Abstract
Airway smooth muscle (ASM) contraction is an important component of the pathophysiology of asthma. Taurine, an agonist of glycine receptor chloride (GlyR Cl−) channels, was found to relax contracted ASM, which led us to question whether functional GlyR Cl− channels are expressed in ASM. Messenger RNA for β (GLRB), α1 (GLRA1), α2 (GLRA2), and α4 (GLRA4) subunits were found in human (Homo sapiens) and guinea pig (Cavia porcellus) tracheal smooth muscle. Immunoblotting confirmed the protein expression of GLRA1 and GLRB subunits in ASM. Electrical activity of cultured human ASM cells was assessed using a fluorescent potentiometric dye and electrophysiological recordings. Glycine increased current and significantly increased fluorescence in a dose-dependent manner. The GlyR Cl− channel antagonist strychnine significantly blocked the effects of glycine on potentiometric fluorescence in ASM cells. Guinea pig airway ring relaxation of ACh-induced contractions by isoproterenol was significantly left-shifted in the presence of glycine. This effect of glycine was blocked by pretreatment with the GlyR Cl− channel antagonist strychnine. Glycine treatment during tachykinin- and acetylcholine-induced contractions significantly decreased the maintenance of muscle force compared to control. GlyR Cl− channels are expressed on ASM and regulate smooth muscle force and offer a novel target for therapeutic relaxation of ASM.—Yim, P. D., Gallos, G., Xu, D., Zhang, Y., Emala, C. W. Novel expression of a functional glycine receptor chloride channel that attenuates contraction in airway smooth muscle.
Keywords: membrane potential, isoproterenol, organ bath, guinea pig, FLIPR
The worldwide incidence of asthma continues to increase, yet the pharmacological approaches to airway smooth muscle relaxation have not improved since the introduction of β-adrenoreceptor agonists many decades ago. Not only are some patients with asthma refractory to β-adrenoreceptor therapy, but significant morbidity and mortality have been associated with the use of long-acting β-adrenoreceptor agonists (1). Thus, the need for alternative approaches to relax airway smooth muscle in asthma is urgent.
Intracellular calcium levels are a key regulator of the contractile tone of airway smooth muscle. The plasma membrane electrical potential influences the calcium sensitivity of contractile proteins and the handling of calcium from both extracellular sources and intracellular stores (2, 3). Thus, the manipulation of plasma membrane potential via ligand-gated ion channels is an attractive therapeutic target for modulation of airway smooth muscle tone. Our laboratory has recently shown that one family of ligand-gated ion channels, the classic neuronal GABAA chloride inhibitory channel, is expressed and is functionally coupled to the relaxation of airway smooth muscle (4). During these studies, it was discovered that taurine, an agonist at both the GABAA channel and GlyR Cl− channel, potentiated isoproterenol-mediated relaxation of airway smooth muscle, but this prorelaxation effect was only partially attenuated by the GABAA channel antagonist, gabazine. Therefore, we questioned whether taurine's prorelaxant effects might also be modulated through GlyR Cl− channels, which have never before been described on airway smooth muscle.
GlyR Cl− channels are inhibitory chloride channels abundantly expressed in the spinal cord and belong to the same pentameric ligand-gated channel family as GABAA (5). GlyR Cl− channel pentamers are made up of 5 known subunits (GLRA1, GLRA2, GLRA3, GLRA4, and GLRB) in either homomeric or heteromeric form. Naturally occurring homomers are made of 5 GLRA2 subunits, and heteromers comprise a combination of GLRA and GLRB subunits (6). Subunit combinational specificity dictates the pharmacokinetic, pharmacodynamic, and binding affinity profiles of individual GlyR Cl− channels, but all GlyR Cl− channels conduct chloride ions, which, in neuronal cells, favor plasma membrane hyperpolarization (5). Interestingly, β-adrenoreceptor-mediated relaxation of airway smooth muscle is due, in part, to plasma membrane hyperpolarization via opening of plasma membrane large-conductance, calcium-activated potassium channels (KCa; ref. 7). GABAA and glycine chloride channels are similar in structure and function, leading us to question whether GlyR Cl− channels are expressed on airway smooth muscle and whether activation of these GlyR Cl− channels would mimic the prorelaxant effects of GABAA channel activation, identifying a novel therapeutic target for relaxation of airway smooth muscle.
MATERIALS AND METHODS
Materials
Glycine, indomethacin, capsaicin, pyrilamine, and acetylcholine were obtained from Sigma (St. Louis, MO, USA). The fluorescent potentiometric probe (FLIPR) membrane potential assay kit was obtained from Molecular Devices (Sunnyvale, CA, USA). The protease inhibitor cocktail set III was purchased from Calbiochem (Gibbstown, NJ, USA). Trypsin 0.05%- EDTA was purchased from Invitrogen (Carlsbad, CA, USA). TRIzol reagent was obtained from Ambion (Austin, TX, USA). Antibodies for the GLRA1 subunit of the GlyR Cl− channel were obtained from Millipore (Billerica, MA, USA). Antibodies for the GLRB subunit of the GlyR Cl− channel were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Cultures of immortalized human airway smooth muscle cells were a kind gift from Dr. William Gerthoffer (University of South Alabama, Mobile, AL, USA) and have previously been characterized (8). The cells were grown to confluence in 75-cm2 flasks for RT-PCR and immunoblot assays, and 96-well black-walled clear bottom plates for fluorescent FLIPR membrane potential assays. All cells were maintained in SmBM2 medium (Lonza, Walkersville, MD, USA) at 37°C in 95% air/5% CO2 as described previously (4).
Isolation of smooth muscle from human trachea and guinea pig
All human airway tissue protocols were reviewed by the Columbia University Institutional Review Board and were deemed not human subjects research under 45 CFR 46. Human tracheal tissue was obtained from the National Disease Research Interchange (NDRI; Philadelphia, PA, USA) or from discarded airway tissue from healthy lung donors during transplantation surgery at Columbia University.
All guinea pig protocols were approved by the Columbia University Institutional Animal Care and Use Committee. Guinea pigs were deeply anesthetized with 50 mg/kg pentobarbital. The chest cavity was opened, and the guinea pigs were exsanguinated. Whole brain (positive controls for RT-PCR and immunoblot), retina (positive control for immunoblot), and the entire trachea were excised and immersed in cold Kreb-Henseleit (KH) buffer (in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.236 MgSO4, 1.3 NaH2PO4, and 5.6 glucose, pH 7.4).
For both human and guinea pig airways, fibrous tissue from the extraluminal side of the trachea was carefully dissected and discarded. The tracheal epithelial layer was removed either by gentle intraluminal abrasion for the guinea pig or by fine dissection of airway epithelium with forceps in the human. The intact trachea was then either divided into rings for muscle force studies performed in organ baths or frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) for laser microdissection-assisted RNA extraction or dissected under a dissecting microscope to remove smooth muscle for protein extraction.
Isolation of RNA by laser capture microdissection (LCMD) and reverse transcription of cDNA
Freshly dissected and excised human and guinea pig tracheal rings were embedded in OCT compound followed by isopentane/dry ice freezing. Six consecutive frozen sections (10 μm) were made under RNase-free conditions and were placed on a single 1-mm PEN-membrane-coated slide (PALM Microlaser Technologies, Westchester, NY, USA) and processed for RNA preservation and cell staining using a LCMD staining kit (Ambion AM1935). Histological confirmation of stained sections guided subsequent laser dissection, harvesting only central portions of smooth muscle to avoid contamination from adjoining cells using a PALM MicroBeam laser microscope. RNA was recovered from LCMD samples using a RNAqueous-micro kit (Ambion) according to manufacturer's recommendations. Recovered RNA was reversed transcribed into cDNA using one of two commercial kits, Advantage RT-for-PCR (BD Biosciences, Mountain View, CA, USA) or SuperScript III (Invitrogen). LCMD-captured RNA (10 μl) in sample buffer was reverse transcribed using random hexamer primers at 42°C for 1 h in 20 μl, according to manufacturers' recommendations. Because the content of RNA in LCMD samples is very small, quantification of RNA was not performed.
Isolation of RNA from cultured airway smooth muscle and reverse transcription of cDNA
RNA was extracted from immortalized cultured human airway smooth muscle cells using the TRIzol reagent (Ambion), according to the manufacturer's recommendations. Using two RT-PCR kits, Advantage RT-for-PCR (BD Biosciences) and Super Script III (Invitrogen), 1 μg RNA from each sample was reverse transcribed using random hexamer primers at 42°C for 1 h in 20 μl according to manufacturers' recommendations.
PCR
Newly synthesized cDNA (5 μl) from RNA isolated by LCMD from native guinea pig or human airway smooth muscle and cultured immortalized human airway smooth muscle cells were used in PCR using the Advantage Polymerase Kit (Clontech, Mountain View, CA, USA). Sense and antisense primers (0.4 μM) were used for corresponding GlyR Cl− channel subunits (Table 1). All cDNA samples were denatured at 94°C for 10 s. Annealing temperatures were all 68°C for 1 min. Each sample underwent 45 cycles of amplification in a PTC-200 Peltier thermal cycler (Bio-Rad, Hercules, CA, USA). PCR products were analyzed on a 5% nondenaturing polyacrylamide gel in Tris acetate, EDTA buffer. The gel was stained with ethidium bromide (Molecular Probes, Eugene, OR, USA) and visualized using a gel imager (Biospectra UVP, Upland, CA, USA) and Visionworks software (Biospectra UVP). PCR products were sequenced to confirm identity (GeneWiz, South Plainfield, NJ, USA; Supplemental Table S1).
Table 1.
Primers used for PCR amplification of glycine receptor chloride channel subunits in guinea pig and human airway smooth muscle
| Name | Primer sequence, 5′–3′ | Product size (bp) |
|---|---|---|
| GLRA1 | GAGAAGGACTTGAGATACTGCACCATGTTGATCCAGAAGGAGATCCATGAG | 163 |
| GLA2 | CCTCCGAAGAAGACAGAAGAGGCAGAATAGCCCGGTCCACAAACTTCTTCTTGATAG | 195 |
| Human GLRA3 | GAATAAGACAGAAGCTTTTGCACTGGAGAAGTTGCTCGGGAGATGGTATCAATCTTCTTGG | 237 |
| Guinea pig GLRA3 | CTTTTGCACCAAGCACTACAACACAGGTCATGCCTAAGAATTTTATAGATAACCCAGTAGA | 292 |
| GLRA4 | GAAGATGCTCCTGCTGTCCAAGTGCAGAAGGAGACCCAGGACAGGATGA | 218 |
| GLRB | TGGAAAAGGTGGAAATGTGGCTAAAAAGAACAATGCTGAAGTCATTAGATCTCAGATCAGACTTAGAA | 146 |
Immunoblot
Confluent cultures of human airway smooth muscle cells were rinsed in cold PBS and mechanically removed from the surface of the culture flask in the presence of protease inhibitor cocktail (Calbiochem protease inhibitor cocktail set III, EDTA free). Cells were centrifuged at 200 g for 10 min at 4°C, and the pellet was resuspended in RIPA buffer as described previously (4). Cell suspensions were homogenized at 4°C using a high-speed homogenizer at top speed for 30 s. Whole-cell lysates were solubilized by heating at 95°C for 10 min in gel loading buffer, as described previously (4).
Native guinea pig and human airway smooth muscle were dissected with a microscope from trachea and mainstem bronchi and were homogenized at top speed for 30 s in RIPA buffer. Homogenized whole tissue lysate was then centrifuged at 500 g for 10 min at 4°C. Supernatant was stored at −80°C. Each sample was then solubilized by heating in 95°C heating block for 10 min in denaturing gel loading buffer (4).
All samples (60–90 μg) and positive controls (60–90 μg) were electrophoresed (8% SDS-PAGE) and transferred to PVDF membranes. Nonfat milk (5%)was used to block nonspecific binding sites for 1 h. Primary antibodies were applied in 1% nonfat milk overnight at 4°C (primary antibodies and dilutions listed in Table 2). HRP-labeled secondary antibody in 1% milk was then applied for 1 h at room temperature. Visualization was obtained by ECL (GE Healthcare, Little Chalfont, UK) or Super Signal Femto (Pierce, Rockford, IL, USA) and was recorded on film (Kodak BioMax light film; Kodak, Rochester, NY, USA) or digital pictures (Biospectra UVP).
Table 2.
Primary antibodies used for immunoblot detection of glycine channel subunits
| Primary antibody | Product number | Source | Dilution | MW (kDa) |
|---|---|---|---|---|
| GLRA1 | Santa Cruz 17283 | Rabbit | 1:1000 | 65 |
| GLRB | Millipore Ab15012 | Goat | 1:1000 | 48 |
Electrophysiology
Immortalized human airway smooth muscle cells were grown to confluence on collagen-treated T25 flasks. Collagenase type IV in SmBM2 medium was used to release cells adherent to the collagen matrix in the flask. Medium with cells in suspension was then harvested in a 10-ml conical tube and centrifuged at 300 g. Supernatant was removed, and the pellet was resuspended in SmBM2 medium and transferred into collagen-treated glass bottom 1-cm Petri dishes at ∼10% confluence. Each dish was then incubated at 37°C 5% CO2 for 1–4 h for reattachment of cells to glass-bottom Petri dishes.
Glass-bottom dishes served as a disposable recording chamber. ALA VM-8, an 8-chamber pressure-driven drug application system, was used in a still bath of extracellular salt solution. Whole-cell intracellular voltage recordings under current clamp conditions were performed with a 2-kHz Bessel filter, recording at 10 kHz using an Axopatch 200b amplifier (Axon Instruments, Foster City, CA, USA). Intracellular solutions consisted of (in mM) 140 KCl, 5 MgATP, 5 EGTA, 1 MgCl2, 10 HEPES, and 5 CaCl2 (pH 7.2). Extracellular solution consisted of (in mM) 134 NaCl, 1.4 KCl, 10 HEPES, 1 MgCl2, 1.8 CaCl2, and 10 glucose (pH 7.4). Electrodes were pulled using a P-97 micropipette puller from 1.5-mm OD borosilicate capillary glass (Sutter Instruments, Novato, CA, USA). Glass electrode resistances ranged from 5 to 10 MΩ with intracellular solution.
Whole-cell intracellular current recordings under voltage-clamp conditions were performed with a 2-kHz Bessel filter, recording at 10 kHz using an Axopatch 200b amplifier. When recording glycine responses, the intracellular solutions consisted of (in mM) 130 CsCl, 5 MgATP, 5 EGTA, 1 MgCl2, 10 HEPES, and 5 CaCl2 (pH 7.2), and the extracellular solution consisted of (in mM) 130 CsCl, 10 HEPES, 1 MgCl2, 1.8 CaCl2, and 10 glucose (pH 7.4). When recording K-gluconate current responses, intracellular and extracellular solutions were the same as the solutions previously mentioned in the voltage recordings protocol. Electrodes were pulled using a P-97 micropipetter puller from 1.5-mm OD borosilicate capillary glass (Sutter Instruments). Glass electrode resistances ranged from 5 to 10 MΩ with intracellular solution. All recordings were analyzed on Clampfit 8.0 software (Molecular Devices).
Membrane potential measurements using FLIPR
The functional channel activity of the GlyR Cl− channels was also characterized using the FLIPR membrane potential assay. Protocols have been described previously (9). Human airway smooth muscle cells were grown to 100% confluency in 96-well black-walled clear-bottom plates. Cells were treated with medium with serum 24 h prior to assay (SmGm2 medium). On the day of the assay, cells were washed with Krebs buffer, as described previously (9). FLIPR membrane potential blue dye was dissolved in Krebs buffer at the concentration of 1 vial (∼125 mg) in 100 ml. Cells were incubated in 100 μl of 50% dye for 20 min in a humidified 37°C cell culture incubator (95% air/5% CO2). Fluorescence was repetitively measured at 2-s intervals in a prewarmed (37°C) Flex Station 3 UV spectrophotometer (Molecular Devices) using an excitation wavelength of 530 nm, an emission wavelength of 565 nm and a cutoff filter of 550 nm. NS1619 (100 μM; potassium channel opener causing hyperpolarization), 40 mM K-gluconate (depolarizing agent) or 1 mM glycine was added at a single concentration. In separate assays, glycine at increasing concentrations (1 μM–1 mM) was automatically injected into individual wells with or without a 400 μM strychnine pretreatment.
Functional studies of airway smooth muscle
Closed guinea pig tracheal rings containing two incomplete cartilagenous rings were suspended at 1 g resting tension in oxygenated KH buffer at 37°C, as described previously (10, 11). Briefly, closed rings were tied with silk suture on the cartilage at the cartilage-smooth muscle border bilaterally. One suture was tied to a fixed hook, while the other suture was tied to a Grass FT03 force transducer (Grass Telefactor, West Warwick, RI, USA). The closed ring remained in a temperature-controlled bath filled with 4 ml KH buffer, and muscle force was continuously digitally recorded. Each tracheal ring was precontracted with a standardized pretreatment paradigm. Rings were first contracted with N-vannilylnonanamide (10 μM), a capsaicin analog that depletes nonadrenergic noncholinergic nerves. KH buffer was exchanged in the organ baths, resting tone was reset to 1 g, and the tracheal rings were subjected to 2 cycles of increasing log concentrations of ACh (100 nM–100 μM). All baths were washed by 6 KH buffer exchanges, and tensions were adjusted to 1 g after each cycle. Following the second cycle of an acetylcholine dose response, the organ bath KH buffer was exchanged 8 times, and resting tension was adjusted to 1 g. To eliminate the confounding effects of airway nerves and histamine receptors, tetrodotoxin (1 μM) and pyrilamine (10 μM) were added to the buffers of all organ baths. Rings then underwent separate experiments in which they were contracted with acetylcholine (EC50 concentration) or 100 nM β-ala NKA fragment 4–10 (a subtype-selective agonist at the neurokinin type 2 receptor) in the absence or presence of glycine or taurine with or without pretreatment with GlyR Cl− channel antagonists (strychnine and ginkoglide B), as detailed below.
In vitro assessment of glycine effects on tension after an acetylcholine EC50 contraction
Following the above standardized pretreatments, each guinea pig tracheal ring was contracted with an EC50 acetylcholine contraction. At peak tension, baths were randomly assigned as control (untreated) or treated (1 mM glycine) rings with measurement of the maintenance of muscle force over 10 min.
In vitro assessment of glycine effects on tension after a β-ala-NKA fragment 4–10 contraction
Following the above standardized pretreatments, each guinea pig tracheal ring was contracted with 100 nM β-ala-NKA fragment 4–10. At steady-state tension (∼5 min) baths were randomly assigned as control (untreated) or treated (1 mM glycine) rings with measurement of the maintenance of muscle force over 20 min.
In vitro assessment of GlyR Cl− channel agonist effects on β2-adrenoreceptor-mediated airway smooth muscle relaxation after an EC50 contraction with acetylcholine
Each guinea pig tracheal ring was contracted by acetylcholine EC50 dose determined by the above precontraction protocol. At steady state tension (∼15 min), each bath was randomly assigned into 3 treatment groups. Control groups were exposed to cumulatively increasing isoproterenol concentrations in half-log increments, as described previously (10). Experimental groups were pretreated with 100 μM taurine or 1 mM glycine 5 s before isoproterenol treatments. In separate experiments, GlyR Cl− channels were pretreated with antagonists before the addition of taurine/isoproterenol or glycine/isoproterenol. Taurine-treated groups were antagonized by pretreatment with 1 μM ginkoglide B and 3 μM strychnine 15 min before the acetylcholine contractile challenge. Glycine-treated groups were antagonized by pretreatment with 3 μM strychnine 15 min before contractile the acetylcholine challenge.
Statistical analysis
Each experimental permutation included intraexperimental vehicle controls. We employed one-way ANOVA with Bonferroni post-test comparisons between appropriate groups. In addition, dose-response curves were evaluated using a sigmoidal dose-response analysis function in Prism 4.0 software (GraphPad, San Diego, CA, USA), which employs a 4-parameter logistic equation, according to the Hill model: Y = min + (Emax − min)/(1 + 10(dose−logEC50)), where min represents the initial resting muscle tension. In cases in which only 2 experimental groups were being compared, a 2-tailed Student's t test was employed. Data are presented as means ± se; P < 0.05 in all cases was considered significant.
Image processing
All RT-PCR and immunoblot gel images have been modified in contrast, brightness, and grayscale level in order to enhance image quality. In all cases, adjustments were applied identically to a single image. Some images have had the order of individual lanes digitally changed to maintain the same order of samples between all images.
RESULTS
Human airway smooth muscle contains mRNA encoding subunits of the glycine chloride channel
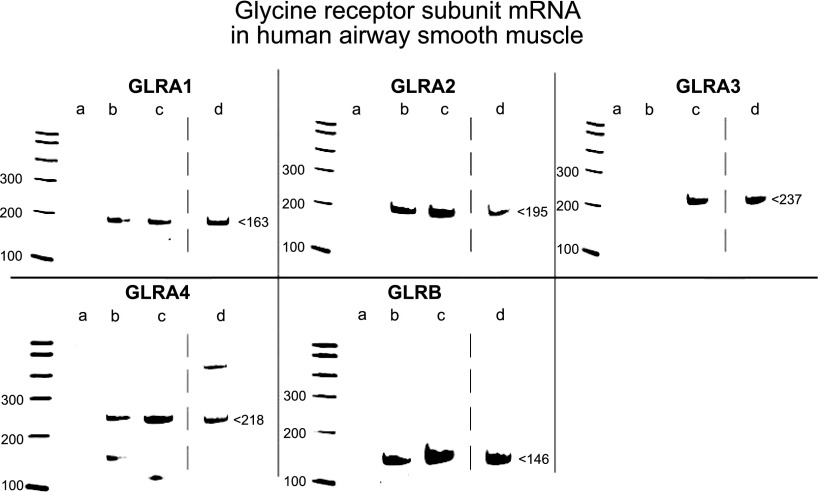
Messsenger RNA isolated by laser microdissection from native airway smooth muscle contained within human trachea (n=3–4), and mainstem bronchi was reverse transcribed into cDNA and amplified by PCR using primers (Table 1) specific for each of the known 4 GLRA subunits and single GLRB subunit of the GlyR Cl− channel. PCR yielded products of predicted sizes for the GLRA1, GLRA2, GLRA4, and GLRB subunits of the GlyR Cl− channel, but not for the GLRA3 subunit (Fig. 1, lane b). RNA isolated from a homogenous cell population from an immortalized human airway smooth muscle cell line (n=10) contained mRNA encoding all 4 known GLRA subunits and the GLRB subunit of the GlyR Cl− channel (Fig. 1, lane c). RNA from whole human brain (positive control) yielded PCR products of predicted sizes for all 4 GLRA subunits and the GLRB subunit (Fig. 1, lane d). Confirmation of sequence was performed on GLRA1, GLRA2, GLRA4, and GLRB (Supplemental Table S1).
Figure 1.
mRNA expression of GlyR Cl− channel subunits in human airway smooth muscle detected by RT-PCR. Representative gel images of RT-PCR products of known glycine subunits. RNA isolated from native human airway smooth muscle by laser capture microdissection includes mRNA that encodes GLRA1, GLRA2, GLRA4, and the GLRB subunit of GlyR Cl− channels. RNA isolated from cultured human airway smooth muscle cells and whole human brain (positive control) express all 4 known GLRA subunits and the GLRB subunit of GlyR Cl− channels. Gel images are representative of the analysis of 3–10 individual samples from native and cultured human airway smooth muscle. Lane a: negative control (no cDNA input); lane b: native human airway smooth muscle; lane c: immortalized human airway smooth muscle cells; and lane d: whole human brain.
GlyR Cl− channel subunit mRNA expression is conserved between humans and guinea pigs
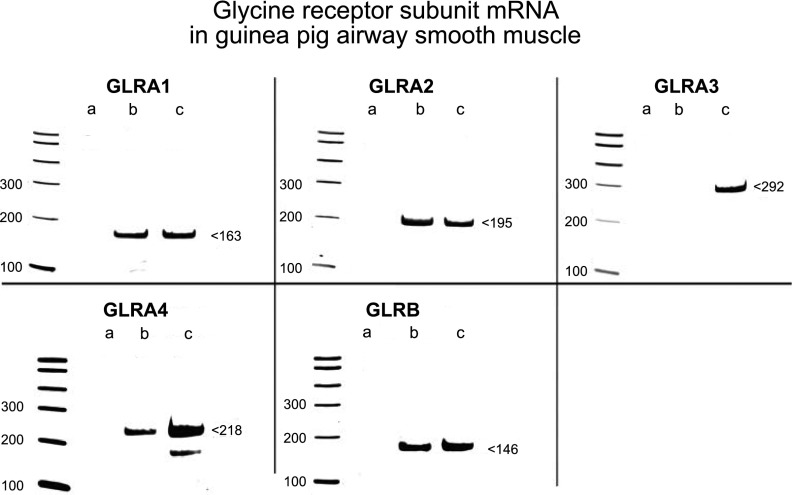
RNA isolated from native guinea pig tracheal airway smooth muscle using laser capture microdissection (n=2–4) also yielded RT-PCR products representing GLRA1, GLRA2, GLRA4, and GLRB subunits (Fig. 2, lane b), but GLRA3 was not expressed, in agreement with results seen in native human airway smooth muscle. RNA isolated from whole guinea pig brain served as a species-specific positive control and yielded RT-PCR products of predicted sizes for all 4 GLRA and the GLRB subunits of the GlyR Cl− channel (Fig. 2, lane c). The conservation of subunit expression between guinea pigs and humans validated the use of guinea pig airway smooth muscle for subsequent glycinergic functional studies. Confirmation of sequence of PCR was performed on GLRA1, GLRA2, GLRA4, and GLRB (Supplemental Table S1).
Figure 2.
mRNA expression of GlyR Cl−-channel subunits in guinea pig airway smooth muscle detected by RT-PCR. Representative gel images of RT-PCR products of known glycine subunits. RNA isolated from native guinea pig airway smooth muscle by laser capture microdissection includes mRNA that encodes GLRA1, GLRA2, GLRA4, and the GLRB subunit of GlyR Cl− channels. RNA isolated from whole guinea pig brain (species-specific positive control) express all 4 known GLRA subunits and the β subunit of GlyR Cl− channels. Gel images are representative of the analysis of 2–4 individual samples from native guinea pig airway smooth muscle. Lane a: negative control (no cDNA input); lane b: native guinea pig airway smooth muscle; and lane c: whole guinea pig brain.
Protein expression of GlyR Cl− channel subunits in human and guinea pig airway smooth muscle
Immunoreactive bands of expected molecular mass were detected by immunoblotting for the GLRA1 and GLRB subunits in native human (Fig. 3, lane b) and guinea pig airway smooth muscle (Fig. 3, lane d), as well as in cultured human airway smooth muscle cells (n=4; Fig. 3, lane c).
Figure 3.
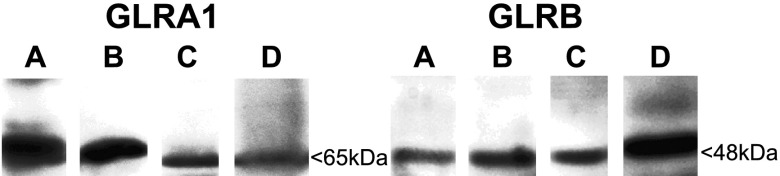
Protein expression of GlyR Cl− channel subunits in human and guinea pig airway smooth muscle detected by immunoblot. Representative gel images of immunoblot of known glycine subunits. Protein was extracted from native human airway smooth muscle, native guinea pig airway smooth muscle, and cultured immortalized human airway smooth muscle cells and probed with antibodies against GLRA1 and the GLRB subunits of GlyR Cl− channels. Protein isolated from human whole brain and retinal lysates was used as positive controls. Gel images are representative of the analysis of 4 individual samples from native guinea pig airway smooth muscle, native human airway smooth muscle, and immortalized human airway smooth muscle cells. Lane A: left panel, guinea pig retina; right panel, guinea pig brain; lane B: native human airway smooth muscle; lane C: cultured immortalized human airway smooth muscle cells; and lane D: native guinea pig airway smooth muscle.
Glycine-induced current measurements in immortalized human airway smooth muscle cells
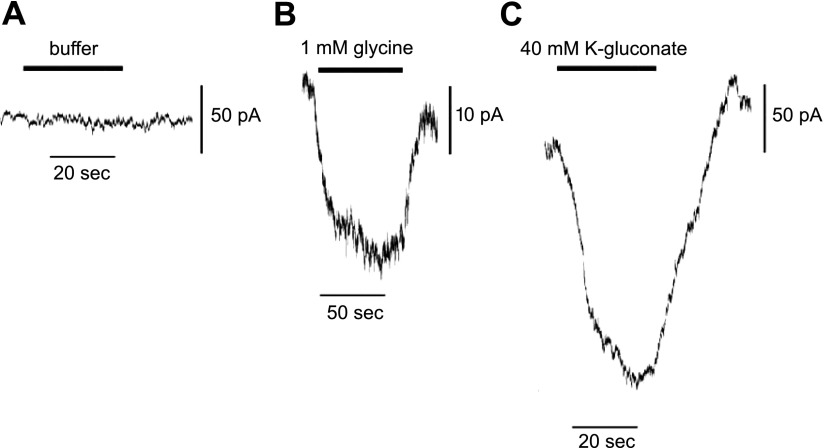
Glycine-gated chloride channel currents were measured using whole-cell inside-out patch-clamp configuration with equimolar cesium chloride both in the internal and external solutions. Three of 7 cells displayed functional increases in current (∼20–30 pA) with treatments of glycine, when voltages were clamped at −60 mV. A representative tracing of a typical glycine induced current is shown (Fig. 4B). To show the relative magnitude of the chloride current of the GlyR Cl− channel, buffer (Fig. 4A) and 40 mM potassium gluconate (Fig. 4C) control current measurements were performed, and currents were measured. Treatment with 40 mM potassium gluconate resulted in a current measurement ranging from ∼120 to 250 pA in 5 of 5 cells.
Figure 4.
Electrophysiological demonstration of GlyR Cl−-channel current activity in airway smooth muscle cells. Representative tracings of a airway smooth muscle in whole-cell configuration under voltage. A) Buffer addition. B) Glycine (1 mM) yielded a 24-pA current. Similar tracings were recorded in 3 of 7 cells patched. C) K-gluconate (40 mM) yielded a 212-pA current. Similar tracings were recorded in 5 of 5 cells patched.
Quantification of glycine-induced plasma membrane potential changes in cultured airway smooth muscle cells in relation to FLIPR emission fluorescence
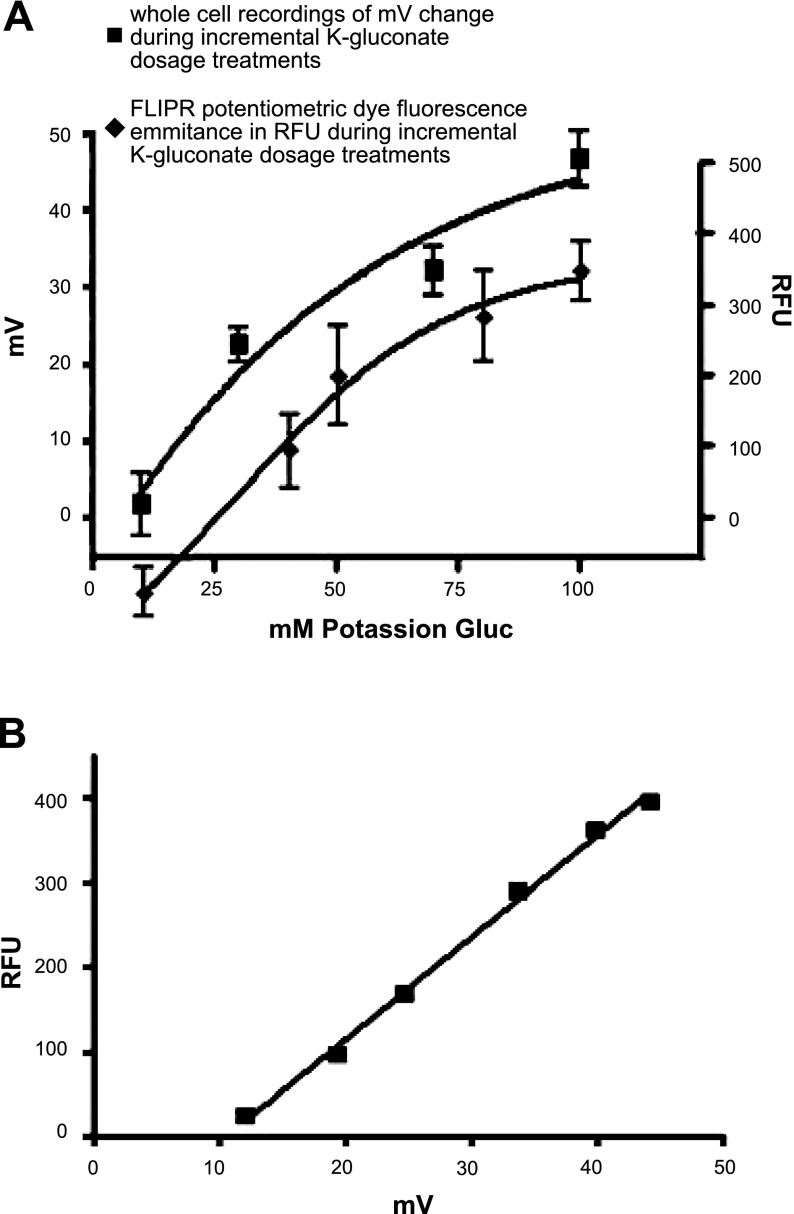
A standard curve was derived using dose responses to potassium gluconate in the FLIPR microplate reader experiments and in electrophysiological recordings of membrane potential. A Boltzmann nonlinear regression curve fit was used to generate dose-response curves (Fig. 5A). A linear plot was then derived from the relationship of these two curves creating a fluorescence [relative fluorescence units (RFU)] vs. membrane potential (mV) relationship plot (Fig. 5B).
Figure 5.
Quantification of glycine-induced plasma membrane potential (mV) changes in cultured airway smooth muscle cells in relation to FLIPR emission fluorescence. A) Dose response to K gluconate, measuring either fluorescence [relative flurorescence units (RFU); bottom curve, right axis] or membrane potential (top curve, left axis) in cultured airway smooth muscle cells. Fluorescence was measured by potentiometric FLIPR dye, and membrane potential was measured by electrophysiological whole-cell recordings. Curves were approximated using a Boltzmann nonlinear regression curve fit. B) Linear regression of matched data points from panel A Boltzmann curves, correlating membrane potential change to fluorescence change.
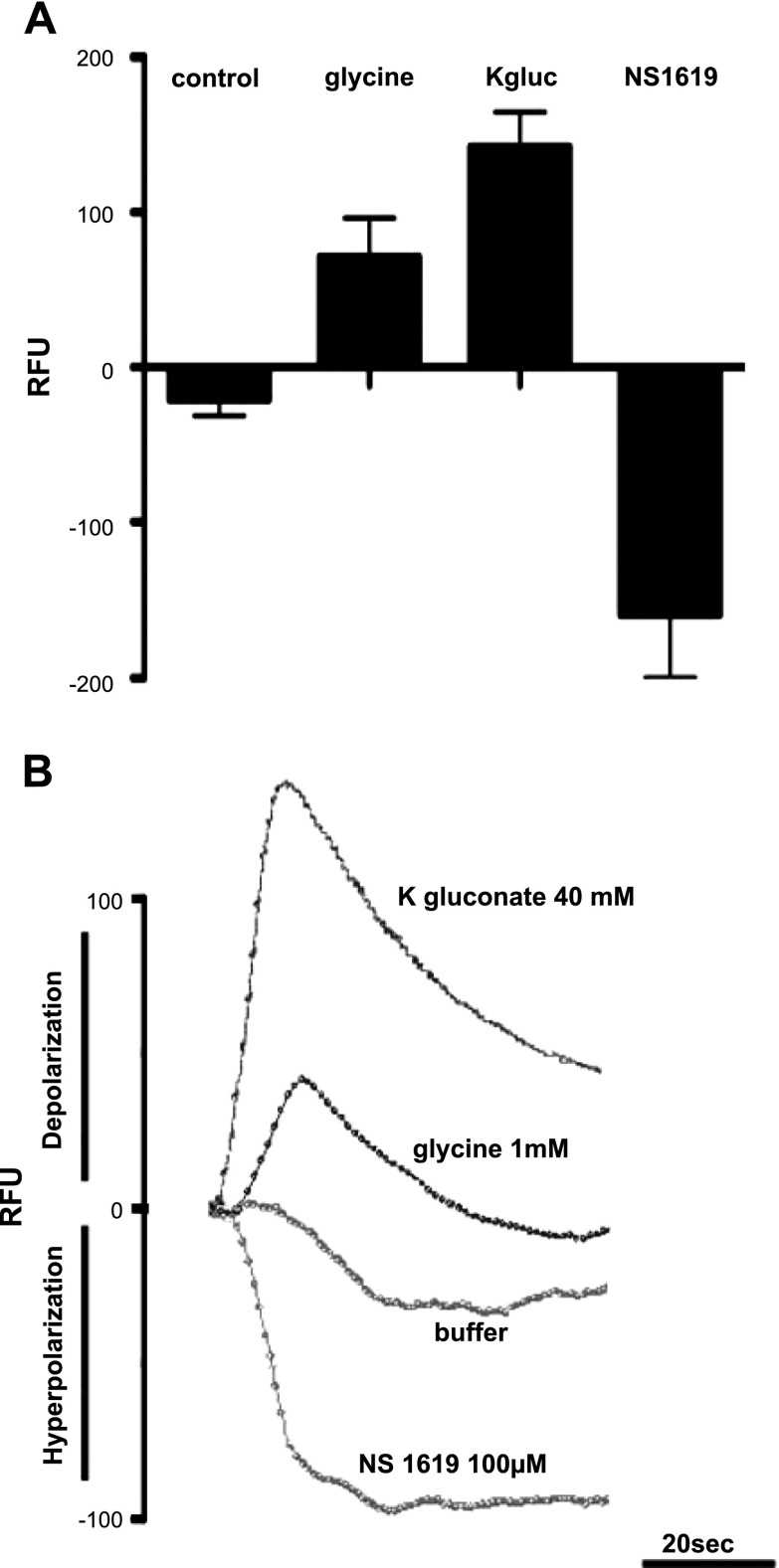
During the FLIPR fluorescent dye studies, 1 mM glycine treatments were performed with hyperpolarization controls (100 μM NS1619) and depolarization controls (40 mM K gluconate) (Fig. 6B). Cells treated with 1 mM glycine displayed a mean ± se value of 71 ± 25 RFU (Fig. 6A). According to the linear plot of fluorescence vs. membrane potential, 71 RFU correlates to a change in membrane potential of 10–15 mV.
Figure 6.
FLIPR dye assessment of depolarization and hyperpolarization stimulation of airway smooth muscle in response to NS1619, K-gluconate and glycine. A) Graphical representation of FLIPR potentiometric dye fluorescence emissions (RFU) after cultured airway smooth muscle cells were treated with either buffer (control), 1 mM glycine, 40 mM K-gluconate, or 100 μM NS1619 (n=6–12). Mean mean ± se fluorescence change for 1 mM glycine is 71 ± 25 RFU; for K-gluconate, 142 ± 22 RFU. B) Representative tracing of real-time FLIPR potentiometric dye fluorescence emissions after cultured airway smooth muscle cells were treated with either buffer, 1 mM glycine, 40 mM K-gluconate, or 100 μM NS1619. Tracings display directionality of fluorescence change in relation to depolarization (upward deflection: glycine K-gluconate) and hyperpolarization (downward deflection: NS1619).
Glycine induces plasma membrane potential changes in cultured airway smooth muscle cells, which is attenuated by a GlyR Cl− channel-specific antagonist
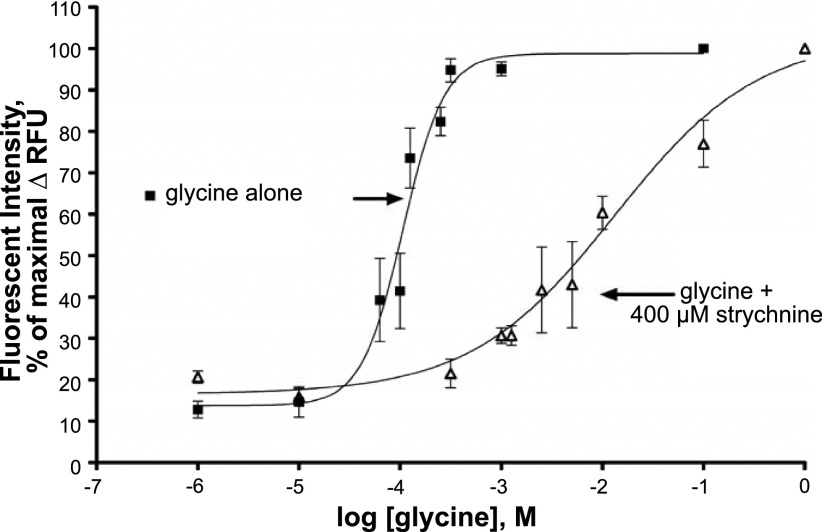
Glycine dose-response curves were performed using a membrane potentiometric fluorescent dye, which yielded an EC50 value for glycine of 106 μM (n=4; Fig. 7), which is a value similar to the EC50 values measured in HEK cell lines overexpressing GlyR Cl− channel subunits (9). Glycine dose-response curves were generated after pretreatment with 400 μM strychnine, which shifted the glycine EC50 over 2 log orders of magnitude (n=3–6). These studies show that glycine dose dependently induced ion movement in human airway smooth muscle cells that was effectively blocked by the specific GlyR Cl− channel antagonist strychnine.
Figure 7.
Dose-dependent glycine effects on membrane potential in cultured human airway smooth muscle cells. Glycine dose-dependently (1 μM to 1 M) increases fluorescent intensity, indicative of a change in membrane potential in cultured human airway smooth muscle cells, yielding an EC50 value of 106 μM for glycine (n=4). This glycine dose-response curve was shifted to the right, increasing the EC50 by 2 log orders of magnitude in the presence of 400 μM strychnine.
Glycine directly relaxes acetylcholine-induced contraction in guinea pig tracheal rings
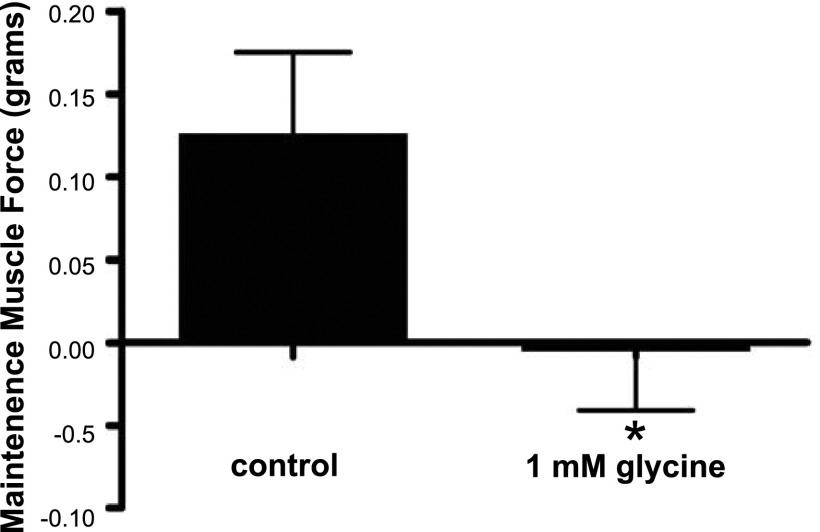
To demonstrate that glycine can directly relax airway smooth muscle contracted by the classic contractile agonist acetylcholine, maintenance of muscle force was measured in guinea pig tracheal rings contracted with acetylcholine. Guinea pig tracheal rings contracted with EC50 dose of acetylcholine had a decrease in the maintenance of muscle force over a 600-s period when 1 mM glycine was added at the peak of the induced contraction, compared to control rings, which actually demonstrated an increase in muscle force over the same period (n=3; P<0.01; Fig. 8).
Figure 8.
Glycine treatment of guinea pig tracheal ring airway smooth muscle inhibits maintenance of muscle force induced by acetylcholine EC50. Guinea pig tracheal rings contracted by EC50 concentration of acetylcholine demonstrated a reduced maintenance of contracted tone over 600 s in the presence of 1 mM glycine. *P < 0.01 (n=3).
Glycine directly relaxes neurokinin A-induced contraction in guinea pig tracheal rings
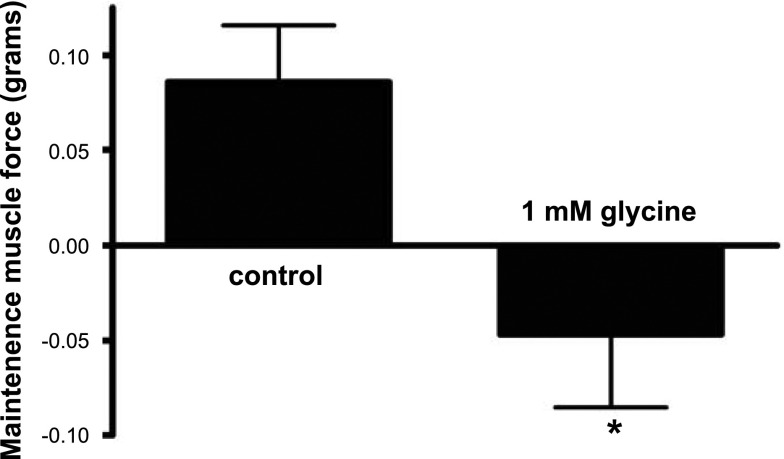
To demonstrate that glycine can directly relax airway smooth muscle (in addition to potentiating isoproterenol-induced relaxation) and to demonstrate that prorelaxant effects of glycine were not limited to one contractile agonist, maintenance of muscle force was measured in guinea pig tracheal rings contracted with neurokinin A. Guinea pig tracheal rings contracted with 100 nM neurokinin A had a decrease in the maintenance of muscle force over a 1200-s period when 1 mM glycine was added at the peak of the induced contraction, as opposed to control rings, which demonstrated an increase in muscle force over the same period (n=8–9; P<0.05; Fig. 9).
Figure 9.
Glycine treatment of guinea pig tracheal ring airway smooth muscle inhibits maintenance of muscle force induced by neurokinin A. Guinea pig tracheal rings contracted by 100 nM neurokinin demonstrated a reduced maintenance of contracted tone over 1200 s in the presence of 1 mM glycine. *P < 0.05 (n=8–9).
Glycine and a glycine channel agonist each potentiate isoproterenol induced relaxation of acetylcholine-contracted guinea pig airway smooth muscle
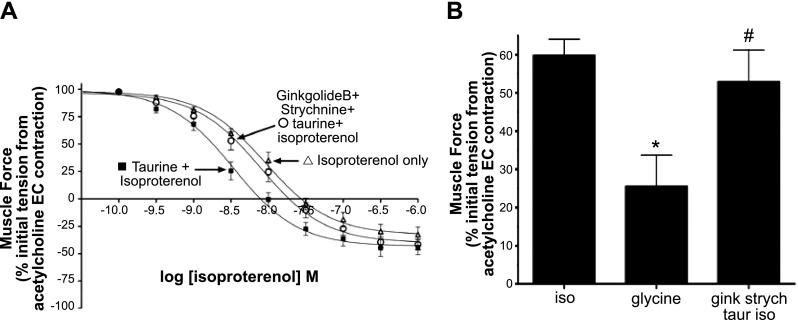
Guinea pig airway smooth muscle rings under isometric tension in organ baths were contracted with an EC50 concentration of acetylcholine and relaxed with cumulatively increasing concentrations of isoproterenol in the absence or presence of glycine (with or without the GlyR Cl− channel antagonist, strychnine) or taurine, a ligand with partial affinity for the GlyR Cl− channel (with or without the antagonsist strychnine). The EC50 for relaxation induced by isoproterenol alone was 9.2 nM (n=13; Fig. 10A). In the presence of the partial agonist taurine (200 μM), the isoproterenol-induced relaxation shifted to the left, yielding an EC50 value of 3.4 nM (n=13; Fig. 10A). To specifically implicate the activation GlyR Cl− channels by taurine, 2 GlyR Cl− channel-specific antagonists were included in organ bath buffers of the tracheal rings; strychnine and ginkoglide B (3 and 1 μM, respectively). The dose response for isoproterenol-induced relaxation in the presence of taurine and the GlyR Cl− channel antagonists was shifted back to the right, yielding an EC50 value (7.8 nM) similar to that obtained with isoproterenol alone (n=14; Fig. 10A). Extrapolated data from the dose-response curves near the isoproterenol EC50 value (5 nM; Fig. 10B), shows that at a dose of 5 nM isoproterenol, taurine increases relaxation significantly (P<0.01) compared to control (n=13–14; Fig. 10B). Treatment with the antagonists strychnine and ginkgolide B blocks taurine-induced enhanced relaxation (P>0.05) compared to control (n=13–14; Fig. 10B).
Figure 10.
Isoproterenol-induced relaxation of acetylcholine-contracted guinea pig airway smooth muscle is enhanced by the partial GlyR Cl− channel agonist taurine. A) Isoproterenol concentration-response curves comparing treatment with isoproterenol only (▵) to isoproterenol after a single concentration of 100 μM taurine (■) or isoproterenol with a single concentration of 100 μM taurine in the presence of 3 μM strychnine and 1 μM ginkgolide B (○). Taurine (100 μM) enhancement of isoproterenol relaxation was reversed in the presence of GlyR Cl− channel-specific inhibitors ginkgolide B and strychnine (n=13–14). B) Relaxation of acetylcholine-induced contraction by a single dose of isoproterenol (iso; 5 nM) is enhanced by taurine (taur; 100 μM). This taurine enhancement of isoproterenol-mediated relaxation was reversed in the presence of strychnine (strych; 3 μM) and ginkgolide B (1 μM). *P < 0.01 vs. control (n=13–14); #P = ns vs. control (n=13–14).
In another group of studies, isoproterenol alone showed a relaxation curve yielding an EC50 value of 11.1 nM (n=11; Fig. 11A). Glycine (1 mM) shifted the relaxation curve to the left, yielding an EC50 value of 4.5 nM (n=16; Fig. 11A). To confirm that glycine's effects were on the GlyR Cl− channels, strychnine (30 μM) was included in the organ bath buffer, and isoproterenol-induced relaxation curves in the presence of glycine with strychnine were shifted to the right, yielding an EC50 value (8.8 nM) similar to that obtained with isoproterenol alone (n=7; Fig. 11A). Extrapolated data from the dose-response curves near the isoproterenol EC50 value (10 nM; Fig. 11A), shows that at a dose of 10 nM isoproterenol, glycine increases relaxation significantly (P<0.01) compared to control (n=11–16; Fig. 11B), while treatments with strychnine counteract glycine-induced enhanced relaxation, yielding isoproterenol EC50 values not significantly different from controls (n=7–11; P>0.05; Fig. 11B).
Figure 11.
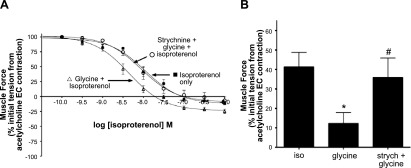
Isoproterenol-induced relaxation of acetylcholine-contracted guinea pig airway smooth muscle is enhanced by glycine. A) Isoproterenol concentration-response curves comparing treatment with isoproterenol only (■) to isoproterenol after a single concentration of 1 mM glycine (▵) or isoproterenol with a single concentration of 1 mM glycine in the presence of 30 μM strychnine (○). This glycine enhancement of isoproterenol relaxation was reversed in the presence of the GlyR Cl− channel-specific inhibitor, strychnine. (n=7–16). B) Relaxation of acetylcholine-induced contraction by a single dose of isoproterenol (iso; 10 nM) is enhanced by glycine (1 mM). This glycine enhancement of isoproterenol-mediated relaxation was reversed in the presence of strychnine (strych; 30 μM). *P < 0.05 vs. control (n=11–16); #P = ns vs. control (n=7–11).
DISCUSSION
The primary findings of the present study are that GlyR Cl− channels are expressed on airway smooth muscle cells, and they function to facilitate relaxation of airway smooth muscle. This novel finding points toward new therapeutic targets for relaxation of constricted airway smooth muscle in diseases such as asthma and chronic obstructive pulmonary disease.
Our laboratory recently discovered the functional expression of the GABAA ligand-gated chloride channels in airway smooth muscle (4). In studies characterizing the GABAA channel, it was noted that taurine, an agonist at both GABAA and GlyR Cl− channels, potentiated relaxation, but this effect was only partially reversed by the GABAA channel antagonist gabazine. This led us to question whether part of the effect of taurine was due to activity at a previously undescribed GlyR Cl− channel in airway smooth muscle. Indeed, in the present study, we show that taurine-induced potentiation of isoproterenol-mediated relaxation of airway smooth muscle is reversed by strychnine and ginkgolide B, GlyR Cl− channel antagonists. Moreover, we also demonstrate that the endogenous ligand glycine also potentiated isoproterenol-mediated relaxation and was also blocked by GlyR Cl− channel antagonism. Furthermore, glycine alone was able to attenuate the maintenance of a contraction initiated by another contractile agonist, β-ala NKA fragment 4–10, in airway smooth muscle.
These functional studies led us to confirm the molecular expression of GlyR Cl− channel subunits in airway smooth muscle. GlyR Cl− channels, like their superfamily relatives, the GABAA channels, are widely expressed in neuronal cells, but their extraneuronal expression and function have been less widely studied. GlyR Cl− channels have been found in human macrophages, endothelial cells, and certain endocrine glands (5, 12, 13). The present study demonstrates mRNA and protein expression of multiple subunits of GlyR Cl− channels in airway smooth muscle. Messenger RNA encoding the GLRA1, GLRA2, GLRA4, and GLRB subunits were identified in native airway smooth muscle from trachea of humans and guinea pigs. The glycine GLRA3 subunit mRNA is not present in native guinea pig or human airway smooth muscle, but it was detected in immortalized human airway smooth muscle cells. Evidence of modulation in glycine subunit expression has previously been demonstrated in cells in culture (14). We hypothesize that culture conditions, serum, or growth factor components may induce glycine GLRA3 subunit expression in cultured human airway smooth muscle cells. Our studies identified mRNA encoding the GLRA4 subunit in guinea pigs as 2 splice variants (data not shown for one variant) and a single variant in human airway smooth muscle. Although GLRA4 subunit mRNA expression was found in the present study in human airway smooth muscle, previous studies have shown that exon 9 of the GLRA4 subunit has a stop codon in humans, preventing the translation of the fourth transmembrane domain of the GLRA4 subunit protein, which would render it nonfunctioning (15).
Analysis of protein expression focused on the GLRA1 and β subunits for 2 reasons. Our study of membrane potential with a potentiometric dye showed the EC50 of glycine to be 106 μM. This is similar to the EC50 of glycine for GlyR Cl− channels composed of GLRA1/GLRB subunits (89 μM; ref. 9) or homomeric GLRA1 subunits (82 μM; ref. 9). In contrast, the glycine EC50 for GLRA2 homomeric GlyR Cl− channels is 290 μM (9). Second, the GLRA1/ GLRB subunit combination is the predominantly expressed combination in adult human neurons, while expression of glycine GLRA2 subunits is predominantly limited to neonatal neurons (6). We did not detect glycine GLRA3 mRNA in native airway smooth muscle, and full-length functional glycine GLRA4 subunits have not been described in human tissues. We identified protein expression of glycine GLRA1 and GLRB subunits in native human and guinea pig airway smooth muscle, as well as cultured human airway smooth muscle cells. The finding of these subunits in a homogeneous population of cultured airway smooth muscle cells further confirms expression of GlyR Cl−-channel subunit proteins directly on airway smooth muscle cells. This conservation of expression between species helps validate the use of guinea pig airway smooth muscle cells in our accompanying functional studies.
GlyR Cl− channels act as ligand-gated pores, which are selective for anion movement (5). Any anion movement in or out of the cell will lead to a change in the membrane potential. Chloride movement is dictated by membrane potential in relation to the chloride equilibrium potential. Therefore, opening of a chloride channel at resting membrane potential, which is below the chloride equilibrium potential in ASM, would allow for chloride efflux, causing depolarization. However, at a depolarized state, following a contractile agonist, the membrane potential is above the chloride equilibrium, resulting in inward chloride flow and relative hyperpolarization.
Using potentiometric dyes, we demonstrate in the present study that glycine dose dependently changes membrane potential in human airway smooth muscle cells, and this effect was significantly blocked, but not fully blocked, by pretreatment with the GlyR Cl− channel antagonist strychnine. Glycine as a ligand is known to act on other channels and have effects on ion movement. For example, NMDA channels use glycine as a coagonist with glutamate and open nonspecific cation channels on airway smooth muscle (16). In addition, cotransport of glycine with sodium and chloride through glycine transporters (GLYT) may be another pathway in which glycine changes the electrophysiological properties of ASM (17); however, GLYT has not yet been demonstrated on ASM. Although we were not able to achieve full blockade of glycine treatment effects using strychnine in the potentiometric dye studies, we were able to fully reverse the effects of glycine with strychnine (GlyR Cl− channel-specific antagonist) in the airway relaxant studies. These results suggest that glycine may have effects on channels and cotransporters other than GlyR Cl− channels, but the relaxation caused by glycine treatments is mainly due to glycine receptor chloride channels.
To determine whether glycine could directly contribute to the relaxation of airway smooth muscle (independently of isoproterenol), the maintenance of muscle force with or without glycine was measured following agonist (acetylcholine or β-ala NKA fragment 4–10) induced contractions. Glycine significantly attenuated the maintenance of these contractions.
Clinical relaxation of constricted airway smooth muscle in diseases, such as asthma and chronic obstructive pulmonary disease, is classically achieved with β-adrenoreceptor agonists. Therefore, our initial functional studies of glycine in isolated guinea pig tracheal rings employed contraction with a primary endogenous airway constrictor (acetylcholine) followed by relaxation with the β-adrenoreceptor ligand (isoproterenol). One mechanism by which β-agonists relax airway smooth muscle is by opening calcium-activated potassium (KCa) channels (7), allowing for the efflux of potassium to hyperpolarize plasma membrane potential, thus reversing the membrane depolarization induced during contraction. We hypothesized that opening of the glycine ligand-gated chloride channel under depolarized (acetylcholine-contracted) conditions should favor chloride influx and contribute to the relative hyperpolarization afforded by β-agonists, further facilitating relaxation. Indeed, our functional studies demonstrate that both glycine and taurine shift the isoproterenol dose-response curve toward enhanced relaxation, and these effects were blocked by the GlyR Cl− channel antagonist strychnine. These results agree with our previous studies using another ligand-gated chloride channel, the GABAA channel (10).
The relative importance of plasma membrane potential in the initiation and maintenance of contraction of an airway smooth muscle cell has been controversial (7, 18). Early studies of the importance of membrane potential in this cell type focused on the role of L-type calcium channels, because this was one of the earliest described voltage-dependent channels in this cell type. Enthusiasm for an important role for the L-type calcium channel (and membrane potential) was diminished when clinical trials of L-type calcium channel antagonists yielded only modest benefits in asthmatics (19) and when it was realized that full opening of the L-type calcium channel requires a membrane potential, threshold to peak, of about −35 to 10 mV, values not believed to be naturally achieved in a depolarized airway smooth muscle cell (3). However, evidence remains for a role of membrane potential in airway smooth muscle contraction and relaxation, including the opening of KCa channels as a component of β-agonist relaxation; the ability to induce airway smooth muscle contraction with blockade of potassium channels (e.g., tetraethylammonium chloride, iberiotoxin); and the identification of T-type calcium channels in airway smooth muscle, which fully open at a more hyperpolarized membrane potential compared to the earlier characterized L-type calcium channel (3).
Traditional understanding of airway smooth muscle contraction has focused on a key second messenger pathway activated by classic contractile agonists: the Gq protein pathway activating protein kinase C and phospholipase C (PLC), resulting in the synthesis of inositol phosphate (IP3) and the liberation of calcium from sarcoplasmic reticulum (SR) stores. A growing amount of evidence suggests that this Gq-PLC-IP3-Ca signaling pathway does not function independently of membrane potential (20, 21). In addition, changes in membrane potential have been shown to activate both M3 muscarinic receptors (Gq coupled; ref. 2) in airway smooth muscle cells and M2 muscarinic receptors (Gi coupled; ref. 22) independent of receptor occupancy by ligand.
Moreover, evidence that SR-released calcium can activate plasma membrane chloride channels, inducing depolarization (23), suggests that second messenger regulation of intracellular SR calcium release and plasma membrane potential do not operate in isolation. Furthermore, membrane potential regulation of Rho kinase activity has been described. Rho kinase-mediated deactivation of myosin light-chain phosphatase increases contractile sensitivity to cytoplasmic calcium influx whether the calcium arises from extracellular or intracellular sources. Depolarization induced by electric field stimulation (24) or pharmacologically (i.e., potassium chloride; ref. 25) have both demonstrated increases in Rho kinase activity and increases in muscle force generation. Thus, depolarization of the cytoplasmic membrane not only has a traditional role in plasma membrane calcium channel activation but also regulates key G-protein-coupled intracellular calcium pathways and phosphorylation of contractile proteins sensitive to calcium, resulting in enhanced muscle force generation.
This novel identification of GlyR Cl− channels in airway smooth muscle can be added to a growing list of chloride channels found on the airway smooth muscle, including the cystic fibrosis transmembrane receptor (CFTR; ref. 26), recently identified calcium-activated chloride channels of the anoctamin or TMEM16 family (27), GABAA channels (4) and now GlyR Cl− channels. The numerous and specific ways in which the airway smooth muscle cell can regulate chloride movement may underscore the previously unrecognized importance of these anions in airway smooth muscle cellular processes. Further studies on the role of chloride and membrane potential modulation may uncover physiological and pathophysiological mechanisms in airway smooth muscle and ultimately may elucidate new pharmacologic targets.
In summary, we demonstrate for the first time the expression of GlyR Cl− channels on human and guinea pig airway smooth muscle that function to change membrane potential and contribute to relaxation of airway smooth muscle from contractions induced by acetylcholine, a muscarinic receptor agonist, or β-ala NKA fragment 4–10, a tachykinin receptor agonist. These ligand-gated chloride channels may be a novel therapeutic target for the regulation of airway smooth muscle tone in diseases such as asthma and chronic obstructive pulmonary disease.
Supplementary Material
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Wijesinghe M., Weatherall M., Perrin K., Harwood M., Beasley R. (2009) Risk of mortality associated with formoterol: a systematic review and meta-analysis. Eur. Respir. J. 34, 803–811 [DOI] [PubMed] [Google Scholar]
- 2. Liu Q. H., Zheng Y. M., Korde A. S., Yadav V. R., Rathore R., Wess J., Wang Y. X. (2009) Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc. Natl. Acad. Sci. U. S. A. 106, 11418–11423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen L. J. (1997) T-type and L-type Ca2+ currents in canine bronchial smooth muscle: characterization and physiological roles. Am. J. Physiol. Cell Physiol. 272, C1757–C1765 [DOI] [PubMed] [Google Scholar]
- 4. Mizuta K., Xu D., Pan Y., Comas G., Sonett J. R., Zhang Y., Panettieri R. A., Jr., Yang J., Emala C. W., Sr. (2008) GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L1206–L1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch J. W. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 [DOI] [PubMed] [Google Scholar]
- 6. Lynch J. W. (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56, 303–309 [DOI] [PubMed] [Google Scholar]
- 7. Kotlikoff M. I., Kamm K. E. (1996) Molecular mechanisms of beta-adrenergic relaxation of airway smooth muscle. Annu. Rev. Physiol. 58, 115–141 [DOI] [PubMed] [Google Scholar]
- 8. Gosens R., Stelmack G. L., Dueck G., McNeill K. D., Yamasaki A., Gerthoffer W. T., Unruh H., Gounni A. S., Zaagsma J., Halayko A. J. (2006) Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L523–L534 [DOI] [PubMed] [Google Scholar]
- 9. Jensen A. A. (2005) Functional characterisation of human glycine receptors in a fluorescence-based high throughput screening assay. Eur. J. Pharmacol. 521, 39–42 [DOI] [PubMed] [Google Scholar]
- 10. Gallos G., Gleason N. R., Zhang Y., Pak S. W., Sonett J. R., Yang J., Emala C. W. (2008) Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L1040–L1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jooste E., Zhang Y., Emala C. W. (2005) Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology 102, 117–124 [DOI] [PubMed] [Google Scholar]
- 12. Froh M., Thurman R. G., Wheeler M. D. (2002) Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G856–G863 [DOI] [PubMed] [Google Scholar]
- 13. den Eynden J. V., Ali S. S., Horwood N., Carmans S., Brone B., Hellings N., Steels P., Harvey R. J., Rigo J. M. (2009) Glycine and glycine receptor signalling in non-neuronal cells. Front. Mol. Neurosci. 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elster L., Banke T., Kristiansen U., Schousboe A., Wahl P. (1998) Functional properties of glycine receptors expressed in primary cultures of mouse cerebellar granule cells. Neuroscience 84, 519–528 [DOI] [PubMed] [Google Scholar]
- 15. Simon J., Wakimoto H., Fujita N., Lalande M., Barnard E. A. (2004) Analysis of the set of GABA(A) receptor genes in the human genome. J. Biol. Chem. 279, 41422–41435 [DOI] [PubMed] [Google Scholar]
- 16. Nassar T., Yarovoi S., Fanne R. A., Akkawi S., Jammal M., Allen T. C., Idell S., Cines D. B., Higazi A. A. (2010) Regulation of airway contractility by plasminogen activators through N-methyl-d-aspartate receptor-1. Am. J. Respir. Cell Mol. Biol. 43, 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roux M. J., Supplisson S. (2000) Neuronal and glial glycine transporters have different stoichiometries. Neuron 25, 373–383 [DOI] [PubMed] [Google Scholar]
- 18. Janssen L. J. (2002) Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L1161–L1178 [DOI] [PubMed] [Google Scholar]
- 19. Ahmed T., D'Brot J., Abraham W. (1988) The role of calcium antagonists in bronchial reactivity. J. Allergy Clin. Immunol. 81, 133–144 [DOI] [PubMed] [Google Scholar]
- 20. Mahaut-Smith M. P., Martinez-Pinna J., Gurung I. S. (2008) A role for membrane potential in regulating GPCRs? Trends Pharmacol. Sci. 29, 421–429 [DOI] [PubMed] [Google Scholar]
- 21. Imtiaz M. S., von der Weid P. Y., van Helden D. F. (2010) Synchronization of Ca2+ oscillations: a coupled oscillator-based mechanism in smooth muscle. FEBS J. 277, 278–285 [DOI] [PubMed] [Google Scholar]
- 22. Ben-Chaim Y., Tour O., Dascal N., Parnas I., Parnas H. (2003) The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J. Biol. Chem. 278, 22482–22491 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y. X., Kotlikoff M. I. (1997) Muscarinic signaling pathway for calcium release and calcium-activated chloride current in smooth muscle. Am. J. Physiol. 273, C509–C519 [DOI] [PubMed] [Google Scholar]
- 24. Sahan-Firat S., Tiftik R. N., Nacak M., Buyukafsar K. (2005) Rho kinase expression and its central role in ovine gallbladder contractions elicited by a variety of excitatory stimuli. Eur. J. Pharmacol. 528, 169–175 [DOI] [PubMed] [Google Scholar]
- 25. Janssen L. J., Tazzeo T., Zuo J., Pertens E., Keshavjee S. (2004) KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L852–L858 [DOI] [PubMed] [Google Scholar]
- 26. Michoud M. C., Robert R., Hassan M., Moynihan B., Haston C., Govindaraju V., Ferraro P., Hanrahan J. W., Martin J. G. (2009) Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 40, 217–222 [DOI] [PubMed] [Google Scholar]
- 27. Huang F., Rock J. R., Harfe B. D., Cheng T., Huang X., Jan Y. N., Jan L. Y. (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. U. S. A. 106, 21413–21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.