Summary
Obesity is associated with infiltration of macrophages into adipose tissue (AT), contributing to insulin resistance and diabetes. However, relatively little is known regarding the origin of AT macrophages (ATMs). We discovered that murine models of obesity have prominent monocytosis and neutrophilia, associated with proliferation and expansion of bone marrow (BM) myeloid progenitors. AT transplantation conferred myeloid progenitor proliferation in lean recipients, while weight loss in both mice and humans (via gastric bypass) was associated with a reversal of monocytosis and neutrophilia. Adipose S100A8/A9 induced ATM TLR4/MyD88 and NLRP3 inflammasome-dependent IL-1β production. IL-1β interacted with the IL-1 receptor (IL-1R) on BM myeloid progenitors to stimulate the production of monocytes and neutrophils. These studies uncover a positive feedback loop between ATMs and BM myeloid progenitors, and suggest that inhibition of TLR4 ligands or the NLRP3-IL-1β signaling axis could reduce AT inflammation and insulin resistance in obesity.
Introduction
Obesity is one of the most prevalent diseases globally, leading to metabolic syndrome (MetS), type 2 diabetes (T2D) and increased risk of cardiovascular disease (CVD) (Haffner et al., 1990). Obesity-associated inflammation is widely regarded as one of the major factors driving insulin resistance (IR) and the onset of T2D (Osborn and Olefsky, 2012). A hallmark of inflammation in obesity is the accumulation of visceral adipose tissue (VAT) macrophages (ATMs) (Weisberg et al., 2003). As VAT pathologically expands in obese subjects, ATMs appear to undergo a phenotypic switch from resident ATMs to what is generally referred to as an inflammatory phenotype (Lumeng et al., 2007). This expansion of inflammatory ATMs along with the decrease in anti-inflammatory T-regulatory cells in the VAT results in an imbalanced environment and is thought to drive IR and the progression to T2D in obese subjects (Osborn and Olefsky, 2012).
Overexpression of the key monocyte/macrophage chemoattractant MCP-1 in the VAT results in macrophage accumulation, increased VAT inflammation and impaired insulin sensitivity (Kamei et al., 2006). In contrast, inhibiting macrophage recruitment using Ccr2−/− mice (receptor for MCP-1) protects mice from IR (Weisberg et al., 2003). This implies that macrophage recruitment pathways are important in inflammation-driven IR. More evidence to support the role of macrophage inflammation in IR has arisen from studies depleting ATMs (Weisberg et al., 2003) or inflammatory CD11c+ ATMs (Patsouris et al., 2008), or restricting their inflammatory capabilities (Arkan et al., 2005; Solinas et al., 2007; Vandanmagsar et al., 2011; Wen et al., 2011). Reduced ATM-driven inflammation protects from diet-induced IR. However, little is known about the source of ATMs and the signaling processes that lead to their accumulation in VAT.
There is a strong association between obesity, diabetes and leukocytosis, particularly of the myeloid lineage (Ford, 2002; Kullo et al., 2002; Ohshita et al., 2004; Schmidt et al., 1999). We recently reported that enhanced myelopoiesis in T1D mouse models significantly impairs the resolution of atherosclerosis (Nagareddy et al., 2013). Increased numbers of circulating leukocytes are prevalent in obesity and predict the development of T2D (Schmidt et al., 1999). Obese children also present with increased levels of circulating monocytes (Schipper et al., 2012). Together these pieces of evidence suggest that there may be a causal relationship between leukocytosis, particularly monocytosis, and the accumulation of ATMs and IR. We previously have shown that expansion and proliferation of bone marrow (BM) hematopoietic stem and progenitors results in monocytosis and is associated with enhanced atherogenesis (Murphy et al., 2011; Yvan-Charvet et al., 2010). However, the role of myeloid progenitor cell proliferation and monocytosis in driving ATM accumulation in obesity has not been examined.
We hypothesized that VAT inflammation results in the release of inflammatory mediators that communicate directly with hematopoietic progenitor cells in the BM to promote leukocytosis. We reasoned that this would set up a feed-forward loop to provide the adipose tissue with more monocytes/macrophages that in turn trigger adipose inflammation and promote IR.
Results
Monocytosis in obesity is associated with enhanced myelopoiesis
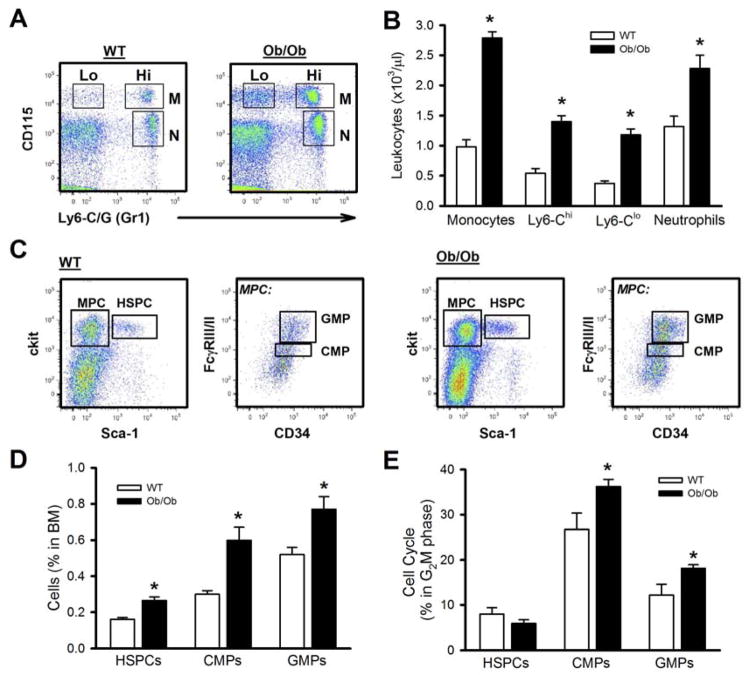
To determine if obesity is associated with leukocytosis, we studied the leptin deficient Ob/Ob mouse. Ob/Ob mice had an approximately 2.5-fold increase in the numbers of circulating monocytes (both Ly6-Chi and Ly6-Clo) and neutrophils compared to their lean controls (Figure 1A, B). The increased numbers of circulating myeloid cells were due to enhanced production by hematopoietic progenitor cells. Ob/Ob mice had a global expansion of hematopoietic stem and multipotential progenitor cells (HSPCs), common myeloid progenitors (CMPs) and granulocyte macrophage progenitors (GMPs) compared to the lean controls (Figure 1C, D, gating Fig S1A, B). This was accompanied by increased proliferation of CMPs and GMPs but not HSPCs (Figure 1E). The expansion of dormant HSPCs is consistent with increased retention in the BM (Ferraro et al., 2011). A similar monocytosis, expansion and proliferation of myeloid progenitors was found in the leptin receptor deficient db/db mouse model (Figure S1C–E).
Figure 1. Leukocytosis in obese mice is due to enhanced myelopoiesis. Ob/Ob or lean controls on a chow diet.
A) Representative flow cytometry plots indicating monocyte and neutrophil populations and B) quantified in the accompanying graph. N: neutrophil, M: monocyte, Lo: Ly6-Clo, Hi: Ly6-Chi.
C) Representative flow cytometry plots depicting the BM progenitor cells and D) quantified. MPC: myeloid progenitor cell.
E) Cell cycle analysis of the hematopoietic cells using DAPI. All data mean ± SEM, n=5/group. *P<0.05.
See also Figure S1.
Increased myelopoiesis is not due to leptin deficiency or hyperglycemia
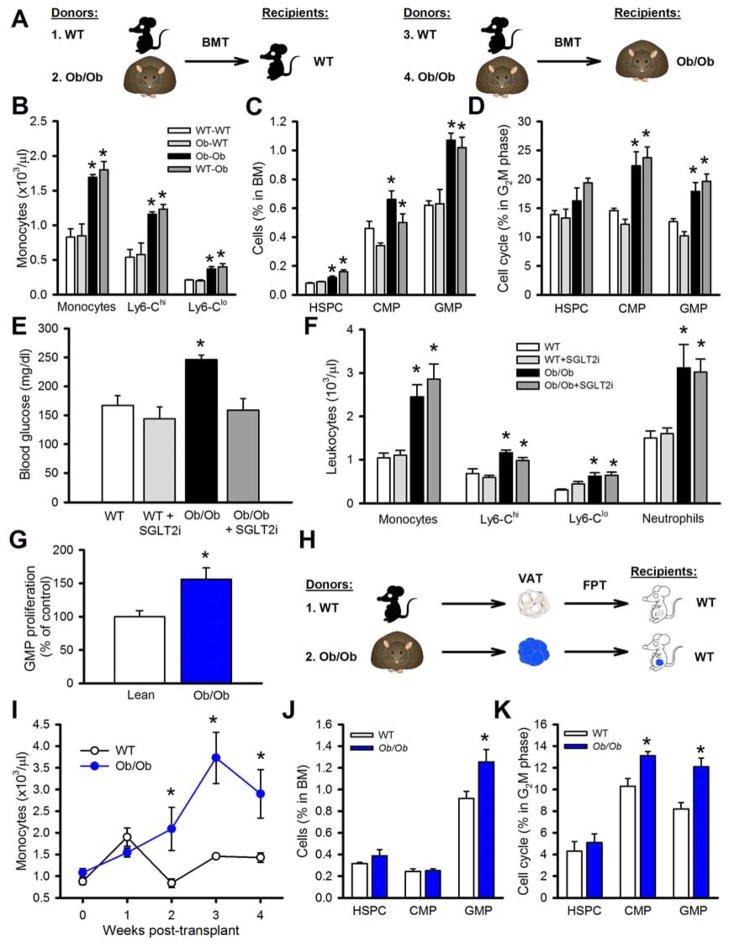
We next examined if leptin deficiency caused monocytosis by replenishing leptin in Ob/Ob mice (i.p; 1μg/kg/day for 7 days). Leptin did not did not reduce blood monocytes (Table S1) or affect plasma cholesterol, but did lower triglycerides, blood glucose and food intake and led to a slight reduction in body weight (the mice were still obese vs. lean controls) (supplemental Table 1 & not shown). To further assess whether leptin deficiency in myeloid cells is the cause of monocytosis, we conducted BM transplant (BMT) studies using BM from WT and Ob/Ob mice (Study Outline - Figure 2A). Transplantation of WT or Ob/Ob BM into WT recipients did not promote monocytosis, while transplantation of WT or Ob/Ob BM into Ob/Ob recipients increased levels of circulating monocytes (Figure 2B). These results were also mirrored in the abundance and proliferation of hematopoietic stem and progenitor cells (Figure 2C, D). These data confirm that defective leptin signaling does not cause monocytosis in obesity.
Figure 2. Adiposity drives leukocytosis.
A) Experimental overview: 1. WT or 2. Ob/Ob BM was transplanted into WT recipients. 3. WT or 4. Ob/Ob BM was transplanted into Ob/Ob recipients. BM was allowed to reconstitute for 5 weeks. B) Monocyte levels, C) BM progenitors and. D) cell cycle analysis of BM progenitors. All data mean ± SEM, n=5/group. *P<0.05.
E, F) WT and Ob/Ob mice were treated with a SGLT2i. E) Blood glucose levels. F) Blood leukocytes. All data mean ± SEM, n=5/group. *P<0.05.
G) Conditioned media from cultured lean or Ob/Ob VAT was incubated (10% v/v) with BM progenitor cells and GMP proliferation was measured by EdU incorporation. n=6 (independent conditioned media treatments from the VAT of 6 mice/group).
H) Experimental overview: FPT: Equal portions of VAT from lean or Ob/Ob mice were transplanted into lean WT recipients. I) Monocyte levels were monitored weekly for 4 weeks. J, K) After 4 weeks the J) abundance and K) cell cycle of the BM progenitor cells were determined by flow cytometry. All data mean ± SEM, n=5/group. *P<0.05.
We have previously shown in mouse models of T1D that hyperglycemia promotes leukocytosis (Nagareddy et al., 2013). To examine if hyperglycemia caused monocytosis in obesity, we treated Ob/Ob mice with a sodium glucose co-transporter 2 inhibitor (SGLT2i; dapagliflozin 25mg/kg, for 4 weeks) to reduce blood glucose. The SGLT2i normalized blood glucose levels (Figure 2E), but failed to lower the number of circulating leukocytes (Figure 2F). These findings are different to those we reported in the STZ T1D model, where glucose lowering did reverse leukocytosis, most likely because glucose levels were much higher (500 mg/dl) than in the current study (250 mg/dl) (Nagareddy et al., 2013).
Adipose tissue from Ob/Ob mice drives leukocytosis
To test whether monocytosis was due to signals emanating from VAT, we first cultured VAT from lean or Ob/Ob mice and used the conditioned media for in vitro BM progenitor proliferation assays. Incubation of BM cells with conditioned media from Ob/Ob mouse VAT significantly increased GMP proliferation compared to media from lean mouse VAT (Figure 2G). To confirm this in vivo we conducted fat pad transplant (FPT) studies using equal amounts of VAT from WT or Ob/Ob mice as described previously (Gavrilova et al., 2000; Klebanov et al., 2005) (Study Outline- Figure 2H). After approximately 10 days, the initial inflammation due to the surgery had subsided and circulating monocyte levels of mice that received fat from WT mice had returned to normal (Figure 2I). In contrast, WT mice that received an equal amount of VAT from Ob/Ob mice developed monocytosis (Figure 2I), which was associated with increased numbers and proliferation of myeloid progenitor cells (Figure 2J, K). We also performed FPT experiments using subcutaneous adipose tissue (SAT) and found that obese SAT induced leukocyte production but to a lesser extent compared to what we observed using VAT (Figure S2A–C), consistent with previous studies suggesting that SAT is less inflammatory than VAT (Osborn and Olefsky, 2012). While we acknowledge that the FPT studies should be interpreted with caution as macrophages are also involved in tissue remodeling and angiogenesis, collectively these data suggest that factors originating from the VAT signal to the BM to promote monocyte production.
Leukocytosis in diet induced obesity (DIO)
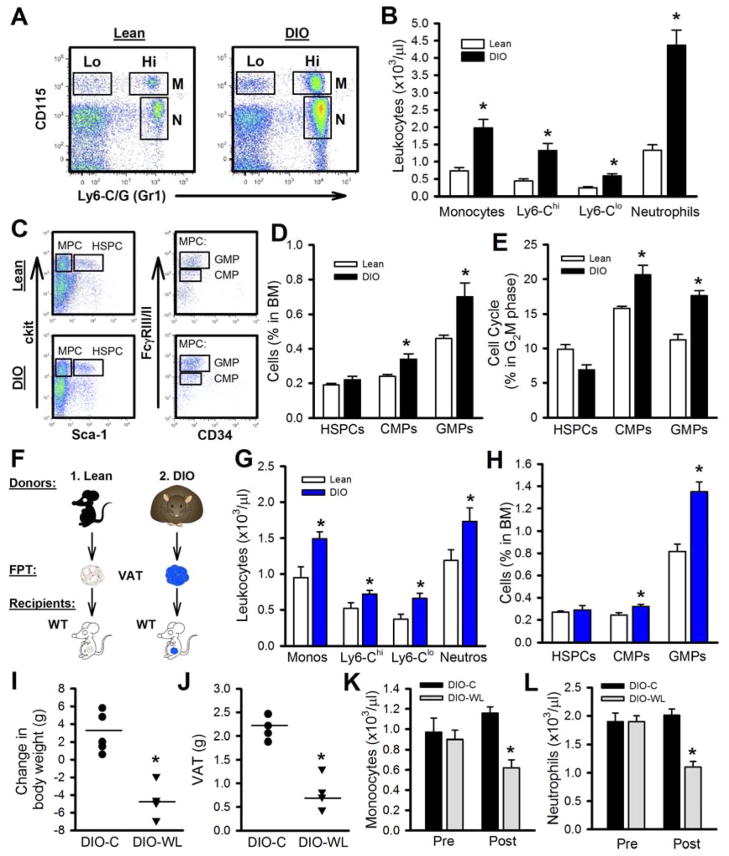
To confirm that monocytosis was due to obesity, we fed WT mice a high fat diet (HFD; 60% kcal) for 20 weeks (DIO) in parallel with lean controls fed a low fat diet (LFD; 10% kcal). DIO mice weighed an average of 55 g, significantly greater than their lean counterparts that averaged 34 g. Similar to Ob/Ob mice, obesity in DIO mice was associated with >2.5-fold increase in the numbers of circulating monocytes and neutrophils (Figure 3A, B). This was accompanied by an increase in the numbers and proliferation of CMPs and GMPs but not HSPCs (Figure 3C–E). We also performed a FPT study using VAT from DIO and lean mice (Figure 3F) and observed monocytosis and neutrophilia along with expansion of CMPs and GMPs in the BM in the DIO FPT recipients (Figure 3G, H). Taken together, these studies confirmed that monocytosis is obesity-dependent.
Figure 3. Adipose tissue of HFD-fed mice promotes monocytosis.
A–E) WT mice were fed a LFD (Lean) or a HFD (DIO) for 20wks. A) Representative flow cytometry plots depicting blood leukocyte populations and B) quantified. C) Representative flow cytometry plots depicting BM progenitor cells and D) the abundance quantified. E) Cell cycle analysis on the BM progenitor cells. All data mean ± SEM, n=6 lean and 12 DIO mice/group. *P<0.05.
F) Experimental overview: FPT: Equal portions of VAT was transplanted into lean WT recipients for 4 weeks. G) Blood leukocyte levels and H) BM progenitors. All data mean ± SEM, n=6/group. *P<0.05.
I–L) 16wk HFD-fed DIO obese mice were randomized into 2 groups; 1) DIO-control (DIO-C): continued on a HFD or 2) DIO-weight loss (WL): switched to a chow diet to promote weight loss for 3 weeks. I) Changes in body weight and J) VAT mass after 3 weeks of weight-loss. Circulating K) monocyte and L) neutrophil levels. All data mean ± SEM, n=6/group. *P<0.05. See also Figure S2 and Table S2.
Weight loss decreases leukocytes in obese mice and humans
VAT inflammation is reduced after weight loss (Berg and Scherer, 2005), thus we examined the effect of weight-loss on leukocyte levels in DIO mice. After 16 weeks of HFD feeding mice were returned to LFD. This led to ~5 g weight loss over 3 weeks and a significant reduction in VAT, comparable to levels observed in lean controls (Figure 3I, J). Associated with weight reduction was a significant decrease of both monocyte and neutrophil levels (Figure 3K, L). To demonstrate the potential to translate our findings to humans, we examined leukocyte levels in 13 obese subjects before and 18 months after bariatric surgery (9 Roux-en-Y gastric bypass; 2 adjustable gastric banding; 2 sleeve gastrectomy). The subjects had an average weight loss of 26.7% of their total body weigh and a significant reduction in monocyte and neutrophil counts (Table S2). The decrease in circulating monocytes correlated with improvement in IR as calculated by HOMA-IR (Figure S2D). While these weight-loss findings cannot show causality, they are consistent with our hypothesis that obesity causes monocytosis and neutrophilia.
Adipose factors that increase BM proliferation
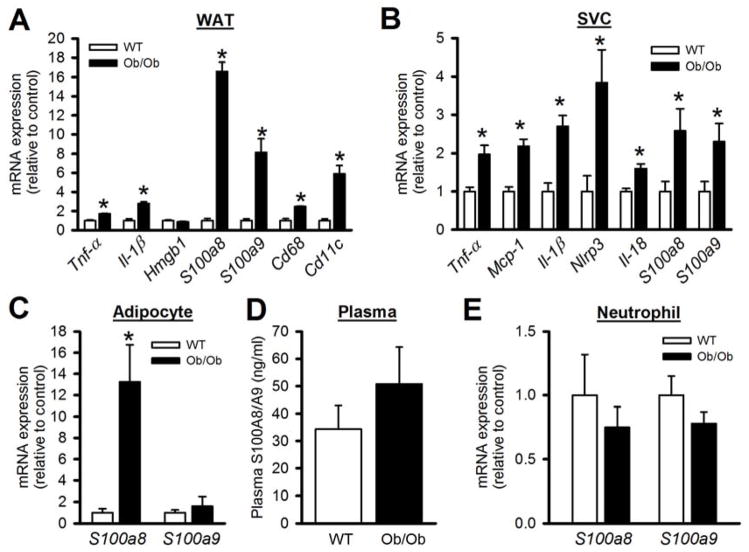
In order to determine the potential factors that are released from VAT, we conducted mRNA analysis of a number of genes. Consistent with previous reports (Berg and Scherer, 2005; Weisberg et al., 2003), the VAT from obese mice displayed increased expression of a number of inflammatory genes and macrophage markers (Figure 4A & SF3A). However the most striking increase was in the expression of S100a8/a9, (Figure 4A & SF3A), the proteins that we recently demonstrated as major players in hyperglycemia-induced monocytosis (Nagareddy et al., 2013). To determine the source of S100a8/a9 within the adipose tissue, we separated the macrophage rich stromal vascular cells (SVC) from adipocytes and found that while S100a8 and S100a9 were equally increased in the SVCs, only S100a8 was upregulated in the adipocytes (Figure 4B, C). As expected Tnf-α, Mcp-1 and genes of the inflammasome/IL-1β pathway were also increased in obese adipose SVCs (Figure 4B). Surprisingly S100A8/A9 levels did not increase in the plasma (Figure 4D). In addition, the expression of S100a8/a9 in circulating neutrophils, the main source of S100A8/A9 in mice with T1D (Nagareddy et al., 2013), was not increased in obese mice (Figure 4E). Together, these findings suggest that if S100A8/A9 are eliciting a biological response it is confined to the adipose tissue.
Figure 4. Inflammatory gene expression in the visceral adipose tissue.
Lean or Ob/Ob mice were fed a chow diet for 12 wks. Gene expression from A) Total VAT, B) VAT-SVCs and C) VAT-adipocytes was quantified by qPCR. D) Plasma levels of S100A8/A9 in lean and obese mice. E) S100a8 and S100a9 mRNA expression in FACS isolated blood neutrophils. All data mean ± SEM, n=6/group. *P<0.05. See also Figure S3.
We also measured the plasma levels of S100A8/A9 in a series of human subjects, which were stratified by their BMI and found no difference between lean and obese subjects (Figure S3B). However, as noted in obese mice, a significant increase in S100A8/A9 levels was observed in the VAT from obese humans with insulin-resistant diabetes compared to healthy lean subjects (lean vs. obese BMI: 22.6±1.3 vs. 25.7±1.4. mean± SD, P<0.05) (Figure S3C, D).
MyD88-dependent toll-like receptor 4 signaling is required for obesity-driven leukocytosis
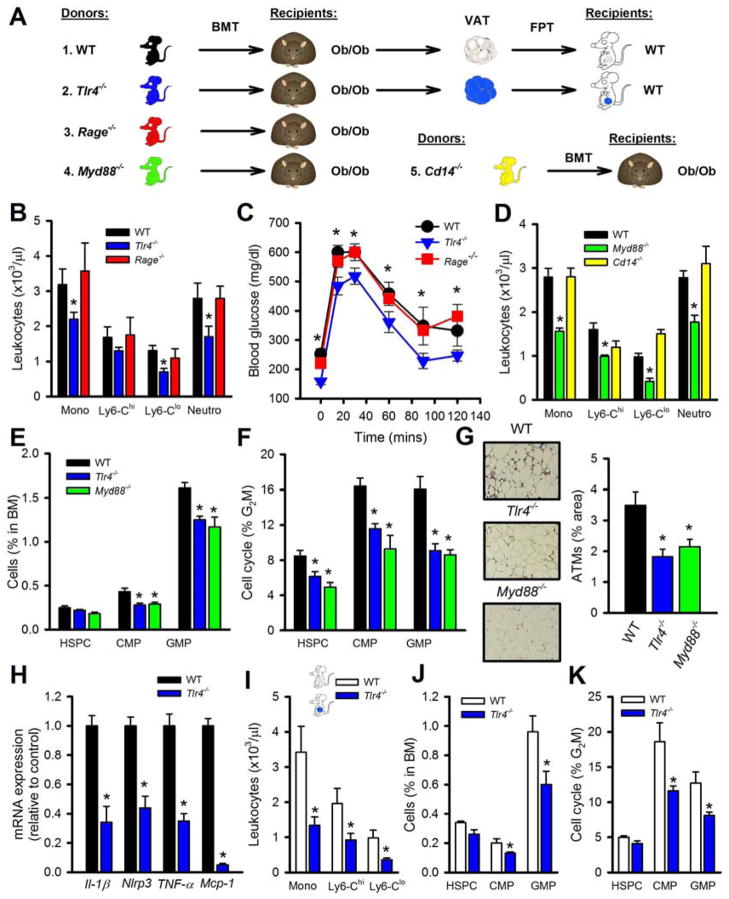
Since S100a8/a9 can induce inflammatory cytokines by engaging pattern recognition receptors (PRRs) including toll-like receptor 4 (TLR4) (Vogl et al., 2007) and the receptor for advanced glycation end products (RAGE) (Boyd et al., 2008), we examined the role of PRRs in obesity-associated leukocytosis. In T1D S100A8/A9 binds to RAGE (but not TLR4) on CMPs initiating myelopoiesis (Nagareddy et al., 2013), however, we did not observe increased RAGE expression on CMPs from either DIO or Ob/Ob mice (data not shown), likely due to these mice only having moderate increases in blood glucose levels. We assessed the importance of TLR4 and RAGE in BM- and hematopoietic-derived VAT cells on leukocytosis in obese mice through a series of BMT and FPTs (see Figure 5A for the experimental outline). All recipient mice remained obese and had similar body weights between the different BM genotypes (body weight at the end of the study; mean±SEM: WT: 55.5±0.9, Rage−/−: 55.9±3.3, Tlr4−/−: 55.6±0.8). Ob/Ob mice transplanted with BM from mice carrying a mutant form of TLR4 (Tlr4−/−) (Poltorak et al., 1998) had significantly less monocytes and neutrophils, while mice transplanted with WT or Rage−/− BM had comparable levels (Figure 5B). The Tlr4−/− BMT mice also had improved glucose tolerance compared to the WT and Rage−/− BMT mice (Figure 5C). We also found positive correlations between monocyte levels, fasting blood glucose and ATMs (Figure S4A–C), but not with body weight as expected since the recipients were Ob/Ob mice (data not shown).
Figure 5. MyD88 dependent TLR4 signaling is required for leukocytosis in obesity.
A) Experimental overview: Ob/Ob mice were transplanted with BM from donor mice (1–5) and allowed to reconstitute for 5 week before blood leukocytes were assessed. VAT was harvested from Ob/Ob mice after BMT (1, 2) and equal portions of VAT were transplanted into lean WT mice. B–C) Ob/Ob mice BMT with WT, Tlr4−/− or Rage−/− BM. B) Blood leukocyte levels and C) OGTT (1g/kg of glucose). D) Blood leukocyte levels in Ob/Ob BMT with WT, Myd88−/− or Cd14−/− BM. E) Abundance of hematopoietic progenitor cells in the BM and F) cell cycle analysis. G) Macrophage abundance in the VAT was assessed by immunohistochemistry using anti-Mac3 antibody. X20 objective. H) Expression of inflammatory genes in the SVCs of the VAT from Ob/Ob BMT mice (1, 2).
I–K) FPT of VAT from Ob/Ob mice (1, 2). I) Blood leukocyte levels, J) BM progenitor abundance and K) cell cycle analysis. All data mean ± SEM, n=6/group. *P<0.05. See also Figure S4.
TLR4 has two main downstream signaling pathways, MyD88 and TRIF/TRAM (Takeuchi and Akira, 2010). To determine which pathway downstream of TLR4 was important in driving leukocytosis in obesity, we preformed BMTs from mice deficient in Myd88 or Cd14 (essential for LPS/TLR4 to internalize and signal via TRIF/TRAM (Kagan et al., 2008; Zanoni et al., 2011), also reported to be involved in microbe-induced IR (Cani et al., 2007)). Ob/Ob mice that received Myd88−/− BM had significantly less circulating monocytes and neutrophils than WT or Cd14−/− BMT mice (Figure 5D). Again, lower levels of these leukocytes were associated with improved glucose tolerance in these mice compared to Ob/Ob mice that received BM from either WT or Cd14−/− BMT mice (Figure S4D). TLR4 and MyD88 deficiency in hematopoietic cells also resulted in fewer progenitor cells that were proliferating at a lower rate compared to WT BM recipients (Figure 5E, F). We also found fewer macrophages in the VAT of the Ob/Ob mice transplanted with Tlr4−/− or Myd88−/− BM (Figure 5G). Next, we profiled the inflammatory genes of the SVCs of the VAT from Ob/Ob mice that received WT or Tlr4−/−. As expected, we observed a significant reduction in the expression of TNF-α, Mcp-1, Il-1β and Nlrp3 a key component of the inflammasome (Figure 5H). There were no changes in the expression of S100a8/a9 in the SVCs (data not shown), suggesting that the expression of these genes is not regulated by TLR4 in the VAT.
To determine the role of hematopoietic TLR4 specifically in the VAT in promoting monocytosis in obesity, we transplanted the VAT from the Ob/Ob mice that received either WT or Tlr4−/− BM into lean WT recipients (see overview Figure 5A–1 & 2). Mice that received the VAT containing Tlr4−/− cells of hematopoietic origin were protected from developing monocytosis and did not display expansion or increased proliferation of their BM progenitor cells (Figure 5I–K). Further, conditioned media from the VAT of Ob/Ob transplanted with Tlr4−/− BM failed to induce myelopoiesis in vitro compared to conditioned media from Ob/Ob mice transplanted with WT BM as measured by incorporation of EdU+ into their GMPs (Figure S4E).
CD11c+ ATMs promote myelopoiesis in obesity
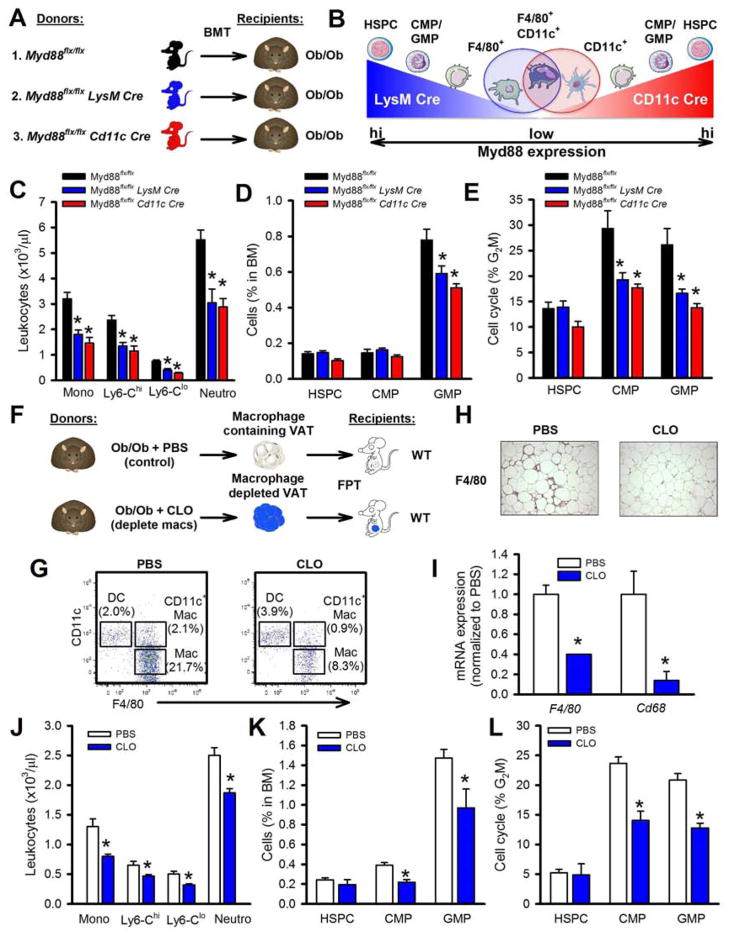
TLR4 is expressed in a variety of hematopoietic cells, including HSPCs, CMPs, GMPs (Nagai et al., 2006), and mature myeloid cells (Takeuchi and Akira, 2010) including ATMs (Nguyen et al., 2007). It is possible that signals arising from the VAT (e.g. S100A8/A9) could bind to TLR4 on any of these cells to promote myelopoiesis. Thus, we used cell specific knockouts of Myd88 in mature myeloid cells transplanted into Ob/Ob recipients to assess a role of macrophages and dendritic cells (outlined in Figure 6A), i.e. Myd88flox/flox (controls), Myd88flox/flox LysM Cre BM (deletion in neutrophils, monocytes and macrophages) and Myd88flox/flox CD11c Cre BM (deletion in dendritic cells, CD11c+ macrophages and to a lesser extent monocytes and neutrophils) (Hoshi et al., 2012) (Figure 6B shows a schematic overview of the two Cres and Myd88 expression levels from hematopoietic cells as they mature). Ob/Ob mice that received BM with Myd88 deletion via LysM Cre or CD11c Cre had fewer circulating monocytes and neutrophils (Figure 6C). These mice also had a reduced number and proliferation of GMPs in the BM (Figure 6D, E). These data confirm that TLR4 signaling in mature myeloid cells especially the inflammatory CD11c+ macrophages (Hoshi et al., 2012) as described by Patsouris et al., but not hematopoietic progenitor cells, is required to promote monocytosis in obesity.
Figure 6. TLR4/MyD88 signaling in CD11c+ ATMs drives leukocytosis in obesity.
A) Experimental outline: Ob/Ob mice were transplanted with BM from Myd88flx/flx, Myd88flx/flx LysM Cre or Myd88flx/flx CD11c Cre and allowed to reconstitute for 5 weeks. B) Schematic overview of the LysM and CD11c Cre induced deletion of Myd88 in the respective myeloid cells and the overlapping CD11c+ macrophage. C) Blood leukocyte levels, D) BM progenitor abundance and E) cell cycle analysis. All data mean ± SEM, n=6/group. *P<0.05.
F) Experimental overview: Ob/Ob mice were injected intraperitoneally with PBS or clodronate (CLO) liposomes (to deplete ATMs). The VAT was harvested washed and transplanted into lean WT mice for 4 weeks. G) Flow cytometry plots of the ATM subsets (parent gate CD45+), H) F4/80 staining of the VAT and I) F4/80 and Cd68 mRNA expression in the VAT. J) Circulating blood monocyte levels, K) BM progenitor abundance and L) proliferation after transplantation. All data mean ± SEM, n=5/group. *P<0.05. See also Figure S5A.
As TLR4 signaling in ATMs appeared to be required for obesity-driven myelopoiesis, we reasoned that depletion of ATMs should prevent VAT-induced monocytosis in FPT experiments. A strategy to deplete ATMs by administration of clodronate (CLO) liposomes directly into the fat failed as injection of CLO liposomes intraperitoneally to Ob/Ob mice (Feng et al., 2011) also decreased circulating monocytes in both Ob/Ob and WT mice (data not shown), suggesting it had reached the circulation. To circumvent this complication, we removed the VAT from Ob/Ob mice pre-treated with either PBS- or CLO-liposomes, extensively washed the isolated VAT (to remove any residual liposomes), and then transplanted equal amounts of VAT into lean mice (see Figure 6F). To confirm that the transplanted fat was free of ATMs, we examined the abundance of dendritic cells (DCs: CD45+CD11c+F4/80−), ATMs (Mac: CD45+CD11c−F4/80+) and CD11c+ ATMs (CD11c+ Mac: CD45+CD11c+F4/80+) by flow cytometry and found a significant decrease in both ATM populations (Figure 6G). We also found fewer F4/80+ cells by immunohistochemistry and a decrease in the mRNA expression of F4/80 and Cd68, confirming ATM depletion (Figure 6H, I). Mice that received CLO-treated VAT had fewer circulating monocytes and neutrophils compared to those that received VAT from PBS-treated donor mice (Figure 6J), which was accompanied by a decrease in number and proliferation of the BM progenitors cells (Figure 6K, L). To support this finding we also cultured BM progenitor cells in DIO adipocyte (ATM devoid)-conditioned media and examined GMP proliferation. We observed significantly less GMP proliferation when the GMPs were incubated in the adipocyte-only conditioned media compared to GMPs incubated in conditioned media from total VAT (Figure S5A). Together these data suggest that ATMs are essential to promote myelopoiesis in obesity.
The IL-1β/NLRP3 inflammasome pathway drives leukocytosis in obesity
Consistent with recent reports (Vandanmagsar et al., 2011; Wen et al., 2011), we found that key genes involved in the IL-1β pathway were increased in the SVCs of obese mice (i.e. Il-1β and Nlrp3: Figure 4B). Moreover, the expression of these same genes was markedly decreased in the SVCs of Tlr4−/− transplanted Ob/Ob mice (Figure 5H). Therefore we hypothesized that the IL-1β pathway was playing a major role in promoting leukocytosis in obesity. More evidence to suggest this comes from the recent finding that the expression of the Nlrp3-inflammasome and Il-1β in VAT are dramatically reduced in mice and humans undergoing weight loss (Vandanmagsar et al., 2011), which we report here also coincides with decreased monocyte and neutrophil levels (Figure 3K, L and supplemental Table 2).
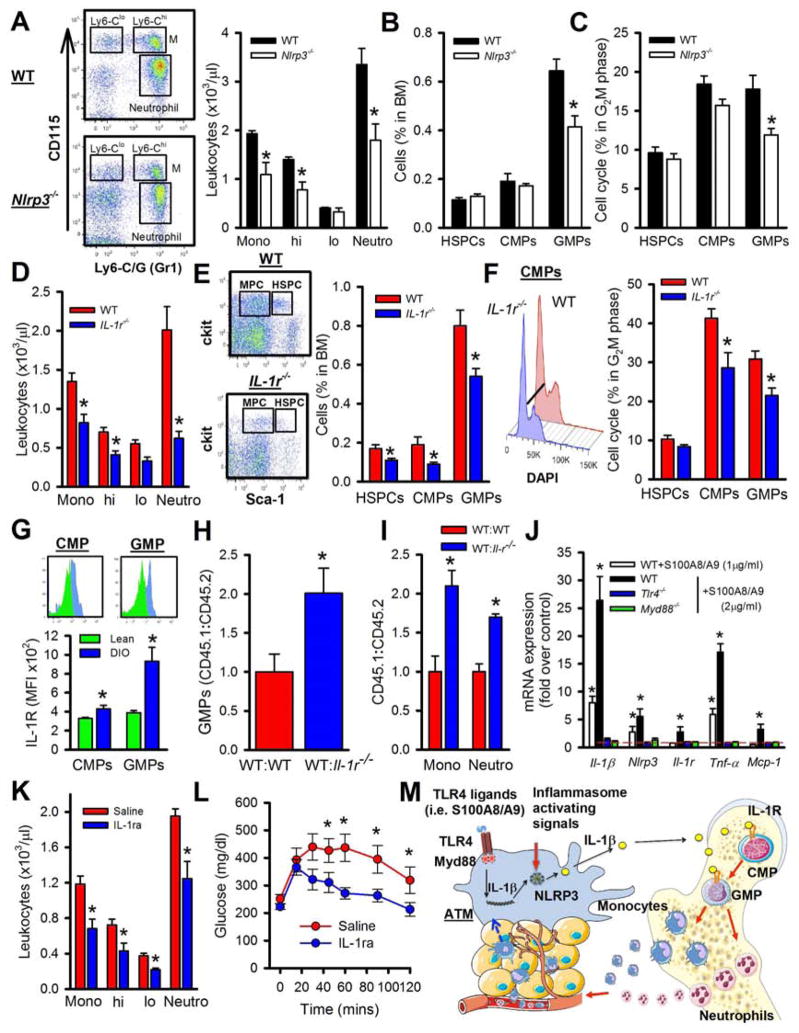
To address the role of IL-1β in obesity-driven monocytosis, we transplanted BM from WT or Nlrp3−/− mice into Ob/Ob recipients. In line with our hypothesis, the Ob/Ob mice that received Nlrp3−/− BM had significantly fewer circulating leukocytes and a reduction in the BM progenitors than Ob/Ob mice that received WT BM (Figure 7A–C).
Figure 7. IL-1β signaling contributes to obesity-driven leukocytosis.
A–C) Ob/Ob mice were transplanted with WT or Nlrp3−/− BM and allowed to reconstitute for 5 weeks. A) Representative flow cytometry plots depicting blood leukocytes and quantified. B) Abundance of BM progenitor cells and C) cell cycle analysis. All data mean ± SEM, n=5/group. *P<0.05.
D–F) WT and IL-1r−/− mice were fed a HFD for 6 months. A) Blood leukocyte levels. B) Representative flow cytometry plots depicting the BM progenitors and quantified. MCP: myeloid progenitor cells. C) Representative cell cycle histograms and quantification. All data mean ± SEM, n=6/group. *P<0.05.
G) IL-1R expression on CMPs and GMPs in the BM from lean or DIO mice. Expression was normalized to an isotype control. All data mean ± SEM, n=6/group. *P<0.05.
H–I) Competitive BMT studies. Ob/Ob mice were transplanted with equal amounts of CD45.1 WT/CD45.2 WT or CD45.1 WT/CD45.2 IL-1r−/− BM and allowed to repopulate for 6 weeks. Ratios of CD45.1:CD45.2 for H) BM progenitor cells and I) blood leukocytes. All data mean ± SEM, n=5/group. *P<0.05.
J) BM derived macrophages from WT, Tlr4−/− of Myd88−/− mice were stimulated with S100A8/A9 for 4hrs and gene expression was assessed using qPCR. Dashed red line indicates control; unstimulated WT macrophages. All data mean ± SEM, n=5/group. *P<0.05.
K–L) WT mice were fed a HFD for 16 weeks and then given daily i.p injections of Anakinra (IL-1ra; 7.5mg/kg) or saline for 10 days. K) Blood leukocytes and L) OGTT. All data mean ± SEM, n=6/group. *P<0.05.
M) Schematic overview of the proposed mechanism of how obesity promotes enhanced myelopoiesis. See also Figure S5.
Next we assessed the IL-1β signaling pathway by placing WT and IL-1 receptor (IL-1R) knockout mice on HFD for 6 months. Both groups were obese (>60 g), with the IL-1r−/− mice weighing slightly more. We observed significantly fewer monocytes and neutrophils, along with BM progenitors in DIO IL-1r−/− mice compared to the DIO WT mice (Figure 7D–F).
IL-1β could be acting locally within the VAT to cause monocytosis via a secondary signal such as a colony stimulating factor or it could be travelling to and interacting with myeloid progenitor cells in the BM. To explore the latter option we profiled the cell surface expression of the IL-1R on the CMPs and GMPs in the BM of lean and DIO WT mice and found an increase on CMPs and GMPs from DIO mice (Figure 7G). No interaction of the IL-1R antibody with the IL-1r−/− BM progenitor cells was seen, confirming specificity (data not shown). While upregulation of the IL-1R on CMPs/GMPs does not confirm that IL-1β is acting directly on the BM progenitor cells to promote their proliferation, we did find that IL-1β could induce the proliferation of GMPs in vitro in an IL-1R dependent manner (Figure S5B) and we were able to detect increased circulating levels of IL-1β in DIO mice (WT: 4.5±0.5 vs. DIO: 7.95±0.6 pg/ml: mean±SEM n=6, P<0.05). Moreover, in an in vitro competitive GMP proliferation experiment we found that WT CD45.1 GMPs outgrew IL-r−/− CD45.2 GMPs in response to IL-1β (Figure S5C). To explore this further we performed in vivo competitive BMT studies (cBMT) in Ob/Ob recipients. We found that WT CD45.1 GMPs outgrew IL-1r−/− CD45.2 progenitors in the BM (Figure 7H), producing ~2-fold more monocytes and neutrophils in the blood (Figure 7I), which resulted in more WT CD45.1 ATMs (Figure S5D). This confirms that IL-1β is acting in a cell extrinsic fashion to promote the proliferation of GMPs and CMPs (which require the IL-1R) in obese mice to produce myeloid cells.
Our data along with others (Vandanmagsar et al., 2011; Wen et al., 2011) suggest that the production of IL-1β in obesity originates from the ATMs. As IL-1β is downstream of TLR4 signaling, we questioned if S100A8/A9, known ligands of TLR4, could induce Il-1β gene expression. We cultured BM-derived macrophages from WT, Tlr4−/− and Myd88−/− mice and stimulated them with S100A8/A9. The expression of Il-1β, Nlrp3 and Il-1r, along with other downstream TLR4 genes such as Tnf-α and Mcp-1 was induced by S100A8/A9 (Figure 7J). Tlr4−/− and Myd88−/− macrophages failed to respond to S100A8/A9 stimulation (Figure 7J).
Finally, to determine if the IL-1β/IL-1R pathway could be therapeutically targeted to reduce circulating leukocytes, we treated DIO mice with the IL-1R antagonist (IL-1Ra, Anakinra). WT mice were fed a HFD for 16wks before being treated with Anakinra (i.p. 7.5mg/kg daily for 10 days). Administration of Anakinra significantly reduced monocyte and neutrophil levels along with fewer BM progenitors compared to vehicle treated mice (Figure 7K, S5E, F). The abundance of ATMS was also significantly reduced in the Anakinra treated mice (Figure S5G). Somewhat surprisingly, the short-term administration of Anakinra also led to a significant improvement in glucose handling as determined by an OGTT (Figure 7L).
Together, these findings point to a role for S100A8/A9 and possibly other TLR4 ligands present in obese VAT to induce the expression of Il-1β and the Nlrp3-inflammasome in ATMs via TLR4/MyD88. It appears that VAT TLR4 agonists, such as S100A8/A9, act locally on ATMs via TLR4-MyD88 to initiate a signaling cascade resulting in the activation of NLRP3 inflammasome and release of IL-1β, which travels to the BM to engage the IL-1R on CMPs and GMPs promoting their proliferation and resulting in monocytosis and neutrophilia (Figure 7M).
DISCUSSION
Our findings suggest that chronically inflamed VAT in obesity can signal to the BM hematopoietic progenitor cells to proliferate, expand and increase the production of myeloid cells. Our data suggest that a feed-forward mechanism exists in obesity such that inflamed adipose tissue stimulates the production of more monocytes, leading to an exacerbation of inflammation and associated disease processes. Our findings are consistent with a model in which obesity-induced leukocytosis is driven by a process where up regulation of S100A8/A9 along with other TLR4 ligands in the VAT signal in macrophages via the MyD88 pathway to induce the expression of Il-1β, which is then processed by the NLRP3-inflammasome (activated by secondary stimuli e.g. extracellular ATP, free fatty acids, etc.) to form mature IL-1β (Masters et al., 2011); IL-1β travels to the BM to induce the proliferation of hematopoietic progenitor cells via the IL-1R, ultimately resulting in monocytosis and neutrophilia (Figure 7M).
VAT inflammation driven by the accumulation of ATMs promotes IR (Osborn and Olefsky, 2012) and modulating macrophage recruitment alters the inflammatory milieu (Kamei et al., 2006; Weisberg et al., 2003). However the source of the ATMs and the specific cellular mechanisms linking adiposity to increased monocyte levels in obesity had not been defined. While M2 macrophages have been reported to proliferate locally under the control of T-helper 2 activation and release of IL-4 (Jenkins et al., 2011), this does not appear to be a major process in obesity as the bulk of ATMs are of the M1 variety. Previously, Oh and colleagues showed that only a small pool of macrophages proliferate in the obese VAT (Oh et al., 2012). Further, the majority of these CD11c+ macrophages were newly recruited CCR2+ monocytes. While another more recent report has also suggested that ATMs proliferate, again this is only a small pool and can likely only sustain the ATM pool for a short period of time (Amano et al., 2013), similar to the findings and explanations from emerging studies on atherosclerotic lesions (Murphy and Tall, 2014; Randolph, 2013; Robbins et al., 2013). Our BMT studies agree with the findings of Oh et al., suggesting that there may be continuous recruitment of blood monocytes into the VAT where they mature into macrophages. Further, our cBMT experiments clearly show that the number of blood monocytes may determine the abundance of ATMs as we observed more WT CD45.1 monocytes and ATMs than Il-r−/− CD45.2 monocytes and ATMs. It appears reasonable to suggest that sheer increases in the abundance of monocytes would likely lead to increased recruitment to the VAT and our cBMT studies where WT progenitors produced more blood monocytes and more ATMs provide evidence for this. Therefore, delineation of the mechanisms of aberrant monocyte production in obesity is likely to be essential to understanding ATM accumulation.
Our studies show that VAT from obese mice can directly induce monocyte production by signaling to the hematopoietic progenitor cells. The failure of glucose reduction to correct the leukocytosis confirmed that a different process was driving leukocyte production in these models of obesity/T2D compared to the mechanism in T1D (Nagareddy et al., 2013). Glucose reduction alone may have been insufficient because the hyperglycemia in these mice was less severe and inflammatory signals emanating from adipose tissue were more robust. We also failed to observe an upregulation of RAGE on CMPs as we reported for T1D, suggesting that in obesity these cells were limited in their response to S100A8/A9. Instead, our studies revealed that hematopoietic TLR4 was playing a major role. While others have shown that BM progenitor cells express functional TLRs that are be able to sense peripheral infections and expand immune cell production (Nagai et al., 2006), using of Myd88flox/flox mice we affirmed a role for TLR4 expressing ATMs. These macrophages sense DAMPs (such as S100A8/A9) and perhaps other ligands in the VAT and produce IL-1β that signal to the BM to manufacture more monocytes. Monocytosis contributes to the pool of ATMs and enhances the inflammatory milieu.
While not known to have a major role in normal hematopoiesis, IL-1β certainly plays an important role in inducing myeloid progenitor cell proliferation in response to inflammation, to produce neutrophils and monocytes (Hsu et al., 2011; Ueda et al., 2009) and triggers progenitor cell proliferation via a number of mechanisms in different diseases (Dinarello, 1996). Our cBMT experiments clearly show that the IL-1R on hematopoietic cells is required for the proliferation of CMPs and GMPs in obesity. However, it is important to note that this experiment cannot test the direct source of IL-1β. That is, IL-1β could also be produced locally in the BM to signal to these progenitor cells. However, collectively our data (FPTs, increased plasma IL-1β levels and in vitro proliferation) does suggest that the signals emanate from the VAT, and are also consistent with recent studies implicating adipose inflammasome activation as a source of IL-1β in mice and humans (Vandanmagsar et al., 2011; Wen et al., 2011).
The endogenous TLR4 ligand(s) responsible for inducing the expression of Il-1β and key genes of the inflammasome in obesity have remained enigmatic. A number of endogenous ligands have been proposed to signal via TLR4, these include lipopolysaccharide (LPS), free fatty acids (FFAs), modified LDL and DAMPs (e.g. S100A8/A9, HMGB1) (Erridge, 2010). In the setting of obesity, other molecules such as extracellular ATP, free fatty acids (Wen et al., 2011) and ceramides (Vandanmagsar et al., 2011) have been suggested as the secondary stimuli to activate the inflammasome in LPS-primed macrophages (Wen et al., 2011). Our data suggest that S100A8/A9, a known ligand for TRL4, may be an initiating ligand that stimulates ATMs to induce the NRLP3-inflammasome-IL-1β pathway. We also found that weight reduction in mice and humans (Poitou et al., 2011)), which has also been reported to decrease the NLRP3-inflammasome and plasma IL-1β levels (Vandanmagsar et al., 2011), decreased monocytes and neutrophils further strengthening the link between monocyte levels and IL-1β. Moreover, S100A8/A9 are also decreased after weight loss, supporting our hypothesis that they are initiating ligands (Catalan et al., 2011).
Not only is monocytosis likely to be involved in promoting IR, it can also contribute to secondary diseases that are quite often observed in obese individuals. Monocytosis is a well-established risk factor of CVD (Danesh et al., 1998; Erlinger et al., 2004; Grimm et al., 1985) and a number of studies in mice have shown that monocytosis and enhanced monocyte recruitment directly promote atherogenesis (Murphy et al., 2011; Swirski et al., 2007; Tacke et al., 2007; Yvan-Charvet et al., 2010) and inhibit lesion regression (Nagareddy et al., 2013; Potteaux et al., 2011). Therefore it is possible that monocytosis contributes to increased CVD risk in humans with obesity and T2D (Haffner et al., 1990). CD16+ monocytes in humans positively correlate with the Framingham risk score and carotid intima media thickness (Rogacev et al., 2010), well-established indicators of CVD. Increased body mass index in children is also associated with monocytosis, with expansion of both the CD14++CD16− and CD14++CD16+ subsets (Schipper et al., 2012).
Currently there is tremendous interest in developing therapies that inhibit inflammatory pathways in obesity to prevent the onset of secondary diseases such as T2D. We show here that adipose inflammation drives monocyte production via IL-1β. Targeting IL-1β, as well as upstream inducers of TRL4 activation, such as S100A8/A9, could potentially break a feed-forward cycle, preventing aberrant monocyte production and recruitment to the VAT or other sites of inflammation (i.e. atherosclerotic lesions). Disruption of IL-1β signaling in subjects with T2D using the IL-1Ra, Anakinra, improved insulin sensitivity and also decreased circulating WBCs (Larsen et al., 2007). In this study we found that short-term administration of Anakinra decreased leukocyte production, resulting in fewer ATMs and significantly improved glucose tolerance. Another emerging idea from our studies is that leukocyte counts could potentially be used as a biomarker for the efficacy of Anakinra and other therapeutics that target the IL-1β pathway, especially for diabetes and CVD. Further support for our findings comes from the observation that Anakinra also decreases leukocyte levels in other inflammatory diseases (Aksentijevich et al., 2009; Goldbach-Mansky et al., 2006; Pascual et al., 2005).
Overall, our study is the first to demonstrate that VAT can signal to the BM to induce monocyte production and illustrates a number of targetable pathways to prevent aberrant monocyte production, systemic inflammation and possibly risk of secondary diseases (e.g. CVD and T2D) in obesity. While our study suggests a major role ATM-derived IL-1β signaling to increase BM myeloid progenitors, it is likely that additional cytokines and mechanisms are also involved in obesity. For example, Tsuchiya and colleagues have shown that FoxO ablation in macrophages leads to myeloid progenitor expansion in the setting of hypercholesterolemia (Tsuchiya et al., 2013), suggesting that excessive insulin signaling associated with hyperinsulinemia could also be a factor contributing to excessive leukocyte production in insulin resistant states. Our study also indicates that therapeutic interventions that block leukocytosis could alleviate secondary complications of obesity. Inhibitors that are more specific for obesity-driven IL-1β could be developed to target NLRP3 inflammasome [i.e. CRID3 (Coll and O’Neill, 2011)] or the upstream ligands of TLR4, such as S100A8/A9. Finally, our study reveals that monocyte and neutrophil levels could be used as an indicator of adipose tissue health in metabolic diseases.
Experimental Procedures
Mice and Treatments
8–12 week old male; wild type (WT), B6.V-Lepob/J (Ob/Ob), B6.B10ScN-Tlr4lps-del/JthJ (Tlr4−/−), Rage−/−, Myd88−/− in C57BL/6J backgrounds were used for this study. WT, Ob/Ob, Tlr4−/−, Myd88−/− and DIO mice were purchased from the Jackson Laboratory. Rage−/− mice were provided by Yasuhiko Yamamoto (Kanazawa University, Japan). BM for studies involving IL-1r−/− and the Nlrp3−/− mice was obtained from a colony maintained at Pennington Biomedical Research Center, Baton Rouge or Walter and Eliza Hall, Melbourne. Myd88flox/flox, Myd88flox/floxLysMCre and Myd88flox/flox CD11c Cre BM was provided by Dr. Ruslan Medzhitov (Yale University). The SGLT2i (Dapagliflozin) was from Bristol Myers Squibb Company and administered in drinking water at 25 mg/kg(b.w)/day for 4 weeks. Recombinant murine leptin was administered via intraperitoneal injection (i.p.) at a dose of 1μg/kg/day for 7 days.
Bone Marrow Transplant (BMT) Studies
Recipient mice were given 100mg/L neomycin 2 weeks before and after BMT. WT mice were lethally irradiated (with 2 × 6.5 Gy from a cesium gamma source) and transplanted with BM (5×106 cells) from the donor mice and allowed to reconstitute over a 5-week period.
Fat Pad Transplant (FPT) Studies
VAT was harvested from the donor mice, washed in PBS cut into 100mg pieces. WT lean recipient mice were anesthetized with ketamine (100mg/kg)/xylazine (10mg/kg). A small incision was made to access the subcutaneous space and the VAT pads were carefully inserted. The wound was sutured closed and monocyte levels were measured over time. Each recipient mouse received a transplant from an individual donor.
Flow Cytometry
Blood leukocytes
Monocytes (total and subsets) and neutrophils were identified from whole blood as previously described (Murphy et al., 2011). Monocytes were identified as CD45hiCD115hi and further identified into Ly6-Chi and Ly6-Clo; neutrophils were identified as CD45hiCD115loLy6-C/Ghi (Gr-1).
Hematopoietic stem cells
Hematopoietic stem and progenitor cells from the BM and spleen were analyzed by flow cytometry as previously described (Murphy et al., 2011). HSPCs were identified as lineage−, Sac1+ and ckit+ (LSK) while the hematopoietic progenitor subsets were separated by using antibodies to CD16/CD32 (FcγRII/III) and CD34. CMPs were identified as lin−, Sca1−, ckit+, CD34int, FcγRII/IIIint, GMPs as lin−, Sca1−, ckit+, CD34int, FcγRII/IIIhi. Cell cycle analysis was performed using DAPI (Sigma). Flow cytometry was performed using an LSRII (for analysis) or FACS Aria (for sorting), both machines running FACS DiVa software. All flow cytometry data were analyzed using FlowJo software (Tree Star Inc.)
In Vitro Proliferation Assay
BM cells progenitor cells were cultured for 16hrs either in the presence of cytokines essential for hematopoietic growth; stem cell factor (100ng/ml, R&D Systems), IL-3 (6ng/ml, R&D Systems) and GM-CSF (2ng/ml, R&D Systems) with the test samples indicated for the respective experiments. For proliferation measurements, cells were incubated with 10μM of EdU for 12hrs. Proliferation was measured by determining the amount of EdU incorporation or percentage of cells in the G2M phase by staining with DAPI and quantified by flow cytometry.
Statistics
2-tailed Student’s t test or 1-way ANOVA with a Bonferroni multiple-comparison post-test was used to analyze data (GraphPad Prism). A P value > 0.05 conferred significance. Data are mean ± SEM unless otherwise indicated (i.e. error bars depict SEM).
Supplementary Material
Highlights.
Obesity associated -monocytosis is caused by myeloid progenitor cell proliferation
Adipose tissue -derived S100A8/A9 induces IL-1β expression via TLR4/MyD88 pathway
Deletion of NRLP3 or IL-1R reduces obesity associated monocytosis
Acknowledgments
We thank Bristol-Myers Squibb for the SGLT2 inhibitor. We thank Melinda Coughlan (Baker IDI) for the db/db mice and the Flow Cytometry and Imaging facilities at Columbia University. This project was funded by grants from NIH: U01-HL087945 & DK095684 (IJG, EAF), HL45095 (IJG), HL87123 (ART), P01HL092969, R01HL097365 and R01HL062887 (KEB), AG031797, AG043608, AI105097 and DK090556 (VDD), NIH/NIDDK RO1DK072011 (JK), UL1TR000117 and P20 GM103527 (AA-L), and Pilot and Feasibility Award from the Diabetes Complications Consortium (DCC to IJG). RWG was supported by NIHT32 training grant DK064584-10. PRN was supported by a post-doctoral fellowship from the Canadian Institutes of Health Research and a Pathway to Independence Award from NIH (1K99HL122505). AJM was supported in part by a post-doctoral fellowship from the American Heart Association (12POST11890019), a Viertel award from Diabetes Australia Research Trust Australia and a NHMRC program grant (APP10363652).
Footnotes
Detailed methods are available online as a supplementary file.
COMPETING FINANCIAL INTERESTS
The authors acknowledge support from BMS Pharmaceutical Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell metabolism. 2013 doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation research. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circulation research. 2008;102:1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Fernandez-Real JM, Salvador J, et al. Increased levels of calprotectin in obesity are related to macrophage content: impact on inflammation and effect of weight loss. Mol Med. 2011;17:1157–1167. doi: 10.2119/molmed.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll RC, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6:e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U.S. adults: results from the Second National Health and Nutrition Examination Survey mortality study. Cancer Epidemiol Biomarkers Prev. 2004;13:1052–1056. [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PloS one. 2011;6:e24358. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Science translational medicine. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES. Leukocyte Count, Erythrocyte Sedimentation Rate, and Diabetes Incidence in a National Sample of US Adults. Am J Epidemiol. 2002;155:57–64. doi: 10.1093/aje/155.1.57. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. The Journal of clinical investigation. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254:1932–1937. [PubMed] [Google Scholar]
- Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, Reizis B, Shen Z, Fox JG, Iwasaki A, et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, Yu GY, Lai LC, Temkin V, Sinzig U, et al. IL-1beta-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKbeta. Nature immunology. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature immunology. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- Klebanov S, Astle CM, DeSimone O, Ablamunits V, Harrison DE. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. The Journal of endocrinology. 2005;186:203–211. doi: 10.1677/joe.1.06150. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30) Am J Cardiol. 2002;89:1441–1443. doi: 10.1016/s0002-9149(02)02366-4. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. The Journal of clinical investigation. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Tall AR. Proliferating macrophages populate established atherosclerotic lesions. Circulation research. 2014;114:236–238. doi: 10.1161/CIRCRESAHA.113.302813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell metabolism. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshita K, Yamane K, Hanafusa M, Mori H, Mito K, Okubo M, Hara H, Kohno N. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care. 2004;27:491–496. doi: 10.2337/diacare.27.2.491. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. The Journal of clinical investigation. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ. Proliferating macrophages prevail in atherosclerosis. Nature medicine. 2013;19:1094–1095. doi: 10.1038/nm.3316. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, Cremers B, Grenner Y, Geisel J, Schlitt A, et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J. 2010;31:369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- Schipper HS, Nuboer R, Prop S, van den Ham HJ, de Boer FK, Kesmir C, Mombers IM, van Bekkum KA, Woudstra J, Kieft JH, et al. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia. 2012;55:2800–2810. doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell metabolism. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of clinical investigation. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. The Journal of clinical investigation. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Westerterp M, Murphy AJ, Subramanian V, Ferrante AW, Jr, Tall AR, Accili D. Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circulation research. 2013;112:992–1003. doi: 10.1161/CIRCRESAHA.112.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. Journal of immunology. 2009;182:6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nature medicine. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.