Abstract
The myxomatous mitral valve is the most common form of valvular heart disease. The pathologic presentation of myxomatous mitral valve disease varies between valve thickness, degree of leaflet prolapse and the presence or absence of flail leaflets. Recent molecular biology studies have confirmed that the myxomatous changes in mitral valve prolapse equals a cartilage phenotype, which is regulated by the Lrp5 receptor. Clinically, echocardiography defines the valve pathology to determine the surgical approach to valve repair or replacement. Furthermore, the timing of surgical valve repair is controversial and is the subject of a current multicenter trial. The results will resolve the timing of whether watchful waiting versus early surgical valve repair decreases morbidity and mortality of this disease process. This review will summarize the current understanding of the cellular and hemodynamic mechanisms of myxomatous mitral valve disease, which may have future implications in the targeted therapy of this disease process.
Keywords: LDL-density-pressure theory, Lrp5 receptor, mitral valve prolapse, mitral valve regurgitation, myxomatous mitral valve, surgery, valvular heart disease
Myxomatous mitral valve regurgitation is the most common indication for surgical valve repair in the world [1]. The syndrome of mitral valve prolapse (MVP) or myxomatous mitral valve disease is the most common form of valvular heart disease, occurring in 0.6–2.4% of the population, thus being more common than bicuspid aortic valve [2]. For years, this disease process was thought to be a passive degenerative phenomenon. Understanding the cellular mechanisms of this valve lesion and the role of traditional cardiovascular clinical risk factors may provide further understanding of this disease and help to risk-stratify this patient population.
Typically, MVP is a diagnosis commonly detected by cardiac auscultation with one or more systolic clicks and/or a mid-to-late systolic murmur detected on a careful auscultation. Diagnosing MVP and timing to surgical correction are some of the most challenging aspects in clinical cardiology. Chronic mitral regurgitation (MR), due to increasing prevalence of myxomatous disease and the increasing mean age of population, is presenting as often as aortic stenosis. Moderate or severe MR is found in 1.7% of the general population in the entire world and in up to 9.3% of those over 75 years [1]. Echocardiography is the main tool for MR evaluation. In modern times, with regard to mitral valve (MV) repair surgery, echocardiography has become even more important not only in diagnosing the valve pathology of MR but also in understanding the mechanisms of disease to guide the decision for timing of surgical repair or replacement. The clinical parameters related to the complexity of MR, which include patient's symptoms, echocardiography findings and hemodynamics, are the primary driving forces to developing an all-encompassing assessment for cardiologists and surgeons to achieve optimal clinical outcomes. This review will outline how to translate cellular signaling from bench to bedside to further risk-stratify the timing of surgical valve repair.
Classification of MR in myxomatous MVP
Characterizing MR in MVP is one of the most frequent and challenging diagnosis in the echo-cardiography lab. Currently, echocardiography has become the main tool to cover not only the MVP diagnosis but also to define by imaging the valvular pathologic lesions and degree of MR, to guide timing of correction of MV regurgitation. In 1961, Reid [3] hypothesized that the midsystolic click and the late systolic murmur were due to MR. In 1963, Barlow et al. [4] confirmed this hypothesis using left ventricular cineangiography. Since then, intracardiac phonocardiography, and in the 21st century, the gold standard echo-cardiography confirms and characterizes the diagnosis. Familial primary MVP is an autosomal dominant trait that exhibits both sex- and age-dependent penetrance [5]. The discovery of genes involved in the pathogenesis of this common disorder is critical to understanding its diversity in presentation [6]. Primary MVP also includes inherited connective tissue diseases, such as Ehlers–Danlos syndrome, Marfan syndrome, pseudoxanthoma elasticum and osteogenesis imperfecta [2].
Non-familial MVP includes the finding of myxomatous MV changes with varying degrees of pathology, from mild thinning of the MV leaflet of fibroelastic deficiency to severe thickening and billowing with and without chordal rupture or Barlow's disease [2]. MVP can be associated with other disorders including coronary artery disease, rheumatic heart disease, cardiomyopathies and flail leaflets [2].
In the 21st century, clinical and pathologic classification of the varying presentations of MVP has become straightforward with the advent of echocardiography and Doppler hemodynamics. The common symptoms of dyspnea and fatigue associated with the physical exam and echo are now the current state-of–the-art for risk-stratifying this disease process. This combination of echocardiography and patient's symptoms is the primary driving force to develop a full assessment of MR to allow the clinician and surgeon to achieve superior clinical outcomes, but is the subject of the controversial timing to repair, which is debated across the continents.
Timing of MV repair
The International Valve Guidelines, including the American College of Cardiology (ACC)/American Heart Association (AHA) [7,8] and European Society of Cardiology (ESC) guidelines [9], define the timing of MV repair in MR. The difference in the timing between AHA and the ESC has been the center of focus of numerous studies. Currently, the treatment strategy for patients with myxomatous MV disease is to monitor the patients by echocardiography and symptoms. Once the patients develop severe symptoms, the current class I indication for therapy is to repair the valve. The guidelines from Europe and America define the timing based on the degree of regurgitation and symptoms, but complexities of the MV pathology, such as pure myxomatous MV disease with and without flail leaflets are not considered in the timing to surgical valve repair. Furthermore, neither is the recent discovery that myxomatous MVs are gradient from cartilage formation [10–13] within the valve leaflet to bone formation in the calcified mitral annulus [14]. The timing of intervention for MV regurgitation is an evolving and controversial topic in the field of valvular medicine. However, over the past decade, there has been a rapid goal toward treating patients with severe MR with early repair prior to the onset of symptoms.
There are two basic approaches in the literature and outlined in the ACC/AHA/ESC [9,15]. Across the globe, and especially the USA and European scientific guidelines, define the timing to repair patients who are asymptomatic with a slight but critical difference between level of clinical decision-making. The presence of absence of symptoms in this patient population has become the criteria that separates the AHA from the ESC guidelines. In the USA, patients who are asymptomatic fall under class IIA category (in favor of the early repair) [16]. In Europe, patients with no symptoms are classified as IIB (not strongly in favor of early repair) [9].
Timing of repair in flail MV leaflet
The most recent publication on the timing of repair in MV regurgitation is from the Mayo Clinic, in which the hypothesis is focused on treating patients with flail leaflet. The Mitral Regurgitation International Database [17] includes 2097 consecutive patients with flail MV regurgitation (from 1980 to 2004) and demonstrates no significant difference in early mortality and new-onset heart failure rates (0.9% for early surgery vs 0.9% for medical management) between treatment strategies at 3 months. In contrast, long-term survival rates were higher for patients with early surgery (86 vs 69% at 10 years; p < 0.001), associated with a 5-year reduction in mortality of 52.6% (p < 0.001). Final conclusions among registry patients with MV regurgitation due to flail mitral leaflets and performance of early mitral surgery compared with initial medical management were associated with greater long-term survival and a lower risk of heart failure, with no difference in new-onset atrial fibrillation. The results of this study further confirm the US guidelines. These guidelines, which recommend early surgery for asymptomatic patients with preserved left ventricular function [18,19], have been recommended by tertiary referral centers and are a class IIA recommendation in the AHA/ACC guidelines [16]. Early surgery may prevent left ventricular dysfunction from the chronic volume overload secondary to the MR.
Outcomes for watchful waiting for myxomatous MV disease
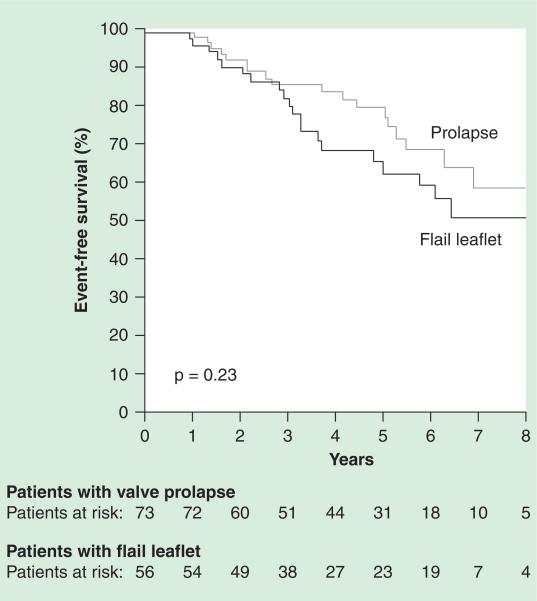
The most important study in differentiating the timing of the surgical intervention has been defined by the degree of MR and the presence and absence of symptoms in patients was published from a European Valve Center [20]. In this study, the authors differentiated the timing for repair between asymptomatic versus symptomatic. The study from Vienna demonstrated a much lower rate of surgical valve repair at 8 years with >50% of patients who did not meet criteria for surgical valve repair. The patient populations are different, as are the valve lesions causing significant MR. In the study from Vienna [20], with regard to watchful waiting for the timing of MV surgical repair, the authors demonstrate that watchful waiting of patients with pure myxomatous MV disease and no symptoms did well. The ESC [9] guidelines support a more conservative strategy of watchful waiting based on careful monitoring of patients for symptoms, left ventricular dimensions and signs of changes in clinical status. The investigators also evaluated the difference between pure myxomatous MV disease with and without flail leaflet and found no significant difference in outcome, but a trend toward a better outcome in the patients without a flail leaflet. Further long-term follow-up of patients may demonstrate significant difference in outcomes for these different valve lesions as shown in Figure 1.
Figure 1. Outcomes of watchful waiting in myxomatous mitral valve disease.
Kaplan-Meier event-free survival for patients with mitral valve prolapse (grey line; n = 74) versus flail leaflet (black line; n = 58).
Reproduced with permission from [20] © Wolters Kluwer Health (2006).
Hemodynamics in patients with myxomatous MV disease
Understanding of hemodynamics will further risk-stratify this patient population. The role of echo is the gold standard not only for the color flow Doppler analysis of severity of MR, but also for quantitative hemodynamics. In the past, cath hemodynamics has been a helpful tool to define severity [21]. Currently, cardiac catheterization hemodynamics has played a lesser role in the pathophysiological understanding of this disease. However, historically, prior to Doppler echo, the role of hemodynamics in different valve pathologies was very important. In a study from 1985 [22], 39 patients with symptomatic severe (MR) were studied by cardiac catheterization and 2D echocardiography prior to MV replacement. In patients with chronic severe MR, flail MV was found at surgery in 23 patients (group 1); 16 patients had intact chordae tendineae (group 2). No difference was found between groups 1 and 2 with regard to hemodynamic findings.
Left atrial volumes in end systole (LAESV) and end diastole (LAEDV) were determined by 2D echocardiography from apical four- and two-chamber views with the use of a biplane area-length method. The LAESV and LAEDV measured 116 ± 66 and 56 ± 48 ml, respectively, in group 1, as compared with 185 ± 101 and 105 ± 62 ml in group 2 (p < 0.025). Ten patients from group 1 with LAESV ≤100 ml (group 1A) were compared with the remaining 13 patients with LAESV >100 ml (group 1B). Patients in group 1A had significantly smaller left ventricular volume and higher mean pulmonary wedge pressure, pulmonary artery and left ventricular enddiastolic pressure compared with patients in groups 1B and 2 (p < 0.05). Thus, a subset of patients with flail mitral leaflets and smaller LAESV have hemodynamic features of acute MR, whereas the remainder with larger LAESV are indistinguishable from patients with chronic MR. The results of this study are demonstrated in Table 1. The results of this study combined with the watchful waiting study indicate that hemodynamic pressures, left atrial size and volume and measurements of pulmonary pressures play a critical role in the understanding of the natural history and also the controversy of timing of surgical valve repair. Patients with acute MR hemodynamics from ruptured chords are at higher risk, as defined by Ling et al. and confirmed by the Mitral Regurgitation International Database, which clearly indicates that early repair is necessary for improved long-term outcomes [17,18]. Patients with or without flail and who have cardiac chambers that have adapted to increased volume abnormalities, may have time to repair, but there will be definitive answers with the results of future randomized clinical studies.
Table 1.
Mitral valve biology versus mitral valve hemodynamics in severe mitral regurgitation.
| Overall biology | Myxomatous mitral valve disease secondary to chondrocyte phenotype | ||
|---|---|---|---|
| Mechanism | Ruptured mitral chords | Intact mitral chords | Ruptured mitral chords |
| Hemodynamics | Small left atrial volume Increase pulmonary pressures |
Large left atrial volume Normal pulmonary pressure |
Large left atrial volume Normal pulmonary pressure |
| Long-term outcome | Similar to acute MR | Equal | Equal |
MR: Mitral regurgitation.
Data taken from [15].
Cellular mechanisms of myxomatous MV disease
To further understand the importance of valve pathology, the recent studies over the past decade have demonstrated that specific pathologic phenotype is a spectrum of valve pathologies that encompass the disease entity itself. The classification is based on the pathologic characterization of the MV, which for years was characterized as degenerative, but in the 21st century, it is clear that the biology of the heart valve is an active cellular biology.
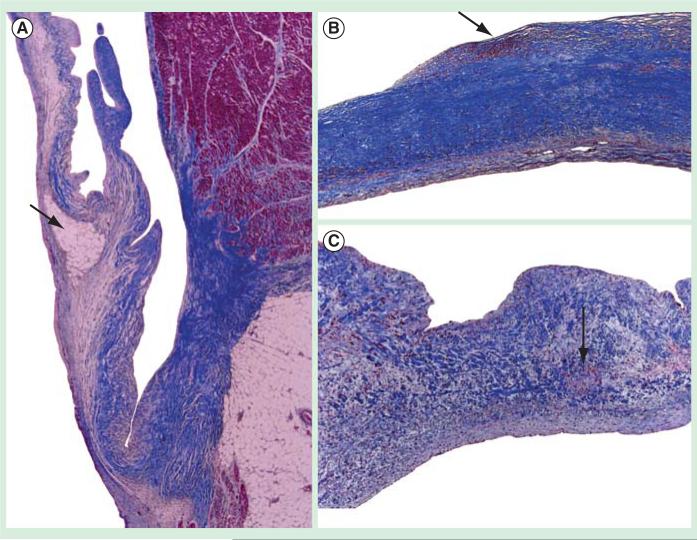
Myxomatous MV lesions causing MR are caused by progressive thickening due to activated myofibroblasts [23], causing car tilage formation in the valve or what is termed myxomatous changes. The risk factors for the development of MV disease and aortic valve disease have been defined in the past 10 years as traditional cardiovascular disease [14]. The human correlation of cholesterol as a risk factor for the development of MV leaflet thickening is first described in a patient case report of familial hypercholesterolemia and MV disease as shown in Figure 2 [24]. The MV demonstrated atherosclerosis in this 7-year-old patient who was diagnosed with familial hypercholesterolemia, which is the loss of the low-density lipoprotein receptor important in lipid metabolism.
Figure 2. Mitral valve atherosclerotic histology from case report of patient with familial hypercholesterolemia.
(A) Mitral valve atherosclerosis (2×). (B) Mitral valve atherosclerosis (10×). (C) Mitral valve atherosclerosis (20×).
Data taken from [43].
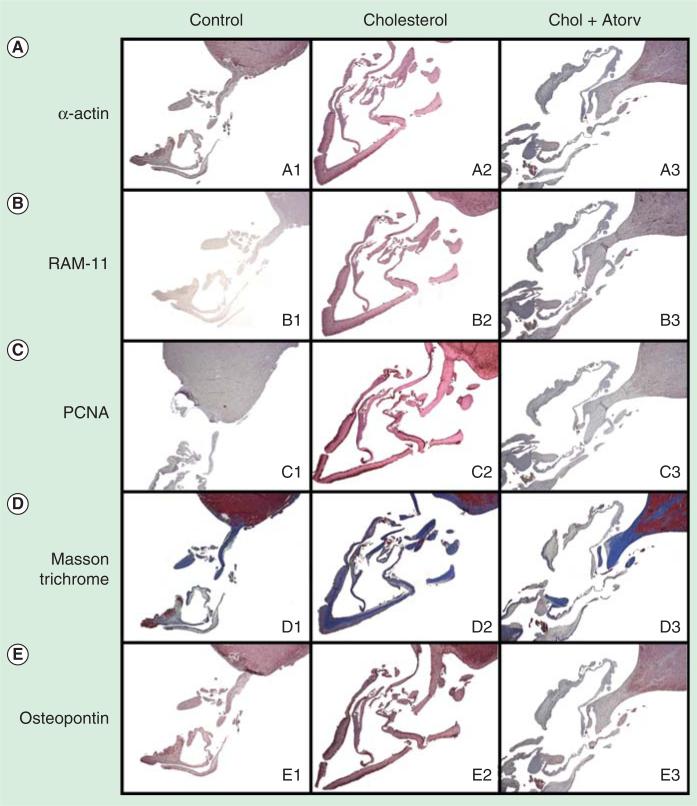
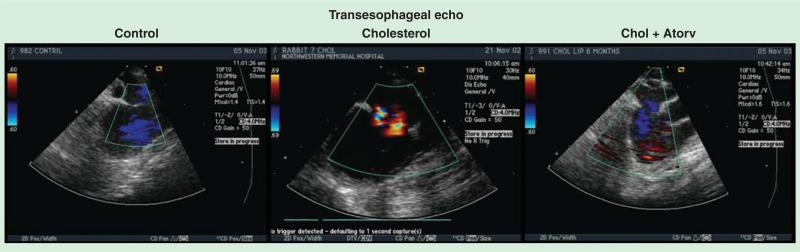
To confirm the initiating event of the activation of MV fibroblasts, this hypothesis was tested in an experimental model of hypercholesterolemia. A 2-month cholesterol diet was fed to rabbits, which confirmed the development of atherosclerosis and MV cellular proliferation in the leaflets as shown in Figure 3 [25]. This model has been tested for 6 months. The 6-month experimental hypercholesterolemia rabbit model developed significant MR by transesophageal echocardiography, which improved in the atorvastatin therapy as shown in Figure 4. These experimental findings implicate the potential for traditional cardiovascular risk factors in the development of MV disease [14].
Figure 3. Immunohistochemistry of 2-month experimental hypercholesterolemia treatment demonstrating atherosclerosis after 2 months and attenuation with atorvastatin.
PCNA: Proliferating cell nuclear antigen.
Data taken from [25].
Figure 4. Transesophageal echocardiography of the 6-month experimental hypercholesterolemia rabbit model demonstrating significant regurgitation in the cholesterol treatment and attenuation with atorvastatin.
Data taken from [30].
Lrp5 signaling mechanism in myxomatous MVP
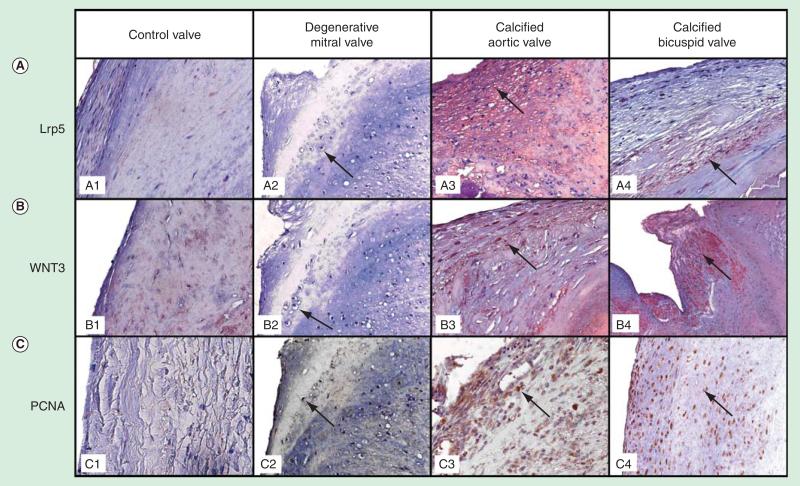
Despite this increase in prevalence and incidence of valvular heart disease, the signaling pathways in human valve disease have not been identified. Studies have demonstrated in experimental animal models that bone matrix protein expression in the aortic valve and vasculature are regulated by the low-density lipoprotein receptor-related protein 5 (Lrp5) pathway in the presence of elevated hypercholesterolemia [26–30]. The Lrp5, a co-receptor of low-density lipoprotein receptor family, has been discovered as an important receptor in the activation of skeletal bone formation via binding to the secreted glycoprotein Wnt and activating bcatenin to induce bone formation. The study further defined the mechanism of myxomatous MV disease, which is caused by osteogenic differentiation secondary to the activation of the Lrp5 receptors in diseased valve leaflets in humans [10]. To test this hypothesis, diseased MVs from surgery, calcified tricuspid and bicuspid aortic valves determine if the Lrp5 signaling pathway is expressed in these tissues [10]. Figure 5 demonstrates the immunohistochemistry stains for the osteoblast signaling markers: Lrp5, Wnt3 and proliferating cell nuclear antigen (PCNA). Figure 5A (panels A1 and A2) and Figure 5B (panels B1 and B2) demonstrate a mild amount of Lrp5 and Wnt3 staining in the control valves and in the areas of hypertrophic chondrocytes in the MVs. The Lrp5 and Wnt3 staining was increased in the calcified aortic valves (Figure 5A [panels A3 and A4] and Figure 5B [panels B3 and B4]). Figure 5C, panels C3 and C4, demonstrates the presence of an increase in PCNA protein expression as compared with Figure 5C, panels C1 and C2, which demonstrates a decrease in PCNA protein staining.
Figure 5. Immunohistochemistry of the human mitral degenerative valves and calcified tricuspid and bicuspid aortic valves for endochondral signaling markers low-density lipoprotein receptor-related protein 5/Wnt and proliferating cell nuclear antigen.
Control valve, degenerative mitral valve (arrow points to hypertrophic chondrocytes), calcified aortic valves (arrow points to positive stain) and bicuspid aortic valve (arrow points to positive stain; magnification 25×). (A) Lipoprotein receptor-related protein 5 stain. (B) Wnt 3 stain. (C) Proliferating cell nuclear antigen stain [10].
Lrp5: Lipoprotein receptor-related protein 5; PCNA: Proliferating cell nuclear antigen.
Until recently, the etiology of valvular heart disease has been thought to be a ‘degenerative’ process related to the passive accumulation of calcium binding to the surface of the valve leaflet. Recent descriptive studies have demonstrated the critical features of aortic valve calcification, including osteoblast expression, cell proliferation and atherosclerosis [29,31] and MV degeneration, glycosaminglycan accumulation, proteoglycan expression and abnormal collagen expression. These studies define the biochemical and histologic characterization of these valve lesions. The most striking phenotypic abnormalities in this study are the formation of cartilaginous structures in the degenerative MVs and bone formation in the calcified aortic valves. The development of endochrondral bone formation progresses in response to the different pressures in the heart chambers via Lrp5 activation in the mesenchymal cells in the valve leaflets.
Biochemistry & pathology of flail myxomatous MV chordae
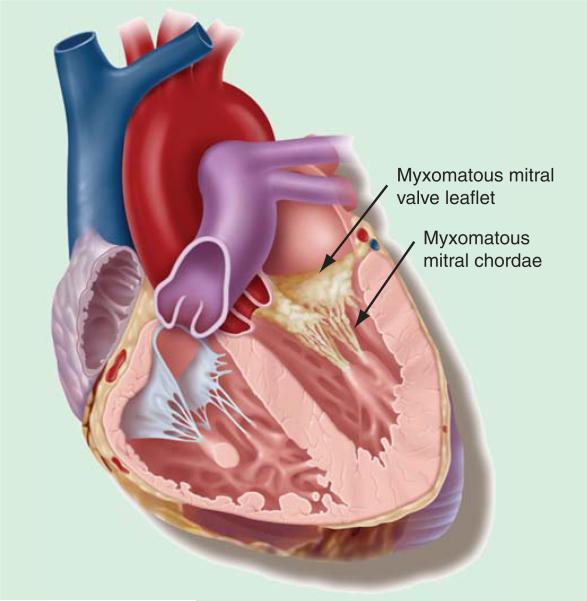
It is well known that myxomatous MV disease is accompanied by lengthening and/or rupture of chordae tendinae. However, the mechanisms and the mode of chordal rupture remain controversial, and the pathologic anatomy of the apparently healthy chordae has mostly been overlooked. Figure 6 demonstrates the cartilaginous phenotype in the MV leaflet in myxomatous MV disease and thickened chordae consistent with similar biochemical abnormalities. The investigators [32] analyzed the structural aspects of both ruptured and intact chordae tendinae from patients with abnormal chordal pathology. Structural and ultrastructural microscopic analyses indicate that both the extracellular matrix and the interstitial cells are severely affected. Myxomatous chordae show alterations in the synthesis and deposition of collagen and elastin, disorganization of collagen bundles and rupture of collagen fibers, accumulation of proteoglycans and of cellular and vesicular remnants and cell transformation into a myofibroblast phenotype. Structural disruption makes the spongiosa and the dense collagenous core separate and break. Degeneration of the chordae is segmental, affecting both chordae that are clearly abnormal and chordae that appear healthy on visual inspection. The authors concluded that surgery corrects the damage, but the underlying causes of the myxomatous changes are not corrected. Thus, progression of the disease and affectation of additional chordae may be the basis for the late complications and the recurrent MR, which occurs several years after surgery. The study investigators concluded that the early surgical repair is necessary for this patient population.
Figure 6.
Photograph of myxomatous mitral valve with ruptured chords.
Translating the molecular biology to surgical repair of the myxomatous MV disease: application of the LDL-density-pressure theory
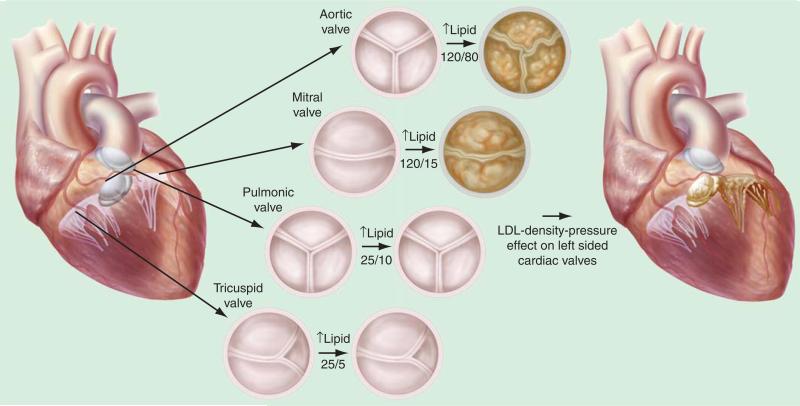
The development of heart valve disease occurs in the left side of the heart, the aortic valve and the MV, which manifests as calcific aortic valve disease and myxomatous MV disease. The LDL-density-pressure theory provides a scientific explanation for the manifestation of this phenotype. In experimental hyper-cholesterolemia, the left-sided aortic heart valves [31,33–36] and the MV [35] develop the atherosclerotic lesion and not the right-sided heart valves: pulmonic and tricuspid valves. The right-sided heart valves in the experimental model are completely normal. Figure 6 demonstrates the differences of the pressures in the heart and the expression of the phenotype of the heart valve. The lipids bind to the Lrp5/6 receptors to activate the canonical Wnt pathway and the bone formation. Since the Lrp5 plays a role in the mechanostat theory [37], this mechanism provides the foundation for the pressure theory on mechanical effects on Lrp5 in the heart. Normal pressures in the heart increase from the right atrium, to the right ventricle, to the left atrium, and finally to the left ventricle for normal cardiac physiology as shown in Figure 7. However, this theory hypothesizes that in the presence of hyperlipidemia, the phenotypic expression of the valve changes in response to the different pressures in the heart. Human phenotypic studies of cardiac valve disease have demonstrated that the aortic valve expresses an osteoblast phenotype [33,38] and the MV expresses a chondrogenic phenotype [38]. It is known that the MV has a lower pressure present in the left atrium as compared with the aorta. Therefore, the pressure on the MV is enough to produce cartilage and the pressure on the aortic valve is higher to drive the Lrp5 mechanostat mechanism to form bone.
Figure 7. LDL-density-pressure theory.
Figure reproduced from [27].
The mitral annulus that has slightly higher pressures than the valve leaflet can form bone if the pressures are high enough to cause mitral annular calcification [39,40]. The results of these studies further confirm the mechanostat theory for the role of Lrp5 in the presence of the different pressures in the heart. The higher pressure aortic valve differentiates to form bone, and the MV, which has lower pressures, only develops calcification at the mitral annulus, and in the leaflets, it develops a cartilage phenotype. Even though the lipids are present throughout the systemic circulation, the right-sided valves with the lowest pressures in the heart do not calcify or form cartilage, as shown in Figure 7.
European surgical intervention trial
Dutch Asymptomatic Mitral Regurgitation (AMR) is the first multicenter surgical trial to test the timing of MV repair [41]. The decision to operate early versus watchful waiting is at the center of a clinical trial entitled Dutch AMR (NCT01708265). To obtain evidence for these approaches, the Dutch AMR trial [42] will be the first multicenter randomized trial on this topic worldwide. Two treatment strategies are compared: early surgery versus watchful waiting. The investigators anticipate developing a foundation for the best clinical strategy for this patient population. Furthermore, the results will provide solid recommendations for clinical guidelines in the future management of MV disease. In addition to identification of the best treatment and evidence-based medicine, it is of utmost importance to have knowledge on the cost–effectiveness of treatment. The investigators expect that the surgical strategy may be cost-effective in comparison with the strategy of watchful waiting.
Conclusion
MV regurgitation is a complex disease process, which requires expertise in imaging, evaluation and symptom management to diagnose, follow and treat this patient population. Current imaging modalities in echocardiography have vastly improved our understanding of the mechanisms of valve regurgitation, valve pathology and the natural history of this disease. The recent understanding of the cartilage phenotype, which develops in the MV secondary to differential pressures in the cardiac chambers, will help physicians to further understand the cellular mechanisms. Furthermore, hemodynamic risk stratification for the different pathologies associated with myxomatous MV disease will also become important in defining the timing of surgery and may help to further delineate the controversies that are present in the literature. The Dutch AMR will further help to delineate the approach and outcomes for this complex patient population and further define for physicians the timing of surgery in the future.
Expert commentary
Currently, the only treatment for severe MR is surgical valve repair with the ongoing question of timing to repair. This important decision-making process is the subject of many ongoing studies and a large multicenter clinical trial in Europe entitled Dutch AMR. The exact timing to surgical valve repair in patients with severe MR is under intense investigation in part due to the rapid knowledge in the field of quantitative echo hemodynamics, cellular biology of the heart valve, which will provide in the future, a more stringent opportunity to risk-stratify this patient population using pathology, echo hemodynamics, and the most important criteria, the presence or absence of symptoms. Timing to repair this disease process will allow clinicians to avoid atrial fibrillation and future left ventricular enlargement. The results of Dutch AMR will play a pivotal role in the surgical management of this disease process.
Five-year view
To date, there is no medical therapy for the treatment of severe MR. The few experimental models in the literature indicate that hypercholesterolemia may contribute to the development of the actual valve leaflet pathology. Activation of the Lrp5 receptor in response to pressure and lipids, as formulated in the LDL-density-pressure theory, will induce endochondral cartilage formation in the MV leaflet. Over time, the thickened leaflet will cause progressive valve regurgitation and left atrial enlargement. If the traditional cardiovascular risk factors are responsible for a subset of this patient population, who do not have a genetic predisposition to this disease, then primary prevention may be an option in the future for this patient population. Traditional cardiovascular risk factors studies are needed to move forward this hypothesis, and the potential opportunity to slow the progression of this disease prior to the development of severe MR and significant symptoms in this patient population.
Key issues.
The European Society of Cardiology and American College of Cardiology/American Heart Association valvular guidelines suggest two different timing to surgical valve repair, based on the presence and absence of symptoms. However, the long-term safety, morbidity and mortality data still remain to be firmly established, in part due to the need for more stringent risk stratification using valve pathology, hemodynamics and clinical symptoms.
A well-designed, adequately powered clinical surgical trial is ongoing to address these shortcomings and provide a more definitive assessment of the indications of timing to surgical valve repair.
Experimental studies indicate the potential for traditional cardiovascular risk factors in the development of myxomatous mitral valve disease; future epidemiologic studies testing this hypothesis are necessary to further define this hypothesis and the potential for targeting this disease with cholesterol-lowering medications and antihypertensive therapy.
Acknowledgments
NM Rajamannan is the inventor on a patent for methods to slow progression of valvular heart disease. This patent is owned by the Mayo Clinic and the author does not receive any royalties from this patent. This work is supported by NIH grant funding: 5R01HL085591 and 3R01HL085591S1.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1••.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–72. doi: 10.1038/nrcardio.2010.202. [Most up-to-date report of the epidemiology of vavular heart disease in Europe.] [DOI] [PubMed] [Google Scholar]
- 2•.O'Rourke RA, Bailey SR. Mitral valve prolapse syndrome. The Heart. 2004:1695–706. (Chapter 68) [This chapter in the textbook Heart is a very nice review to characterize the cardiac cath hemodynamics for this disease process.] [Google Scholar]
- 3•.Reid JV. Mid-systolic clicks. S Afr Med J. 1961;35:353–7. [Historical record of auscultation of the mid-systolic click.] [PubMed] [Google Scholar]
- 4.Barlow JB, Pocock WA, Marchand P, Denny M. The significance of late systolic murmurs. Am Heart J. 1963;66:443–52. [Google Scholar]
- 5.Freed LA, Acierno JS, Jr, Dai D, et al. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am J Human Genet. 2003;72:1551–9. doi: 10.1086/375452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou HT, Shi YR, Hsu Y, Tsai FJ. Association between fibrillin-1 gene exon 15 and 27 polymorphisms and risk of mitral valve prolapse. J Heart Valve Dis. 2003;12:475–81. [PubMed] [Google Scholar]
- 7.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the society of cardiovascular anesthesiologists endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello B, de Leon AC., Jr Guidelines for the management of patients with valvular heart disease: executive summary. A report of the american college of cardiology/american heart association task force on practice guidelines (committee on management of patients with valvular heart disease). Circulation. 1998;98:1949. doi: 10.1161/01.cir.98.18.1949. [DOI] [PubMed] [Google Scholar]
- 9••.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68. doi: 10.1093/eurheartj/ehl428. [The current European Society of Cardiology valvular guidelines indicating the class II indication for careful observation to symptoms in patients with severe mitral regurgitation.] [DOI] [PubMed] [Google Scholar]
- 10••.Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [First published report of LDL receptor-related protein 5 receptor expression in the mitral valve with cartilage formation and hypertrophic chrondrocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grande-Allen KJ, Borowski AG, Troughton RW, et al. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study.[see comment]. J Am Coll Cardiol. 2005;45(1):54–61. doi: 10.1016/j.jacc.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 12.Grande-Allen KJ, Calabro A, Gupta V, et al. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14(7):621–33. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 13••.Grande-Allen KJ, Griffin BP, Calabro A, et al. Myxomatous mitral valve chordae. Ii: selective elevation of glycosaminoglycan content. J Heart Valve Dis. 2001;10:325–32. discussion 332-323. [The first published report of selective glycosaminoglycan expression in myxomatous mitral valve chordae.] [PubMed] [Google Scholar]
- 14.Lazaros G, Toutouzas K, Drakopoulou M, et al. Aortic sclerosis and mitral annulus calcification: a window to vascular atherosclerosis? Expert Rev Cardiovasc Ther. 2013;11:863–77. doi: 10.1586/14779072.2013.811978. [DOI] [PubMed] [Google Scholar]
- 15••.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): Developed in collaboration with the society of cardiovascular anesthesiologists: Endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. Circulation. 2006;114:e84–23. doi: 10.1161/CIRCULATIONAHA.106.176857. [The current American College of Cardiology/American Heart Association valvular guidelines indicating the class II indications of the timing of surgical valve repair in patients who do not have symptoms.] [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the society of cardiovascular anesthesiologists endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 17•.Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–16. doi: 10.1001/jama.2013.8643. [The most recent study assessing the timing of surgical valve repair.] [DOI] [PubMed] [Google Scholar]
- 18.Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation. 1997;96:1819–25. doi: 10.1161/01.cir.96.6.1819. [DOI] [PubMed] [Google Scholar]
- 19.Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417–23. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 20•.Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–44. doi: 10.1161/CIRCULATIONAHA.105.599175. [The European study indicating watchful waiting is the most prudent approach toward the timing of surgical valve repair in patients with severe mitral regurgiation.] [DOI] [PubMed] [Google Scholar]
- 21.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 22.Ren JF, Panidis IP, Kotler MN, et al. Flail mitral valve syndrome: comparison with chronic mitral regurgitation of other etiologies. Am Heart J. 1985;109:435–42. doi: 10.1016/0002-8703(85)90544-7. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin E, Aikawa M, Stone JR, et al. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–32. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 24.Rajamannan NM, Spelsberg TC, Moura LM. Mitral valve disease in a patient with familial hypercholesterolemia. Rev Port Cardiol. 2010;29:841–2. [PMC free article] [PubMed] [Google Scholar]
- 25••.Makkena B, Salti H, Subramaniam M, et al. Atorvastatin decreases cellular proliferation and bone matrix expression in the hypercholesterolemic mitral valve. J Am Coll Cardiol. 2005;45:631–3. doi: 10.1016/j.jacc.2004.11.023. [First experimental model of experimental cholesterolemia causing atherosclerosis in the mitral valve.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajamannan NM. The role of lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the apoe−/−/lrp5−/−mice. J Cell Biochem. 2011;112:2987–91. doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamannan NM. The role of lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem. 2011;112:2222–9. doi: 10.1002/jcb.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in lrp5 bone formation. Cardiovasc Pathol. 2011;20:168–76. doi: 10.1016/j.carpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajamannan NM, Subramaniam M, Caira F, et al. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the lrp5 receptor pathway. Circulation. 2005;112:I229–34. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajamannan NM, Rosenhek RA, Rahimtoola SH. Atorvastatin attenuates severe myxomatous mitral regurgitation via lrp5/sox9 formation, European Society of Cardiology. Eur Heart J. 2009;30(Suppl) Abstract 997. [Google Scholar]
- 31.Rajamannan NM, Subramaniam M, et al. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–5. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Icardo JM, Colvee E, Revuelta JM. Structural analysis of chordae tendineae in degenerative disease of the mitral valve. Int J Cardiol. 2013;167:1603–9. doi: 10.1016/j.ijcard.2012.04.092. [DOI] [PubMed] [Google Scholar]
- 33.Rajamannan NM, Edwards WD, Spelsberg TC. Hypercholesterolemic aortic-valve disease. N Engl J Med. 2003;349:717–18. doi: 10.1056/NEJMc031360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajamannan NM, Sangiorgi G, Springett M, et al. Experimental hypercholesterolemia induces apoptosis in the aortic valve. J Heart Valve Dis. 2001;10:371–4. [PubMed] [Google Scholar]
- 35.Makkena B, Salti H, Subramaniam M, et al. Atorvastatin decreases cellular proliferation and bone matrix expression in the hypercholesterolemic mitral valve. J Am Coll Cardiol. 2005;45:631–3. doi: 10.1016/j.jacc.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajamannan NM, Subramaniam M, et al. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91:806–10. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson ML, Summerfield DT. Parameters of lrp5 from a structural and molecular perspective. Crit Rev Eukaryot Gene Expr. 2005;15:229–42. doi: 10.1615/critreveukargeneexpr.v15.i3.50. [DOI] [PubMed] [Google Scholar]
- 38.Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boon A, Lodder J, Cheriex E, Kessels F. Mitral annulus calcification is not an independent risk factor for stroke: a cohort study of 657 patients. J Neurol. 1997;244:535–41. doi: 10.1007/s004150050140. [DOI] [PubMed] [Google Scholar]
- 40.Aronow WS, Ahn C, Kronzon I. Association of mitral annular calcium with symptomatic peripheral arterial disease in older persons. Am J Cardiol. 2001;88:333–4. doi: 10.1016/s0002-9149(01)01657-5. [DOI] [PubMed] [Google Scholar]
- 41••.Tietge WJ, de Heer LM, van Hessen MW, et al. Early mitral valve repair versus watchful waiting in patients with severe asymptomatic organic mitral regurgitation; rationale and design of the Dutch AMR trial, a multicenter, randomised trial. Neth Heart J. 2012;20:94–101. doi: 10.1007/s12471-012-0249-y. [The first randomized clinical trial Dutch Asymptomatic Mitral Regurgitation testing the timing of surgical valve repair in patients with severe mitral regurgitation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tietge WJ, de Heer LM, van Hessen MW, et al. Early mitral valve repair versus watchful waiting in patients with severe asymptomatic organic mitral regurgitation; rationale and design of the Dutch AMR trial, a multicenter, randomised trial. Neth Heart J. 2012;20:94–101. doi: 10.1007/s12471-012-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajamannan NM, Spelsberg TC, Moura LM. Mitral valve disease in a patient with familial hypercholesterolemia. Rev Port Cardiol. 2010;29:841–2. [PMC free article] [PubMed] [Google Scholar]