Abstract
Bacterial cells are surrounded by a crosslinked polymer called peptidoglycan (PG), the integrity of which is necessary for cell survival. The carbohydrate chains that form the backbone of PG are made by peptidoglycan glycosyltransferases (PGTs), highly conserved membrane-bound enzymes that are thought to be excellent targets for the development of new antibacterials. Although structural information on these enzymes recently became available, their mechanism is not well understood due to a dearth of methods to monitor PGT activity. Here we describe a direct, sensitive and quantitative SDS-PAGE method to analyze PGT reactions. We apply this method to characterize the substrate specificity and product length profile for two different PGT domains: PBP1A from Aquifex aeolicus and PBP1A from Escherichia coli. We show that both disaccharide and tetrasaccharide diphospholipids (Lipid II and Lipid IV) serve as substrates for these PGTs, but the product distributions differ significantly depending on which substrate is used as the starting material. Reactions using the disaccharide substrate are more processive and yield much longer glycan products than reactions using the tetrasaccharide substrate. We also show that the SDS-PAGE method can be applied to provide information on the roles of invariant residues in catalysis. A comprehensive mutational analysis shows that the biggest contributor to turnover of 14 mutated residues is an invariant glutamate located in the center of the active site cleft. The assay and results described provide new information about the process by which PGTs assemble bacterial cell walls.
The characteristic shape of a bacterial cell is a function of the three dimensional architecture of the cell wall (1). The major rigid component of the cell wall is peptidoglycan, a mesh-like polymer comprising linear glycan chains held together by peptide crosslinks (2). The glycan chains are assembled from a diphospholipid-linked disaccharide-peptide precursor, Lipid II, by peptidoglycan glycosyltransferases (PGTs) (Fig. 1). These glycan chains are then crosslinked via the attached peptides by transpeptidases (TP) (3, 4).
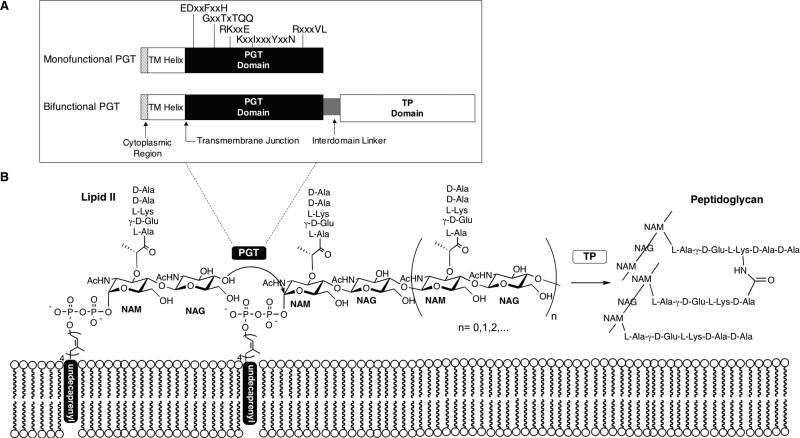
Fig. 1.
PGT topology and the glycosyltransfer reaction. (A) Topology of monofunctional and bifunctional PGTs. Highly conserved residues, arranged in the motifs that typify the PGTs, are indicated above. (B) PGTs catalyze a glycosyltransfer reaction that involves attack by the C4-OH of a terminal NAG residue on the anomeric carbon of a NAM unit of the diphospholipid donor (Lipid II: n = 0; Lipid IV: n = 1); the direction of elongation has not been rigorously established but is thought to occur via addition of new subunits to the reducing end of the growing polymer (2). The glycans are then cross-linked at the peptide stem by bacterial TPs.
The transpeptidases are targets for several families of clinically important antibiotics, including the penicillins, cephalosporins, and imipenems (5,6), and the PGTs are thought to be promising antibacterial targets as well. There is a potent family of natural products, represented by the phosphoglycolipid moenomycin, that inhibit the PGTs with lethal cellular effects (7-10). However, there are still no drugs in clinical use that operate by binding directly to the PGTs. A better understanding of the PGTs and how they can be inhibited is required to exploit them as antibiotic targets and may also shed light on their cellular functions.
PGTs are membrane-anchored polymer-ases that exist naturally in two forms: as N-terminal domains in bifunctional penicillin-binding proteins (PBPs), called Class A high molecular weight PBPs, which contain C-terminal TP domains, and as monofunctional enzymes that do not contain transpeptidase domains (Fig. 1A) (2,11-13). All PGTs contain five conserved motifs comprised of a number of invariant residues (14). Notable progress towards understanding PGTs has been made recently. For example, structures of two PGTs, one with moenomycin bound in the active site cleft, are now available and provide a starting point for inhibitor design (11,15) However, we still lack answers to fundamental questions about the reactions catalyzed by PGTs, which limits our ability to think about their roles in bacterial cell growth and division. Questions that remain unanswered include: How long are the glycan chains made by particular PGTs? Do different PGTs within the same organism show significant differences in glycan product distributions? Are the glycan chain length distributions compatible with current models for the three dimensional structure of the bacterial cell wall? Do other cellular factors affect chain length distributions? One of our goals is to develop facile in vitro methods to analyze PGT reactions so that these and other fundamental questions about their behavior can be addressed.
PGTs catalyze the conversion of the peptidoglycan precursor, Lipid II, into glycan chains. The process is proposed to involve the coupling of Lipid II units to form a tetrasaccharide, Lipid IV (Fig. 1, n=1), followed by successive addition of Lipid II units to the reducing end of the growing polymer (16). Understanding the PGT reaction requires having both Lipid II and Lipid IV substrates, combined with an assay that reports on glycan chain lengths. We have developed methods to obtain useful quantities of Lipid II and Lipid IV substrates (17-21), so the necessary starting materials to study PGTs are now available (Fig. 2). In this paper, we describe a facile assay to analyze PGT reactions that provides information on reaction rates as well as glycan chain lengths. This assay has enabled us to compare two PGT domains from different organisms, PBP1A from Aquifex aeolicus and PBP1A from Escherichia coli, with respect to their ability to utilize disaccharide and tetrasaccharide substrates. For the A. aeolicus PGT domain, for which we recently reported the structure (15), we have also carried out a comprehensive mutational analysis of the conserved residues in the five signature motifs that typify PGT domains (14,22). We identify the most important residues for activity, which is pertinent to interpreting the crystal structures of PGTs, and we also show that the gel electrophoresis assay provides information about the products of mutant enzymes that cannot be obtained using other types of assays.
Fig. 2.
Structure of the substrate analogues heptaprenyl-Lipid II (1a) and heptaprenyl-Lipid IV (2a). Radiolabeled Lipid II (1b) is made chemoenzymatically using [14C]-GlcNAc as described in (20). Alternatively, an acetyl group can be incorporated on the lysines of the substrate peptide chains, with no effect on substrate utility, by treatment with 14C-acetic anhydride to give (1c) or 14C-acetic anhydride to give radiolabeled (2b) as described in (21).
The methods reported here provide new insights into the reactions catalyzed by PGTs and should make it possible to address many questions about how these enzymes behave. Information on the in vitro biochemistry of PGTs, both alone and in conjunction with other components of the peptidoglycan biosynthetic machinery, will be useful for understanding the roles of PGTs in bacterial morphogenesis and division.
EXPERIMENTAL PROCEDURES
Reagents
Vectors and expression hosts were obtained from Novagen (EMD Biosciences). Primers were synthesized by Integrated DNA Technologies (IDT). DNA sequencing was performed at the Dana-Farber/Harvard Cancer Center DNA Resource Core. Radiolabeled heptaprenyl-Lipid II was synthesized as described by Ye et al. (20) and radiolabeled heptaprenyl-Lipid IV was synthesized as described by Zhang et al. (21). Moenomycin A was extracted and purified from the feed stock flavomycin and purified as described (23). Non-stick PCR tubes used for enzymatic reactions were obtained from VWR. An electrophoresis grade acrylamide stock solution [30% (w/v) acrylamide: 0.8% (w/v) bisacrylamide] was purchased from National Diagnostics. TEMED was purchased from American Bioanalytical. The Quick Change site-directed mutagenesis kit was obtained from Stratagene. All other reagents and buffer components were purchased from Sigma-Aldrich. Cloning, expression and purification of PGT constructs - The cloning, expression and purification of the PGT domain of A. aeolicus ΔPBP1A[N29-K243] and full-length E. coli PBP1A[M1-F850] have been previously described (15,21). The isolated PGT domain of E. coli PBP1A was obtained as follows. The gene encoding the truncated E. coli PBP1A (M1-N251) was PCR amplified from pET22b::ponA containing full length E. coli PBP1A using the appropriate primer pairs (restriction sites are underlined) (Supplemental Data, Table 1). The PCR product was then digested and inserted into the Nde I and Not I restriction sites of pET22b(+) vector to produce pWTS12. The inserted ponA(M1-N251) gene was confirmed by sequencing. Expression and purification followed procedures in (21) with changes described in the Supplemental Data.
Site-directed mutagenesis of ΔPBP1A[N29-K243]
Quick Change Site-Directed mutagenesis kit was used to make all the mutants from the parent plasmid, ΔPBP1A[N29-K243], using the primer pairs given in the Supplemental Data, Table 1. Expression and purification of mutants was carried out as reported in (15).
Gel analysis conditions for the separation of glycan chains
Gels were prepared as described by Lesse et al. and Schägger et al. (24,25) with the following modifications: anode buffer (0.1 M Tris, adjusted to pH 8.8 with HCl), cathode buffer (0.1 M Tris-base, 0.1 M Tricine, 0.1% SDS, pH 8.25) and 3X gel buffer (1.5 M Tris, 0.4% SDS, pH 8.45). Gels were prepared using the Protean xii system (BioRad). Separating gels, 200×200×1 mm, were prepared (stacking gel omitted) in a final concentration of 9%T, 2.6%C in 1X gel buffer, where T is the total percentage concentration of both acrylamide and bisacrylamide and C represents the percentage of bisacrylamide (cross-linker) relative to T. Gel solutions were filtered and degassed before addition of TEMED.
Samples were prepared by vacuum centrifuging the reaction mixtures to 1 μl or dryness and reconstituting them in 2 μl of sample buffer (60 mM Tris-HCl, pH 8.8, 25% glycerol, 2% SDS, 10% saturated solution of bromophenol blue). Samples were then loaded under the cathode buffer as thin bands with filling height less than 3 mm. Electrophoresis was performed on a Protean II xi vertical gel apparatus set at 30 mA constant current with a maximum voltage of 200 V. Gels were run for approximately 4 h or until the bromophenol blue dye front was 0.5 cm from the end of the gel. Gels were dried overnight without fixing and imaged by autoradiography using a tritium storage phosphor screen and scanner (Typhoon 9400, GE Healthcare). Densitometric band quantitations were carried out with the ImageQuant TL software package (GE Healthcare) using the included 1D-gel analysis program. The regularity in the distribution of the ladder of oligomers was also verified by SDS-PAGE separation of a Lipid II and Lipid IV reaction mixture in parallel (Fig. 3).
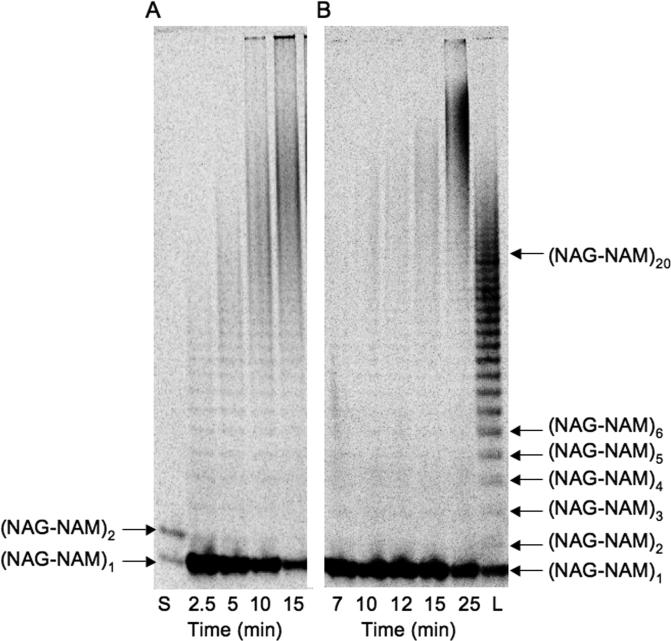
Fig. 3.

SDS-PAGE separation of NAG-NAM oligomers. Lane 1: oligomers generated from reaction of Lipid IV (2b, 110 μCi/μmol) under standard assay conditions (Experimental procedures) and quenched at 45 min. Lane 2: oligomers generated from reaction of 10 μM acetylated Lipid II (1c, 72 μCi/μmol) and A. aeolicus PBP1A PGT domain (3.3 μM) quenched at 3 min. The use of high enzyme: substrate ratios biases the product distribution to shorter oligomers, ≤ (NAG-NAM)20, but oligomers up to (NAG-NAM)30 can be resolved under these conditions (see Fig. S1).
Reaction time courses with isolated PGT domains
All reactions using enzymes from the hyperthermophile, A. aeolicus, were prepared on ice and initiated with a rapid temperature ramp to 55°C in a PCR cycler (Eppendorf Mastercycler). Reactions with enzyme from E. coli were performed at room temperature. All reactions were quenched with excess (200 μM) moenomycin A and kept on ice before gel analysis.
When [14C]-Lipid II (1b) was used as substrate, reactions (5 μL) were carried-out in buffer A (20% DMSO, 50 mM HEPES, pH 7.5, 10 mM CaCl2) containing 8 μM 1b, and 0.08 μM PGT. Reactions were quenched at 2.5, 5, 10, 12, 15 and 25 min for the A. aeolicus PGT domain and 7, 10, 12, 15 and 25 min for the E. coli PGT domain.
When [14C]-Lipid IV (2b) was used as substrate with E. coli PGT domain, conditions were as follows: reactions (5 μL) contained buffer A with 8 μM 2b and 0.8 μM PGT and were quenched at 2, 5, 10, 20, 30 and 45 min. For A. aeolicus PGT domain reactions (12 μl) contained buffer A with 5 μM 2b and 1.7 μM PGT and were quenched at 1, 2, 5, 10, 15 and 30 min.
Elongation of oligomers with Lipid II
Oligomers were prepared by incubating 0.8 μM full-length E. coli PBP1A with 8 μM [14C]-2b in buffer B (buffer A supplemented with 1 kU/mL penicillin G) for 2 h. Boiling for 10 min terminated the reaction and precipitated protein was pelleted by centrifugation at 14,000 rpm for 20 min. The supernatant containing the oligomeric mix was separated into 4.5 μL aliquots. One aliquot of untreated starting material was saved as a control. Reactions (10 μL each) in buffer B contained 4.5 μl oligomeric starting material and 0.8 μM of either full-length E. coli PBP1A or A. aeolicus PGT domain in the presence or absence of 4 μM Lipid II (1a) incubated for 10 min. A second control sample was prepared by incubating full-length E. coli PBP1A and 8 μM ([14C]-GlcNAc)-Lipid II (1b) in buffer B for 5 min.
Mutant activity assays
Reactions (5 μL) were prepared in buffer A using 4 μM radiolabeled Lipid II (1b), 0.06 μM enzyme and time-points taken at 30 and 60 min. Product analysis was performed using a paper chromatography assay as described (15,26).
When mutants were retested at higher enzyme concentrations (2 μM) the reactions were quenched after 30 min. E83Q had time-points taken at 30, 60, and 600 min. Product analysis was then performed using the SDS-PAGE assay described here.
RESULTS
Establishing a gel electrophoresis assay for the separation of glycan chains
Most assays to monitor PGT activity rely on detecting the presence of polymeric product (18,27-30), but cannot distinguish different glycan chain lengths, which greatly limits the information they provide about the reaction. Since the glycan products of a PGT reaction are composed of repeating disaccharide units that have a net negative charge, we investigated gel electrophoresis methods to analyze glycan chain length distributions. Radiolabeled Lipid II (Fig. 2, 1b and 1c) and Lipid IV (Fig. 2, 2b) substrates were prepared as described (20,21) and radiolabels were either incorporated enzymatically or via acetylation. Previous work has established that substrates with substituents at the third position of the pentapeptide chain, such as acetyl groups on the lysine amine (e.g. 1c and 2b) have comparable kinetic parameters to unmodified substrates (e.g. 1b) (21,30). At high substrate:enzyme (~1:3) ratios, the A. aeolicus PBP1A PGT domain converts the substrates into mixtures of products representing a range of glycan chain lengths. Optimal gel conditions for separating PGT products utilized a modified tricine-SDS-PAGE system (24,25). The bromophenol blue dye migrates ahead of Lipid II under the standard running conditions, allowing the progress of the runs to be visually tracked. A 9% gel was optimal for separating uncrosslinked glycan strands containing from one to thirty repeat units [i.e., from (NAG-NAM)1 to (NAG-NAM)30] as determined from the size standards generated using the substrates (Fig 3; see also Supplemental Data, Fig. S1). The separation of glycan chains is indifferent to the presence of proteins, salts at the concentrations used in typical reaction buffers, and other additives. Moreover, since only labeled glycan chains are detected, sample cleanup is unnecessary. The use of radiolabeled substrates in the reaction obviates the need for post-reaction labeling to detect products. Finally, it should be possible to adapt this assay to detect products of reactions carried out with substrates labeled in other ways, e.g., with fluorophores or biotin moieties on the peptide side chains.
Product distributions with Lipid II as the substrate
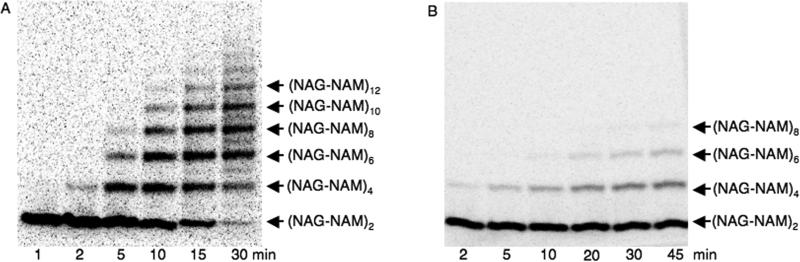
With an assay in place to separate glycan chains, we examined the product distributions for the PGT domains of A. aeolicus PBP1A (Fig. 4A) and E. coli PBP1A (Fig. 4B) using the radiolabeled Lipid II substrate 1b and an enzyme:substrate ratio of 1:100. There is a delay in the formation of products, which is particularly pronounced for the PGT domain of E. coli PBP1A. This lag phase has also been observed for E. coli PBP1B using other assays (26,30) and has been attributed either to a slow initial coupling step to form a “primer” or to a slow rearrangement to the active conformation. Following the lag phase, relatively long glycan chains appear without significant accumulation of short products, consistent with a processive mechanism in which coupling of Lipid II subunits can occur without release of the elongating product. Shorter products are less apparent early in the reaction and the distribution of glycan chains is narrower for the E. coli PBP1A PGT domain than for the A. aeolicus PGT domain. Furthermore, shorter products are less evident early in the reaction for the E. coli enzyme than for the A. aeolicus PGT domain. These results suggest that the E. coli PGT domain is more processive than the A. aeolicus PGT domain. The SDS-PAGE method described here should make it possible to establish whether differences in processivity, and thus glycan chain length distribution, exist among different PGTs encoded in the genome of the same organism.
Fig. 4.
SDS-PAGE analysis of Lipid II (1b, 288 μCi/μmol) reactions catalyzed by the PGT domains of (A) A. aeolicus PBP1A and (B) E. coli PBP1A. Reactions were carried out as described in Experimental Procedures and quenched at the indicated time-points. Lane L: oligomer ladder; lane S: Lipid II (1, 2 pmol) and Lipid IV (2b, 2 pmol) run as standards.
Product distributions using Lipid IV
We recently reported that E. coli PBP1A can self-couple Lipid IV (2b) to generate elongated products (21). This result was surprising because it was previously thought that only the disaccharide substrate (Lipid II) is capable of reacting with the growing glycan polymer. Since the paper chromatography assay used previously did not provide information about glycan chain length, we have used the newly developed SDS-PAGE assay to examine the product lengths for the A. aeolicus PBP1A (Fig. 5A) and E. coli PBP1A (Fig. 5B) PGT domains with the tetrasaccharide substrate 2b. For both enzymes, this substrate reacts more slowly than Lipid II (Table 1) even at high enzyme concentrations. In the case of the tetrasaccharide substrate, short products accumulated before longer products appeared, a pattern consistent with a distributive mechanism in which products are released after each coupling cycle and must rebind before undergoing further elongation. In contrast, even at 1:10 enzyme:substrate ratios, Lipid II reacts to form mainly long polymers (see Fig 6C)
Fig. 5.
SDS-PAGE product distribution of Lipid IV oligomers. Time-courses was carried out using 2b and (A) A. aeolicus PBP1A PGT domain (1.65 μM) or (B) E. coli PBP1A PGT domain (0.8 μM) quenched at the indicated time-points as described in Experimental Procedures.
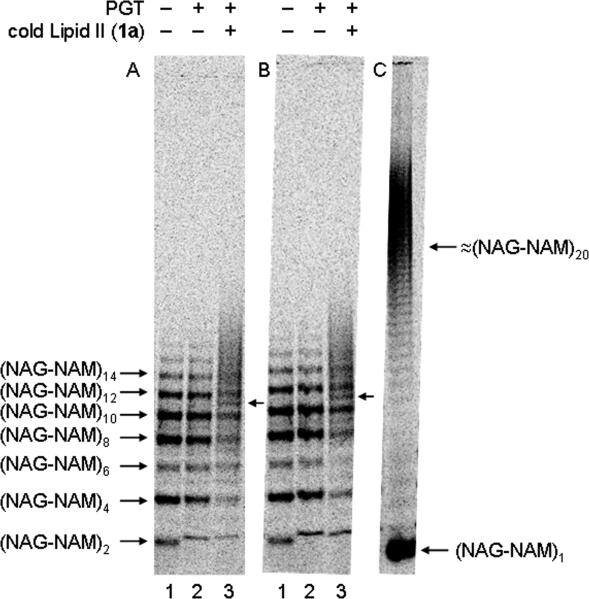
Fig. 6.
Radiolabeled oligomers incubated (+) or not (−) with 4 μM cold Lipid II (1a) and (A) 0.8 μM A. aeolicus PBP1A PGT domain or (B) 0.8 μM E. coli PBP1A. Lane 1: radiolabeled oligomeric starting material generated from 8 μM [14C]-Lipid IV (2b); lane 2: oligomeric starting material incubated with enzyme alone; lane 3: oligomeric starting material incubated with enzyme and 4 μM cold Lipid II (1a) for 10 min. New bands are indicated by arrows. (C) Control reaction using 4 μM radiolabeled ([14C]-GlcNAc)-Lipid II (1b) incubated with 0.4 μM full-length E. coli PBP1A under standard assay conditions for 5 min.
Elongation of exogenously added oligomers
The finding that PGT domains can homopolymerize Lipid IV as the sole substrate raised the possibility that PGTs may couple oligomers longer than Lipid IV. To address this possibility, we enzymatically generated a mixture of radiolabeled oligomers from 14C-Lipid IV (2b) to use as starting materials (Fig. 6A and 6B, lane 1). The oligomer mixture was then treated with the PGT domain from A. aeolicus PBP1A (Fig. 6A, lane 2) or with E. coli PBP1A (Fig. 6B, lane 2). No change in the distribution of oligomers was observed in the presence of either PGT (Fig. 6A and 6B, compare lanes 1 and 2), indicating negligible self-coupling of glycan chains longer than Lipid IV. However, the oligomers were chased into longer products when cold Lipid II (1a) was added to the reactions (Fig. 6A and 6B, lane 3), as indicated by the appearance of new bands between oligomeric starting materials (see arrows, Fig. 6A and 6B) and a smear of radioactivity above the highest band of starting material. These results show that glycan strands containing multiple NAG-NAM repeat units are capable of binding to PGTs and undergoing elongation. Since the radiolabeled oligomers are extended by only a few disaccharide units under reaction conditions in which Lipid II used as the sole substrate produces very long glycan chains (Figure 6C), we have concluded that rebinding and extension of long glycan strands is slower than initiation and processive elongation of new chains.
Gel electrophoretic analysis of selected mutants
PGTs show modest structural homology to lambda lysozyme (11,15), an enzyme that cleaves the β-(1,4)-NAM-NAG glycosidic linkages of peptidoglycan. The coupling reaction is proposed to involve general acid-base catalysis. There are two conserved glutamate residues in PGTs, one in motif 1 (E83 in the A. aeolicus PGT domain) and the other in motif 3 (E140 in the A. aeolicus PGT domain). Both have been proposed to play key roles in catalysis (11,22,28,30,31), although evidence for the importance of the motif 3 glutamate is contradictory. There is also a conserved aspartate (D84) and a conserved histidine (H87). Perhaps because most PGTs tend to be difficult to handle, only a small number of the conserved residues in the five invariant domains have been mutated to examine their relative importance in catalysis. Because the isolated PGT domain of A. aeolicus PBP1A can be easily overexpressed and purified in active form and remains stable for a prolonged period of time, it is a good candidate for mutational analysis (15). Therefore, we replaced fourteen of the conserved residues in the A. aeolicus PGT domain with alanine or with more conservative substitutions. All mutants could be expressed in soluble form and purified at levels comparable to the wild-type truncated construct, suggesting that none of the mutations interfere with protein expression or folding.
The activity of the mutant enzymes was compared to that of the wild-type enzyme using a paper chromatography assay that relies on separation of polymeric products from Lipid II (15,26). The substrate (1b) concentration used in these assays was 4 μM, which is just below the Km for the parent enzyme (15). All mutants showed reduced activity compared to the wild-type enzyme (Fig. 7A), consistent with the conservation of the mutated residues. Several mutants, including four with conservative side chain replacements (E83Q, D84N, R136K, and R218K), had negligible activity under the reaction conditions (Fig. 7A), implying that the catalytic efficiency of these mutants is at least 20-fold lower than the parent enzyme. All other mutants showed significant activity, indicating that these other residues play more modest roles in the catalytic function. We note that the conflicting data (22,28) on the importance of the conserved glutamate in the third motif (E140) is likely a function of the different mutations that were previously made. Whereas alanine is not acceptable at this position, the E140Q mutant is only 2.5 fold less active than the parent enzyme (Fig. 7A). These results rule out an essential role for this glutamate in the catalytic mechanism (11) and suggest that it plays a supporting role, perhaps in substrate recognition.
Fig. 7.
Activity profile for PBP1A point mutants. (A) Standard assay conditions: 60 nM enzyme, 4 μM radiolabeled Lipid II (1b), 55°C, buffer A, 30 min ( ) and 60 min (■) reactions. Residue numbers and substitutions are given along the x-axis. The % conversion was determined using a paper chromatography assay as described in (28); under these assay conditions enzyme activity ≤ 2% conversion is not distinguished over background. (B) Product distribution analysis by SDS-PAGE of low activity mutants. Reactions performed at higher concentrations of enzyme: 2 μM enzyme, 4 μM radiolabeled Lipid II (1b) under standard assay conditions (Experimental Procedures). (C) Reaction of E83Q with Lipid II (1b) was reexamined by SDS-PAGE using an 8-16% Tris-HCl gradient gel under the conditions described in (B). Reactions were quenched at 30, 90 and 600 min. The asterisked reaction was carried out at using 4 μM ([14C]-GlcNAc)- Lipid II (1b) and 4 μM ([12C]-GlcNAc)- Lipid II (1a). Lane L: ladder of oligomers.
) and 60 min (■) reactions. Residue numbers and substitutions are given along the x-axis. The % conversion was determined using a paper chromatography assay as described in (28); under these assay conditions enzyme activity ≤ 2% conversion is not distinguished over background. (B) Product distribution analysis by SDS-PAGE of low activity mutants. Reactions performed at higher concentrations of enzyme: 2 μM enzyme, 4 μM radiolabeled Lipid II (1b) under standard assay conditions (Experimental Procedures). (C) Reaction of E83Q with Lipid II (1b) was reexamined by SDS-PAGE using an 8-16% Tris-HCl gradient gel under the conditions described in (B). Reactions were quenched at 30, 90 and 600 min. The asterisked reaction was carried out at using 4 μM ([14C]-GlcNAc)- Lipid II (1b) and 4 μM ([12C]-GlcNAc)- Lipid II (1a). Lane L: ladder of oligomers.
Mutants that showed negligible activity in the paper chromatography assay were examined again at high enzyme:substrate ratios (30X higher enzyme concentrations) and the product distributions were analyzed using the SDS-PAGE assay (Fig. 7B). The E83Q and D84A mutants were virtually inactive (Fig. 7B, lanes 1 and 2 respectively, see also Fig. 7C); however, good turnover was observed for D84N, R136K, Q120A, and both R218A and R218K under the more forcing concentrations (Fig. 7B, lanes 3, 4, and 5).
Based on the mutational studies, we have concluded that four of the fourteen mutated residues (E83, D84, R136 and R218) play especially important roles in enzymatic function because even conservative mutations (e.g., R to K; D to N; E to Q) decrease enzymatic activity under standard assay conditions by >20-fold. One of these four residues, E83, is particularly important because almost no turnover is observed even for the conservative glutamine mutation under highly forcing conditions (Fig. 7C). We conclude from the above experiments that E83 is the single most important residue for catalysis, and that the carboxylate side chain is critical.
The sensitivity and size resolution of the gel electrophoresis assay makes it possible to detect small amounts of short coupling products, and provides far more information than the traditional paper chromatography assay. Therefore, this SDS-PAGE assay is capable of providing detailed information about individual mutants.
DISCUSSION
Knowing what an enzyme is capable of doing in vitro is fundamental to thinking about what it may do in cells. Therefore, we have established an SDS-PAGE method that allows us to monitor the formation of the glycan chain products of in vitro PGT reactions. Separation of the glycan strands by gel electrophoresis allows for the analysis of the product distributions of PGTs.
Using this SDS-PAGE assay, we have compared the products formed by two different PGTs with two different substrates, Lipid II and Lipid IV. Lipid II is the preferred substrate, with a catalytic efficiency twenty to forty times greater than Lipid IV. Lipid II also reacts processively -- that is, without release of the growing polymer chain – to yield long glycan products with minimal accumulation of short intermediates. In contrast, Lipid IV reacts in a distributive manner. A crystal structure of the A. aeolicus PGT domain suggests that a mobile flap may cover part of the active site cleft during the reaction, and we have speculated that this flap functions to hinder release of the coupled product from the active site (15). Following the chemical coupling step, the product can either translocate in the active site and undergo another round of coupling or it can dissociate. Translocation of the product of Lipid IV coupling to position the new reactive terminus for another round of reaction may be slower than for Lipid II. Alternatively, or in addition, the chemical coupling step of Lipid IV with the growing polymer may be slower than for Lipid II. A decrease in the relative rate of translocation or coupling compared with the rate of dissociation would make dissociation more likely to occur prior to elongation, explaining the observed distributive pattern of products when Lipid IV is the substrate. The finding that Lipid IV can function as a sole substrate, but longer polymers cannot, suggests that PGTs recognize the diphospholipid moiety of the acceptor as well as the donor. Recognition of both the non-reducing and the reducing end of the acceptor may explain the preference for Lipid II over Lipid IV as a substrate.
The results reported above have implications for the incorporation of new disaccharide subunits into the cell wall. Two models for how this process may occur have been proposed (2). In one model, nascent chains synthesized by PGTs are handed off to partner transpeptidases for cross-linking into the fabric of the cell wall; in the other, the disaccharide units themselves are directly integrated into the fabric of the cell wall through PGT-mediated glycosyltransfer. Our in vitro results show that PGTs couple Lipid II to give long oligomers, but are also capable of rebinding and elongating relatively long oligomers, showing that both models are possible. Nevertheless, the in vitro studies reported here show that the processive synthesis of long glycan chains from Lipid II is faster than the addition of Lipid II subunits to long oligomers, suggesting that the bulk of PG synthesis involves the incorporation of nascent chains into the existing framework of the cell wall.
In some organisms different PGTs appear to be required at different points in the cell cycle, although it is not known whether there are differences in their enzymatic properties. The results presented here show that there are differences in processivity in PGTs from different organisms. It is possible that there are also differences in processivity in PGTs from the same organism. If so, there may be differences in the distribution of product lengths, with more processive PGTs producing longer products. The assay described here will make it possible to determine whether the different PGTs show significant differences in processivity; this may, in combination with information on the spatial and temporal localization of the enzymes, help explain their different cellular roles. This assay also enables detailed studies of the factors that affect product lengths. For example, it will be possible to investigate the effects of other proteins or domains that are involved in peptidoglycan synthesis on the length of the glycan chains that are produced. It will also be possible to assess how enzyme to substrate ratios influence product lengths. These types of studies are important to do because glycan polymer length has profound implications for the structure of bacterial peptidoglycan. At the moment, two competing models are being actively considered, one in which the PG chains are perpendicular to the cell membrane (the scaffold model) and one in which the PG chains are parallel to the cell membrane (32). The product lengths observed in this study using Lipid II as a substrate are too long to be compatible with the scaffold model. Therefore, either the scaffold model is incorrect or limited to small regions of the bacterial cell (e.g., the poles), or there are other factors operative in cells that restrict the lengths of the glycan polymers. This assay provides a systematic way to evaluate how sensitive PGT product length distributions are to other factors, including variations in substrate availability and the presence of other components of the biosynthetic machinery.
Supplementary Material
Acknowledgments
We thank Timothy Meredith and Mark Leibman for helpful discussions and technical assistance. This work was supported by the National Institutes of Health (NIH Grant GM076710) and D. Barrett by a UNCF•Merck Graduate Science Research Fellowship.
REFERENCES
- 1.Höltje JV. Microbiol. Mol Biol. Rev. 1998;62(1):181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Heijenoort J. Glycobiology. 2001;11(3):25R–36R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- 3.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. FEMS Microbiol. Rev. 2006;30(5):673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 4.van Heijenoort J. Nat. Prod. Rep. 2001;18(5):503–519. doi: 10.1039/a804532a. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JF, Meroueh SO, Mobashery S. Chem. Rev. 2005;105(2):395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 6.Kahne D, Leimkuhler C, Lu W, Walsh C. Chem. Rev. 2005;105(2):425–448. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- 7.Ostash B, Saghatelian A, Walker S. Chem. Biol. 2007;14(3):257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostash B, Walker S. Curr. Opin. Chem. Biol. 2005;9(5):459–466. doi: 10.1016/j.cbpa.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JG, Li X, Oberthur M, Zhu W, Kahne DE. J. Am. Chem. Soc. 2006;128(47):15084–15085. doi: 10.1021/ja065907x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welzel P. Chem. Rev. 2005;105(12):4610–4660. doi: 10.1021/cr040634e. [DOI] [PubMed] [Google Scholar]
- 11.Lovering AL, de Castro LH, Lim D, Strynadka NC. Science. 2007;315(5817):1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 12.Spratt BG, Zhou J, Taylor M, Merrick MJ. Mol. Microbiol. 1996;19(3):639–640. doi: 10.1046/j.1365-2958.1996.442924.x. [DOI] [PubMed] [Google Scholar]
- 13.Terrak M, Nguyen-Disteche M. J. Bacteriol. 2006;188(7):2528–2532. doi: 10.1128/JB.188.7.2528-2532.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goffin C, Ghuysen JM. Microbiol. Mol. Biol. Rev. 1998;62(4):1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Y, Barrett D, Zhang Y, Kahne D, Sliz P, Walker S. Proc. Natl. Acad. Sci. USA. 2007;104(13):5348–5353. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward JB. Pharmac. Ther. 1984;25(3):327–369. doi: 10.1016/0163-7258(84)90004-4. [DOI] [PubMed] [Google Scholar]
- 17.Breukink E, van Heusden HE, Vollmerhaus PJ, Swiezewska E, Brunner L, Walker S, Heck AJ, de Kruijff B. J. Biol. Chem. 2003;278(22):19898–19903. doi: 10.1074/jbc.M301463200. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz B, Markwalder JA, Wang Y. J. Am. Chem. Soc. 2001;123(47):11638–11643. doi: 10.1021/ja0166848. [DOI] [PubMed] [Google Scholar]
- 19.VanNieuwenhze MS, Mauldin SC, Zia-Ebrahimi M, Winger BE, Hornback WJ, Saha SL, Aikins JA, Blaszczak LC. J. Am. Chem. Soc. 2002;124(14):3656–3660. doi: 10.1021/ja017386d. [DOI] [PubMed] [Google Scholar]
- 20.Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. J. Am. Chem. Soc. 2001;123(13):3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Fechter EJ, Wang TS, Barrett D, Walker S, Kahne DE. J. Am. Chem. Soc. 2007;129(11):3080–3081. doi: 10.1021/ja069060g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrak M, Ghosh TK, van Heijenoort J, Van Beeumen J, Lampilas M, Aszodi J, Ayala JA, Ghuysen JM, Nguyen-Disteche M. Mol. Microbiol. 1999;34(2):350–364. doi: 10.1046/j.1365-2958.1999.01612.x. [DOI] [PubMed] [Google Scholar]
- 23.Adachi M, Zhang Y, Leimkuhler C, Sun B, LaTour JV, Kahne DE. J. Am. Chem. Soc. 2006;128(43):14012–14013. doi: 10.1021/ja065905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesse AJ, Campagnari AA, Bittner WE, Apicella MA. J. Immunol. Meth. 1990;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 25.Schägger H, von Jagow G. Anal. Biochem. 1987;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Walker D, Sun B, Hu Y, Walker S, Kahne D. Proc. Natl. Acad. Sci. USA. 2003;100(10):5658–5663. doi: 10.1073/pnas.0931492100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harz H, Burgdorf K, Höltje JV. Anal. Biochem. 1990;190(1):120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- 28.Offant J, Michoux F, Dermiaux A, Biton J, Bourne Y. Biochim. Biophys. Acta. 2006;1764(6):1036–1042. doi: 10.1016/j.bbapap.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JS, Meadow PM, Haskin MA, Strominger JL. Arch. Biochem. Biophys. 1966;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz B, Markwalder JA, Seitz SP, Wang Y, Stein RL. Biochemistry. 2002;41(41):12552–12561. doi: 10.1021/bi026205x. [DOI] [PubMed] [Google Scholar]
- 31.Schiffer G, Höltje JV. J. Biol. Chem. 1999;274(45):32031–32039. doi: 10.1074/jbc.274.45.32031. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer W, Höltje JV. J. Bacteriol. 2004;186(18):5978–5987. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.