Abstract
Purpose
To assess cardiac safety and potential cardiac risk factors associated with trastuzumab in the NCCTG N9831 Intergroup adjuvant breast cancer trial.
Patients and Methods
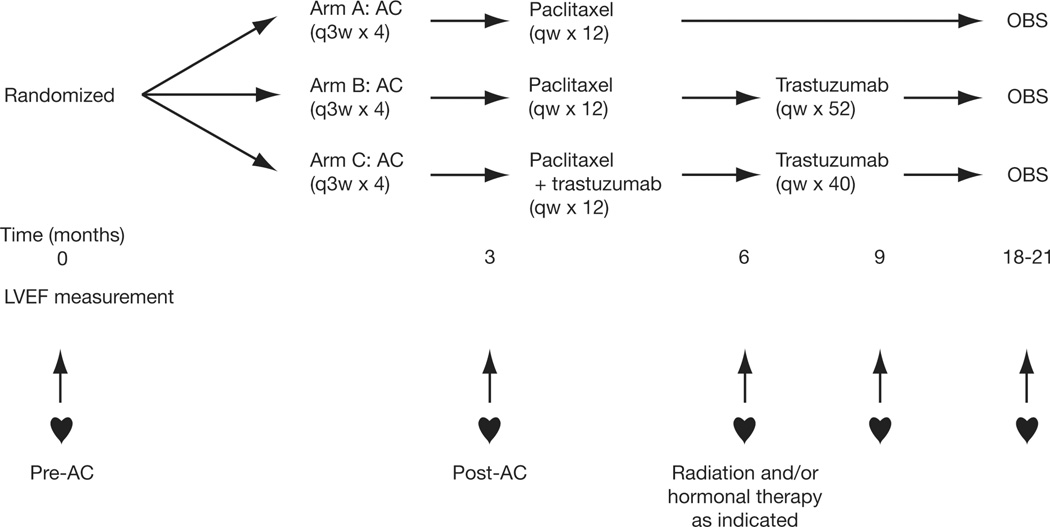
Patients with HER2-positive operable early breast cancer were randomized to 1 of 3 treatment arms: doxorubicin plus cyclophosphamide (AC) followed by either weekly paclitaxel (Arm A); paclitaxel then trastuzumab (Arm B); or paclitaxel plus trastuzumab then trastuzumab alone (Arm C). Left ventricular ejection fraction (LVEF) was evaluated at registration and 3, 6, 9, and 18–21 months post-registration.
Results
A total of 1944 patients completed AC with a satisfactory LVEF and proceeded to post-AC therapy. Post-AC cardiac events (congestive heart failure [CHF] or cardiac death [CD]) were: Arm A, n=3 (2 CHF, 1 CD); Arm B, n=19 (18 CHF, 1 CD); Arm C, n=19 (all CHF); 3-year cumulative incidence was 0.3%, 2.8%, and 3.3%, respectively. Cardiac function improved in most CHF cases following trastuzumab discontinuation and cardiac medication. Factors associated with increased risk of a cardiac event after AC in Arms B and C were older age (P<.003), prior/current antihypertensive agents (P=.005), and lower registration LVEF (P=.033). Incidence of asymptomatic LVEF decreases requiring trastuzumab to be held was 8–10%; LVEF recovered and trastuzumab was restarted in approximately 50% of these patients.
Conclusion
The cumulative incidence of post-AC cardiac events at 3 years was higher in the trastuzumab-containing arms versus control arm but by <4%. Older age, lower registration LVEF, and antihypertensive medications increase the risk of cardiac dysfunction in patients receiving trastuzumab following AC.
Keywords: HER2, trastuzumab, adjuvant, breast cancer, doxorubicin, cyclophosphamide, paclitaxel, cardiac safety
Introduction
Approximately 25% of primary invasive breast tumors exhibit overexpression of the human epidermal growth factor receptor (HER2) protein or amplification of the HER2 oncogene.1,2 Patients with HER2-positive breast tumors are subject to a more aggressive disease course, have a worse prognosis, and are more susceptible to recurrence than patients with HER2-normal breast tumors.1,2
The combination of the anthracycline doxorubicin with cyclophosphamide (AC) is standard adjuvant therapy for early-stage breast cancer, as it significantly improves disease-free and overall survival, particularly when administered sequentially with paclitaxel.3,4 Trastuzumab (Herceptin®, Genentech, Inc, CA) is an anti-HER2 monoclonal antibody that was recently approved for the adjuvant treatment of HER2-positive early breast cancer, in combination with paclitaxel following standard AC therapy. The joint efficacy analysis of the pivotal North Central Cancer Treatment Group (NCCTG) N9831 and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trials demonstrated that adding trastuzumab to this chemotherapy regimen reduced disease recurrence by 52% and risk of death by 33% compared with chemotherapy alone.5
In the adjuvant setting, it is important that the benefits outweigh the risks of short-term and long-term toxicity. Trastuzumab is generally well tolerated and not associated with common cytotoxic chemotherapy side effects; however, cardiac dysfunction (asymptomatic decreases in left ventricular ejection fraction [LVEF] and congestive heart failure [CHF]) have been observed, particularly when trastuzumab is given concomitantly with anthracyclines.6–8
The primary safety objective of the NCCTG N9831 trial was to assess the cardiac safety of AC followed by paclitaxel with or without sequential or concurrent trastuzumab. The effects of AC on cardiac function in this trial have been published9 and the third interim safety analysis in April 2005 showed that 2.2%–3.3% of patients experienced a clinically significant cardiac event during the course of trastuzumab treatment.10 This report presents updated cardiac event incidence rates, potential risk factors, and follow-up of patients who experienced cardiac events after beginning post-AC treatment.
Patients and Methods
Study Design
The NCCTG N9831 Intergroup trial is a 3-arm phase III randomized trial. Eligible patients were randomized to AC followed by: paclitaxel (control arm, Arm A); paclitaxel followed by trastuzumab (sequential arm, Arm B); or paclitaxel plus trastuzumab followed by trastuzumab alone (concurrent arm, Arm C) (Figure 1). Radiation therapy (RT) and/or hormonal therapy were given after completion of chemotherapy, when indicated.
Figure 1. Trial Schema.
Treatment schedule. Doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) given 3-weekly for 4 cycles followed by; weekly paclitaxel (80 mg/m2) for 12 weeks (Arm A); paclitaxel, followed by weekly trastuzumab (4 mg/kg loading dose, followed by 2 mg/kg) for 52 weeks (Arm B); or weekly paclitaxel plus trastuzumab for 12 weeks, followed by weekly trastuzumab alone for 40 weeks (Arm C). AC, doxorubicin plus cyclophosphamide; q3w, every 3 weeks; qw, weekly; OBS, observation; LVEF, left ventricular ejection fraction.
Cardiac events were defined as symptomatic CHF, with objective findings such as rales, S3 gallop rhythm, or evaluated jugular venous pressure, confirmed by multigated acquisition scan [(MUGA]/echocardiogram [ECHO] or electrocardiogram and a chest X-ray, definite cardiac death (due to CHF, myocardial infarction, or primary arrhythmia) or probable cardiac death (sudden death without documented etiology).
Three cardiac safety interim analyses were planned for when 100, 300, and 500 patients per arm had been followed at least 6 months after the start of post-AC treatment. Enrollment to a trastuzumab-containing arm was to be discontinued if there was a difference of ≥4% in the incidence of cardiac events between the trastuzumab-containing arm and the control arm.
Eligibility
Women aged ≥18 years with primary, operable, and histologically confirmed node-positive or high-risk node negative invasive breast cancer, with no evidence of metastases, were eligible. Tumors had to be strongly HER2-positive (immunohistochemistry [IHC] score of 3+ or positive by fluorescence in situ hybridization (FISH), confirmed at central or reference laboratories.11
Patients could not have received >4 weeks of hormonal therapy for breast cancer, any other prior systemic therapy for breast cancer, or prior anthracycline or taxane for any malignancy. LVEF, assessed by MUGA or ECHO scan, needed to be within the institutional normal range, and patients were to have no active cardiac disease, prior myocardial infarctions, history of CHF, arrhythmia or valvular disease, uncontrolled hypertension, or other cardiovascular disorders. All patients gave written, informed consent.
Methods
Patients were randomized to 1 of the 3 treatment arms at trial entry and AC treatment began within 7 days of registration. LVEF was evaluated by MUGA or ECHO scan within 3 weeks prior to registration, 3, 6, and 9 months following registration, and 3 months after discontinuation/completion of study treatment (18 months after registration for Arms A and C, and 21 months after registration for Arm B). It was recommended that patients be monitored using the same method and radiology facility throughout the study. Patients were clinically followed for symptoms of cardiovascular (CV) disease.
Trastuzumab was not permitted for patients whose post-AC (3-month) LVEF relative to their registration LVEF had either (1) decreased >15% points (absolute change), irrespective of whether the post AC LVEF fell above or below the institutional lower limit of normal (LLN), or decreased ≤15% points to a value below LLN. Trastuzumab was not permitted in patients who showed symptoms related to left ventricular dysfunction, cardiac ischemia, or arrhythmia while receiving AC. During the paclitaxel plus trastuzumab or trastuzumab alone portions of the treatment, trastuzumab treatment was held if LVEF decreased >15 percentage points, or 10–15 percentage points to below the LLN, relative to registration LVEF level. If the repeat LVEF level at 4 weeks again met the criteria to hold trastuzumab, trastuzumab was discontinued.
LVEF changes, cardiac events, and the number of patients who had trastuzumab treatment held or discontinued were reviewed monthly by an independent Cardiac Safety Monitoring Committee (3 Board-certified cardiologists, a breast oncologist, the study principal investigator, and the study statistician). Probable cardiac events were investigated independently by the 3 committee cardiologists; if agreement was reached between ≥2 cardiologists, the event was confirmed as a cardiac event.
Statistical Analyses
For all eligible patients enrolled, point and interval estimates for the proportion of patients whose post-AC LVEF level precluded them from receiving trastuzumab were constructed using the binomial distribution. Fisher’s exact tests and logistic regression modeling were used to assess the association between patient characteristics and preclusion of trastuzumab.
For patients whose post-AC LVEF met the criteria to receive trastuzumab, the cumulative incidence of cardiac events after starting post-AC treatment was estimated non-parametrically where patients were considered at risk from day 1 of cycle 5 until recurrence, second primary cancer (including contralateral breast disease), non-cardiac deaths or last follow-up. In the subset of these patients who were randomized to trastuzumab-containing regimens, log-rank tests were used to assess potential risk factors for cardiac events where time for patients who did not develop a cardiac adverse event was censored at their last follow-up date or a maximum of 3 years. Point and interval estimates of associated hazard ratios were constructed using results of fitting a univariate Cox’s model.
Results
Accrual to Arm C was temporarily suspended in January 2002 because of concerns regarding cardiotoxicity. However, following extensive internal review by an independent cardiac safety monitoring committee, the incidence of cardiac events in Arm C was found to be <4% higher than in Arm A, and accrual to Arm C resumed in September 2002. All patients enrolled during this period were randomized to Arms A and B only, and patients who had been randomized to Arm C prior to 2002 were permitted to receive either trastuzumab concurrent with or following paclitaxel.
A total of 3505 patients enrolled onto the trial between May 19, 2000 and April 29, 2005; 62 were ineligible and 28 withdrew consent prior to receiving treatment. After the implementation of central HER2 testing in March 2002, 283 patients were removed from the study either due to lack of tissue to complete central review or because tumors were not IHC 3+ or FISH-positive by both central and reference laboratory testing.
Of the 3132 eligible patients who began AC treatment (all 3 treatment arms), 68 (2.2%) completed 4 cycles of AC but had no post-AC LVEF assessment. Seventy-two patients (2.3%) did not complete 4 cycles of AC due to refusal, excessive toxicity, co-morbid conditions, progression and/or death. Among these 72 patients, 2 died of cardiac arrest 31 and 45 days post-registration, respectively, and 1 patient developed CHF 14.5 months post registration and died of unknown causes 14 months later.
Among the remaining 2992 patients, 151 patients (5.0%) had post-AC LVEF decreases from registration levels that met the criteria disallowing the administration of any trastuzumab treatment: post-AC LVEF level had decreased >15% in 79 patients (2.6%), and ≤15% to below the LLN in 72 patients (2.4%). Changes in post-AC LVEF levels are shown in Table 1.
Table 1.
Changes in Left Ventricular Ejection Fraction Levels Following AC Treatment in the Entire Cohort
| Change in Post-AC LVEF Level From Registration Level |
Post-AC LVEF Level in Relation to LLN |
Prohibited From Receiving Trastuzumab |
Patients (N=2992), % |
|---|---|---|---|
| Decrease 10–15% points | Above | No | 8.5 |
| Decrease or increase <10% points | Above | No | 81.1 |
| Increase ≥10% points | Above | No | 5.4 |
| Decrease >15% points | Above | Yes | 1.7 |
| Decrease >15% points | Below | Yes | 0.9 |
| Decrease 10–15% points | Below | Yes | 1.4 |
| Decrease <10% points | Below | Yes | 1.0 |
AC, doxorubicin plus cyclophosphamide; LVEF, left-ventricular ejection fraction; LLN=lower limit of normal.
Univariate analysis revealed that a high body mass index (BMI) (P=.185) and current or prior use of antihypertensive medication (P=.392) were not significantly associated with an increased likelihood of experiencing decreases in post-AC LVEF levels that would disallow trastuzumab administration. Multivariate analysis indicated that the likelihood of experiencing decreases in post-AC LVEF levels that would disallow trastuzumab administration was lower for patients aged <50 years versus ≥50 years (P=.047; RR=.71; 95% CI,.51–.99), and lower both in patients whose registration LVEF level was ≥65% (P=.004; RR=.48; 95% CI,.29–.79) or 55–65.9% (P<.001; RR=.35; 95% CI,.21–.59) versus <55% but above the LLN.
Changes in LVEF in the Post-AC Treatment Phase Among Patients not Precluded From Receiving Trastuzumab
The 2148 eligible patients entered on study prior to April 25, 2004 were not affected by treatment modifications instituted following the release of the joint efficacy analysis results in April 2005. Of these 2148 patients, 1944 patients began post-AC treatment either having had a post-AC LVEF level that allowed trastuzumab to be administered (n=1876) or no post-AC LVEF evaluation (n=68). Demographics were similar across all treatment arms (Table X, online only). The median age at enrollment in each arm was 49 years. Approximately 17% of patients had prior or current use of antihypertensive medication. Approximately 73% of patients received RT following paclitaxel but prior to progression, new primary, or CHF. Of these 1944 patients, 1801 were alive at the most recent time of contact and had been followed for a median of 3.75 years.
LVEF levels were to be measured at 6, 9, and 18–21 months post-registration unless a cardiac event, recurrence, second primary, or death had occurred prior to the prescribed time point. LVEF levels within 45 days of these prescribed timepoints were included in the analysis. Table 2 shows median value and range of LVEF levels for each arm at each prescribed evaluation point and the percentage of patients whose change in LVEF met the criteria for withholding trastuzumab (LVEF drop of >15% points, or 10–15% points to below the LLN, relative to registration LVEF level). The percentage of patients who met the criteria to withhold trastuzumab were 4.0–5.1% at evaluations where patients had not been exposed to trastuzumab (Arm A, months 6, 9, and 18; Arm B, month 6), 7.8–10.4% at evaluations during trastuzumab administration (Arm B, month 9; Arm C, months 6 and 9), and 5.4–5.8% following trastuzumab (Arm B, month 21; Arm C, month 18). The LVEF recovered at the next evaluation and trastuzumab was restarted in approximately 50% of the patients who had trastuzumab held at these timepoints.
Table 2.
Changes in Left Ventricular Ejection Fraction During Post-AC Therapy
| Arm A | Registration Evaluation (N=664) |
6-Month Evaluation (N=627) |
9-Month Evaluation (n=595) |
18-Month Evaluation (n=554) |
|---|---|---|---|---|
| Patients not evaluated | 10.8% | 16.5% | 21.5% | |
| Median LVEF level, % (range) | 63 (50, 85) | 61 (43, 87) | 61 (40, 83) | 61 (38, 78) |
| Median absolute LVEF change from registration level, % (range) | −2 (−24, 31) | −2 (−25, 24) | −2.5 (−32, 24) | |
| Patients with satisfactory LVEF | 84.1% | 79.5% | 74.0% | |
| Patients with LVEF decrease requiring re-evaluation | 5.1% | 4.0% | 4.5% | |
| Recovered at re-evaluation | 1.4% | 1.0% | 0.4% | |
| Did not recover at re-evaluation | 0.5% | 0% | 0% | |
| Not re-evaluated | 3.2% | 3.0% | 4.1% |
| Arm B | Registration Evaluation (N=710) |
6-Month Evaluation (N=701) |
9-Month Evaluation (N=637) |
21-Month Evaluation (N=514 |
|---|---|---|---|---|
| Patients not evaluated | 2.0% | 7.1 | 13.4% | |
| Median LVEF level, % (range) | 63 (45, 86) | 62 (35, 87) | 60 (32, 87) | 60 (25, 82) |
| Median absolute LVEF change from registration level, % (range) | −2 (−27, 22) | −4 (−29, 17) | −3 (−27, 23) | |
| Patients with satisfactory LVEF | 94.0% | 85.1% | 81.1% | |
| Patients with LVEF decrease requiring re-evaluation | 4.0% | 7.8% | 5.4% | |
| Recovered at re-evaluation | 1.9% | 3.0% | 0.4% | |
| Did not recover at re-evaluation | 1.7% | 3.3% | 0.2% | |
| Not re-evaluated | 0.4% | 1.5% | 4.7% |
| Arm C | Registration Evaluation (N=570) |
6-Month Evaluation (N=546) |
9-Month Evaluation (N=469) |
18-Month Evaluation (N=400) |
|---|---|---|---|---|
| Patients not evaluated | 3.3% | 6.8% | 8.5% | |
| Median LVEF level, % (range) | 63 (50, 87) | 60 (30, 82) | 60 (30, 86) | 60 (42, 91) |
| Median absolute LVEF change from registration level, % (range) | −4 (−34, 18) | −4 (−32, 17) | −3 (−27, 27) | |
| Patients with satisfactory LVEF | 86.3% | 83.8% | 87.5% | |
| Patients with LVEF decrease requiring re-evaluation | 10.4% | 9.4% | 5.8% | |
| Recovered at re-evaluation | 5.1% | 4.9% | 0.3% | |
| Did not recover at re-evaluation | 3.8% | 2.8% | 0.5% | |
| Not re-evaluated | 1.5% | 1.7% | 5.0% | |
LVEF, left ventricular ejection fraction.
Clinically Significant Cardiac Events during Post-AC Treatment among Patients not Precluded from Receiving Trastuzumab
Of the 1944 patients who began post-AC treatment either having no post-AC LVEF evaluation or their post-AC LVEF level had not met the criteria disallowing trastuzumab, 39 patients had confirmed CHF (Arm A, n=2; Arm B, n=18; Arm C, n=19). A further 2 patients died of cardiac causes: complications related to vascular surgery (Arm A, n=1) and cardiac arrest of unclear etiology (Arm B, n=1). One patient died of unknown causes (Arm B, n=1).
The 1-year cumulative incidence rate for cardiac events after start of AC treatment was 0.0% in Arm A, 1.6% in Arm B, and 3.3% in Arm C. The 2-year cumulative incidence rates were 0.2% in Arm A, 2.7% in Arm B, and 3.3% in Arm C, and corresponding 3-year cumulative incidence rates were 0.3%, 2.8%, and 3.3%, respectively (Figure 2).
Figure 2. Cumulative Incidence of Cardiac Events.
AC, doxorubicin plus cyclophosphamide; T, paclitaxel; H, trastuzumab.
The LVEF levels at registration, time of CHF diagnosis, and at time of the most recent LVEF evaluation for the 39 patients who experienced CHF are shown in Figure 3. All patients with CHF discontinued trastuzumab at time of CHF diagnosis, and the majority received cardiac medication, including diuretics, beta-blockers and anti-arrhythmic agents. Improvements in cardiac function were observed in the majority of patients. Six of these 39 patients have subsequently died due to metastatic disease (n=4), second primary (n=1), and complications during left ventricular assist device surgery (n=1).
Figure 3. Recovery of Cardiac Function in Patients With Congestive Heart Failure.
A, Arm A (n=2); B, Arm B (n=18); C, Arm C (n=19); LVEF, left ventricular ejection fraction; AC, doxorubicin plus cyclophosphamide
Risk Factors for Cardiac Events among Patients Randomized to Trastuzumab-containing Regimens
In patients for whom RT was indicated, RT was to be initiated within 5 weeks of completion of paclitaxel (with or without trastuzumab). Of the 37 patients randomized to a trastuzumab-containing regimen who developed CHF, 13 did not receive RT, 9 received RT after CHF was diagnosed, 2 had CHF during RT, and 13 developed CHF after completion of RT (right-sided post-mastectomy [n=4]; right-sided post-breast sparing [n=3]; left-sided post-mastectomy [n=2]; left-sided post-breast sparing [n=4]).
Factors found to be univariately associated with an increased risk of a cardiac event within 3 years of starting post-AC treatment with a trastuzumab containing regimen included age ≥60 years (P=.003), prior/current use of antihypertensive medication (P=.005), and registration LVEF <55% but above LLN (P=.033) (Table 3). BMI (P=.161) and post-AC LVEF level (P=.134) were not found to be significant risk factors. The cumulative incidence of cardiac events in relation to demographics and registration and post-AC LVEF levels is shown in Table 4.
Table 3.
Risk Factors for a Cardiac Event After Completion of AC in Patients in Arms B and C
| Patients, n |
Cardiac Events, n (%) |
Log-rank P Value |
Univariate Hazard Ratio (95% CI) |
|
|---|---|---|---|---|
| Age, years | ||||
| ≥60 | 212 | 14 (6.6) | 3.2 (1.55, 6.81) | |
| 50–59 | 397 | 11 (2.8) | 0.003 | 1.3 (0.60, 2.93) |
| <50 | 671 | 14 (2.1) | Reference | |
| BMI at registration | ||||
| ≤24.9 | 439 | 8 (1.8) | ||
| 25.0–29.9 | 364 | 12 (3.3) | 0.161 | |
| ≥30.0 | 477 | 19 (4.0) | ||
| Current or prior antihypertensive medications | ||||
| Yes | 216 | 13 (6.0) | 0.005 | 2.5 (1.29, 4.87) |
| No | 1063 | 26 (2.4) | Reference | |
| LVEF at registration | ||||
| ≥65% | 575 | 10 (1.7) | 0.31 (0.11, 0.90) | |
| 55–64.9% | 615 | 24 (3.9) | 0.033 | 0.70 (0.27, 1.84) |
| Above LLN but <55% | 90 | 5 (5.6) | Reference | |
| Post-AC LVEF | ||||
| ≥65% | 565 | 10 (1.8) | ||
| 55–64.9% | 612 | 24 (3.9) | 0.134 | |
| Above LLN but <55% | 90 | 5 (5.6) |
AC, doxorubicin plus cyclophosphamide; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; LLN, lower limit of normal.
Table 4.
Cumulative Incidence Rates of Cardiac Events in Relation to Demographics and Left Ventricular Ejection Fraction Levels
| Arm B | Arm C | |||||
|---|---|---|---|---|---|---|
| 1 Year | 2 Years | 3 Years | 1 Year | 2 Years | 3 Years | |
| Age, years | ||||||
| <50 | 1.1 (0.4–2.8) | 2.4 (1.3–4.6) | 2.4 (1.3–4.6) | 1.7 (0.7–4.1) | 1.7 (0.7–4.1) | 1.7 (0.7–4.1) |
| ≥50 | 2.1 (1.0–4.4) | 3.0 (1.6–5.6) | 3.3 (1.9–6.0) | 5.1 (3.0–8.4) | 5.1 (3.0–8.4) | 5.1 (3.0–8.4) |
| BMI | ||||||
| Underweight/Normal | 0 | 0.8 (0.2–3.2) | 0.8 (0.2–3.2) | 3.2 (1.4–7.0) | 3.2 (1.4–7.0) | 3.2 (1.4–7.0) |
| Overweight | 1.5 (0.5––4.6) | 2.5 (1.1–6.0) | 2.5 (1.1–6.0) | 4.3 (2.1–8.9) | 4.3 (2.1–8.9) | 4.3 (2.1–8.9) |
| Obese | 3.1 (1.6–6.1) | 4.6 (2.7–8.1) | 5.1 (3.0–8.6) | 2.8 (1.2–6.1) | 2.8 (1.2–6.1) | 2.8 (1.2–6.1) |
| Antihypertensive medications | ||||||
| Yes | 2.6 (0.9–8.1) | 5.3 (2.4–11.5) | 5.3 (2.4–11.5) | 6.9 (3.3–14.1) | 6.9 (3.3–14.1) | 6.9 (3.3–14.1) |
| No | 1.3 (0.7–2.7) | 2.2 (1.3–3.8) | 2.4 (1.4–4.0) | 2.6 (1.5–4.5) | 2.6 (1.5–4.5) | 2.6 (1.5–4.5) |
| LVEF at registration | ||||||
| <55% | 2.2 (0.3–15.8) | 4.5 (1.1–17.7) | 4.5 (1.1–17.7) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) |
| 55–59% | 1.3 (0.3–5.3) | 4.5 (1.1–17.7) | 4.5 (1.1–17.7) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) |
| <55% | 2.2 (0.3–15.8) | 4.5 (1.1–17.7) | 4.5 (1.1–17.7) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) | 6.7 (2.2–20.1) |
| Post-AC LVEF | ||||||
| <55% | 1.3 (0.2–9.5) | 2.7 (0.7–10.6) | 2.7 (0.7–10.6) | 3.0 (0.8–12.0) | 3.0 (0.8–12.0) | 3.0 (0.8–12.0) |
| <55% | 2.8 (1.1–7.3) | 2.7 (0.7–10.6) | 2.8 (1.1–7.3) | 5.8 (2.9–11.3) | 5.8 (2.9–11.3) | 5.8 (2.9–11.3) |
| <55% | 1.3 (0.2–9.5) | 2.7 (0.7–10.6) | 2.7 (0.7–10.6) | 3.0 (0.8–12.0) | 3.0 (0.8–12.0) | 3.0 (0.8–12.0) |
Discussion
Results of these analyses showed that a higher proportion of patients in the trastuzumab-containing arms developed CHF or died from cardiac causes after starting their post-AC treatment. This was <4% above that of the non-trastuzumab-containing regimen (3-year cumulative incidences were 2.8%, 3.3% versus 0.3% for Arms B, C, and A, respectively). In the NSABP B-31 trial, patients received the same concurrent paclitaxel and trastuzumab after AC schedule as patients in Arm C of NCCTG N9831. The 3-year cumulative incidences of cardiac events in the NSABP B-31 trial were 4.1% and 0.8% (a differential of 3.3% between arms) for the concurrent paclitaxel/trastuzumab and chemotherapy alone arms, respectively.12
In NCCTG N9831, the cardiac function of the majority of patients experiencing CHF improved after receiving standard medical treatment. Similarly, of the patients who developed NYHA Class III or IV CHF in the NSABP B-31 trial, 95% in the trastuzumab arm were without symptoms of cardiac dysfunction at least 6 months after CHF diagnosis.12
It is well documented13,14 that doxorubicin induces cardiotoxicity, particularly at cumulative doses >300 mg/m2, and recent studies suggest that anthracycline- and trastuzumab-related cardiomyopathy differ.15–17 Ewer and colleagues recently demonstrated that anthracycline-associated cardiac dysfunction is dose-related and appears to cause permanent myocardial damage, whereas trastuzumab-associated cardiac dysfunction is reversible, not related to dose, and does not appear to be related to the same anthracycline morphological changes.16,17 The study showed that trastuzumab treatment can often be continued or restarted in patients who develop cardiac dysfunction with no subsequent cardiac events.17
Whether concurrent or sequential treatment is advisable is often a subject of debate in breast cancer. This trial will allow for the evaluation of trastuzumab given in combination with or following paclitaxel therapy (after completion of AC). A slightly higher 3-year cumulative incidence of cardiac events was observed when paclitaxel and trastuzumab were given concurrently (3.3%) compared with sequential treatment (2.8%). The efficacy of the sequential and concurrent treatment regimens in this NCCTG N9831 trial in terms of disease-free survival demonstrate a trend towards improved outcome for the concurrent approach, but further follow up is necessary before definite conclusions can be reached.18
The HERceptin Adjuvant (HERA) trial is comparing 1 or 2 years of trastuzumab administered following surgery, radiation therapy, and standard neoadjuvant or adjuvant chemotherapy versus observation only in patients with HER2-positive early breast cancer and adequate cardiac function. A significant reduction in disease recurrence and improvement in overall survival was observed with 1 year of trastuzumab therapy compared with observation, at a median follow up of 2 years.19 The incidence (after 2 years follow up) of severe cardiac events (cardiac death or NYHA Class III or IV CHF) in the 1-year trastuzumab arm was 0.6% compared with 0.0% in the observation arm; incidence of symptomatic CHF only was 2.0% in the trastuzumab versus 0.1% in the observation arm.19 After 2 years follow up in NCCTG N9831, the incidence of cardiac events in Arm B (sequential arm) was 2.7% versus 0.2% in Arm A (control arm).
The Breast Cancer International Research Group (BCIRG) 006 trial is assessing standard AC followed by docetaxel, compared with AC followed by docetaxel plus trastuzumab, or carboplatin plus docetaxel plus trastuzumab (TCH). Results from the second interim analysis (median follow up, 36 months) demonstrated that both trastuzumab-containing regimens significantly improved clinical outcomes compared with chemotherapy alone.20 The incidences of grade 3/4 (symptomatic) CHF were 1.9%, 0.4%, and 0.4% in the AC followed by docetaxel/trastuzumab, TCH, and AC/docetaxel arms, respectively; the incidence was significantly different between the AC followed by docetaxel/trastuzumab arm and TCH arm (P=.0015)20 Direct comparisons between the BCIRG 006 trial and the NCCTG N9831 and NSABP B-31 trials are difficult due to differences in the time points for analysis, eligibility criteria, and definition of a cardiac event. The BCIRG 006 trial includes patients aged ≤70 years of age only, whereas the NCCTG N9831 and NSABP B-31 trials have no upper age limit. Approximately 15% of patients in the NCCTG N9831 trial were aged ≥60 years, and an association between increasing age and risk of cardiac toxicity was observed. Increased age (≥60 years) was also found to be a risk factor in the NSABP B-31 trial.12 The proportion of patients aged > 60 years and analysis of the association between demographics and cardiac toxicity have not been reported in the BCIRG 006 trial.
As in the NSABP B-31, a possible link between antihypertensive medications and risk of cardiac dysfunction was found. However, in contrast to NSABP B-31, post-AC LVEF levels that had not fallen more than 15% points or below LLN did not appear to be associated with an increased risk of cardiac dysfunction. Radiation therapy was not correlated with increased risk of cardiac toxicity in either trial.12,21 A prospective substudy of the NCCTG N9831 trial, evaluating 8 circulating markers previously reported to be associated with cardiac injury, is ongoing and will be reported separately.
The improved efficacy and acceptable cardiac safety observed when trastuzumab is added to adjuvant chemotherapy suggest that trastuzumab contributes a significant therapeutic advantage.5,18,20,22 It is important to accurately assess a patient’s cardiac function before, during, and after trastuzumab-based treatment. The development of cardiac monitoring guidelines will enable oncologists to identify patients who would derive the maximum benefit from trastuzumab-based therapy while minimizing the risk of cardiac complications.23
Although longer-term follow up is required to determine the full effect of adverse cardiac events, it is clinically important for physicians and patients to be aware of the findings to date. Future analysis of patients who experienced cardiac dysfunction in this trial may reveal new prognostic or predictive indicators of cardiac dysfunction to aid treatment selection for patients with HER2-positive early breast cancer.
Supplementary Material
Acknowledgements
The authors wish to acknowledge assistance and review of this manuscript by Frances Palmieri, RN, OCN, MSN, and Genentech, Inc.
Financial acknowledgments: Supported in part by a grant from the National Cancer Institute CA25224; Genentech, Inc; and The Breast Cancer Research Foundation
Footnotes
These data were presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 13–17, 2005, and at the 28th San Antonio Breast Cancer Symposium, San Antonio, TX, December 8–11, 2005.
References
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 4.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 8.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Suman VJ, Davidson NE, et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the NCCTG N9831 Intergroup adjuvant trial. J Clin Oncol. 2004;22:3700–3704. doi: 10.1200/JCO.2004.03.516. [DOI] [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, et al. Interim cardiac safety analysis of NCCTG N9831 Intergroup adjuvant trastuzumab trial. Proc Am Soc Clin Oncol. 2005;23:17s. doi: 10.1200/JCO.2015.61.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 12.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 14.Buzdar AU, Marcus C, Smith TL, et al. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55:2761–2765. doi: 10.1002/1097-0142(19850615)55:12<2761::aid-cncr2820551206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Valero V, Gill E, Paton V, et al. Normal cardiac biopsy results following co-administration of doxorubicin (A), cyclophosphamide (C) and trastuzumab (H) to women with HER2 positive metastatic breast cancer. Proc Am Soc Clin Oncol. 2004;23:20. [Google Scholar]
- 16.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 17.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Suman VJ, Davidson N, et al. NCCTG N9831: May 2005 update. Presented at the 41st Annual Meeting of the American Society of Clinical Oncology; Orlando, FL. 2005. [Google Scholar]
- 19.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 20.Slamon D, Eiermann W, Robert N, et al. BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. Breast Cancer Res Treat. 2006;100 (abstr) [Google Scholar]
- 21.Halyard MY, Pisansky TM, Solin LJ, et al. Adjuvant radiotherapy and trastuzumab in stage I–IIA breast cancer: Toxicity data from North Central Cancer Treatment Group Phase III trial N9831. Proc Am Soc Clin Oncol. 2006;24:8s. (abstr) [Google Scholar]
- 22.Smith IE. Trastuzumab following adjuvant chemotherapy in HER2-positive early breast cancer (HERA trial): Disease-free and overall survival after 2-year median follow-up. Presented at the 42nd Annual Meeting of the American Society of Clinical Oncology; Atlanta, GA. 2006. (late-breaking abstract), (abstr) [Google Scholar]
- 23.Ewer MS, Perez EA, Baselga J, et al. Cardiac safety guidelines for the adjuvant use of trastuzumab (Herceptin) in HER2-positive early breast cancer. Breast. 2007;10(Suppl 1):S63. (abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.