunits encoded by individual subunit genes [16]. β2-Containing nAChRs mediate nicotine reward that results from presynaptic DA release following activation of α-conotoxin MII-sensitive nAChRs including α6β2β3*, α4α6β2β3*, α6β2* subtypes and α-conotoxin MII-insensitive α4β2* and α4α5β2* subtypes [17-20]. In accumbens shell, α-conotoxin MII-sensitive nAChRs are critical for nicotine reward [21]. In contrast, nicotine-evoked NE release is mediated by α3β4* nAChRs [22, 23]. Thus, distinct nAChRs subtypes mediate nicotine-evoked DA and NE release, resulting in nicotine reward and hypertension, respectively.
Pathology in the renin-angiotensin system (RAS) is known to underlie some types of cardiovascular diseases [24, 25]. The majority of physiological actions of angiotensin II (Ang II) are mediated by angiotensin type-1 (AT1) receptors, including, vasoconstriction, thirst, activation of the sympathetic nervous system, cellular differentiation and proliferation [26]. Angiotensinogen is the precursor for the RAS system [26, 27]. Importantly, both angiotensinogen mRNA as well as strong glial angiotensinogen immunoreactivity have been demonstrated in striatum and hypothalamus [28, 29]. Furthermore, both striatal and hypothalamic neurons express AT1 and angiotensin type-2 (AT2) receptors [30, 31]. The brain RAS mediates Ang II effects on fluid balance, thirst, blood pressure and cognitive function via AT1 receptors [31, 32]. Ang II activation of AT1 receptors results in release of NE from the hypothalamus [33]. AT1 receptor effects on blood pressure and water intake are counteracted by AT2 receptors [34, 35].
In addition to activation of the noradrenergic system, Ang II also modulates DA function as evidenced by decreases in expression of AT1 and AT2 receptors in the substantia nigra in Parkinson's patients and by Ang II-evoked striatal DA release via AT1 receptor activation [30, 36, 37]. Previous research shows that Ang II (0.1-1 μM ) increased striatal DA released from superfused striatal slices in vitro and that Ang II (1-10 μM) increased striatal DA release into microdialysate in freely moving rats [37]. Losartan, an AT1 antagonist (1μM; administered via the microdialysis probe), inhibited the Ang II (10 μM)-induced increase in extracellular DA [37]. Other work shows that striatal DA levels were decreased following acute subcutaneous administration of losartan (10 mg/kg) [38]. The purpose of the current study was to extend the previous work by determining whether Ang II receptors serve as potential targets for intervention to ameliorate the addictive and cardiovascular effects of nicotine. Specifically, AT1 and AT2 receptor involvement in mediating nicotine-evoked DA and NE release from striatal and hypothalamic slices, respectively, was determined. Also, effects of Ang II-receptor ligands at nAChRs mediating DA and NE release were evaluated.
2. MATERIALS AND METHODS
2.1. Chemicals
Ang II, angiotensin peptides, cytisine, D-glucose, ethylenediaminetetraacetic acid (EDTA), L-ascorbic acid, mecamylamine hydrochloride, nomifensine maleate, pargyline hydrochloride, polyethyleneimine, S-(−)nicotine ditartrate and sodium chloride were purchased from Sigma-Aldrich (St. Louis, MO). [3H]DA (3,4-ethyl-2 [N-3H]dihydroxyphenylethylamine; specific activity, 33.7 Ci/mmol), [3H]NE (levo-1-(3,4-dihydroxy-[ring-2,5,6-3H]phenyl)-2-aminoethanol; specific activity, 14.0 Ci/mmol), [3H]nicotine (L-(−)[N-methyl-3H]; specific activity, 78.4 Ci/mmol) and tissue solubilizer (TS-2) were purchased from PerkinElmer Life Sciences (Boston, MA). [3H]Methyllycaconitine ([1α4(S),6β,14α,16β]-20-ethyl-1,6,14,16-tetramethoxy-4-[[[2-([3-3H]-methyl-2,5-dioxo-1-pyrrolidinyl)benozyl]-oxy]methyl]-aconitane-7,8-diol; specific activity, 25.4 Ci/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Losartan and PD123319 were generous gifts from Merck & Co. Inc. (Whitehouse Station, NJ) and Parke-Davis & Co. (Detroit, MI), respectively. Acetonitrile, calcium chloride, magnesium chloride, methanol, monosodium phosphate, potassium chloride, sodium bicarbonate and trifluoroacetic acid were purchased from Fisher Scientific (Pittsburgh, PA). Sep Pak C18 columns were purchased from Waters (Milford, MA). Ang II polyclonal antibody was purchased from Abcam (Cambridge, MA).
2.2. Animals
Adult male Sprague Dawley rats (200-225 g) were obtained from Harlan Laboratories (Indianapolis, IN). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky. Upon arrival, rats received standard rat chow (Teklad mouse/rat diet, Harlan Laboratories Inc., Indianapolis, IN) and water ad libitum.
2.3. [3H]DA and [3H]NE release assays
Nicotine-evoked [3H]DA and [3H]NE release using superfused striatal and hypothalamic slices, respectively, was determined using previously described methods with minor modifications [39-42]. Briefly, coronal striatal or hypothalamic slices were incubated for 30 min in Krebs' buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 1 mM NaH2PO4, 1.3 mM CaCl2, 11.1 mM glucose, 25 mM NaHCO3, 0.11 mM L-ascorbic acid, and 0.004 mM EDTA, pH 7.4, saturated with 95% O2/5% CO2 at 34°C). Incubation continued for an additional 30 min in buffer containing 0.1 μM [3H]DA or [3H]NE. Then, each slice was transferred to a superfusion chamber and superfused (1 ml/min) with Krebs' buffer containing pargyline (10 μM), a monoamine oxidase inhibitor. Nomifensine (10 μM), a specific dopamine transporter inhibitor, was included only in the superfusion buffer for [3H]DA release assays. After 60 min of superfusion, three samples (5 ml/sample) were collected to determine basal [3H]DA or [3H]NE outflow.
To determine the nicotine concentration response in hypothalamus, superfusion continued in the absence (control) or presence of nicotine (10, 30 and 100 μM) for 45 min. Using a repeated-measures design, each chamber containing a single slice was exposed to one nicotine concentration which remained in the buffer until the end of the experiment.
To evaluate AT1 and AT2 receptor-mediated modulation of nicotine-evoked [3H]DA and [3H]NE release, a within-subject design was employed such that each concentration of antagonist was evaluated in striatum or hypothalamus from each rat, and different groups of rats were employed for each antagonist. Initially, the effect of mecamylamine on nicotine-evoked [3H]DA and [3H]NE release was determined as a positive control, since this drug inhibits all known nAChRs. Subsequently, effects of losartan and PD123319, AT1 and AT2 receptor antagonists [43, 44], respectively, on nicotine-evoked [3H]DA and [3H]NE release were determined. Following collection of basal samples, superfusion continued for 45 min in the absence (control) or presence of mecamylamine (0.1-10 μM), losartan (1-30 μM) or PD123319 (0.01-10 μM), in order to determine intrinsic effects of the antagonists alone on basal [3H]DA and [3H]NE release. Subsequently, nicotine (10 μM for [3H]DA release assays; 100 μM for [3H]NE release assays) was added to the buffer and superfusate samples continued to be collected further for 45 min to determine inhibition of nicotine-evoked [3H]DA and [3H]NE overflow. Each superfusion chamber containing a single slice was exposed to only one concentration of mecamylamine, losartan or PD123319, which remained in the buffer until the end of the experiment. At the end of the experiment, each slice was solubilized with TS-2 tissue solubilizer. Radioactivity in the superfusate samples and slices was determined by liquid scintillation spectrometry (Model B1600TR, Perkin Elmer Inc., Downers Grove, IL).
2.4. [3H]Nicotine and [3H]methyllycaconitine binding assays
The interaction of losartan and PD123319 with α4μ2* and α7* nAChRs was evaluated in [3H]nicotine and [3H]methyllycaconitine binding assays, respectively, using a previously described method [45]. Membrane suspensions (100-140 μg protein/100 μl) were prepared from whole brain (excluding cortex and cerebellum) and incubated for 60 min (250 μl final volume) at room temperature in tubes containing losartan (1 nM-1 mM) or PD123319 (1 nM-1 mM) with either 3 nM [3H]nicotine or 3 nM [3H]methyllycaconitine. Nonspecific binding was determined in the presence of 10 μM cytisine for the [3H]nicotine binding assay and 10 μM nicotine for the [3H]methyllycaconitine binding assay. Reactions were terminated by addition of ice-cold buffer and rapid filtration through Whatman GF/B glass fiber filters presoaked in 0.5% polyethylenimine. Bound radioactivity was determined using liquid scintillation spectrometry (TopCount NXT; PerkinElmer Inc.).
2.5. Electrically-evoked Ang II release
Coronal striatal slices were transferred to superfusion chambers and superfused (1 ml/min) for 60 min with Krebs’ buffer. Three samples (5 min intervals) were collected to determine basal Ang II outflow. Slices were electrically stimulated (30 volts, 3 msec duration, 5 Hz, 5 min) and 12 samples were collected to determine stimulation-evoked Ang II overflow. Superfusates from striatal slices were partially purified over Sep Pak C18 columns (changed every 30 minutes) preequilibrated with 4 ml methanol, 4 ml water, and 10 ml of Krebs’ buffer. After washing columns with 10 ml of 0.1% trifluoroacetic acid, angiotensin peptides were eluted with 2 ml of 90% acetonitrile and 0.1% trifluoroacetic acid, followed by a second elution with 2 ml of 67% methanol, 33% acetonitrile, and 0.1% trifluoroacetic acid. Eluate was evaporated using vacuum centrifugation and reconstituted in radioimmunoassay buffer. Ang II was quantified using a polyclonal Ang II antibody as previously described [46]. This same method was employed to quantify Ang II content in striatal tissue homogenates.
2.6. Ang II-evoked [3H]NE release from hypothalamus
Coronal slices from rat hypothalamus were incubated with [3H]NE (0.01 μM) for 30 min. Slices were transferred to superfusion chambers and superfused (1 ml/min) for 60 min. Three samples (5 min intervals) were collected to determine basal [3H]NE outflow. Slices were electrically stimulated (S1, 30 volts, 3 msec duration, 5Hz, 5 min) in the absence of Ang II, and 6 samples were collected to determine stimulation-evoked [3H]NE overflow. Slices were superfused with buffer only, or buffer containing Ang II (1 nM–1 μM) for 30 min, and then, the electrical stimulus was repeated (S2). The ratio S2/S1 evoked [3H]NE overflow was calculated. In separate studies, the same experimental design was employed to determine if losartan inhibited evoked [3H]NE overflow from superfused hypothalamic slices pre-incubated with [3H]NE. Radioactivity in the superfusate samples and slices was determined by liquid scintillation spectrometry.
2.7. Data analysis
Data are presented as mean values ± S.E.M., and ‘n’ represents the number of animals tested in each experiment. Data were analyzed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA) and SPSS version 9.0 (SPSS Science, Chicago, IL) software. Fractional [3H]DA and [3H]NE release were calculated by dividing the amount of [3H] in each superfusate sample by the tissue-[3H] at the time of sample collection; data are expressed as a percentage of basal. Basal [3H]DA outflow was determined from the three samples collected prior to antagonist addition to the superfusion buffer. Nicotine-evoked total [3H]DA and [3H]NE overflow were calculated by summing the increases in fractional release above basal for an equivalent period of exposure to nicotine. Concentration- and time-dependent effects of the antagonists on nicotine-evoked [3H]DA and [3H]NE release were determined using two-way repeated measures ANOVA, with concentration and time as within-subject factors. One-way ANOVAs were used to determine concentration-dependent effects of the antagonists on total [3H]DA and [3H]NE release and concentration dependent effects of Ang II and losartan on [3H]NE release. Dunnett's post hoc analyses were employed for subsequent comparisons of treatment samples to control. Nicotine and antagonist concentration-response data were fit by nonlinear least-squares regression using a variable slope, sigmoidal function. IC50 values and values for percent maximal inhibition (Imax) were determined. Student's t-test compared log IC50 values for mecamylamine inhibition of nicotine-evoked [3H]DA and [3H]NE release as well as basal and electrically-evoked Ang II release. For the binding assays, specific [3H]nicotine and [3H]methyllycaconitine binding were determined by subtracting nonspecific binding from total binding. Inhibition constants (Ki values) were determined using the Cheng-Prusoff equation [47].
RESULTS
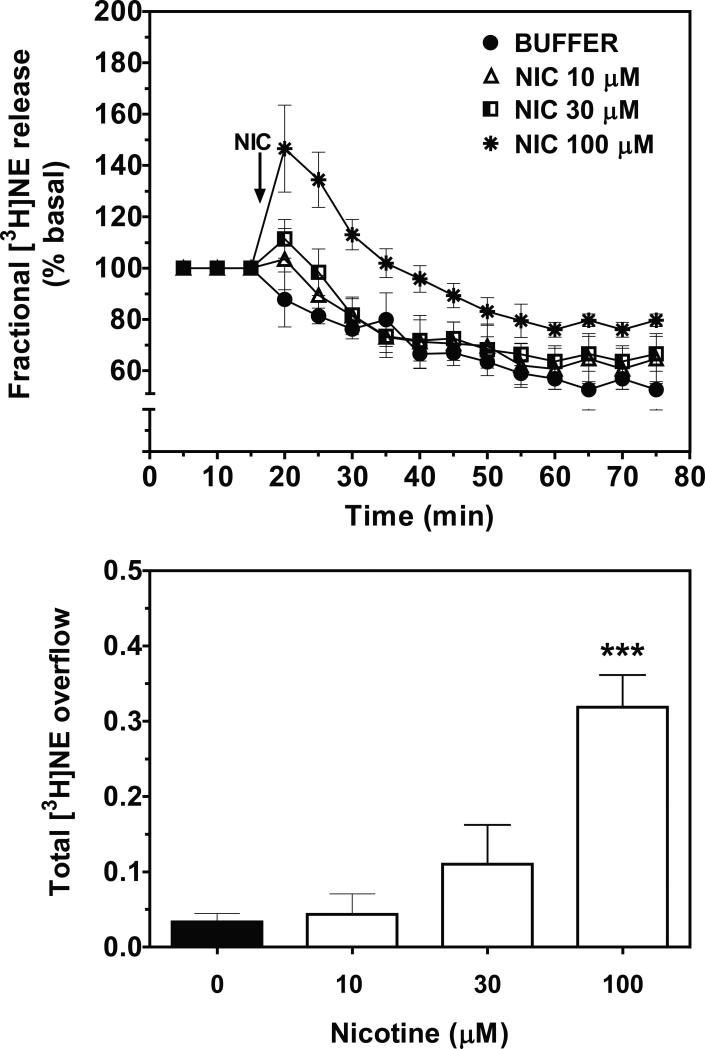
3.1. In a concentration-dependent manner, nicotine evoked [3H]NE overflow from superfused rat hypothalamic slices
Concentration- and time-dependent effects of nicotine on [3H]NE overflow from superfused rat hypothalamic slices are illustrated in Fig. 1. Two-way ANOVA revealed a concentration × time interaction (F33, 176 = 1.73, p < 0.05; Fig 1, top). Across the time-course of the experiment, fractional [3H]NE release increased following nicotine addition to the buffer, peaked 5 min after nicotine addition, and then declined towards basal over time despite the continued presence of nicotine in the buffer. The pattern of nicotine-evoked fractional [3H]NE release was similar for each of the nicotine concentrations (10, 30 and 100 μM) evaluated, although the peak effect was dependent on concentration. Of the concentrations evaluated, only the highest nicotine concentration (100 μM) evoked a significant increase in total [3H]NE overflow compared to the buffer control (F3, 20 = 17.32, p < 0.001; Fig.1, bottom).
Figure 1.
Nicotine (NIC) stimulates fractional [3H]NE release (top) and total [3H]NE overflow (bottom) from superfused rat hypothalamic slices in a time-and concentration-dependent manner. Arrow indicates the time point at which NIC was added to the superfusion buffer. Data are expressed as mean ± S.E.M. Fractional release data are expressed as a percentage of basal. Basal fractional [3H]NE release was 0.83 ± 0.098% tissue-[3H]. n = 5 rats; ***p < 0.001, different from control (0 NIC; buffer only condition).
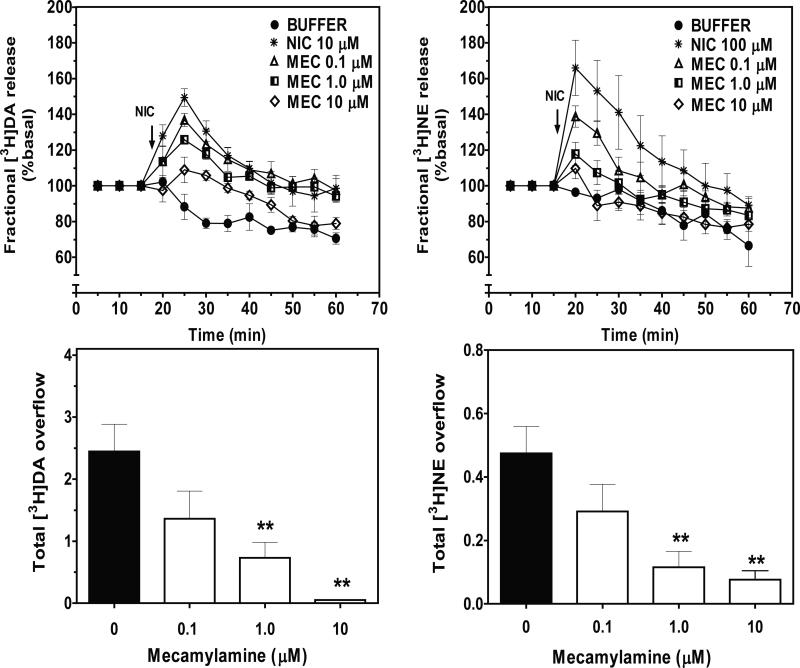
3.2. Mecamylamine and losartan inhibited nicotine-evoked [3H]DA and [3H]NE overflow
Concentration- and time-dependent effects of mecamylamine and losartan on nicotine-evoked [3H]DA and [3H]NE overflow from superfused striatal and hypothalamic slices are illustrated in Figs. 2 and 3, respectively. Based on results from the nicotine concentration-response studies (Smith et al., 2010; current results), antagonist inhibition of nicotine-evoked [3H]DA and [3H]NE fractional release was evaluated using 10 and 100 μM nicotine, respectively. Under control conditions (in the absence of mecamylamine), fractional [3H]DA and [3H]NE release increased following nicotine addition to the buffer, peaked after 10 and 5 min, respectively, and then declined over time towards basal despite the continued presence of nicotine in the buffer. In a concentration-dependent manner, mecamylamine inhibited nicotine-evoked fractional [3H]DA and [3H]NE release. Two-way ANOVA revealed a concentration × timeinteraction for mecamylamine inhibition of nicotine-evoked fractional [3H]DA release. (F24, 112 = 1.68, p < 0.05; Fig 2: top-left). With respect to total [3H]DA overflow, mecamylamine at 1 and 10 μM inhibited the effect of nicotine (F3, 22 = 8.661, p < 0.001; Fig 2: bottom-left). The IC50 value for mecamylamine was 1.0 ± 0.4 μM, and the Imax was 95 ± 1%. A concentration X time interaction (F24, 112 = 2.954, p < 0.001; Fig 2: top-right) was found also for mecamylamine inhibition of nicotine-evoked fractional [3H]NE release. With respect to total [3H]NE overflow, mecamylamine at 1 and 10 μM inhibited the effect of nicotine (F3, 25 = 7.125, p < 0.001; Fig 2: bottom-right). The IC50 for mecamylamine was 0.2 ± 0.03 μM, and the Imax was 85 ± 8%. Log IC50 values for mecamylamine inhibition of nicotine-evoked [3H]DA and [3H]NE overflow were not different (t (9) = 1.187; p > 0.05).
Figure 2.
Mecamylamine (MEC) inhibits NIC-evoked fractional [3H]DA and [3H]NE release (top) and total [3H]DA and [3H]NE overflow (bottom) from superfused rat striatal and hypothalamic slices, respectively. Arrow indicates the time point at which NIC was added to the superfusion buffer. Data are expressed as mean ± S.E.M. Fractional release data are expressed as a percentage of basal. Basal fractional [3H]DA and [3H]NE release were 0.86 ± 0.062 and 0.41 ± 0.034% tissue-[3H], respectively. n = 5 rats; **p < 0.01, indicates different from control (0 MEC; effect of NIC in the absence of MEC).
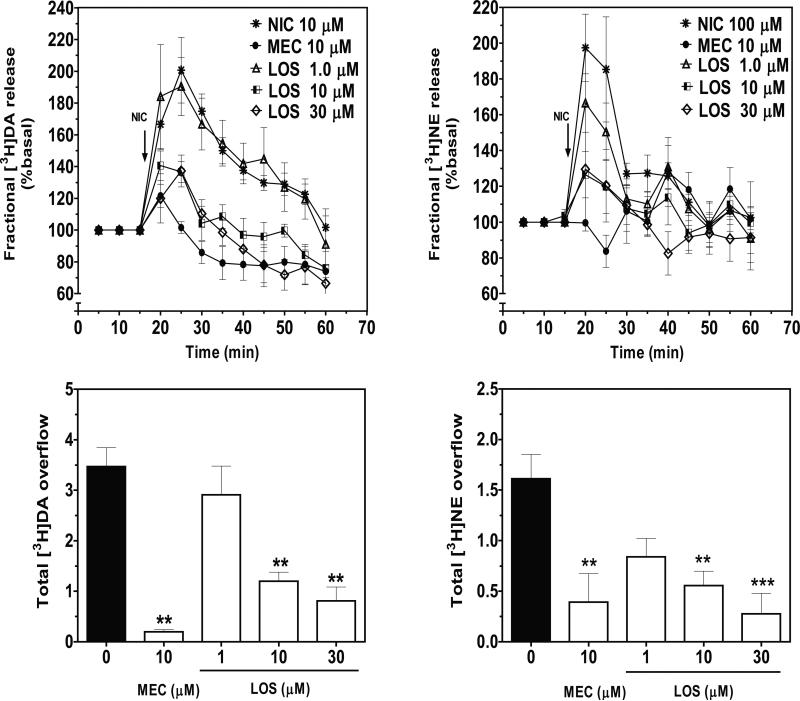
Figure 3.
Losartan (LOS) inhibits fractional NIC-evoked [3H]DA and [3H]NE release (top) and total [3H]DA and [3H]NE overflow (bottom) from rat striatal and hypothalamic slices, respectively. Arrow indicates the time point at which NIC (10 and 100 μM, for [3H]DA and [3H]NE release assays, respectively) was added to the superfusion buffer. Data are expressed as mean ± S.E.M. Fractional release data are expressed as a percentage of basal. Basal values for fractional [3H]DA and [3H]NE release were 0.76 ± 0.053 and 0.39 ± 0.028% tissue-[3H],respectively. n = 6 rats; **p < 0.01, indicates different from control (0 LOS; effect of NIC in the absence of LOS or MEC).
In a concentration- and time-dependent manner, losartan inhibited nicotine-evoked fractional [3H]DA and [3H]NE release (Fig. 3). No intrinsic effects of losartan (1-30 μM) alone on fractional [3H]DA (F4, 29 = 1.85; p > 0.05) and [3H]NE release (F4, 28 = 0.64; p > 0.05) from striatum and hypothalamus, respectively, were found (Table 1). In these experiments, mecamylamine (10 μM) served as a positive control. Two-way ANOVA of the fractional [3H]DA release data revealed main effects of concentration (F3, 25= 3.293, p < 0.001) and time (F8, 200 = 11.728, p < 0.001); however, a concentration X time interaction was not found (F24, 200 = 1.063, p > 0.05; Fig 3: top-left). Losartan at 10 and 30 μM inhibited nicotine-evoked [3H]DA overflow (F3, 37 = 14.57, p < 0.001; Fig 3: bottom-left). The IC50 for losartan was 3.9 ± 1.2 μM, and the Imax was 82 ± 3%. With respect to inhibition of nicotine-evoked fractional [3H]NE release, two-way repeated measures ANOVA revealed a concentration X time interaction (F24, 240 = 1.698, p < 0.05; Fig 3: top-right), and a main effect of time (F8, 240 = 7.851, p < 0.001); however a main effect of concentration was not found (F3, 30 = 2.24, p > 0.05). Losartan also inhibited nicotine-evoked [3H]NE overflow at 10 and 30 μM (F3, 31 = 6.409, p < 0.001; Fig 3: bottom-right). The IC50 for losartan was 2.2 ± 0.7 μM, and the Imax was 89 ± 6%.
Table 1.
Fractional [3H]DA and [3H]NE release from striatal and hypothalamic slices, respectively, in the presence of losartan.
| Fractional release | Control | MEC 10 μM | LOS 1 μM | LOS 10 μM | LOS 30 μM | F-statisticsa |
|---|---|---|---|---|---|---|
| [3H]DA | 0.98 ± 0.021 | 0.97 ± 0.054 | 0.99 ± 0.097 | 0.99 ± 0.093 | 1.22 ± 0.103 | F[4, 29] = 1.85 |
| [3H]NE | 0.54 ± 0.022 | 0.47 ± 0.055 | 0.61 ± 0.060 | 0.63 ± 0.163 | 0.47 ± 0.089 | F[4, 28] = 0.641 |
p>0.05
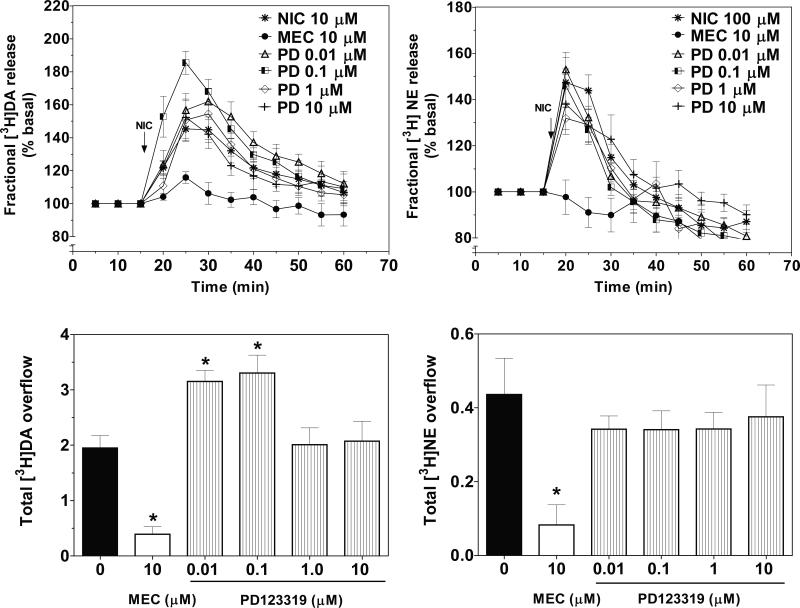
3.3. PD123319 did not inhibit nicotine-evoked [3H]NE overflow, but potentiated nicotine-evoked [3H]DA overflow
Time course of the effect of PD123319 on nicotine-evoked [3H]DA and [3H]NEfractional release is illustrated in Fig. 4. No intrinsic effects of PD123319 (0.01-10 μM) alone on fractional [3H]DA (F = 0.31; p > 0.05) and [3 (F5, 46 H]NE release (F5, 40 = 0.63; p > 0.05) from striatum and hypothalamus, respectively, were found (Table 2). Two-way ANOVA of the fractional [3H]NE release data revealed a PD123319 concentration X time interaction (F32, 312 = 2.26, p < 0.0001; Fig 4: top-right) and a main effect of time (F8, 312= 48, p < 0.001); however, no main effect of concentration (F4, 39 = 2.19, p > 0.05) was found. PD123319 (0.001-10 μM) did not inhibit nicotine-evoked [3H]NE overflow (F4, 38 = 0.3573, p > 0.05; Fig 4: bottom-right).
Figure 4.
PD123319 (PD), an AT2 antagonist, does not inhibit NIC-evoked [3H]NE overflow from rat hypothalamic slices, but potentiates nicotine-evoked [3H]DA overflow from rat striatal slices. Time course of PD effects on NIC-evoked fractional [3H]DA (top-left) and fractional [3H]NE (top-right) release. Effects of PD on total [3H]DA (bottom-left) and total [3H]NE (bottom-right) overflow. Arrow indicates the time point at which NIC was added to the superfusion buffer. Data are expressed as mean ± S.E.M. Fractional release data are expressed as a percentage of basal. Basal fractional [3H]DA and [3H]NE release are 0.86 ± 0.019 and 0.38 ± 0.013% tissue-[3H], respectively. n = 6 rats; *p < 0.05, indicates different from NIC. (0 PD; effect of NIC in the absence of PD or MEC).
Table 2.
Fractional [3H]DA and [3H]NE release from striatal and hypothalamic slices, respectively, in the presence of PD123319.
| Fractional release | Control | MEC 10 μM | PD 0.01 μM | PD 0.1 μM | PD 1 μM | PD 10 μM | F-statisticsa |
|---|---|---|---|---|---|---|---|
| [3H]DA | 3.2 ± 0.60 | 3.6 ± 0.34 | 3.8 ± 0.90 | 3.5 ± 0.74 | 3.5 ± 0.60 | 2.8 ± 0.29 | F[5,46] = 0.31 |
| [3H]NE | 2.6 ± 0.22 | 2.9 ± 0.69 | 1.9 ± 0.31 | 2.7 ± 0.55 | 2.3 ± 0.49 | 2.7 ± 0.26 | F[5,40] = 0.63 |
p>0.05
With respect to nicotine-evoked fractional [3H]DA release, two-way ANOVA revealed main effects of concentration (F4, 53 = 4.139, p < 0.001) and time (F8, 424 = 43.602, p < 0.001); however, the concentration X time interaction was not significant (F32, 424 = 0.953, p > 0.05; Fig 4: top-left). Surprisingly, low concentrations of PD123319 (0.01 and 0.1 μM) potentiated (about 40%) the effect of nicotine to increase [3H]DA overflow (F4, 39 = 3.328, p < 0.05; Fig 4: bottom-left).
3.4. Losartan and PD123319 lacked affinity for α4β2* and α7* nAChRs
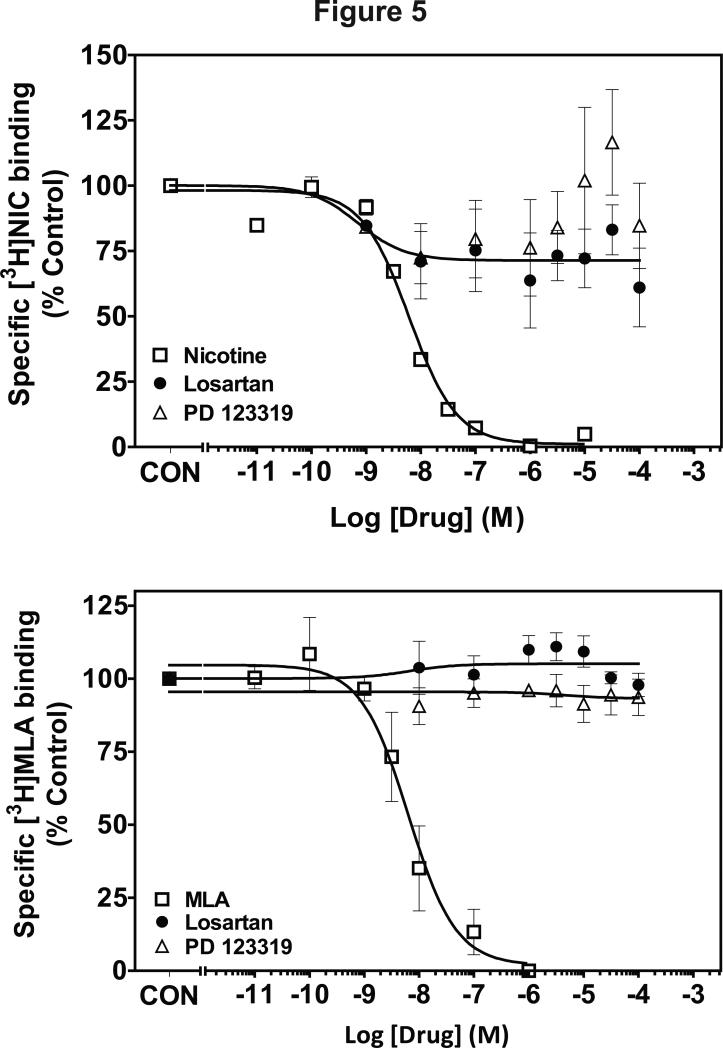
Affinity of losartan and PD123319 for α4β2* and α7* nAChRs was evaluated using [3H]nicotine and [3H]methyllycaconitine binding to whole brain membranes (Fig. 5). Ki values for nicotine were 3 nM and 370 nM at the [3H]nicotine and [3H]methyllycaconitine binding sites, respectively, consistent with previous reports [48]. At the concentrations evaluated, both losartan and PD123319 did not inhibit [3H]nicotine and [3H]methyllycaconitine binding (Fig. 5).
Figure 5.
Losartan and PD123319 concentration-response curves for inhibition of [3H]nicotine (top) and [3H]methyllycaconitine (bottom) binding. Data are expressed as mean ± S.E.M as a percentage of control. Control [3H]nicotine and [3H]MLA binding was (47.9 ± 5.18 and 54.9 ± 3.43 fmol/mg protein, respectively). n = 3-5 rats.
3.5. Ang II in striatal homogenates and superfusates
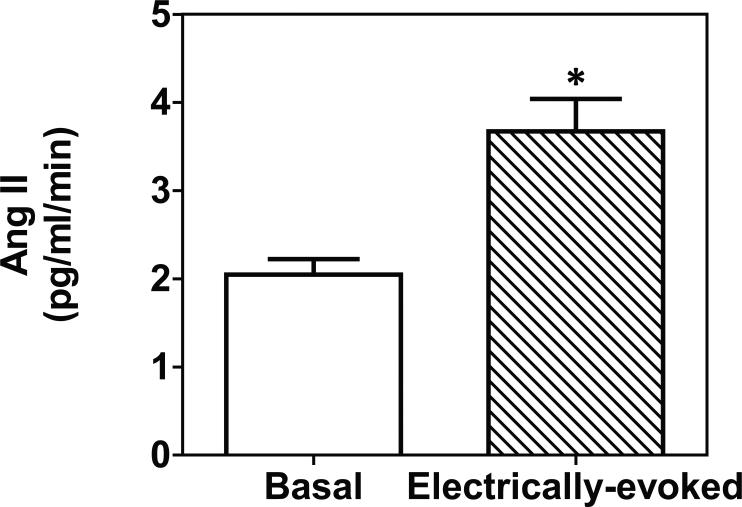
Ang II content in striatal tissue was 237 ± 22.7 pg/g tissue. Electrical stimulation of striatal slices evoked Ang II release in amounts (3.6 ± 0.36 pg/ml/min) significantly greater than basal (2.1 ± 0.18 pg/ml/min) (t(4 )= p < 0.05; Fig. 6). Basal Ang II release during each minute of superfusion constituted 1% of the striatal tissue content.
Figure 6.
Electrical stimulation of striatal slices induces Ang II release. Data are expressed as mean ± S.E.M. n = 3 rats; *p < 0.05, indicates different from basal.
3.6. Ang II evokes [3H]NE release from hypothalamus
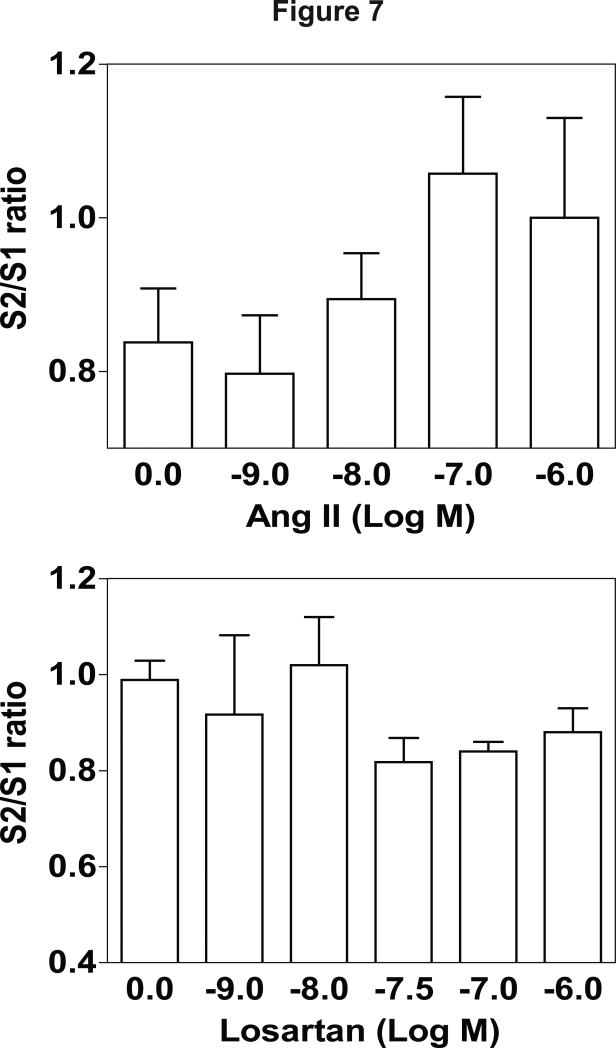
Ang II (1 nM-1 μM) evoked [3H]NE overflow following electrical stimulation of hypothalamic slices; however, one way ANOVA did not reveal a significant effect of concentration (F(4, 34) = 1.631, p > 0.05; Fig. 7 top). Higher concentrations of losartan appeared to inhibit Ang II-evoked [3H]NE overflow; however, this effect was not significant (F(5, 37) = 1.279, p > 0.05; Fig. 7 bottom).
Figure 7.
Ang II evokes [3H]NE release from rat hypothalamic slices (top). Losartan inhibits Ang II-evoked [3H]NE release (bottom). Data are expressed as mean ± S.E.M. n = 5-6 rats; p < 0.05, indicates different from control (0).
DISCUSSION
The role of AT1 receptors in Ang II-induced modulation of the DA reward system was evident from previous findings that losartan inhibits Ang II-mediated DA release from striatum [37]. The current study extends these previous findings by showing that losartan inhibits nicotine-evoked [3H]DA and [3H]NE release from rat striatum and hypothalamus, respectively, suggesting that AT1 receptors mediate, at least in part, both nicotine-evoked DA and NE release. PD123319 potentiates nicotine-evoked [3H]DA release from rat striatum, suggesting that AT2 receptors exert tonic inhibition of nicotine-evoked DA release.
Since losartan and PD 123319 did not inhibit [3H]nicotine and [3H]methyllycaconitine binding, these antagonists do not appear to interact with α4μ2* and α7* nAChRs to elicit the inhibition and potentiation of nicotine-evoked neurotransmitter release. However, inhibition of the binding of these radioligands only interrogates interactions with the agonist binding site on nAChRs. Thus, losartan may be inhibiting the effect of nicotine at these nAChRs through a negative allosteric mechanism of action. On the other hand, PD123319 may be potentiating the effect of nicotine in striatum through a positive allosteric interaction at nAChRs. Considerable evidence demonstrating the ability of pharmacological agents to allosterically modulate nAChRs supports the current interpretation. For example, desformylflustrabromine, a compound isolated from Flustra foliacea, has been identified as a positive allosteric modulator of α4β2 nAChRs [49]. Low micromolar concentrations of desformylflustrabromine potentiated (2–3-fold) agonist-evoked currents associated with human α4β2 nAChRs expressed in Xenopus oocytes, while higher desformylflustrabromine concentrations inhibited this α4β2-mediated response [49]. Also, the anthelminthic agent, ivermectin, was identified as a positive allosteric modulator of the α7 nAChR [50]. Another drug, UCI-30002 [N-(1, 2, 3, 4-tetrahydro-1-naphthyl)-4-nitroaniline], that reduces nicotine self-administration in rats, acts as a negative allosteric modulator at α4β2 nAChRs [51]. Thus, additional mechanistic studies are needed to rule out nAChR involvement in the response to these AT1 and AT2 antagonists.
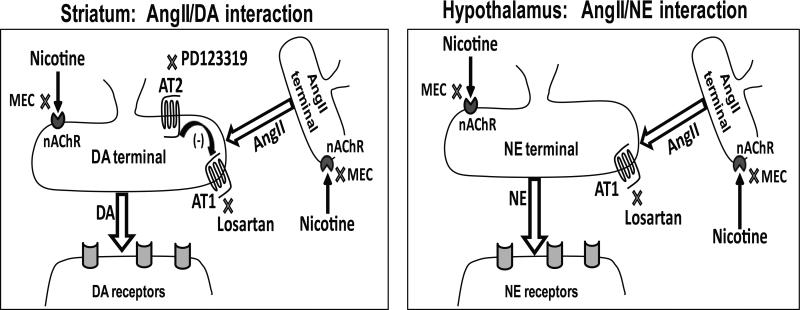
The presence of Ang II in the striatum (22.7 pg/g tissue; current study) and hypothalamus (125 pg/g tissue [30]) indicates that the effects of losartan and PD123319 on nicotine-evoked DA and NE release may be through inhibition of the effects of endogenous and released (nicotine-evoked) Ang II. Ang II-containing neurons are present in striatum and hypothalamus [30, 31], and are the likely site of antagonist action. To summarize, a schematic of the tentative mechanism of action of losartan and PD123319 in striatum and hypothalamus is illustrated in Fig. 8.
Figure 8.
Model for AT1 and AT2 receptors modulation of nicotine-evoked DA and NE release in striatum and hypothalamus, respectively. In the striatum (left) and hypothalamus (right), nicotine is shown to induce Ang II release via activation of nAChRs. Ang II stimulates DA and NE release from striatum and hypothalamus, respectively, in a AT1 receptor-mediated manner. Mecamylamine, a non-selective nAChR antagonist, inhibits nicotine-evoked neurotransmitter release by acting at nAChRs located on DA and NE terminals and/or at nAChRs located on Ang II containing neurons. Losartan inhibits nicotine-evoked neurotransmitter release via AT1 receptors located on DA and NE terminals. In striatum, low concentrations of PD123319 specifically inhibit AT2 receptors; thereby abolishing AT2-mediated tonic inhibition of AT1 receptors.
In the hypothalamus, Ang II increases NE release as demonstrated previously as well as in the current study [33]. Further, losartan inhibits electrically-evoked NE release, although the level of inhibition did not reach statistical significance. Taken together, these results suggest that endogenous Ang II augments NE release in the hypothalamus. Further, losartan appears to inhibit the effect of endogenous Ang II and nicotine-stimulated Ang II release, collectively leading to inhibition of nicotine-evoked NE release (Fig. 8, right).
In contrast to in vitro binding studies that report losartan IC50 values in the nanomolar range [52], higher concentrations of losartan (10 and 30 μM) were required to inhibit the effect of nicotine on [3H]DA and [3H]NE release in the current study. Although, losartan 1 μM decreased nicotine-evoked [3H]NE release from hypothalamic slices, statistical significance for this concentration was not obtained. Differences between the tissue preparations employed in the previously reported binding studies and the current neurotransmitter release study likely contribute to the observed differences in IC50 values for losartan. Such differences in IC50 values have been reported for other antagonists, including for example S-sulpiride, a DA D2 receptor antagonist. S-Sulpiride-induced changes in electrically-evoked [3H]DA release from superfused striatal slices was reported at 10-fold greater concentrations than its affinity for DA D2 receptors determined using striatal membrane preparations [53, 54]. Membrane homogenate preparations allow greater access of drug to the receptor sites; whereas in comparison, intact striatal slice preparations restrict drug access to receptors, such that greater drug concentrations are required to penetrate the slice, reach the receptors and inhibit the functional response [54]. Apart from methodological differences, higher losartan concentrations may be required for inhibiting nicotine-evoked DA and NE release to overcome a putative nicotine-stimulated Ang II release, that further increases DA and NE release in an AT1-dependent manner (Fig. 8).
In contrast to losartan, the selective AT2 antagonist PD123319 did not inhibit nicotine-evoked NE release from hypothalamus, suggesting that AT2 receptors are not involved in this response. Receptor autoradiography studies reveal a low density of AT2 receptors in rat hypothalamus relative to AT1 receptors [55, 56]. Furthermore, in situ hybridization in the hypothalamus detected AT1 receptor mRNA, but not AT2 receptor mRNA [57, 58]. Thus, the lack of inhibitory effect of PD123319 on nicotine-evoked NE release in hypothalamus is likely due to the low expression of AT2 receptors in this brain region.
In the striatum, electrical stimulation increases basal Ang II release, suggesting that neuronal stimulation, such as nicotinic receptor activation, may also increase Ang II release. Further, Ang II is shown to increase DA release in striatum via activation of AT1 receptors [37]. Thus, losartan may partially inhibit nicotine-evoked DA release via inhibition of nicotine-evoked Ang II release. In contrast to nicotine-evoked hypothalamic [3H]NE release, losartan1 μM failed to decrease nicotine-evoked striatal [3H]DA release. Differences in results between brain regions, may, in part, be due to differences in the amount of Ang II released by nicotine, such that greater concentration of losartan is required to attenuate nicotine-evoked striatal [3H]DA release as compared to hypothalamic [3H]NE release.
In contrast to hypothalamus, greater expression of AT2 receptors is observed in striatum [32]. Current results show that at low concentrations (0.01 and 0.1 μM), PD123319 potentiated nicotine-evoked DA release in striatum. The current study also demonstrates that 1% of striatal tissue content of Ang II constituted basal release from striatal slices during each minute of superfusion. Thus, activation of AT2 receptors by endogenous Ang II may suppress nicotine-evoked DA release (Fig. 8 left). Further, AT2 receptor activation counteracts AT1 receptor activation in striatum. Precedence for such counterbalances in the Ang II system comes from studies investigating effects of Ang II in the periphery in which AT2 receptor activation produces systemic vasodilation, counteracting AT1 receptor-mediated vasoconstriction induced by Ang II activation [26]. At high concentrations (1 and 10 μM), PD123119 did not alter nicotine-evoked DA release. Since selective inhibition of AT2 receptors occurs at low PD123319 concentrations, high concentrations of PD123319 may be acting non-selectively to also inhibit AT1 receptors. In support of this interpretation, PD123319 at nM concentrations has been shown to selectively inhibit AT2 receptors expressed in cultured rat mesangial cells, whereas μM concentrations of PD123319 also inhibited AT1B receptors [59]. Further, nonspecific actions of high concentrations of PD123319 at AT1 receptors have been suggested with respect to the peripheral Ang II system [60]. Taken together, PD123319-induced potentiation of the effect of nicotine on DA release in the current study appears to be due to inhibition of AT2 receptors, and the non-selective inhibition of AT1 receptors at high PD123319 concentrations appears to overcome the AT2 effect, such that the potentiation is not observed.
An alternative mechanism that may underlie the effects of losartan and PD123319 on nicotine-evoked DA release includes the presence of non-AT1 and non-AT2 receptors. In rat striatum and hypothalamus, alternate Ang II binding sites have been identified using membrane binding assays and autoradiography [61, 62]. However, losartan and PD123319 have been reported to lack affinity for these alternate binding sites [61]. Nevertheless, the effects of losartan and PD123319 on nicotine-evoked striatal DA release and hypothalamic NE release may be mediated, in part, by an interaction at non-AT1 and non-AT2 receptors. Alternatively, effects of AT antagonists on DA and NE release may be secondary to effects on blood vessels.
In summary, the current findings suggest that AT1 receptors modulate nicotine-evoked DA and NE release in striatum and hypothalamus. However, in striatum, but not hypothalamus, AT2 receptors also appear to be involved. Importantly, crosstalk between AT1 and AT2 receptors in striatum appears to complicate Ang II modulation of the effect of nicotine on DA release. Importantly, the current results identify Ang II receptors as novel targets for intervention in the rewarding effects and cardiovascular complications of tobacco use.
Acknowledgements
The authors acknowledge Dr. Kiran Babu Siripurapu for technical assistance. This research was supported by NIH P50 DA05312, NIH HL73085 and P20RR021954, and a Pre-doctoral Fellowship from the American Heart Association, AHA 715489B.
Abbreviations
- Ang II
angiotensin II
- DA
dopamine
- MEC
mecamylamine
- nAChR
nicotinic acetylcholine receptor
- NE
norepinephrine
- PD123319
1-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Center for Disease Control (CDC) Report. 2010 http://www.cdc.gov/tobacco/data_statistics/sgr/2010/index.htm.
- 2.Balfour DJ. The neurobiology of tobacco dependence: A commentary. Respiration. 2002;69:7–11. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- 3.Corrigall WA, Franklin KBJ, Coen KM, Clark PBS. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 4.Brunzell DH, Picciotto MR. Molecular mechanisms underlying the motivational effects of nicotine. Nebr Symp Motiv. 2009;55:17–30. doi: 10.1007/978-0-387-78748-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54(2):65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- 9.McBride PE. The health consequences of smoking. Cardiovascular diseases. Med Clin North Am. 1992;76(2):333–353. doi: 10.1016/s0025-7125(16)30356-x. [DOI] [PubMed] [Google Scholar]
- 10.Piano MR, Benowitz NL, Fitzgerald GA, Corbridge S, Heath J, Hahn E, et al. American Heart Association Council on Cardiovascular Nursing. Impact of smokeless tobacco products on cardiovascular disease: Implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122(15):1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- 11.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the para-ventricular and supraoptic nuclei in the rat. Brain Res. Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Harland D, Gardiner SM, Bennett T. Paraventricular nucleus injections of noradrenaline: cardiovascular effects in conscious Long-Evans and Brattleboro rats. Brain Res. 1989;496:14–24. doi: 10.1016/0006-8993(89)91047-0. [DOI] [PubMed] [Google Scholar]
- 13.Sharp BM, Matta SG. Detection by in vivo microdialysis of nicotine-induced norepinephrine secretion from the hypothalamic paraventricular nucleus of freely moving rats: dose-dependency and desensitization. Endocrinology. 1993;133(1):11–19. doi: 10.1210/endo.133.1.8391419. [DOI] [PubMed] [Google Scholar]
- 14.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Changeux JP. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem. 2012;287(48):40207–40215. doi: 10.1074/jbc.R112.407668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwoskin LP, Pivavarchyk M, Joyce BM, Neugebauer NM, Zheng G, Zhang Z, et al. Targeting reward-relevant nicotinic receptors in the discovery of novel pharmacotherapeutic agents to treat tobacco dependence. Nebr Symp Motiv. 2009;55:31–63. doi: 10.1007/978-0-387-78748-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;39:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 18.Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of alpha 6-containing nicotinic receptors analyzed with mutant mice. J. Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65(6):1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 20.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71(6):1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 21.Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35(3):665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, et al. α-Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-stimulated norepinephrine release. J Neurosci. 1998;18:8571–9. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall JE. Pathophysiology of obesity hypertension. Curr Hypertens Rep. 2000;2(2):139–147. doi: 10.1007/s11906-000-0073-4. [DOI] [PubMed] [Google Scholar]
- 25.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 26.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24(3):261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 27.Lippoldt A, Paul M, Fuxe K, Ganten D. The brain renin-angiotensin system: molecular mechanisms of cell to cell interactions. Clin Exp Hypertens. 1995;17(1-2):251–266. doi: 10.3109/10641969509087069. [DOI] [PubMed] [Google Scholar]
- 28.Lynch KR, Simnad VI, Ben-Ari ET, Garrison JC. Localization of preangiotensinogen messenger RNA sequences in the rat brain. Hypertension. 1986;8(6):540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Phillips MI, Stenstrom B. Angiotensin II in rat brain comigrates with authentic angiotensin II in blood pressure liquid chromatography. Circ Res. 1985;56:212–219. doi: 10.1161/01.res.56.2.212. [DOI] [PubMed] [Google Scholar]
- 30.Allen AM, MacGregor DP, Chai SY, Donnan GA, Kaczmarczyk S, Richardson K, et al. Angiotensin II receptor binding associated with nigrostriatal dopaminergic neurons in human basal ganglia. Ann Neurol. 1992;32:339–344. doi: 10.1002/ana.410320306. [DOI] [PubMed] [Google Scholar]
- 31.Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78(1-3):1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 32.Culman J, Blume A, Gohlke P, Unger T. The renin-angiotensin system in the brain: possible therapeutic implications for AT (1)-receptor blockers. J Hum Hypertens. 2002;16(3):S64–70. doi: 10.1038/sj.jhh.1001442. [DOI] [PubMed] [Google Scholar]
- 33.Stadler T, Veltmar A, Qadri F, Unger T. Angiotensin II evoked noradrenaline release from the paraventricular nucleus in conscious rats. Brain Res. 1992;569(1):117–122. doi: 10.1016/0006-8993(92)90377-l. [DOI] [PubMed] [Google Scholar]
- 34.Siegl PK, Kivlighn SD, Broten TP. Pharmacology of losartan, an angiotensin II receptor antagonist, in animal models of hypertension. J Hypertens Suppl. 1995;13(1):S15–21. doi: 10.1097/00004872-199507001-00002. [DOI] [PubMed] [Google Scholar]
- 35.Unger T. The angiotensin type 2 receptor: variations on an enigmatic theme. J Hypertens. 17(12 Pt 2):1775–1786. doi: 10.1097/00004872-199917121-00001. [DOI] [PubMed] [Google Scholar]
- 36.Ge J, Barnes NM. Alterations in angiotensin AT1 and AT2 receptor subtype levels in brain regions from patients with neurodegenerative disorders. Eur J Pharmacol. 1996;297:299–306. doi: 10.1016/0014-2999(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 37.Brown DC, Steward LJ, Ge J, Barnes NM. Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br J Pharmacol. 1996;118(2):414–420. doi: 10.1111/j.1476-5381.1996.tb15418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dwoskin LP, Jewell AL, Cassis LA. DuP 753, a nonpeptide angiotensin II-1 receptor antagonist, alters dopaminergic function in rat striatum. Naunyn Schmiedebergs’ Arch Pharmacol. 1992;345(2):153–159. doi: 10.1007/BF00165730. [DOI] [PubMed] [Google Scholar]
- 39.Teng L, Crooks PA, Dwoskin LP. Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: comparison with d-amphetamine. J Neurochem. 1998;71(1):258–265. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]
- 40.Miller DK, Crooks PA, Dwoskin LP. Lobeline inhibits nicotine-evoked [(3)H]dopamine overflow from rat striatal slices and nicotine-evoked (86)Rb(+) efflux from thalamic synaptosomes. Neuropharmacology. 2000;39(13):2654–2662. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 41.Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [(3)H]overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine. J Pharmacol Exp Ther. 2002;302(3):1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- 42.Smith AM, Pivavarchyk M, Wooters TE, Zhang Z, Zheng G, McIntosh JM, et al. Repeated nicotine administration robustly increases bPiDDB inhibitory potency at alpha6beta2-containing nicotinic receptors mediating nicotine-evoked dopamine release. Biochem Pharmacol. 2010;80(3):402–409. doi: 10.1016/j.bcp.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blankley CJ, Hodges JC, Klutchko SR, Himmelsbach RJ, Chucholowski A, Connolly CJ, et al. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem. 1991;34(11):3248–3260. doi: 10.1021/jm00115a014. [DOI] [PubMed] [Google Scholar]
- 44.Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, et al. Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990;252(2):719–725. [PubMed] [Google Scholar]
- 45.Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, et al. N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity. J Pharmacol Exp Ther. 2008;326(2):563–576. doi: 10.1124/jpet.108.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassis LA, Dwoskin LP. Presynaptic modulation of neurotransmitter release by endogenous angiotensin II in brown adipose tissue. J Neural Transm Suppl. 1991;34:129–137. doi: 10.1007/978-3-7091-9175-0_17. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 48.Flammia D, Dukat M, Damaj MI, Martin B, Glennon RA. Lobeline: structure-affinity investigation of nicotinic acetylcholinergic receptor binding. J Med Chem. 1999;42(18):3726–3731. doi: 10.1021/jm990286m. [DOI] [PubMed] [Google Scholar]
- 49.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor targeted therapeutics: Advantages and limitations. Biochem Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, et al. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, et al. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- 52.Siegl PK, Kivlighn SD, Broten TP. Pharmacology of losartan, an angiotensin II receptor antagonist, in animal models of hypertension. J Hypertens Suppl. 1995;13(1):S15–21. doi: 10.1097/00004872-199507001-00002. [DOI] [PubMed] [Google Scholar]
- 53.Zahniser NR, Dubocovich ML. Comparison of dopamine receptor sites labeled by [3H]-S-sulpiride and [3H]-spiperone in striatum. J Pharmacol Exp Ther. 1983;227(3):592–599. [PubMed] [Google Scholar]
- 54.Dwoskin LP, Zahniser NR. Robust modulation of [3H]dopamine release from rat striatal slices by D-2 dopamine receptors. Pharmacol Exp Ther. 1986;239(2):442–453. [PubMed] [Google Scholar]
- 55.Saylor DL, Perez RA, Absher DR, Baisden RH, Woodruff ML, Joyner WL, et al. Angiotensin II binding sites in the hamster brain: localization and subtype distribution. Brain Res. 1992;595:98–106. doi: 10.1016/0006-8993(92)91457-p. [DOI] [PubMed] [Google Scholar]
- 56.MacGregor DP, Murone C, Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin II receptor subtypes in the human central nervous system. Brain Res. 1995;675:231–240. doi: 10.1016/0006-8993(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 57.Jöhren O, Imboden H, Häuser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res. 1997;757(2):218–227. doi: 10.1016/s0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- 58.Jain P, Armando I, Juorio AV, Barden N, Benicky J, Saavedra JM. Decreased hypothalamic and adrenal angiotensin II receptor expression and adrenomedullary catecholamines in transgenic mice with impaired glucocorticoid receptor function. Neuroendocrinology. 2004;80(3):171–180. doi: 10.1159/000082358. [DOI] [PubMed] [Google Scholar]
- 59.Ernsberger P, Zhou J, Damon TH, Douglas JG. Angiotensin II receptor subtypes in cultured rat renal mesangial cells. Am J Physiol. 1992;263(3 Pt 2):F411–416. doi: 10.1152/ajprenal.1992.263.3.F411. [DOI] [PubMed] [Google Scholar]
- 60.Macari D, Bottari S, Whitebread S, De Gasparo M, Levens N. Renal actions of the selective angiotensin AT2 receptor ligands CGP 42112B and PD 123319 in the sodium-depleted rat. Eur J Pharmacol.;1993;249(1):85–93. doi: 10.1016/0014-2999(93)90665-5. [DOI] [PubMed] [Google Scholar]
- 61.Karamyan VT, Speth RC. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 62.Karamyan VT, Speth RC. Distribution of the non-AT1, non-AT2 angiotensin-binding site in the rat brain: Preliminary characterization. Neuroendocrinology. 2008;88(4):256–265. doi: 10.1159/000140635. [DOI] [PubMed] [Google Scholar]