Abstract
The Wnt gene family consists of structurally related genes encoding secreted signaling molecules that have been implicated in many developmental processes, including regulation of cell fate and patterning during embryogenesis. Previously, we found that Wnt signaling is required for primitive or yolk sac-derived-erythropoiesis using the murine embryonic stem cell (ESC) system. Here, we examine the effect of Wnt signaling on the formation of early hematopoietic progenitors derived from human ESCs. The first hematopoietic progenitor cells in the human ESC system express the pan-hematopoietic marker CD41 and the erythrocyte marker, glycophorin A or CD235. We have developed a novel serum-free, feeder-free, adherent differentiation system that can efficiently generate large numbers of CD41+CD235+ cells. We demonstrate that this cell population contains progenitors not just for primitive erythroid and megakaryocyte cells but for the myeloid lineage as well and term this population the primitive common myeloid progenitor (CMP). Treatment of mesoderm-specified cells with Wnt3a led to a loss of hematopoietic colony-forming ability while the inhibition of canonical Wnt signaling with DKK1 led to an increase in the number of primitive CMPs. Canonical Wnt signaling also inhibits the expansion and/or survival of primitive erythrocytes and megakaryocytes, but not myeloid cells, derived from this progenitor population. These findings are in contrast to the role of Wnt signaling during mouse ESC differentiation and demonstrate the importance of the human ESC system in studying species-specific differences in development.
Keywords: ESC, iPSC, hematopoiesis, Wnt
Introduction
Embryology has offered important insights into key pathways regulating embryonic stem cell (ESC) differentiation and efficient induction of endoderm, mesoderm, and ectoderm (reviewed in (Gadue, Huber, Nostro, Kattman, & Keller, 2005; Loebel, Watson, De Young, & Tam, 2003)). By modulating developmentally important signaling pathways, it is possible to mimic the process of embryogenesis to drive the differentiation of stem cells through discreet developmental intermediaries (Murry & Keller, 2008). The Wnt pathway has been shown to be especially important in regulating multiple stages of development of hematopoietic cells from pluripotent stem cells (PSC) including mesoderm formation and hematopoietic specification in both mouse and human (Gadue, Huber, Paddison, & Keller, 2006) (Gertow et al., 2013; Hwang et al., 2009; Lengerke et al., 2008; Lindsley, Gill, Kyba, Murphy, & Murphy, 2006; Nostro, Cheng, Keller, & Gadue, 2008; Vijayaragavan et al., 2009; Woll et al., 2008).
The Wnt pathway is evolutionary conserved and diversifies into two main branches, canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) (reviewed in (Wodarz & Nusse, 1998)). Both branches play critical roles in specifying cellular fate and movement during embryonic development and adult tissue regeneration. Activation of the canonical Wnt signaling pathway occurs through ligand binding of Wnts to a Frizzled family receptor that is associated with a co-receptor belonging to the LRP-5/6/arrow family. This activation inhibits the destruction complex, APC/Axin/CK1/GSK3β, leading to the stabilization of β-catenin and its translocation to the nucleus where it interacts with TCF/LEF family transcription factors (reviewed in (Logan & Nusse, 2004)). The secreted antagonists, sFRPs, WIF-1, and DKKs, can inhibit this pathway by blocking receptor binding. In the absence of a Wnt ligand, GSK3β in the destruction complex phosphorylates β-catenin, which marks it for degradation. The non-canonical Wnt signaling pathways appear to function in a β-catenin independent manner, designated as Wnt/Calcium and Wnt/JNK in vertebrates and Wnt/planar cell polarity pathway and does not utilize the LRP co-receptors (reviewed in (Veeman, Axelrod, & Moon, 2003)).
While there is consensus that Wnt signaling plays important roles in hematopoietic development from PSCs, there is conflicting data especially on the role of canonical Wnt signaling in hematopoietic specification or survival. We have previously shown that activation of the Wnt pathway in murine ESC-derived hematopoiesis is essential for establishment of the primitive erythroid, but not definitive, lineages (Nostro et al., 2008). These data are supported by a publication examining human ESC-derived hematopoiesis suggesting that the canonical Wnt family member, Wnt3a, functions by expanding ESC-derived hematopoietic progenitors (Vijayaragavan et al., 2009). In contrast, a study by Gertow et al. demonstrates that canonical Wnt signaling is inhibitory for hematopoietic colony formation from human ESCs (Gertow et al., 2013). To address these seemingly contradictory results, the role of Wnt signaling in the specification and expansion of hematopoietic cells derived from human ESCs was examined.
For differentiating PSCs into hematopoietic progenitor cells, we developed a novel 2-dimensional system that avoids the use of feeder cells, serum or embryoid body (EB) formation. We show that the earliest population of hematopoietic progenitor cells have erythroid, megakaryocyte, and myeloid potentials. The first hematopoietic cells co-express CD41 and CD235, typical markers of megakaryocyte and erythrocyte progenitors, respectively. By using this system, we examined the effect of Wnt signaling at various stages of hematopoietic development of human ESCs. Unlike the murine system, we found that canonical Wnt signaling inhibits the development and expansion of this progenitor population. This inhibitory effect also impacts the expansion of erythrocytes and megakaryocytes, but not the myeloid lineage. These studies highlight the importance of working with human ESCs as species-specific differences can be addressed when translating findings from the murine system.
Materials and Methods
Human ESC line maintenance
The human ESC line used in this study was H9 (NIH code WA09 from Wicell Research Institute, Madison, WI) and the iPS cell line, CHOPWT2.2, has been described previously (Mills et al., 2013). Minor modifications were made to the basic protocols for growth and maintenance of human ESC lines that have been previously described (Amit et al., 2000). Briefly, cells were grown on irradiated mouse embryonic feeder cells in maintenance medium consisting of DMEM/F12 supplemented with 20% knock-out serum replacement, 100 μM non-essential amino acids, 0.075% sodium bicarbonate, 1 mM sodium pyruvate, 2 mM glutamine, 50 U/ml penicillin, 50 g/ml streptomycin (all from Invitrogen, Grand Island, NY), 10−4 M β–mercaptoethanol (Sigma, St Louis, MO), and 10 ng/ml human bFGF (Stemgent) in 6-well tissue culture plates at 37°C, 5% CO2 and atmospheric O2. Medium was changed daily or every 2 days and colonies were typically passaged every week following size and morphology assessment. Cells were passaged to new feeders as small clusters in human ESC medium containing ROCK inhibitor (10 μM) using TrypLE (Invitrogen) and gentle scraping using a 25 cm, 2-position blade cell scraper (Sarstedt, Newton, NC). The enhanced green fluorescent protein (GFP) expressing H9 sub-line was generated using a previously published strategy to target the construct into the AAVS1 locus with a zinc finger nuclease (Hockemeyer et al., 2009). The construct contained a chicken actin promoter which drives the expression of GFP.
Differentiation of human ESCs
Prior to the induction of differentiation, cells (2-4 × 105/well) were feeder depleted by culturing on Matrigel coated wells (BD Biosciences, Bedford, MA) (6-well tissue culture plate, Falcon 3046) in human ESC maintenance medium for 24 to 48 hours or until the cells reached ~70% confluence. When the cells were ready for differentiation, one well of cells was used for cell count and pluripotency assessment by analysis of the surface markers, SSEA3 and SSEA4 (>90% co-expression). Three different base media were used in the differentiation protocol and all were supplemented with 2 mM glutamine, 50 μg/ml ascorbic acid (Sigma, St. Louis, MO), 150 μg/ml transferrin (Roche Diagnostics), and 4 × 10−4 M monothioglycerol (MTG) (Sigma). The base media were RPMI (Invitrogen), StemPro-34 (SP-34) (Invitrogen), and serum free differentiation media (SFD) (Gadue et al., 2006). Cultures were maintained at 37°C in an environment of 5% CO2, 5% O2, and 90% N2. The medium and cytokines that were used each day of differentiation are as follows: Days 0-1 RPMI with 5 ng/ml BMP4, 50 ng/ml VEGF and 25 ng/ml Wnt3a; Day 2 RPMI with 5 ng/ml BMP4, 50 ng/ml VEGF and 20 ng/ml bFGF; Day 3 SP34 with 5 ng/ml BMP4, 50 ng/ml VEGF and 20 ng/ml bFGF; Days 4-5 SP34 with 15 ng/ml VEGF and 5 ng/ml bFGF; Day 6 SFD with 50 ng/ml VEGF, 100 ng/ml bFGF, 100 ng/ml SCF, and 25 ng/ml Flt3L; Days 7-9 SFD with 50 ng/ml VEGF, 100 ng/ml bFGF, 100 ng/ml SCF, 25 ng/ml Flt3L, 50 ng/ml TPO, 10 ng/ml IL-6, and 0.05-2 U EPO. Fresh media mixes (2 ml/well) were added each day. Extensive cell death occurred up to day 2 of differentiation followed by the formation of a monolayer of cells. By day 6, cellular proliferation was apparent and the volume of medium was increased to 4 ml/well. From days 7 to 9, single cells shed off of the adherent layer into the medium and were collected for further analysis. During differentiation, cells in the adherent layer were analyzed for surface markers by dissociation into single cells using 0.25% trypsin-EDTA (1 ml/well, 5 mins at room temperature). Mouse Wnt3a 50 ng/ml, mouse Wnt5a 50 ng/ml, human DKK1 150 ng/ml, and CHIR99021 1 uM were added to differentiation cultures as time points indicated in the text. In Figure 4, the day 9 sorted cells were cultured in SFD media containing 50 ng/ml VEGF, 100 ng/ml bFGF, 100 ng/ml SCF, 25 ng/ml Flt3L, 50 ng/ml TPO, 10 ng/ml IL-6, 10ng/ml IL3, and 0.05 U EPO. In Figure 5, sorted megakaryocytes were cultured in 50 ng/ml TPO and 25 ng/ml SCF, sorted erythroid cells were cultured in 2U EPO and 25 ng/ml SCF, and sorted myeloid cells were cultured in 10 ng/ml IL-3 and 25 ng/ml SCF. The neutrophil expansion media was as described in (Choi, Vodyanik, & Slukvin, 2011) and included 3 days of culture with 200 ng/ml GM-SCF followed by an additional two days of culture in 100 ng/ml G-CSF on an OP9 feeder layer. Primitive CMPs were cryopreserved in an ice cold serum mixture (90% FBS + 10% DMSO) and slowly frozen in a styrofoam box at −80°C.
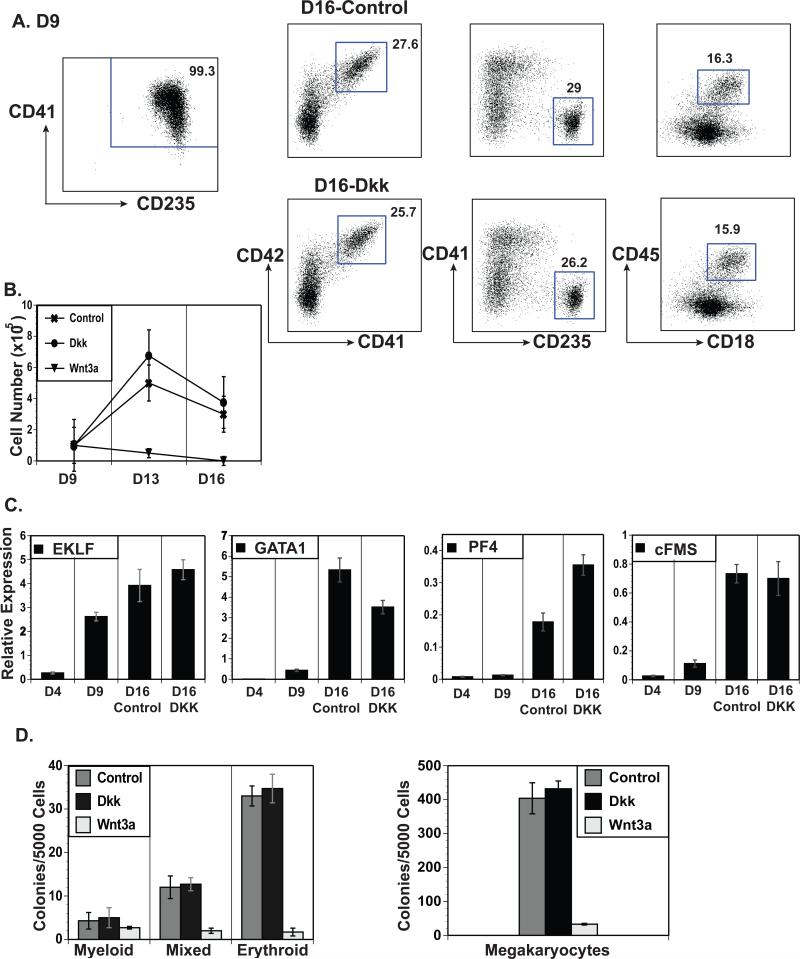
Figure 4.
Wnt signaling inhibits the expansion or survival of CD235+CD41+ CMPs. (A) The left flow cytometric profile shows sorted day 9 CD235+CD41+ H9-derived CMPs. The right profiles show these cells cultured for 7 days in hematopoietic cytokines ± DKK1 (Dkk) or Wnt3a and analyzed for markers of megakaryocytes (CD41 vs. CD42), erythrocytes (CD41 vs. CD235), and myeloid cells (CD45 vs. CD18). (B) Cell counts of the cells treated as described in A at days 9, 13 and 16 compared to the untreated (Control) cells. (C) QRT-PCR expression analyses of lineage specific genes in day 4 mesoderm, day 9 sorted CD235+CD41+ cells, and cells treated with DKK1/Wnt3a as described in A. (D) Sorted day 9 CD235+CD41+ cells as described in A plated in methylcellulose and Mega-cult based colony assays with the addition of DKK1 (Dkk) or Wnt3a.
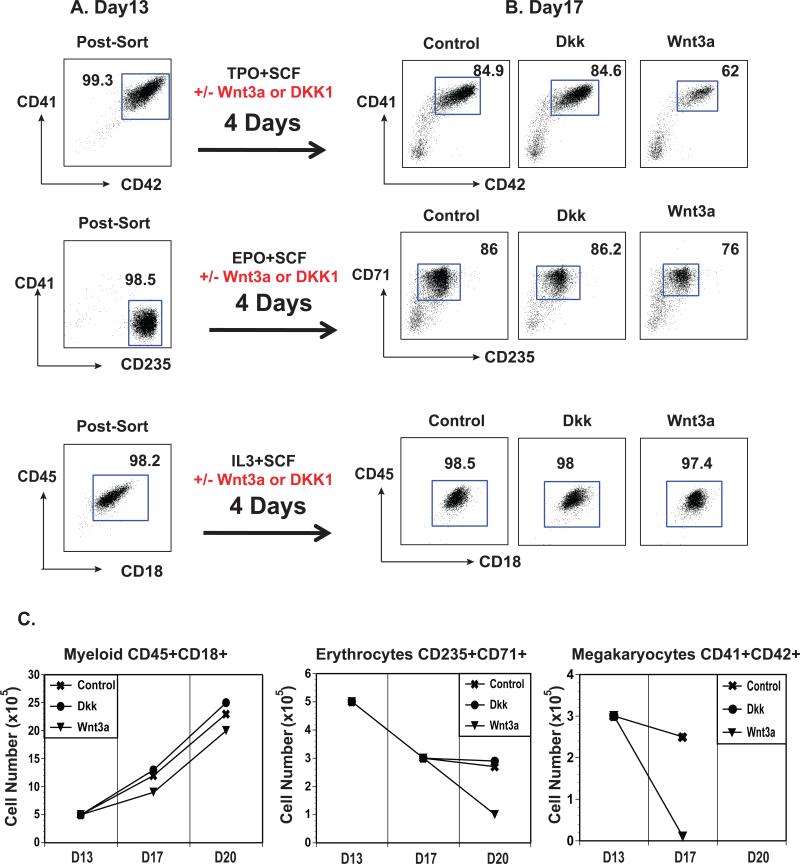
Figure 5.
Wnt signaling affects megakaryocyte and erythroid survival/expansion. (A) Flow cytometric profiles of sorted erythroid (CD41−CD235+), megakaryocyte (CD41+CD42+) and myeloid (CD45+CD18+) cells. (B) Sorted cells from A were cultured for 4 days in lineage specific cytokines ± DKK1 (Dkk) or Wnt3a and analyzed by flow cytometry for CD42 vs. CD41, CD235 vs. CD41, and CD18 vs. CD45. (C) Cell counts of two time points for cultures treated as described in B.
Flow Cytometry, Cell Sorting, and Colony Assays
Analysis of the cells was performed using a Cantos flow cytometer (Becton Dickinson, San Jose, CA). Cell sorting was performed using a FACS Aria II (Becton Dickinson). Flow cytometric data were analyzed using the FlowJo (Treestar, San Carlos, CA) software program. The antibodies used in this study-included anti-KDR-PE (R&D Systems), anti-CD235-APC, anti-CD31-PE-Cy7, anti-CD43-FITC, anti-CD41a-PE, anti-CD42a-PE, anti-CD18-APC, and anti-CD45-pacific blue (BD Pharmingen). The analysis of hematopoietic progenitor cells was performed using methylcellulose (Stem Cell Technologies H4230) supplemented with the cytokines interleukin-3 (IL-3), erythropoietin (EPO), GM-CSF, and stem cell factor (SCF) per manufacturer's instructions. The analysis of megakaryocyte progenitor cells was performed using Megacult (Stem Cell Technologies 04900) supplemented with the cytokines thrombopoietin (TPO), IL-3, and interleukin-6 (IL-6), as per manufacturer's instructions.
RT-PCR and Quantitative Real-time PCR
Total RNA was prepared using the RNeasy mini or micro kits (Qiagen, Valencia, CA) in which samples were treated with RNase-free DNase. The reverse transcription of RNA (100 ng-1 μg) into cDNA was performed using random hexamers with Superscript II Reverse Transcriptase (RT) (Life technologies, Grand Island, NY), according to the manufacturers instructions. Real-time quantitative polymerase chain reaction (PCR) was performed on the LightCycler-480 (Roche-Genentech, South San Francisco, CA). All experiments were done in triplicate with SYBR-GreenER pPCR SuperMix (Life technologies, Grand Island, NY), according to the manufacturer's instructions. Primers for all the genes (Supplemental Table 1) were prepared by Integrated DNA Technologies, Inc. (www.idtdna.com). Dilutions of human genomic DNA standards ranging from 100 ng/μl to 50 pg/μl were used to evaluate PCR efficiency of each gene relative to the housekeeping gene CYCLOPHILIN.
Results
Generation of a single population of hematopoietic progenitors
To differentiate human ESC and iPSCs to hematopoietic lineages, a number of assays have been developed that utilize either EB formation (Kennedy, D'Souza, Lynch-Kattman, Schwantz, & Keller, 2007; Ng, Davis, Azzola, Stanley, & Elefanty, 2005; Zambidis, Peault, Soon Park, Bunz, & Civin, 2005) or cell culture in the presence of stromal cell lines (Kaufman, Hanson, Lewis, Auerbach, & Thomson, 2001; Vodyanik, Bork, Thomson, & Slukvin, 2005). We developed a serum and stromal-free cell differentiation protocol using adherent conditions in which a homogenous population of non-adherent hematopoietic progenitor cells is produced (see materials and methods). This system is designed to follow distinct stages of development including the induction of primitive streak (PS) formation and specification of mesoderm followed by the maturation and expansion of hematopoietic progenitor cells (Figure 1A). The induction and specification phases of the protocol involve the plating of undifferentiated ESCs onto Matrigel in the presence of BMP4 and Wnt3a, conditions that lead to efficient mesoderm development (Figure 1A). The cells are then cultured in VEGF and bFGF to expand the hematopoietic mesoderm population and subsequently in a combination of bFGF, VEGF, SCF, and Flt3L to expand the hematopoietic progenitors. As an indicator of mesoderm differentiation, possibly hemogenic endothelium, the co-expression of KDR and CD31 on the total adherent cell population was analyzed by flow cytometry. Figure 1A shows that approximately 50% of the cells co-expressed these surface markers by day 4. From days 7-9 of differentiation, non-adherent cells began appearing in the culture supernatants. Approximately 90% of these cells expressed CD43 (data not shown), a marker of early hematopoietic progenitors (Vodyanik, Thomson, & Slukvin, 2006), and co-expressed CD41 and CD235 (Figure 1B); markers that have been used to define a megakaryocyte-erythroid progenitor (MEP) derived from human ESCs (Klimchenko et al., 2009; Vodyanik et al., 2006). To characterize the progenitor potential of these MEP-like cells, gene expression and colony assays were performed. The hematopoietic genes RUNX1 (Kumano & Kurokawa, 2010) and SCL (Lécuyer & Hoang, 2004) were expressed in non-adherent cells collected from days 7-9 and GATA1 (Crispino, 2005) was expressed in cells collected at days 8 and 9 (Figure 1C). The red cell transcription factor EKLF (Yien & Bieker, 2013) was highly expressed in cells collected on day 9 of differentiation. These data correlated with the potential of these cells to generate erythroid colonies, which increased at each time point and was maximal at day 9 of differentiation (Figure 1D). We also tested the expression of globin chains in colonies picked from methycellulose and from CD41+CD235+ cells at days 7, 8, and 9 of differentiation (Supplemental Figure 1). We found mainly expression of epsilon globin, with little gamma globin and no beta globin expression, indicative of primitive erythrocytes. Of interest was the observation that mixed and myeloid colonies were also generated from cells taken at each time point. The myeloid colony count remained constant while the mixed colony count gradually decreased at the later time points. Cytospins of each colony type showed primitive erythrocyte cells in erythroid colonies; monocyte/macrophages and neutrophils in the myeloid colonies; and macrophage, primitive erythrocytes, and megakaryocytes in the mixed colonies (Figure 1E). To confirm the neutrophil forming ability of the CD41+CD235+ population, we expanded these cells for five days in neutrophil inducing conditions (Choi et al., 2011) (Supplemental Figure 2) and demonstrate robust co-expression of CD15 and CD66b along with distinct neutrophil morphology as demonstrated by cytospin.
Figure 1.
Monolayer differentiation of human ESCs generates multipotent hematopoietic progenitors. (A) Representative flow cytometric analyses of ES cells (SSEA4 vs. SSEA3), day 5 hemogenic mesoderm (CD31 vs. KDR) and day 9 hematopoietic progenitors (CD235 vs. CD41) induced in a serum free/feeder free adherent culture system. (B) Flow cytometric analysis of CD235 vs. CD41 expression on the non-adherent population obtained from days 7, 8 and 9 of the adherent differentiation cultures. (C) QRT-PCR gene expression analyses of ES cells and the day 7, 8, and 9 non-adherent cells shown in B. (D) Day 7, 8 or 9 non-adherent cells shown in B were assayed for progenitor colony forming potential and myeloid, mixed (muti-potential) and erythroid colonies were quantified. (E) Cytospins showing May-Grünwald Giemsa staining of myeloid, erythroid and mixed colonies. Scale bars represent 20 μm. Arrows in the myeloid cytospin indicate cells with neutrophil morphology. (F) Non-adherent cells from H9 and H9-GFP ESC lines at days 7, 8 and 9 of differentiation were mixed at a 1:1 ratio and plated in a methylcellulose colony assay. Mixed colonies were scored based upon GFP expression (GFP+: all cells in a colony GFP positive, GFP−: all cells in a colony GFP negative, GFP+/−: mixture of GFP positive and negative cells in a colony). Scale bars represent 500 μm.
The presence of mixed and myeloid colonies from the CD41+CD235+ MEP-like population of cells was somewhat unexpected because previous studies have suggested that these cells are restricted in progenitor potential to the erythroid and megakaryocyte lineages only (Klimchenko et al., 2009; Vodyanik et al., 2006). To confirm that the mixed colonies were clonal in nature, mixing experiments were performed using normal H9 human ESCs and a sub-line that constitutively expresses GFP (see materials and methods). Non-adherent cells from days 7-9 of differentiation were mixed 1:1 and plated in colony assays (Figure 1F). The numbers of mixed colonies that were GFP+, GFP−, or containing a mixture of GFP+ and GFP− cells were counted at each time point. Only GFP+ or GFP− mixed colonies were present from all time points examined with a complete absence of GFP+/− colonies demonstrating that the mixed colonies were derived from a single cell. The colony numbers were greatest from the day 7 progenitors and decreased with cells from later time points. These data demonstrate that the CD41+CD235+ cells generated using our adherent differentiation protocol have multipotent progenitor activity. These cells will be designated as primitive common myeloid progenitors (CMP)s instead of MEPs in the remainder of this manuscript.
One advantage of this differentiation system is that it enables purification of primitive CMPs by simply harvesting the CD41+CD235+ non-adherent progenitor cells in the culture supernatant. These CMPs can then be analyzed in downstream assays including colony assays (Figure 1D). Importantly, this CMP population can be cryopreserved with 80% viability after 6 months and 70% viability after one year (Supplemental Figure 3A). These cryopreserved cells can be induced to differentiate into myeloid, erythroid, and megakaryocytes in liquid expansion media and perform in colony assays with similar efficiency to non-cryopreserved cells (Supplemental Figure 3B and C). The advantage of being able to cryopreserve CMPs is that stocks of progenitors can be collected to use at later time points without the need to perform laborious and time-consuming in vitro differentiation assays for downstream analyses.
Inhibition of human ESC-derived primitive CMPs by Wnt3a
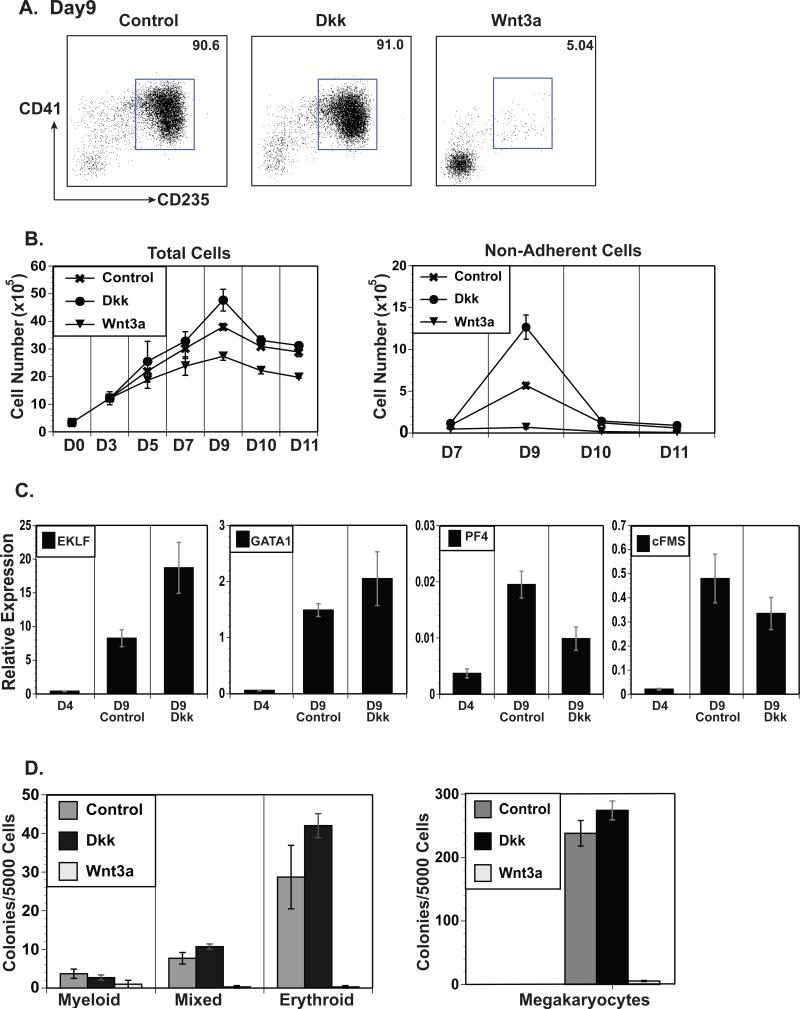
We have previously demonstrated the absolute requirement of the Wnt signaling pathway in murine primitive erythropoiesis during the in vitro differentiation of mouse ES cells (Nostro et al., 2008). To examine the role of this pathway in human primitive hematopoiesis, Wnt3a was added to the differentiation cultures starting at day 4 of differentiation. This time point was selected because typically ~50% of the cells were KDR+CD31+ (Figure 1A), indicative of mesoderm specification; and are negative for CD43, CD235, and CD41 (data not shown); suggesting that hematopoietic lineage specification had not yet occurred. The non-adherent cells generated in the differentiation cultures at day 9 were analyzed for surface markers, gene expression, and progenitor potential. One of the first observations was that the day 9 primitive CMP population in the presence of Wnt3a was greatly reduced with only ~5% of the non-adherent cells expressing CD41+CD235+ (Figure 2A). In addition, the absolute number of non-adherent cells was decreased by over 10-fold with Wnt3a treatment (Figure 2B, right), leading to a >200-fold decrease in primitive CMPs. To more precisely determine the timing of the Wnt mediated inhibition, we added Wnt3a for a single 24 hour period starting at day 3, prior to the expression of CD31 but after KDR up-regulation (Supplemental Figure 4A). We found that treatment even at this early stage inhibited the generation of CD41+CD235+ cells, though to a slightly lessor degree than when given at day 4 (Supplemental Figure 4B and C). To confirm that this finding was due to Wnt signaling, the Wnt antagonist DKK1 (Glinka et al., 1998) was added to the cultures also starting at day 4 of differentiation. The addition of DKK1 had little impact on the percentage of CD41+CD235+ cells in the non-adherent population but resulted in a 2-3-fold increase in the number of total non-adherent cells (Figure 2A and B). Cell numbers in the adherent layer demonstrated a mild effect of either Wnt3a or DKK1 treatment (Figure 2B, left), suggesting that these effects were not due to general toxicity. To ensure that DKK1 treatment only affected the number of progenitors but not their developmental potential, gene expression analyses and colony assays were performed. The expression of hematopoietic and lineage-specific genes; EKLF, GATA1, PF4 and cFMS; and the numbers of myeloid, mixed, erythroid, and megakaryocyte colonies were comparable between DKK1-treated and control cells (Figure 2C and 2D). In addition, the few non-adherent cells generated in the Wnt3a treated samples failed to form hematopoietic colonies (Figure 2D). Similar results were found with a wild-type human iPS cell line, where Wnt3a addition led to a loss of CD41+CD235+ cells and hematopoietic colony forming ability while DKK1 treatment increased the number of these cells (Supplemental Figure 5A, B and C). These studies demonstrate that Wnt signaling is inhibitory to the generation of the primitive CMP population derived from human PSCs.
Figure 2.
Wnt signaling is inhibitory to the generation of CD235+CD41+ early hematopoietic progenitors from human ESCs. (A) Flow cytometric analyses of CD235 vs. CD41 on day 9 non-adherent cells treated with DKK1 (Dkk) or Wnt3a starting on day 4 of differentiation. (B) Cell yields from the entire culture (adherent and non-adherent cells) or non-adherent cells treated as described in A. (C) QRT-PCR gene expression analyses of lineage specific genes in control and DKK1 treated cells. (D) Day 9 non-adherent cells treated as described in A assayed for progenitor potential in methylcellulose and Mega-cult colony assays.
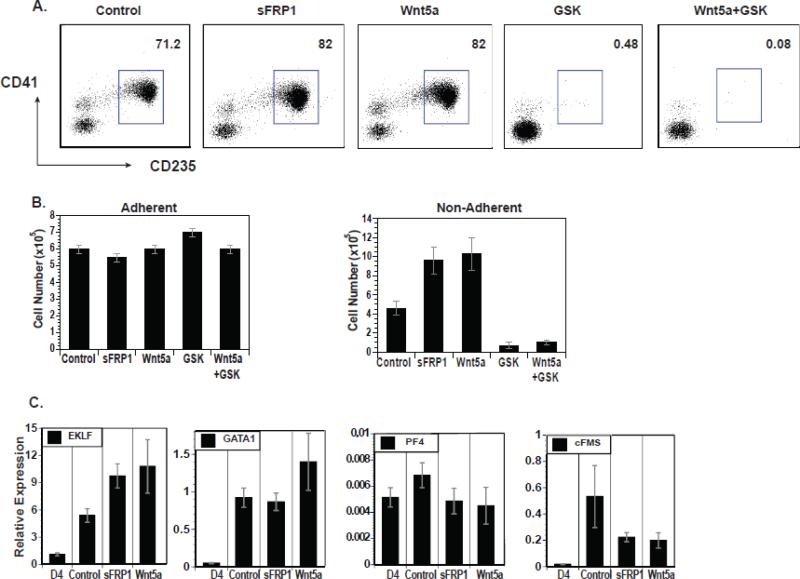
Because Wnt3a had such a dramatic effect on the number of primitive CMPs, we further examined the contributions of canonical and non-canonical Wnt signaling in this developmental pathway. To activate the canonical Wnt pathway directly and bypass the receptor, a small molecule inhibitor of GSK3β (GSK), CHIR99021 (Murray et al., 2004), was used. To activate the non-canonical pathway, Wnt5a was used. To inhibit both pathways, a soluble inhibitor of Wnt, sFRP1, was employed (Yamamoto, Sakane, Yamamoto, Michiue, & Kikuchi, 2008). Cells were treated at day 4 of differentiation with these various agents and the non-adherent cells at day 9 of differentiation were analyzed for CD41 and CD235 expression (Figure 3A). Approximately 80% of the non-adherent cells were CD41+CD235+ in the cultures treated with both Wnt5a and sFRP1 while <5% were CD41+CD235+ in the cultures treated with the GSK inhibitor alone or in combination with Wnt5a. Wnt5a and sFRP1 both increased the number of non-adherent cells in a manner similar to DKK1 treatment, suggesting a possible role of non-canonical Wnt signaling in this developmental stage (Figure 3B). GSK inhibitor treatment led to a drastic decrease in the number of non-adherent cells both alone and in combination with Wnt5a. Because the GSK inhibitor was dominant over Wnt5a, these data suggest that enhanced CMP generation induced by Wnt5a may have been due to inhibition of the canonical pathway. Gene expression studies show that the CD41+CD235+ cells generated following treatment with sFRP1 and Wnt5a were comparable to the untreated control population of cells (Figure 3C). These data suggest that the canonical Wnt pathway inhibits the production of the primitive CMP population of cells.
Figure 3.
Canonical Wnt signaling inhibits hematopoiesis. (A) Flow cytometric analyses of CD235 vs. CD41 on day 9 non-adherent cells treated on day 4 of differentiation with sFRP1, Wnt5a, GSK3β inhibitor (GSK), and GSK3β inhibitor + Wnt5a starting. (B) Cell yields for the entire culture (adherent and non-adherent) and non-adherent cells treated as described in A. (C) QRT-PCR expression analyses of lineage specific genes in non-adherent cells treated as described in A.
Inhibition of lineage maturation by Wnt3a
Because Wnt3a had such a profound effect on the generation of primitive CMPs, the effect of Wnt3a on CMP-derived lineage maturation was examined. To ensure purity of the starting CMP population, day 9 CD235+CD41+ cells were sorted (Figure 4A, left). The sorted cells were expanded for one week in culture with a mixture of cytokines to generate erythroid, megakaryocyte, and myeloid lineages in the presence or absence of Wnt3a or DKK1. The addition of Wnt3a led to a decrease in cell number with a complete loss of cells after one week in culture (Figure 4B). The addition of DKK1 to the liquid cultures did not statistically affect either cell expansion or the proportions of megakaryocyte, erythroid or myeloid cell generation. The DKK1-treated and control cells also had similar gene expression and progenitor profiles (Figure 4C and 4D). The addition of Wnt3a directly into the methylcellulose in colony forming assays led to a drastic decrease in mixed, erythroid and megakaryocyte colony production with a smaller effect on myeloid colony production while DKK1 treatment had no effect (Figure 4D). These data suggest that Wnt3a signaling acts on primitive CMP cells and causes cell death and/or impaired cell proliferation.
To address the question of whether Wnt signaling affects the committed primitive CMP-derived hematopoietic lineages, megakaryocytes, erythrocytes, and myeloid cells were generated from primitive CMPs, purified by cell sorting and cultured in the presence or absence of Wnt3a or DKK1 (Figure 5). Cell counts and cell surface marker analyses were performed. The erythroid and megakaryocyte lineages remained sensitive to the effects of Wnt3a as shown by a steady decrease in percentage and absolute numbers of cells compared to the untreated control cultures (Figure 5B and 5C). The myeloid lineage was not affected by the presence of Wnt3a (Figure 5B and 5C). DKK1 treatment had no effect on any of these lineages. Taken together, these data suggest that canonical Wnt signaling acts to disrupt primitive CMP generation and expansion or survival of megakaryocytes and erythrocytes derived from primitive CMPs.
Discussion
The first hematopoietic progenitor population derived from the in vitro differentiation of human pluripotent stem cells expresses the cell surface antigens CD41 and CD235 and has been described to have primitive erythroid and megakaryocyte potential (Klimchenko et al., 2009; Vodyanik et al., 2006). In our culture system, this population of cells is comprised of tri-potent progenitors for the erythroid, megakaryocyte, and myeloid lineages. We demonstrate that over time in the differentiation cultures the colony forming potential of the CD41+CD235+ population changes, with an increase in erythroid potential and a loss of mixed colony forming potential while maintaining expression of the CD41 and CD235 cell surface markers (Figure 1). These data suggest kinetic changes in the developmental potential of the CD41+CD235+ population over time and may explain apparent discrepancies between our work and previously published reports. It will be critical in the future to establish additional markers that correlate more closely with lineage specification. While the generation of a human primitive CMP population described here is novel, it is not necessarily unexpected from our understanding of yolk sac hematopoiesis in the mouse system. A bi-potent population that generates primitive erythrocytes and megakaryocytes has been isolated from the early yolk sac and termed the primitive MEP (Tober et al., 2007). But it has been well established that primitive erythroid, megakaryocyte, and myeloid lineage progenitors are all present in the early mouse yolk sac, prior to circulation (Palis, Robertson, Kennedy, Wall, & Keller, 1999; Xu M et al., 2001). Therefore, it is possible that a tri-potent primitive CMP population is also present in the early mouse embryo. Further experimentation is needed to determine whether this is the case or human primitive hematopoiesis is divergent from the mouse. Species-specific differences in primitive hematopoiesis have been described. For example, zebrafish primitive erythrocytes and myeloid cells are derived from distinct anatomical locations (Chen & Zon, 2009).
Here, we show a dramatic inhibitory effect of canonical Wnt signaling on the development and/or survival of human primitive CMP and CMP-derived erythrocytes and megakaryocytes. This is in stark contrast to the murine system where we demonstrated that Wnt signaling enhanced primitive erythrocyte development (Nostro et al., 2008). Gertow et al also demonstrated that the addition of Wnt3a into colony forming assays of human ES cells suppressed the generation of both blast and hematopoietic colonies (Gertow et al., 2013). These findings suggest that there are species specific differential requirements for Wnt signaling in early hematopoiesis. Our work extends the findings of Gertow et al. by demonstrating an inhibitory effect of Wnt on the survival or expansion of purified erythrocytes and megakaryocytes derived from primitive CMPs.
Wnt signaling has been previously studied during hematopoietic differentiation from human ES cells by Vijayaragavan et al. (Vijayaragavan et al., 2009). While our work and Gertow et al. demonstrate an important role of Wnt signaling in the inhibition of primitive hematopoiesis, this earlier study suggests that canonical Wnt signaling can enhance hematopoietic progenitor expansion. This may be due to the effects of Wnt signaling on distinct hematopoietic populations. The primitive CMP we describe here is CD45− (data not shown) and may represent an early primitive yolk sac hematopoietic program while the CD45+ cells examined by Vijayaragavan et al. may represent a later definitive yolk sac program. The generation of two progenitor populations has been documented in human ES cell differentiation cultures (Vodyanik et al., 2006) and two populations also arise from the mouse yolk sac during normal embryogenesis (review in (McGrath & Palis, 2005)). The differential requirement of Wnt signaling in these two programs will be an important future area of research. The use of Wnt or small molecule Wnt agonists to deplete cultures of primitive CMPs may represent a powerful tool for the purification of more mature hematopoietic precursors and repopulating hematopoietic stem cells from human ES cell differentiation cultures.
In summary, we demonstrate that the first hematopoietic progenitor derived from human PSCs has tri-lineage potential, which we term the primitive CMP. We established a novel serum-free/stroma free differentiation system for the generation of large numbers of a homogeneous population of CMPs that can be isolated by harvesting the supernatant of the differentiation cultures. These primitive CMPs can be cryopreserved, enabling the storage of large numbers of progenitors for easy access to use in down stream applications. Lastly, we establish the negative impact of canonical Wnt signaling in CMP generation and/or survival as well in CMP-derived megakaryocytes and erythroid cells. This system represents a powerful and flexible model to study early human hematopoiesis in vitro.
Supplementary Material
Highlights.
Novel serum/feeder free system creates hematopoietic cells from human ES/iPS cells
CD235+CD41+ human hematopoietic progenitors are tri-potent primitive CMPs
Human primitive CMP formation and survival is negatively affected by Wnt signaling
Abbreviations
- ESC
Embryonic stem cell
- PSC
pluripotent stem cell
- iPSC
induced pluripotent stem cell
- CMP
common myeloid progenitor
- GFP
green fluorescent protein
- SFD
serum free differentiation media
- IL-3
interleukin-3
- EPO
erythropoietin
- GM-CSF
granulocyte-macrophage colony stimulating factor
- SCF
stem cell factor
- TPO
thrombopoietin
- IL-6
interleukin-6
- RT
Reverse Transcriptase
- PCR
polymerase chain reaction
- EB
embryoid body
- MEP
megakaryocyte-erythroid progenitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental biology. 2000;227(2):271–278. doi: 10.1006/dbio.2000.9912. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Chen AT, Zon LI. Zebrafish blood stem cells. Journal of cellular biochemistry. 2009;108(1):35–42. doi: 10.1002/jcb.22251. doi: 10.1002/jcb.22251. [DOI] [PubMed] [Google Scholar]
- Choi K-D, Vodyanik M, Slukvin II. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nature protocols. 2011;6(3):296–313. doi: 10.1038/nprot.2010.184. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino JD. GATA1 in normal and malignant hematopoiesis. Seminars in cell & developmental biology. 2005;16(1):137–147. doi: 10.1016/j.semcdb.2004.11.002. [Review] doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. Experimental hematology. 2005;33(9):955–964. doi: 10.1016/j.exphem.2005.06.009. [Review] doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16806–16811. doi: 10.1073/pnas.0603916103. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K, Hirst CE, Yu QC, Ng ES, Pereira LA, Davis RP, Elefanty AG. WNT3A Promotes Hematopoietic or Mesenchymal Differentiation from hESCs Depending on the Time of Exposure. Stem Cell Reports. 2013;1(1):53–65. doi: 10.1016/j.stemcr.2013.04.002. doi: 10.1016/j.stemcr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, Dekelver RC, Jaenisch R. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nature biotechnology. 2009;27(9):851–857. doi: 10.1038/nbt.1562. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y-S, Chung BG, Ortmann D, Hattori N, Moeller H-C, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(40):16978–16983. doi: 10.1073/pnas.0905550106. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(19):10716–10721. doi: 10.1073/pnas.191362598. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller GM. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimchenko O, Mori M, Distefano A, Langlois T, Larbret F, Lecluse Y, Debili N. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell- derived primitive hematopoiesis. Blood. 2009 doi: 10.1182/blood-2008-09-178863. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- Kumano K, Kurokawa M. The role of Runx1/AML1 and Evi-1 in the regulation of hematopoietic stem cells. Journal of cellular physiology. 2010;222(2):282–285. doi: 10.1002/jcp.21953. [Review] doi: 10.1002/jcp.21953. [DOI] [PubMed] [Google Scholar]
- Lécuyer E, Hoang T. SCL: from the origin of hematopoiesis to stem cells and leukemia. Experimental hematology. 2004;32(1):11–24. doi: 10.1016/j.exphem.2003.10.010. [Review] [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Daley GQ. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2(1):72–82. doi: 10.1016/j.stem.2007.10.022. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development (Cambridge, England) 2006;133(19):3787–3796. doi: 10.1242/dev.02551. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Loebel DAF, Watson CM, De Young RA, Tam PPL. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Developmental biology. 2003;264(1):1–14. doi: 10.1016/s0012-1606(03)00390-7. [Review] [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [Review] doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Experimental hematology. 2005;33(9):1021–1028. doi: 10.1016/j.exphem.2005.06.012. [Review] doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Mills JA, Wang K, Paluru P, Ying L, Lu L, Galvão AM, Gadue P. Clonal genetic and hematopoietic heterogeneity among human induced pluripotent stem cell lines. Blood. 2013 doi: 10.1182/blood-2013-02-484444. doi: 10.1182/blood-2013-02-484444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Cohen P. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. [Comparative Study]. The Biochemical journal. 2004;384(Pt 3):477–488. doi: 10.1042/BJ20041057. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller GM. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [Review] doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–1603. doi: 10.1182/blood-2005-03-0987. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2(1):60–71. doi: 10.1016/j.stem.2007.10.011. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, Keller GM. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development (Cambridge, England) 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KKL, Palis J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109(4):1433–1441. doi: 10.1182/blood-2006-06-031898. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Developmental cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [Review] [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K, Szabo E, Bossé M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4(3):248–262. doi: 10.1016/j.stem.2008.12.011. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell- derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617–626. doi: 10.1182/blood-2004-04-1649. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual review of cell and developmental biology. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [Review] doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, Kaufman DS. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111(1):122–131. doi: 10.1182/blood-2007-04-084186. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M,J, Matsuoka S, Yang FC, Ebihara Y, Manabe A, Tanaka R, Tsuji K. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97(7):2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Developmental cell. 2008;15(1):37–48. doi: 10.1016/j.devcel.2008.04.015. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Yien YY, Bieker JJ. EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Molecular and cellular biology. 2013;33(1):4–13. doi: 10.1128/MCB.01058-12. [Review] doi: 10.1128/MCB.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Soon Park T, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106(3):860–870. doi: 10.1182/blood-2004-11-4522. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.