Abstract
The spiral ganglion is an essential functional component of the peripheral auditory system. Most types of hearing loss are associated with spiral ganglion cell degeneration which is irreversible due to the inner ear's lack of regenerative capacity. Recent studies revealed the existence of stem cells in the postnatal spiral ganglion, which gives rise to the hope that these cells might be useful for regenerative inner ear therapies. Here, we provide an in-depth analysis of sphere-forming stem cells isolated from the spiral ganglion of postnatal mice. We show that spiral ganglion spheres have characteristics similar to neurospheres isolated from the brain. Importantly, spiral ganglion sphere cells maintain their major stem cell characteristics after repeated propagation, which enables the culture of spheres for an extended period of time. In this work, we also demonstrate that differentiated sphere-derived cell populations not only adopt the immunophenotype of mature spiral ganglion cells but also develop distinct ultrastructural features of neurons and glial cells. Thus, our work provides further evidence that self-renewing spiral ganglion stem cells might serve as a promising source for the regeneration of lost auditory neurons.
Introduction
Loss of sensory hair cells due to hereditary or environmental factors is the most common reason for hearing loss, which affects more than 30% of adults over 65 years of age (http://www.nidcd.nih.gov). In the majority of patients with severe or profound sensorineural hearing loss, hearing can be restored by the use of cochlear implants, which convert sound into electrical signals and thereby functionally replace lost hair cells in the inner ear. Spiral ganglion cells are essential for the process of hearing in the healthy ear as well as in the cochlear-implanted ear because they transmit electrical signals from the cochlea to the brain. Loss of afferent innervation has been observed in mice after exposure to levels of noise that do not damage hair cells1 and also occurs in human ears.2 Loss of spiral ganglion cells is also observed as a secondary consequence of hair cell loss especially in patients with long-term deafness.3
A number of studies have identified factors that protect existing spiral ganglion neurons including neurotrophic factors, antioxidants and electrical stimulation.4–6 However, once spiral ganglion cells are lost, the inner ear does not regenerate this cell type. In recent years, various types of stem cells have been proposed as a potential source for replacement cells (for review, see Shi and Edge7).
Several research groups have shown that the mammalian spiral ganglion harbors sphere-forming stem cells that can be isolated using a modified neurosphere assay.8–12 Spheres were also isolated from the cochlea,8,10,13–20 the utricle,8,10,21,22 and most recently from the cochlear nucleus23,24 as a method to isolate multipotent stem cells. When this assay is applied to cells of the postnatal spiral ganglion, cells with sphere-forming capacity grow into neurosphere-like cell colonies. Further analysis of these sphere-forming cells revealed that they exhibit the distinct features of stem cells: they are self-renewing and can differentiate into cells with some characteristics of the mature cells in the original tissue.8–12
Stem cells from the spiral ganglion are therefore considered as a highly promising cellular source to regenerate neural structures of the inner ear. A basic requirement for a future use of spiral ganglion stem cells in animal models or in a clinical trial is a detailed knowledge of the properties of these cells. In this study, we characterize sphere-forming stem cells derived from the spiral ganglion and mature cell populations differentiated from these cells. Since transplantation experiments require the generation of sufficient numbers of stem/progenitor cells in vitro, we were interested in the properties of spheres that were propagated using the neurosphere assay. Our data show that a subset of spiral ganglion-derived sphere cells conserves major characteristics such as proliferative activity and expression of stem/progenitor cell markers even after repeated propagation.
Several studies have demonstrated that specific culture conditions can lead to up-regulation of neuronal and glial markers in cell populations derived from spiral ganglion stem cells.8–12 Here, we show that the cells differentiated from spiral ganglion stem cells not only express mature markers but can also develop specific ultrastructural features of neurons and glia. Collectively, our data provide additional evidence for the suitability of spiral ganglion stem cells as a renewable source for the generation of neurons and glial cells.
Materials and Methods
Animals
Early postnatal (P1–3) Balb/c mice (Janvier Labs, St. Berthevin Cedex, France) were used for our experiments. The Animal Research Committee of the Goethe University of Frankfurt/Main (Germany) approved our procedures which were carried out in accordance with the German guidelines for care and use of laboratory animals (§4(3)).
Isolation, culture and propagation of sphere-forming stem cells from the early postnatal spiral ganglion
Mice were decapitated, and the temporal bone was dissected out after removal of the brain and transferred into ice-cold Hank's balanced salt solution (Invitrogen, Carlsbad, CA). The otic capsule was freed from the otic bulla, opened, and removed to visualize the membranous labyrinth of the cochlea. The cochlear duct (organ of Corti, spiral ligament, and stria vascularis) was microdissected from the modiolus where the spiral ganglion resides. For isolation of sphere-forming stem cells, we used a modified neurosphere assay protocol that has been published previously.18,22,25 In brief, spiral ganglia were enzymatically digested in 0.125% trypsin/EDTA (Invitrogen) in phosphate-buffered saline (PBS; Life Technologies, Carlsbad, CA) for 5 min at 37°C. This process was terminated by a cocktail of 10 mg/mL soybean trypsin inhibitor (Worthington, Lakewood, NJ) and 1 mg/mL DNase I (Worthington) in Dulbecco's modified Eagle's medium and Nutrient Mixture F-12 (DMEM/F12; Sigma, St. Louis, MO) followed by trituration with pipette tips (Starlab, Hamburg, Germany) to achieve mechanical dissociation and the use of a 70-μm cell strainer (BD Falcon, Franklin Lakes, NJ) to obtain a homogeneous single cell suspension. The cells were then cultured in polyHEMA-coated suspension culture six-well plates (Sigma, Greiner Bio-One, Kremsmünster, Austria) in a volume of 2 mL of DMEM/F12 supplemented with N2 (Invitrogen), B27 (Invitrogen), epidermal growth factor (EGF; 20 ng/mL), basic fibroblast growth factor (bFGF; 10 ng/mL), insulin-like growth factor-1 (IGF-1; 50 ng/mL), heparan sulfate (50 ng/mL) (all growth factors were from Sigma-Aldrich), and ampicillin (50 μg/mL; Sigma-Aldrich). For propagation of spiral ganglion-derived spheres, spheres were harvested after 3–5 days, mechanically dissociated following enzymatic digestion by treatment with Accumax (PAA Laboratories, Cölbe, Germany), and replated for 3–5 days to allow for formation of the subsequent generation of spheres. Analysis of sphere size was determined by measuring the maximum sphere diameter (d) and the sphere volume was calculated using the formula 1/6πd3.
Differentiation of spiral ganglion-derived spheres
Individual secondary spheres were plucked and exposed to differentiative conditions by transferring them to plastic four-well tissue culture plates (Greiner Bio-One) with a 0.1% gelatin (Millipore, Billerica, MA)-coated surface containing 100 μL of DMEM/F12 per well supplemented with N2 (Invitrogen), B27 (Invitrogen), brain-derived neurotrophic factor (BDNF, 50 ng/mL), NT-3 (50 ng/mL) (both from R&D Systems, Minneapolis, MN), and ampicillin (50 μg/mL; Sigma-Aldrich). Three quarters of the medium were replaced by fresh, prewarmed medium every other day.
Analysis of marker expression (BrdU, Ki-67, Nestin) in sphere cells was done after attachment of the spheres to tissue culture plates. For quantification of Ki-67 expression only cells with a strong nuclear staining were counted. Immunohistochemical and ultrastructural analysis of differentiated sphere cultures was performed after a period of 8–10 days in vitro.
Immunohistochemistry
Cells were washed with PBS, fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS for 15 min, and incubated with PBT-1 (PBS, 0.1% Triton X-100, 1% bovine serum albumin, 5% heat-inactivated goat serum) for 15 min to permeabilize the cell membranes and block nonspecific binding sites. Next, the cells were incubated with primary antibodies overnight at 4°C. Two washes with PBT-1 and PBT-2 (PBS, 0.1% Triton X-100, 0.1% bovine serum albumin), respectively, were followed by incubation with species-specific, fluorophore-conjugated secondary antibodies for 2 h at room temperature. Cells were washed twice with PBT-2 and counterstained with the blue-fluorescent nucleic acid stain 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). For immunodetection of BrdU, cells were exposed to 2 N HCl after fixation. Images of immunostained samples were acquired with an epifluorescence microscope (Axio Imager.M2, Zeiss, Oberkochen, Germany).
Antibodies
We used various primary antibodies in this study to characterize marker expression in spheres and sphere-derived cells: anti-nestin (Developmental Studies Hybridoma Bank [Iowa City, IA], Rat-401, mouse monoclonal, 1:200), anti-BrdU (Novus Biological [Littleton, CO], NB500-169, rat monoclonal, 1:500; and Sigma, B2531, mouse monoclonal, 1:500), anti-Ki-67 (DCS [Hamburg, Germany], Ki681C01, rabbit monoclonal, 1:100), anti-GFAP (Dako [Glostrup, Denmark], Z0334, rabbit polyclonal, 1:500), and anti-MAP-2 (Sigma, M4403, mouse monoclonal, 1:2000). FITC-conjugated, TRITC-conjugated, and Cy5-conjugated species-specific secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) were diluted 1:200.
Scanning electron microscopy
For ultrastructural analysis of whole spiral ganglion-derived spheres and differentiated sphere-derived cells, cells were fixed in 2% glutardialdehyde (Merck, Darmstadt, Germany) in PBS for 2 h and dehydrated in a graded ethanol series (25%, 50%, 70%, 95%, 99% ethanol) for 10 min per condition. The cells were then coated overnight with hexamethyldisilazane (Merck), dried, and sputter-coated with Au/Pd with an Agar Sputter Coater (Agar Scientific, Stansted, Essex, United Kingdom). Cells were visualized using a Hitachi S-4500 SEM Cold Field Emission SEM System operated at 5 kV at a working distance of 10–15 mm.
Transmission electron microscopy
Spheres were fixed for 2.5 h in Yellow Fix (2% paraformaldehyde, 0.1 M sodium cacodylate buffer (pH 7.2, 0.02% picric acid) with 2% glutaraldehyde in PBS. The specimens were rinsed three times with 0.1 M sodium cacodylate buffer, fixed for 30–40 min with 1% osmic acid (Carl Roth, Karlsruhe, Germany) and rinsed another three times with 0.1 M sodium cacodylate buffer. Dehydration was carried out by treatment with a graded ethanol series (50%, 70%, 80%, 96%, 100% ethanol, 15 min each and 100%, 100% ethanol, 30 min each) followed by treatment with Xylol (10 min), Epon/Xylol (1:1, overnight), and Epon (4–5 h). Finally, spheres were cured with Epon Resin (Serva, Heidelberg, Germany) at 60°C for 20 h. For orientation, semithin sections (1 μm) were prepared using a Reichert Ultracut S Microtome and stained with Richardson's stain (1% methylene blue and 1% Borax in distilled water, and 1% Azur II in distilled water, mixed 1:1) for 15 sec at 60°C. After this, ultrathin sections were prepared using a Reichert Ultracut S Microtome and stained with 0.5% uranylacetate (30 min) and 0.2% lead citrate (1 min). Sections were viewed with a Zeiss EM 109 transmission electron microscope.
Time-lapse live cell imaging
Time lapse microscopy of differentiating spheres was performed using a Zeiss Observer.Z1 microscope equipped with an incubation system (Temp Module S1, a CO2 Module S1) and an AxioCam MRm. Images were captured in 15-min intervals over 45.5 h.
Statistics
Data are presented as mean values and standard deviations. Statistical analyses were performed with IBM SPSS Statistics 21 (International Business Machines Corp., Armonk, NY) using a one-way ANOVA followed by Scheffé's post hoc test. Results were considered statistically significant at a level of p<0.05.
Results
The morphological features of spiral ganglion-derived spheres are strikingly similar to those of neurospheres from the brain
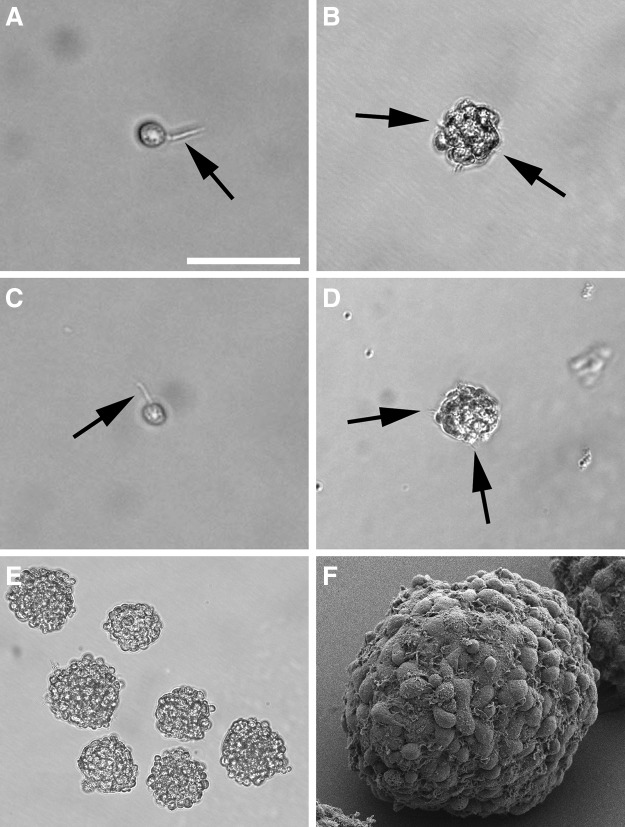
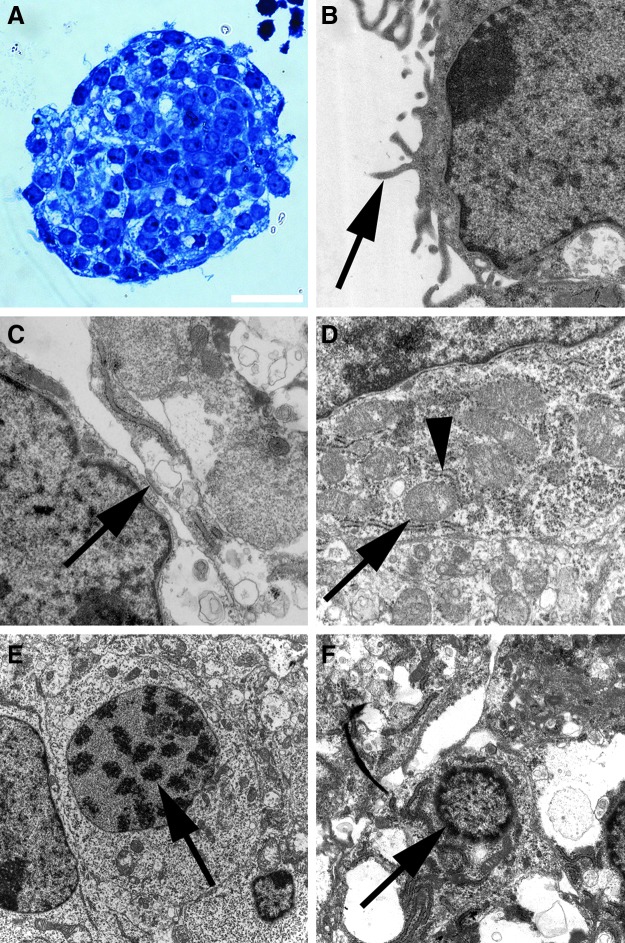
We microdissected the spiral ganglia of early postnatal mice (Fig. 1A–D) and isolated stem cells from this inner ear organ by their ability to form neurospheres. After 3–5 days free-floating spherical clusters formed from single cells in this defined, serum-free suspension culture system. The morphology of the spiral ganglion-derived spheres resembled the appearance of neurospheres that can be isolated from distinct areas of the brain. The spheres consisted of densely packed cells and displayed a round or oval shape and a grape-like structure (Fig. 2B,E,F). When we screened the surface of single progenitor cells and spheres at high magnification, we detected microspikes (filopodia) (Fig. 2A,B), which have been previously described as a typical morphological feature of neural progenitors and young, viable neurospheres from the brain (Fig. 2C,D).26–28 Ultrastructural analysis of spiral ganglion-derived spheres showed pseudopodia-like cell processes on cells in the spheres' periphery and plentiful mitochondria in the sphere cells indicative of high proliferative activity of the cell colonies (Fig. 3).
FIG. 1.
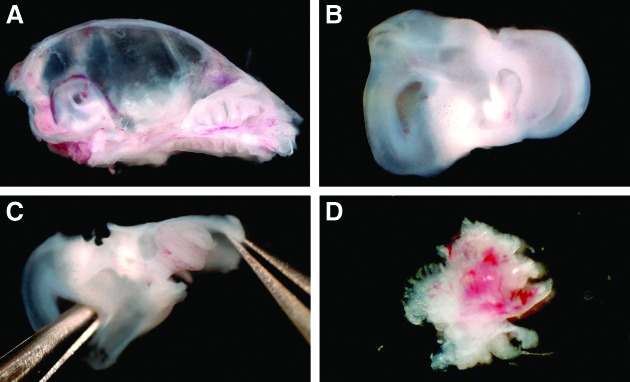
Critical steps of spiral ganglion dissection from early postnatal mice. (A) The temporal bone of this left hemiskull is exposed by removal of the cerebrum. (B) Inner ear with the cochlea and the vestibular apparatus. (C) Opening and careful removal of the cartilaginous otic capsule reveals the membranous labyrinth. (D) The modiolus harbors the spiral ganglion whose neurites can be seen after detachment of the cochlear duct.
FIG. 2.
Morphological features of neural progenitors and spheres. (A, B) High magnification of single spiral ganglion-derived progenitor cell and a developing spiral ganglion sphere reveals the presence of microspikes on their surfaces (arrows)—a characteristic feature of neural progenitors and young neurospheres isolated from the murine brain. To demonstrate the analogy, examples of a neural progenitor cell and a neurosphere derived from the brain is shown in (C) and (D). Images are taken on day in vitro (DIV) 1 (progenitor cells) and after 4 DIV (spheres). (E) Typical appearance of spiral ganglion spheres after a 7-day culture period. (F) Scanning electron microscopy of a spiral ganglion sphere composed of many proliferating single cells. Scale bar=100 μm in B and D, 50 μm in A, C, and F, 200 μm in E.
FIG. 3.
Ultrastructural characterization of spiral ganglion spheres. (A) Semithin section of a spiral ganglion sphere stained with Richardson's stain to highlight nuclear and cytoplasmic details of sphere cells. (B) Cells on the spheres' surface are characterized by pseudopodia (arrow). These pseudopodia can also be observed in the intercellular spaces within the spheres. (C) Adherens junction (arrow) that links the actin cytoskeletons of two adjacent sphere cells. (D) Areas with considerable amounts of rough endoplasmatic reticulum (arrowhead) and mitochondria (arrow) indicate the high energy metabolism in proliferative sphere cells. (E) A sphere cell's nucleus harbors condensed heterochromatin (arrow), which might indicate that this cell already underwent mitosis. (F) The early stage of a sphere cell's apoptosis is associated with the shrinkage of the cell and the disaggregation of the nucleus, which exhibits condensed chromatin (arrow). Scale bar=20 μm in A, 1000 nm in B, C, and D, 3000 nm in E, and 1500 nm in F.
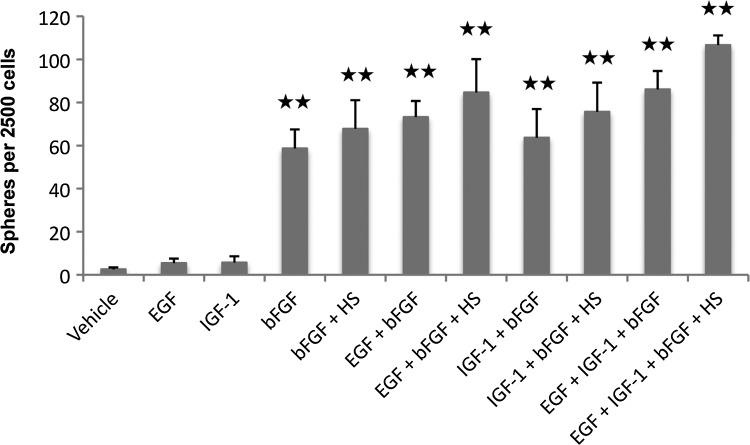
To systematically evaluate the neurosphere assay, which has been previously used to isolate inner ear stem cells,8–10,18,21,22 we analyzed the effects of EGF, IGF-1, bFGF, and heparan sulfate on sphere formation alone and in various combinations (Fig. 4). Sphere formation occurs at a very low level without growth factors. When we tested EGF, IGF-1, and bFGF as single factors, bFGF turned out to be the most potent stimulator of sphere formation. The highest number of spheres could be obtained when using EGF, IGF-1, bFGF, and heparan sulfate in combination. This condition was therefore used for all further sphere isolation experiments.
FIG. 4.
Isolation of spheres from the spiral ganglion of early postnatal mice. Quantification of floating cell colonies with a maximum diameter ≥40 μm that form from dissociated spiral ganglion cells in the presence of growth factors and without growth factors (vehicle) after 7 days in a nonadherent petri dish. Data are the mean±SD from three independent experiments; **p<0.01 compared with vehicle (bovine serum albumin [BSA]). bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; IGF-1, insulin-like growth factor-1; HS, heparan sulfate.
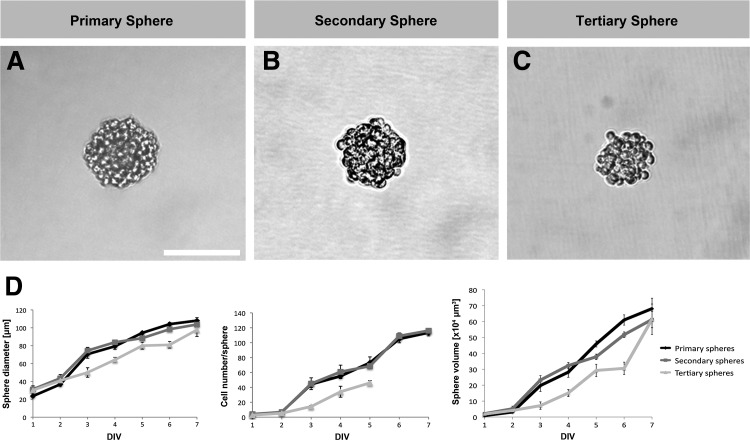
Analysis of primary sphere diameter, cell number, and volume revealed that small cell colonies appear already 1 day after plating the single cells and that these clusters grow continuously throughout the 7-day culture period (Fig. 5A,D). After propagation, the second and third sphere generations showed very similar growth characteristics (Fig. 5B–D).
FIG. 5.
Primary spheres (A) and propagated spheres (B, C) show similar morphology and growth characteristics. (D) Analysis of the size (maximum diameter), the cell number, and the volume of primary, secondary, and tertiary spheres during a 7-day culture period using the neurosphere assay. After DIV 5 tertiary spheres showed a reduced ability to attach to coated culture dishes, which was required for cell counting. The sphere cell number was therefore only analyzed until DIV 5. Data are the mean±SD from three independent experiments. Scale bar=100 μm.
Spheres develop from proliferating cells and maintain their stem/progenitor cell status after propagation
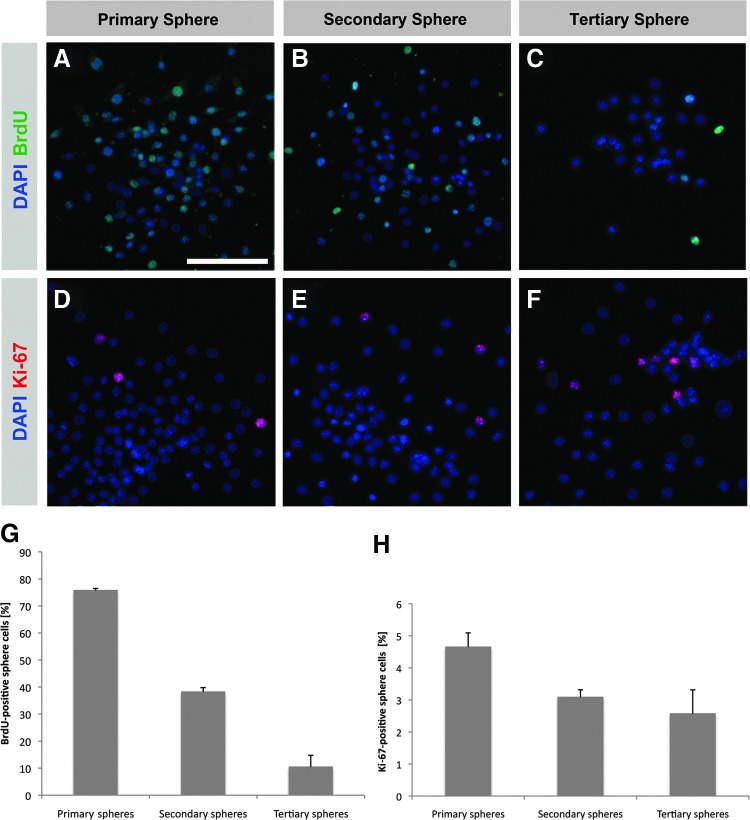
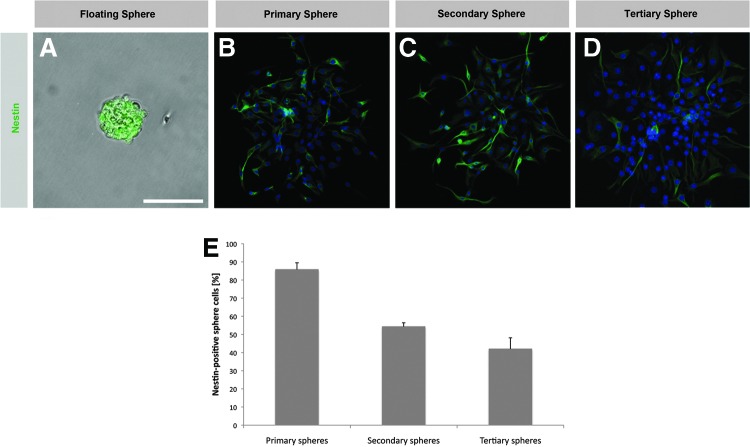
Spheres isolated from the central nervous system or the inner ear arise from proliferating cells and sphere cells express various neural stem/progenitor cell markers.8,9,18,21,22,29 We quantitatively analyzed and compared proliferation activity and marker expression in primary spheres and spheres obtained by repeated propagation (secondary and tertiary spheres). We found robust BrdU incorporation in the nuclei of primary sphere cells (75.7±0.8%) indicating that these cells underwent cell division during sphere formation in the neurosphere assay (Fig. 6A,G). The detection of Ki-67 expression, a marker for cell proliferation, in 4.7±0.4% of the primary sphere cells is a further indication of their proliferative activity (Fig. 6D,H). A significant number of primary sphere cells expressed the neural stem cell marker nestin (85.8±3.7%) (Fig. 7A,B,E). To find out whether spheres maintain their stem/progenitor cell status and proliferative activity after propagation, we analyzed the secondary and tertiary sphere generation. Secondary and tertiary spheres also showed significant proliferative activity as indicated by BrdU incorporation (38.2±1.6% and 10.4±4.3% of the sphere cells) (Fig. 6B,C,G) and Ki-67 expression (3.1±0.2% and 2.6±0.7% of the sphere cells) (Fig. 6E,F,H). Likewise, propagated spheres seem to keep their stem/progenitor cell status since we detected robust expression of nestin in the sphere cells (54.3±2.2% of the secondary sphere cells, 42.0±6.1% of the tertiary sphere cells) (Fig. 7C–E).
FIG. 6.
Spiral ganglion-derived spheres are floating colonies with high proliferative capacity. Spheres that form in culture media containing BrdU show incorporation of the thymidine analog in a significant proportion of the sphere cells which can be observed in primary spheres (A). Following propagation, BrdU is also incorporated during the formation of secondary (B) and tertiary spheres (C). Nuclear expression of Ki-67, a marker associated with the active phases of the cell cycle, indicates the proliferative activity of primary spheres (D) and subsequent sphere generations (E, F). (G, H) Quantification of BrdU and Ki-67 expression in the primary, secondary, and tertiary generation of spiral ganglion-derived spheres. Data are the mean±SD from three independent experiments. Scale bar=100 μm.
FIG. 7.
Spiral ganglion spheres conserve the expression of the neural stem/progenitor cell marker nestin after propagation. (A, B) Nestin expression is abundantly expressed in the cytoplasm of primary sphere cells. Nestin expression can also be detected in secondary (C) and tertiary spheres (D). (E) Quantification of nestin expression in primary spiral ganglion-derived spheres and subsequent sphere generations. Data are the mean±SD from three independent experiments. Nuclei in B–D are visualized with DAPI (blue). Scale bar=60 μm in A, 100 μm in B–D.
Sphere-derived differentiated cells not only express neuronal and glial markers but also acquire ultrastructural properties of neurons and glial cells
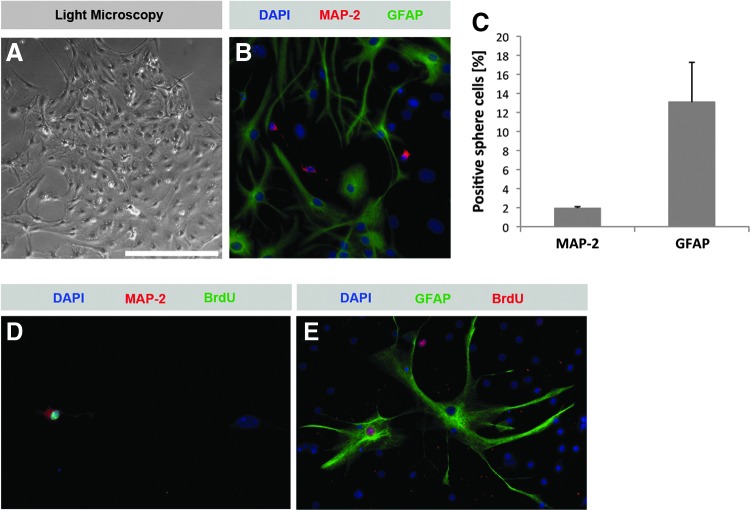
It has been shown previously that spiral ganglion-derived spheres have the potential to differentiate into cells that express the neuronal markers TUJ and NF-M and the glial cell marker GFAP.8–10 These studies suggest that specific culture conditions can promote sphere differentiation and lead to the acquisition of a mature neuronal or glial immunophenotype. Our data corroborate these findings: when we transferred floating spheres to gelatin-coated plates with media containing BDNF and NT-3, spheres attached, flattened, and started to differentiate (Fig. 8A, Supplementary Movie S1). After a 10-day differentiation period we found 1.9±0.2% MAP-2-expressing cells and 13.1±4.2% GFAP-expressing cells in sphere-derived cell colonies (Fig. 8B, C). MAP-2 is a stringent marker of neurons in the central nervous system, but has also been shown to be expressed in the perikarya and neurites of spiral ganglion neurons.30 To find out whether the neurons and glial cells differentiated from spiral ganglion sphere cells that were newly created after culture, we added BrdU for 48 h during sphere generation. When we analyzed differentiated cell populations deriving from these spheres, we observed nuclear BrdU incorporation in MAP-2–expressing neuron-like cells (Fig. 8D) and GFAP-expressing glia-like cells (Fig. 8E). This finding indicates that neurons and glia cells developed from dividing progenitors in vitro.
FIG. 8.
Differentiation of spiral ganglion-derived sphere populations into mature cells. (A) Light microscopic appearance of an adherently growing, differentiating spiral ganglion sphere. (B, C) After a 10-day differentiation period neuron-like cells expressing the neuronal marker MAP-2 can be found among plenty of GFAP-positive glia-like cells. When a BrdU-pulse is added to sphere cultures, nuclear BrdU incorporation can be found in neuron-like (D) and glia-like (E) cells differentiated from these spheres, indicating that spiral ganglion sphere-derived differentiated cells arise from dividing stem/progenitor cells. Scale bar=250 μm in A, 100 μm in B and E, and 120 μm in D.
Our data raised the question whether these differentiated cells also acquired the distinct morphological features of neurons and glia cells. We therefore analyzed differentiated cell populations by scanning electron microscopy (Fig. 9A–C). Our ultrastructural analysis revealed the presence of neuron-like cells with outgrowth of cellular processes that appeared to be neurites. These neuron-like cells were typically surrounded by glia-like cells with a flat morphology (Fig. 9A). In addition, spiral ganglion stem cell–derived neuron-like cells formed various types of cellular contacts indicating a close cell–cell interaction (Fig. 9B,C). Our findings provide evidence that mature cells differentiated from spiral ganglion spheres in vitro not only express mature neural markers but also develop specific morphological features of mature spiral ganglion cells. Interestingly, cell differentiation only occurred when we plated whole spheres on a substrate. Differentiation of dissociated sphere cells, a commonly used method for differentiation of brain-derived neurospheres, was not possible because most cells died after a few days.
FIG. 9.
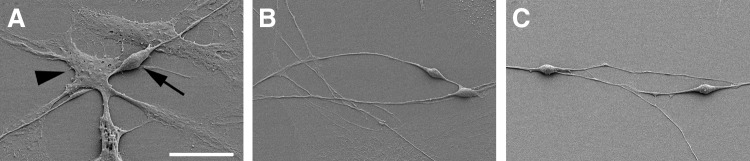
Scanning electron microscopic observation of sphere-derived cell populations reveals the distinct morphology of neuron-like and glia-like cells. Note the bipolar appearance of the neuron-like cell (arrow) with two neurites growing on a monolayer composed of cells with the characteristics of glial cells (arrowhead) shown in (A). Close interaction of spiral ganglion stem cell-derived neuron-like cells is suggested by axosomatic (B) and dendro-dendritic (C) contacts. Scale bar=20 μm in A and 30 μm in B and C.
Discussion
More than 20 years ago, Reynolds and Weiss31 introduced the neurosphere assay, a defined serum-free suspension culture technique, as a tool to isolate neural stem cells from the brain. A large number of studies have since used this method to characterize neural stem cells and to show their distinct ability to proliferate, self-renew, and differentiate into the major cell types of the central nervous system (for review, see Rietze and Reynolds26). Cells with similar features occur in the mammalian inner ear.8,13,21 This is also true for the spiral ganglion which harbors multipotent stem cells that divide, form neurospheres, and differentiate into neurons and glial cells.9–12
In this study, we present an in-depth characterization of stem cells derived from the early postnatal spiral ganglion using immunohistochemical and ultrastructural methods as well as time-lapse live cell imaging. We were especially interested in the features of propagated spheres and those of cells differentiated from spheres as a way to extend and refine previous studies that focused on the characteristics of these stem cells.8–12
Re-evaluation of the modified neurosphere assay that has been used in recent years to isolate inner ear stem cells8–10,18,21,22 revealed that bFGF was the most potent mitogen for isolation of sphere-forming stem-like cells from the spiral ganglion. This finding might also indicate that bFGF-responsive cells account for the major portion of the stem cell pool that resides in the postnatal spiral ganglion. The spheres that arose from dissociated spiral ganglion cells resembled neurospheres from the brain. The process of sphere formation and the ability to attach to a substrate and to flatten and form a monolayer of differentiating cells were found to be strikingly similar to the behavior of neural stem cells of the brain as compared by time-lapse microscopy. Moreover, we found neurosphere typical microspikes26–28 on the surface of spiral ganglion spheres. These actin-rich filopodia serve as cellular antennae and are involved in cell migration and neurite outgrowth.32 The abundant presence of mitochondria in spiral ganglion sphere cells as demonstrated by electron microscopy has previously been described as a typical attribute of proliferative neurosphere cells isolated from the brain.33
The secondary and tertiary spheres (obtained by propagation of spheres) not only maintained considerable proliferative activity but also conserved their stem/progenitor cell phenotype. Expression of nestin, a neural stem cell marker expressed in neurospheres from the brain,29 was maintained in many sphere cells even after repeated propagation/expansion of spheres in vitro. We conclude that spiral ganglion-derived spheres can be maintained and expanded in vitro for use in transplantation studies or for use as a model to screen and evaluate drugs. It was important to test whether spheres maintained their proliferative capacity and stem/progenitor status after propagation because stem/progenitor cells for these studies would be required in sufficient numbers. Our findings corroborate recent data by Lou and colleagues19 who reported the retention of the progenitor cell phenotype in passaged sphere cultures isolated from the postnatal cochlear epithelium.
When floating spheres are transferred to a substrate that allows adherent cell culture, spheres attach and start to differentiate into mature cell types specific for the organ from which spheres were isolated. We detected MAP-2–positive neuron-like cells and GFAP-positive glia-like cells in the differentiated sphere cell populations. Using a BrdU-incorporation assay, we confirmed that neuronal and glial cell populations were derived from proliferating progenitor cells. The potential of spiral ganglion-derived spheres to differentiate into mature cells that express neuronal and glial markers has been well described in recent studies.8–12 We showed here that these cells also acquire characteristic features of cells of the spiral ganglion. The majority of spiral ganglion neurons in the mammalian inner ear have a bipolar morphology with two neurites at the two subtending poles of the neuronal cell body. One neurite extends towards the organ of Corti and the other leads to the brainstem.34 In deaf patients, a healthy population of bipolar spiral ganglion neurons is believed to be crucial for hearing restoration with cochlear implants because these cells transmit the electrical signals from the electrode to the brain. We therefore screened our spiral ganglion stem cell–derived differentiated cultures using electron microscopy and found neuron-like cells with a bipolar appearance. In most cases, these cells appeared to grow in clusters of cells with a glia-like morphology similar to the microarchitecture in the cochlea, where close neuron–glial interactions are observed.35
In summary, our study provides a detailed characterization of sphere-forming spiral ganglion stem cells and cell populations differentiated from these cells. We show that spiral ganglion spheres conserve their major characteristics after propagation and that sphere cells give rise to mature cells with the distinct phenotypes of neurons and glial cells. Our data provide a promising vantage point for future experiments focusing on the electrophysiological features of spiral ganglion stem cell–derived neurons to show that they are functioning. The generation of functional neurons from a self-renewing cellular source would offer a highly attractive perspective for future attempts to regenerate neural elements of the inner ear.
Supplementary Material
Abbreviations Used
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BrdU
bromodeoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco's modified Eagle's medium
- EDTA
ethylenediaminetetraacetic acid
- EGF
epidermal growth factor
- GFAP
glial fibrillary acidic protein
- IGF-1
insulin-like growth factor-1
- MAP-2
microtubule-associated protein 2
- NF-M
neurofilament-M
- NT-3
neurotrophin-3
- PBS
phosphate-buffered saline
- TUJ
neuronal class III β-tubulin
Acknowledgments
We thank Mingjie Tong of the Massachusetts Eye & Ear Infirmary and Stefan Momma of the Edinger Institute (Frankfurt/M.) for expert advice, Hanns Ackermann for help with the statistics, and Manfred Ruppel (Goethe University Frankfurt/M.) for expert assistance with the electron microscopy. This work was supported by a Young Investigator Grant in the Frankfurt Research Promotion Program (FFF) of the Faculty of Medicine of the Goethe University Frankfurt am Main and a research grant from the LOEWE Center for Cell and Gene Therapy Frankfurt (funded by Hessian Ministry of Higher Education, Research and the Arts) (to M.D.). M.D. is a fellow of the Alexander-von-Humboldt Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makary CA, Shin J, Kujawa SG, et al. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165 [DOI] [PubMed] [Google Scholar]
- 4.Shinohara T, Bredberg G, Ulfendahl M, et al. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama J, Yamagata T, Ulfendahl M, et al. Effects of antioxidants on auditory nerve function and survival in deafened guinea pigs. Neurobiol Dis. 2007;25:309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562 [DOI] [PubMed] [Google Scholar]
- 7.Shi F, Edge AS. Prospects for replacement of auditory neurons by stem cells. Hear Res. 2013;297:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima K, Grimm CM, Corrales CE, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oshima K, Teo DT, Senn P, et al. LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol. 2007;7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senn P, Oshima K, Teo D, et al. Robust postmortem survival of murine vestibular and cochlear stem cells. J Assoc Res Otolaryngol. 2007;8:194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rask-Andersen H, Boström M, Gerdin B, et al. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–191 [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20:1723–1731 [DOI] [PubMed] [Google Scholar]
- 13.Malgrange B, Belachew S, Thiry M, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002;112:79–88 [DOI] [PubMed] [Google Scholar]
- 14.Zhai S, Shi L, Wang BE, et al. Isolation and culture of hair cell progenitors from postnatal rat cochleae. J Neurobiol. 2005;65:282–293 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhai SQ, Shou J, et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J Neurosci Methods. 2007;164:271–279 [DOI] [PubMed] [Google Scholar]
- 16.Savary E, Hugnot JP, Chassigneux Y, et al. Distinct population of hair cell progenitors can be isolated from the postnatal mouse cochlea using side population analysis. Stem Cells. 2007;25:332–339 [DOI] [PubMed] [Google Scholar]
- 17.Savary E, Sabourin JC, Santo J, et al. Cochlear stem/progenitor cells from a postnatal cochlea respond to Jagged1 and demonstrate that notch signaling promotes sphere formation and sensory potential. Mech Dev. 2008;125:674–686 [DOI] [PubMed] [Google Scholar]
- 18.Diensthuber M, Oshima K, Heller S. Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J Assoc Res Otolaryngol. 2009;10:173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou XX, Nakagawa T, Ohnishi H, et al. Otospheres derived from neonatal mouse cochleae retain the progenitor cell phenotype after ex vivo expansions. Neurosci Lett. 2013;534:18–23 [DOI] [PubMed] [Google Scholar]
- 20.Waldhaus J, Cimerman J, Gohlke H, et al. Stemness of the organ of Corti relates to the epigenetic status of Sox2 enhancers. PLoS One. 2012;7:e36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299 [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Monedero R, Yi E, Oshima K, et al. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68:669–684 [DOI] [PubMed] [Google Scholar]
- 23.Rak K, Wasielewski NV, Radeloff A, et al. Isolation and characterization of neural stem cells from the neonatal rat cochlear nucleus. Cell Tissue Res. 2011;343:499–508 [DOI] [PubMed] [Google Scholar]
- 24.Volkenstein S, Oshima K, Sinkkonen ST, et al. Transient, afferent input-dependent, postnatal niche for neural progenitor cells in the cochlear nucleus. Proc Natl Acad Sci U S A. 2013;110:14456–14461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23 [DOI] [PubMed] [Google Scholar]
- 27.Azari H, Sharififar S, Rahman M, et al. Establishing embryonic mouse neural stem cell culture using the neurosphere assay. J Vis Exp. 2011;47 pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladiwala U, Basu H, Mathur D. Assembling neurospheres: dynamics of neural progenitor/stem cell aggregation probed using an optical trap. PLoS One. 2012;7:e38613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritti A, Parati EA, Cova L, et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci. 1996;16:1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hafidi A, Fellous A, Ferhat L, et al. Developmental differentiation of MAP2 expression in the central versus the peripheral and efferent projections of the inner ear. J Comp Neurol. 1992;323:423–431 [DOI] [PubMed] [Google Scholar]
- 31.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710 [DOI] [PubMed] [Google Scholar]
- 32.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454 [DOI] [PubMed] [Google Scholar]
- 33.Lobo MV, Alonso FJ, Redondo C, et al. Cellular characterization of epidermal growth factor-expanded free-floating neurospheres. J Histochem Cytochem. 2003;51:89–103 [DOI] [PubMed] [Google Scholar]
- 34.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101 [DOI] [PubMed] [Google Scholar]
- 35.Hansen MR, Vijapurkar U, Koland JG, et al. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.