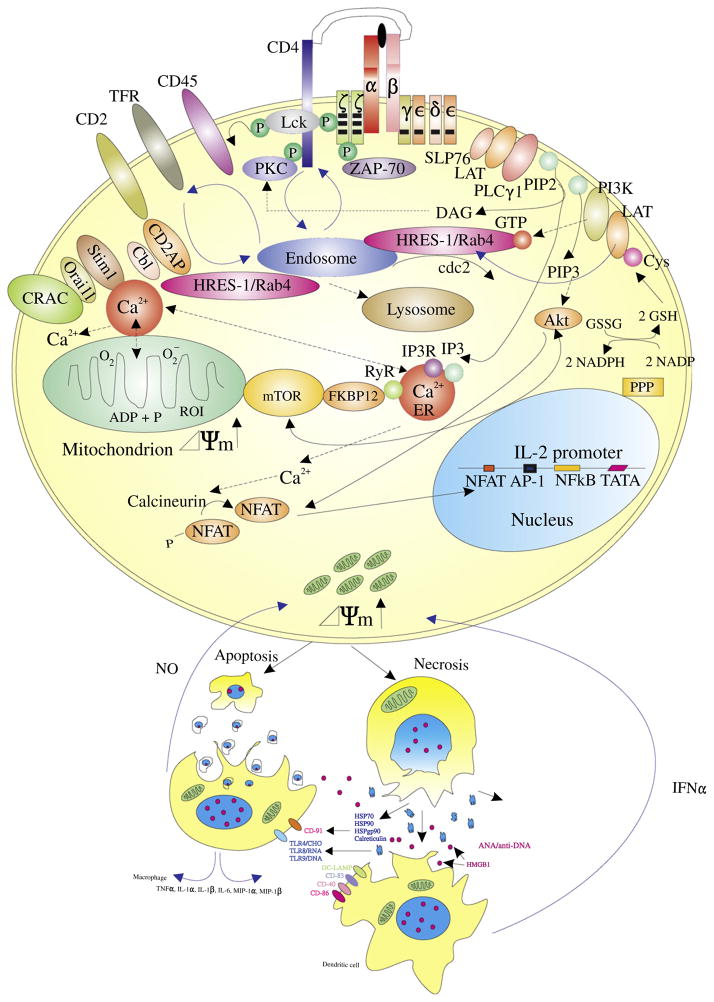
The pathogenesis systemic lupus erythematosus (SLE) is attributed to genetic and environmental factors that cumulatively elicit the dysfunction of T and B lymphocytes and dendritic cells, resulting in the formation of antinuclear antibodies (ANA) and immune complexes of ANA with nuclear DNA, RNA, and proteins [1]. The production of ANA is thought to be driven by the release of necrotic materials from lupus T cells, exhibiting mitochondrial dysfunction characterized by a persistent elevation of the mitochondrial transmembrane potential (Δψm) or mitochondrial hyperpolarization (MHP) [2] and ATP depletion which predispose to death by necrosis. The increased release of necrotic materials from T cells may drive disease pathogenesis by activating macrophages and dendritic cells to produce nitric oxide (NO) and interferon α (IFN α) in SLE. Within T cells, MHP leads to the activation of the mammalian target of rapamycin (mTOR) that plays key roles in metabolic pathways that control activation and lineage specification [3].
Dysregulated signaling through the T-cell receptor (TCR) is a critical determinant of abnormal T-cell activation in patients with SLE [4,5]. The binding of antigen to the αβ or γδTCR is associated with the formation of multimeric receptor modules centered around the signal-transducing TCR ζ chain (Fig. 1). The cytoplasmic domain of TCRζ harbors an immunoglobulin receptor family tyrosine-based activation motif (ITAM) which is crucial for the recruitment of intracellular tyrosine kinases [6]. Binding of Lck to the TCR-CD3 complex through CD4 or CD8 initiates the phosphorylation of ITAM. This in turn triggers the SH-2-mediated binding of zeta-associated protein-70 (ZAP-70) or the related spleen tyrosine kinase (Syk). ZAP-70 is activated via phosphorylation by Lck. Phosphorylated ZAP-70 and Syk recruit the adaptor proteins, linker for activation of T cells (LAT) and SH2 domain containing leukocyte protein of 76kD (SLP-76); the latter is also known as lymphocyte cytosolic protein 2 (LCP2) [7]. Phosphorylated LAT binds directly to phospholipase C-γ1 (PLC γ1) and thus controls hydrolysis of phosphatydilinositol-4,5-biphosphate (PIP2) to inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Phosphorylation of inositol lipid second messengers is mediated by phosphatidylinositol 3′hydroxyl kinase (PI3K). The stimulatory effect of the TCR alone on PI3K activity is small. Concurrent triggering of the CD28 co-stimulatory molecule by its ligands CD80 or CD86 is required for optimal PI3K activation. IP3 binds to its receptors in the endoplasmic reticulum (ER), opening Ca2+ channels that release Ca2+ from the ER and connected mitochondria to the cytosol. Decreased Ca2+ concentration in the ER activates the Ca2+ release-activated Ca2+ channel (CRAC) in the plasma membrane. Such TCR-induced rapid Ca2+ flux through CRAC is increased in lupus T cells [8]. The replacement of TCRζ with FcεRIγ at the level of the T-cell synapse has been identified as the most apical event in enhanced signaling through the TCR in SLE patients [9]. Changes in TCRζ protein levels have been attributed to altered transcription [10] and translation [11] and increased lysosomal degradation [12]. Alternatively, increased mitochondrial Ca2+ stores may also contribute to enhanced signaling through the CRAC channel [13].
Figure 1.
Schematic diagram of the metabolic pathways regulating oxidative stress and endosome traffic controlling the recycling of receptor and adaptor proteins between the cell surface and lysosomes. The pentose phosphate pathway (PPP), reduced glutathione (GSH), and nitric oxide (NO) regulate mitochondrial electron transport, the transmembrane potential (Δψm) and the production of reactive oxygen intermediates. The mammalian target of rapamcyin (mTOR) senses Δψm and regulates endosome traffic, protein homeostasis through balancing translation and lysosomal degradation via autophagy. Necrosis-prone T cells release oxidized DNA and HMGB1 which stimulate macrophages, and dendritic cells to produce NO and interferon alpha (IFN-α). Oxidation of cysteine residues and endocytic recycling are proposed to mediate the depletion of the linker for activation of T cells (LAT) in lipid rafts of T lymphocytes in patients with SLE.
Abdoel and colleagues have now discovered that LAT is displaced from lipid rafts and decreass in lupus T cells after TCR activation [14]. The reasons for the accelerated decrease of LAT following activation in lupus T cells are not clear but proposed to be related to increased ubiquitinylation and degradation in the proteasome [14]. These findings are plausible although somewhat discordant with previous observations reporting similar levels of LAT in lipid rafts of lupus and control T cells [15] but either decreased association of LAT with ZAP-70 [16] or increased association of LAT with Lck in lupus T cells [17]. As Lck and ZAP70 levels are reduced and Syk levels are increased in lupus T cells, the latter being critical in conferring enhanced Ca2+ flux [18], changes in LAT or partitioning of LAT to lipid rafts could be crucial to abnormal signal transduction in SLE.
The rapid depletion of LAT, as soon as 1 min after CD3 stimulation, and a lack of changes in its state of phosphorylation, strongly argue that this change result from differences in partitioning and degradation rather than diminished production through transcription and translation. Interestingly, LAT is ubiquitinylated and targeted to endosomes also harboring transferrin receptor (TFR) and the TCR or CD3ζ chain [19]. The trafficking of such endosomes is regulated by small GTPases, in particular, HRES-1/Rab4 that targets the TFR, CD4, as well as the TCRζ chain for lysosomal degradation in Jurkat cells [20] and in peripheral blood CD4 T cells [12]. As overexpression of HRES-1/Rab4 in Jurkat cells and lupus T cells inhibits the recycling of endosomes carrying TFR, CD4, and TCRζ and targets them for lysosomal degradation, conceivably, this mechanism may also account for depletion of LAT in the lipid raft of lupus T cells (Fig. 1). Curiously, rapamycin inhibits T-cell activation-induced LAT expression in healthy donors [21]. As rapamycin blocks the expression of HRES-1/Rab4, it also reverses the depletion of CD4, TCRζ and Lck along with the compensatory up-regulation of FcεRIγ and Syk in lupus T cells [12]. It would be telling to formally determine the impact of mTOR blockade on LAT and whether or not reconstitution of LAT could limit T-cell activation-induced Ca2+ flux in SLE.
Alternatively, the depletion of LAT in lipid rafts may not conform with the direction of changes in CD4 and TCRζ and rather reflect the consequences of mitochondrial dysfunction, oxidative stress, and glutathione (GSH) depletion in lupus T cells [22,23]. Creating oxidative stress by lowering intracellular GSH levels, resulted in the membrane displacement of LAT in human peripheral blood T cells [24]. Targeted mutation of redox-sensitive cysteine residues within LAT prevented the displacement of LAT under conditions of chronic oxidative stress. It would be important to determine if such mutations also prevent localization of LAT to endosomes.
In summary, the recently discovered depletion of LAT potentially represents a new biomarker for rewiring of the T-cell synapse, mitochondrial dysfunction, oxidative stress, and enhanced endocytic recycling in lupus T cells. Although these novel findings require confirmation, they already represent an inviting new target for therapeutic intervention in SLE.
Footnotes
This work was supported in part by grants AI 048079 and AI 072648 from the National Institutes of Health, the Alliance for Lupus Research, and the Central New York Community Foundation.
References
- 1.Perl A, Fernandez DR, Telarico T, Doherty E, Francis L, Phillips PE. T-cell and B-cell signaling biomarkers and treatment targets in lupus. Curr Opin Rheumatol. 2009;21(5):454–464. doi: 10.1097/BOR.0b013e32832e977c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gergely PJ, Grossman C, Niland B, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez DR, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med. 2010;9(46):173–178. [PMC free article] [PubMed] [Google Scholar]
- 4.Nambiar MP, Fisher CU, Warke VG, et al. Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1948–1955. doi: 10.1002/art.11072. [DOI] [PubMed] [Google Scholar]
- 5.Kyttaris VC, Krishnan S, Tsokos GC. Systems biology in systemic lupus erythematosus: Integrating genes, biology and immune function. Autoimmunity. 2006;39(8):705–709. doi: 10.1080/08916930601061363. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Garvin JE, Koretzky GA, Jordan MS. T Cell Activation. Annu Rev Immunol. 2009;27(1):591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koretzky GA, Boerth NJ. The role of adapter proteins in T cell activation [Review] [137 refs] Cell Mol Life Sci. 1999;56:1048–1060. doi: 10.1007/s000180050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J Immunol. 1995;155(4):2269–2281. [PubMed] [Google Scholar]
- 9.Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Juang YT, Wang Y, Jiang G, et al. PP2A Dephosphorylates Elf-1 and Determines the Expression of CD3{zeta} and FcR {gamma} in Human Systemic Lupus Erythematosus T Cells. J Immunol. 2008;181(5):3658–3664. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury B, Tsokos CG, Krishnan S, et al. Decreased Stability and Translation of T Cell Receptor ζ mRNA with an Alternatively Spliced 3′-Untranslated Region Contribute to ζ Chain Down-regulation in Patients with Systemic Lupus Erythematosus. J Biol Chem. 2005;280(19):18959–18966. doi: 10.1074/jbc.M501048200. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez DR, Telarico T, Bonilla E, et al. Activation of mTOR controls the loss of TCR in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric Oxide-Dependent Mitochondrial Biogenesis Generates Ca2+ Signaling Profile of Lupus T Cells. J Immunol. 2004;173(6):3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdoel N, Brun S, Bracho C, Rodríguez MA, Blasini AM. Linker for activation of T cells is displaced from lipid rafts and decreases in lupus T cells after activation via the TCR/CD3 pathway. Clin Immunol. 2012;142:243–251. doi: 10.1016/j.clim.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Juang YT, Chowdhury B, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181(11):8145–8152. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabouridis PS, Isenberg DA, Jury EC. A negatively charged domain of LAT mediates its interaction with the active form of Lck. Mol Membr Biol. 2011;28(7–8):487–494. doi: 10.3109/09687688.2011.624990. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh D, Tsokos GC. Spleen tyrosine kinase (Syk): a src family of non-receptor kinase has multiple functions and represents a valuable therapeutic target in the treatment of autoimmune and inflammatory disease. Autoimmunity. 2010;43:48–55. doi: 10.3109/08916930903374717. [DOI] [PubMed] [Google Scholar]
- 19.Brignatz C, Restouin A, Bonello G, Olive D, Collette Y. Evidences for ubiquitination and intracellular trafficking of LAT, the linker of activated T cells. Biochim Biophys Acta. 2005;1746(2):108–115. doi: 10.1016/j.bbamcr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Nagy G, Ward J, Mosser DD, et al. Regulation of CD4 Expression via Recycling by HRES-1/RAB4 Controls Susceptibility to HIV Infection. J Biol Chem. 2006;281:34574–34591. doi: 10.1074/jbc.M606301200. [DOI] [PubMed] [Google Scholar]
- 21.Cho CS, Chang Z, Elkahwaji J, et al. Rapamycin antagonizes cyclosporin A and tacrolimus (FK506) mediated augmentation of linker for activation of T cell expression in T cells. Int Immunol. 2003;15(11):1369–1378. doi: 10.1093/intimm/dxg138. [DOI] [PubMed] [Google Scholar]
- 22.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez D, Perl A. Metabolic control of T cell activation and death in SLE. Autoimmun Rev. 2009;8:184–189. doi: 10.1016/j.autrev.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gringhuis SI, Papendrecht-van der Voort E, Leow A, Levarht EWN, Breedveld FC, Verweij CL. Effect of Redox Balance Alterations on Cellular Localization of LAT and Downstream T-Cell Receptor Signaling Pathways. Mol Cell Biol. 2002;22(2):400–411. doi: 10.1128/MCB.22.2.400-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]