Abstract
Hematopoietic stem cells (HSCs) are defined by their ability to repopulate the bone marrow of myeloablative conditioned and/or (lethally) irradiated recipients. To study the repopulating potential of human HSCs, murine models have been developed that rely on the use of immunodeficient mice that allow engraftment of human cells. The NSG xenograft model has emerged as the current standard for this purpose allowing for engraftment and study of human T cells. Here, we describe adaptations to the original NSG xenograft model that can be readily implemented. These adaptations encompass use of adult mice instead of newborns and a short ex vivo culture. This protocol results in robust and reproducible high levels of lympho-myeloid engraftment. Immunization of recipient mice with relevant antigen resulted in specific antibody formation, showing that both T cells and B cells were functional. In addition, bone marrow cells from primary recipients exhibited repopulating ability following transplantation into secondary recipients. Similar results were obtained with cryopreserved human bone marrow samples, thus circumventing the need for fresh cells and allowing the use of patient derived bio-bank samples. Our findings have implications for use of this model in fundamental stem cell research, immunological studies in vivo and preclinical evaluations for HSC transplantation, expansion, and genetic modification.
Key words: : human bone marrow, stem cells, xenograft model, thymus
Introduction
Transplantation of hematopoietic stem and progenitor cells (HSPCs) is applied for a variety of diseases, including malignant diseases or congenital immune deficiencies. In spite of major improvements, HSPC transplantation is still associated with high morbidity and mortality.1–3 Therefore, gene therapy has emerged as an alternative for treatment of severe combined immunodeficiency (SCID).4
Repopulation of human HSPCs is difficult to study experimentally in humans; therefore, studies rely primarily on the use of xenotransplantation in mice. The most widely used mouse model for this purpose was the nonobese diabetic (NOD)-scid mouse strain, which can be engrafted with human HSPCs.5 This mouse is deficient in B cells and T cells but develops functional natural killer (NK) cells. However, this model gives low levels of human blood cell chimerism and lacks proper human T-cell development. Another disadvantage is the relatively short life-span of the mice due to development of thymic lymphomas.6 With the development of mouse strains with more severe immune deficiency it has become possible to transplant human HSPCs with higher efficiency. The first such mouse strain that became available was the Rag2−/−γc−/− mouse that is on a mixed background, in which both peripheral blood lymphocytes7 and CD34+ cells isolated from cord blood8 could be engrafted. This was followed by a report in which CD34+ HSPCs were transplanted in newborn BALB/c-Rag2−/−Il2rg−/−.9 In these mice both human myeloid and lymphoid cells developed, and it was demonstrated that the human adaptive immune system was functional.
Further improvement was obtained by crossing the NOD-scid mouse to an Il2rg−/− mouse (NOG mouse, NOD/Shi-scidIl2rg−/−)10 and later on by development of the NSG mice (NOD/LtSz-scidIl2rg−/−).11,12 These mice differ from the NOG mice10 in the substrain of NOD-scid that was used and their mutation in Il2rg. While the NOG mouse has a truncated nonsignaling form of Il2rg, the NSG mouse has a null mutant of Il2rg and therefore is lacking the ability to bind cytokines. Both mouse strains were compared side by side, which revealed higher engraftment of human cells in the bone marrow (BM) of NSG mice, especially at limiting cell doses.13 This can either be caused by the difference in background or the different mutation in Il2rg.
In this study, we show the feasibility of using cryopreserved human BM following a short ex vivo culture in NSG mice. Engrafted cells differentiated into different cell lineages and were also functional. Furthermore, we introduced a short culture that would allow for genetic modification of HSPCs. Thus, we provide an adaptation of the original NSG protocol that can be readily implemented and allows for wider and more robust use of this promising xenograft model.
Material and Methods
Isolation of human CD34+cells
Umbilical cord blood (UCB) was obtained from the Diaconessenhuis Hospital Leiden (Leiden, The Netherlands) after informed consent of the parents. Human BM was obtained from healthy pediatric BM donors at the Leiden University Medical Center (Leiden, The Netherlands). Informed consent was obtained from the parents for use of leftover samples for research purposes. The mononuclear cell fraction was isolated using Ficoll gradient centrifugation, frozen in fetal calf serum (FCS) and 10% dimethyl sulfoxide (Greiner Bio-One B.V., Alphen aan den Rijn, The Netherlands and Sigma-Aldrich, St. Louis, MO, respectively), and stored in liquid nitrogen until use. CD34+ progenitors were isolated using the CD34 Microbead Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Isolated cells were cultured overnight (unless indicated differently) in StemSpan serum-free expansion medium (StemSpan-SFEM, StemCell Technologies Inc., Vancouver, BC, Canada) in the presence of 10 ng/mL stem cell factor (a gift from Amgen, Thousand Oaks, CA), 20 ng/mL recombinant human thrombopoietin (R&D Systems, Abingdon, UK), 20 ng/mL recombinant mouse insulin-like growth factor 2 (R&D Systems) and 10 ng/mL recombinant human fibroblast growth factor-acidic (Peprotech, Rocky Hill, NJ). After overnight culture, cells were washed and resuspended in Iscove's modified Dulbecco's medium (IMDM) without phenol red (Gibco, Life Technologies, Bleiswijk, The Netherlands).
Mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were obtained from Charles River Laboratories (UK) and bred in the animal facility at the Leiden University Medical Center. Experimental procedures were approved by the Ethical Committee on Animal Experiments of the Leiden University Medical Center. Mice aged 5–6 weeks were sublethally irradiated with 1.91 Gy using orthovoltage X-rays. Within 24 h after irradiation, CD34+ cells were transplanted by intravenous injection (200 μL) in the tail vein. The first 4 weeks, mice were maintained on water containing 0.07 mg/mL polymixin B (Bupha, Uitgeest, The Netherlands), 0.0875 mg/mL ciprofloxacin (Bayer, Mijdrecht, The Netherlands), and 0.1 mg/mL amphotericin B (Bristol-Myers Squibb, Woerden, The Netherlands) with ad libitum food pellets and DietGel Recovery (Clear H2O, Portland, ME). After 4 weeks, mice were maintained on ad libitum water and regular chow. Peripheral blood was drawn from the tail vein every 4 weeks. At the end of experiments, mice were sacrificed by CO2 inhalation and thymus, spleen, peripheral blood, femurs, and tibiae were obtained. Single cell suspensions were made from thymus and spleen using a 70-μm nylon cell strainer (BD Falcon, Franklin Lakes, NJ). Bone marrow was obtained by flushing femurs and tibiae with IMDM (Gibco, Life Technologies) 2.5% FCS (Greiner Bio-One B.V., Alphen aan den Rijn, The Netherlands) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Life Technologies).
For secondary transplantations, half of the BM from a donor was thawed and transplanted via intravenous injection in the lateral tail vein of irradiated NSG recipients.
Flow cytometry
Erythrocytes from spleen and peripheral blood samples were lysed using NH4Cl (8.4 g/L)/KHCO3 (1 g/L) solution. Mononuclear cells were stained in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]/0.2% bovine serum albumin/0.1% sodium azide). Staining was performed for 30 min at 4°C. The following anti-human antibodies were used: CD3-PECy5 (UCHT1), CD4-APCCy7 (RPA-T4), CD8-PECy7 (SK1), CD13-APC (WM15), CD14-PE (M5E2), CD16-PE (B73.1), CD19-APCCy7 (SJ25C1), CD33-APC (WM53), CD34-PE (8G12), CD45-V450, CD45RA-FITC (L48), and CD56-PE (MY31) (all from BD Biosciences, San Jose, CA), and CD38-PECy7 (HIT2), CD49f PerCP-efluor710 (eBioGoH3), and CD90-APC (eBio5e10) (all from eBioscience, San Diego, CA). Data were acquired on a Canto II (BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, OR).
Immunization
Mice were immunized by intraperitoneal injection of 100 μg of trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH; Biosearch Technologies Inc., Novato, CA) in 50% Imject Alum (Thermo Scientific, Rockford, IL). The animals were boosted with 100 μg of TNP-KLH in PBS by intraperitoneal injection 3 weeks after the first injection. Serum was collected 1 week after the last injection. TNP-specific IgG antibodies were determined by sandwich enzyme-linked immunosorbent assay (ELISA). Plates coated with TNP-KLH were incubated for 3 h at room temperature with serial dilutions of the obtained sera. After washing with PBS/0.05% Tween 20 (Sigma Aldrich, St. Louis, MO), plates were incubated with goat anti-human IgG conjugated to biotin (Biosource, Life Technologies, Bleiswijk, The Netherlands, kindly provided by Dr. A. Mulder, Leiden University Medical Center, Leiden, The Netherlands) for 30 min at room temperature. After being washed, plates were incubated with streptavidin conjugated to horseradish peroxidase (Jackson ImmunoResearch, Newmarket, Suffolk, UK) for 30 min at room temperature. Azino-bis-ethylbenzthiazoline sulfonic acid (ABTS, Sigma Aldrich) was used as a substrate for detection using a Bio-Rad Model 680 microplate reader (Bio-Rad Laboratories B.V., Veenendaal, The Netherlands) at a wavelength of 415 nm.
Immunohistochemistry
Paraffin sections of spleens obtained from NSG mice transplanted with human CD34+ cells were stained with hematoxylin and eosin. Final magnifications of images were 100×.
Results
Similar engraftment levels of cultured HSPCs and fresh HSPCs
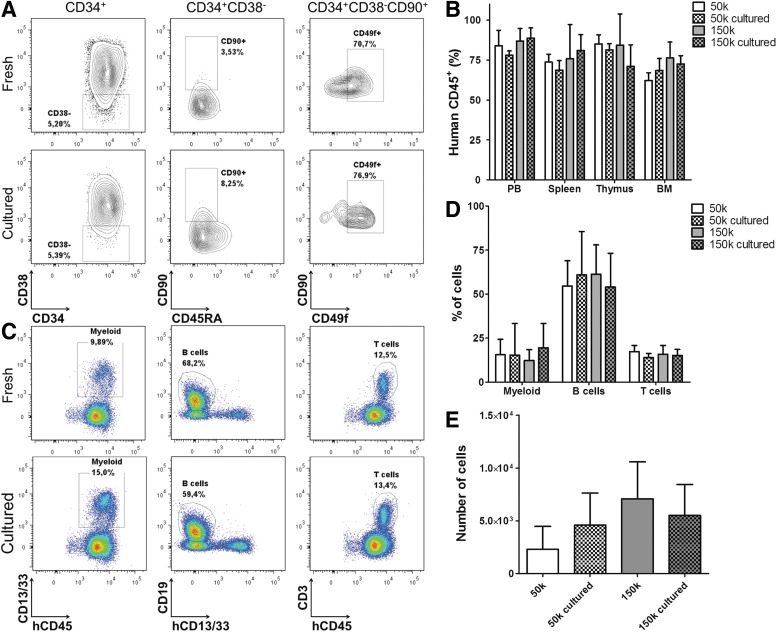
For genetic modification (e.g., gene therapy) HSPCs need to be cultured for a short period. To test whether this is feasible for the NSG model, we cultured HSPCs isolated from UCB overnight to determine the effect on engraftment potential. NSG mice were transplanted with 0.5×105 or 1.5×105 HSPCs directly after isolation or after overnight culture in StemSpan supplemented with cytokines. The transplanted cells were analyzed for expression of surface markers that define human HSC subsets.14 An increase in the percentage of CD90+CD45RA− cells and, within this population, CD49f+ cells was observed after overnight culture (Fig. 1A). This increase of more primitive HSC subsets was associated with similar engraftment of human cells in lymphoid organs (Fig. 1B) and similar contribution of cell lineages to engraftment in peripheral blood between recipients of fresh or cultured HSPCs (Fig. 1C and 1D). Engraftment of human cells and lineage contribution were both unaffected by the number of transplanted cells. No differences in numbers of cells belonging to the most primitive human HSC subset were found in the BM of recipients of fresh and cultured HSPCs, although there was a trend at lower transplanted cell numbers (0.5×105, 50,000) for more primitive stem cells to be detected in the BM of recipients of cultured HSPCs (Fig. 1E). This led us to conclude that the short overnight culture, as needed for genetic modification, does not compromise engraftment potential and lineage contribution of human HSPCs in primary recipients. Furthermore, the described protocol allows for a high level of engraftment in different organs (Fig. 1B).
FIG. 1.
Engraftment capacity of hematopoietic stem and progenitor cells (HSPCs) is not affected by a short in vitro culture. (A) Phenotype of transplanted cells. Plots are gated on the population indicated on top. (B) Engraftment as measured by percentage of human CD45 expressing cells in peripheral blood of humanized NSG mice transplanted with different cell doses of either fresh or cultured HSPCs isolated from umbilical cord blood (UCB). (C) Different cell lineages in peripheral blood of mice transplanted with 1.5×105 noncultured HSPCs isolated from UCB (top) or 1.5×105 cultured HSPCs (bottom). Myeloid cells (left), B cells (middle), and T cells (right) were gated within human CD45+ cells. (D) Contribution of different cell lineages in peripheral blood at 18 weeks after transplantation. Percentages are gated within human CD45+ cells. (E) Numbers of long-term-HSC (hCD45+CD34+CD38−CD90+CD45RA−CD49f+) present in bone marrow (BM) of NSG recipients transplanted with different cell doses of either fresh or cultured HSPCs isolated from UCB. Data are represented as mean±standard deviation (SD) in (B, D, E). 50k, 0.5×105 cells; 150k, 1.5×105cells. HSPCs were isolated from a pool of seven UCB donors, three mice per group.
Specific antibodies are produced after immunization
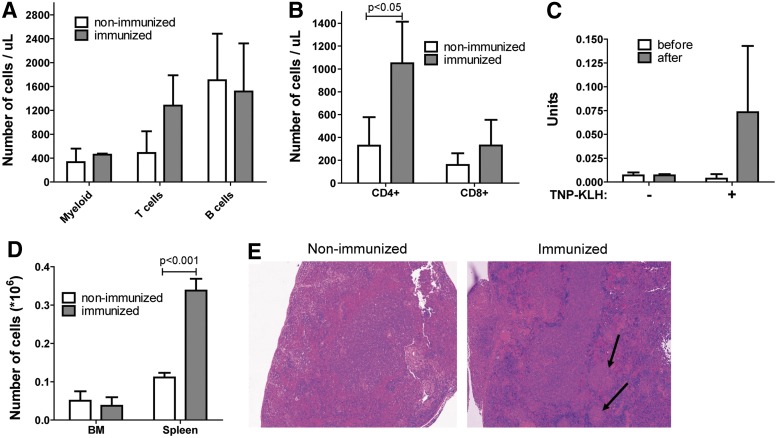
To test the functionality of both T and B cells, we immunized the mice that were transplanted with cultured human HSPCs with TNP-KLH, a T cell–dependent B-cell antigen. One week after the last challenge an increase in the number of T cells in the peripheral blood of immunized mice was observed as compared to nonimmunized mice (Fig. 2A). This increase in number of T cells was caused by an expansion of CD4+ T helper cells, which are the cells to provide crosstalk to B cells (p<0.05, Fig. 2B). To determine the functionality of the B cells, an ELISA for TNP-specific antibodies was performed. An increase of TNP-specific IgG was only measured in the serum of immunized animals (Fig. 2C). In addition, more plasma cells were observed in the spleens, but not in the BM, of immunized animals as compared to nonimmunized mice (Fig. 2D). Germinal centers could also be detected by hematoxylin and eosin staining of sections of the spleen of immunized mice (Fig. 2E). In conclusion, immunization of young NSG mice transplanted with overnight cultured human HSCs showed that both T cells and B cells are functional.
FIG. 2.
Immunization of humanized NSG mice shows the functionality of both T cells and B cells. (A) Expansion of T cells in peripheral blood after immunization. (B) Number of CD4+ and CD8+ T cells in peripheral blood. (C) Quantification of trinitrophenyl (TNP)-specific IgG in serum of immunized mice. (D) Number of human plasma cells that were present in spleens after immunization. (E) Detection of germinal centers in spleens of immunized mice by hematoxylin and eosin staining (100× final magnification). Data are represented as mean±SD in (A–D). HSPCs were isolated from a pool of five UCB donors, three mice per group.
Secondary transplantation results in high multilineage engraftment
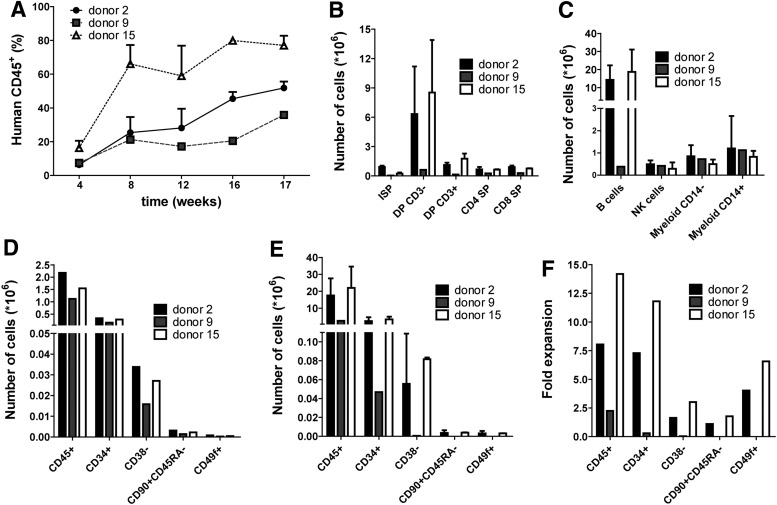
To determine the long-term repopulating ability of stem cells residing in the BM of humanized mice, we performed a secondary transplantation in a total of five mice. Half of the BM of primary recipients that had received cultured HSPCs, was thawed and transplanted via intravenous injection in one (donor 9) or two recipients (donors 2 and 15) depending on the number of cells that was recovered. Yet the recipient of donor 9 received half of the number of HSCs as compared to recipients from cells of the other two donors (Fig. 3D). Good engraftment was observed in the peripheral blood of all secondary recipients (Fig. 3A). The effect of cell number was reflected in the level of engraftment as measured in peripheral blood. Also in all secondary recipients normal development of T cells was present (Fig. 3B, for gating strategy see Supplementary Fig. S1) and all major cell lineages were present in the BM (Fig. 3C). These data show that with this protocol engraftment can be observed in secondary recipients, indicating that there was no stem cell exhaustion in the primary recipients that were transplanted with cultured HSPCs.
FIG. 3.
Secondary transplantation results in high engraftment and development of all lineages. (A) Engraftment over time as measured by human CD45-expressing cells in peripheral blood of secondary recipients. (B) Development of human T cells in the thymus of secondary recipients. Gated on human CD45+ cells; ISP, immature single positive; DP, double positive; SP, single positive. (C) Development of lineages in the BM of secondary recipients. Gated on human CD45+ cells. (D) The number of cells that were transplanted into secondary recipients and (E) the number of cells that were obtained from both hind legs of secondary recipients. Gated on human CD45+ cells. Data are represented as mean±standard deviation (SD). (F) When the numbers in (D and E) are divided, this results in the fold expansion of HSCs in both hind legs of secondary recipients. Data are represented as mean±SD in (A–E). Half of total BM of three primary recipients was transplanted in two secondary recipients (donors 2 and 15) or one recipient (donor 9).
We compared the number of HSCs that were transplanted with the number of HSCs that were obtained 17 weeks after secondary transplantation. Cells were analyzed for different phenotypes that have been described to stage human hematopoietic stem and progenitor cells.14 Using this method, the CD34+CD38−CD90+CD45RA−CD49f+ population contains the highest frequency of long-term (LT) reconstituting HSCs. For all different phenotypes we observed an expansion in the BM obtained from both hind legs of the secondary recipient as compared to the number of cells that was transplanted (Fig. 3D–3F, for gating strategy see Supplementary Fig. S2). This was observed for two out of three donors.
Transplantation of HSCs from both UCB and BM results in high engraftment and development of all lineages
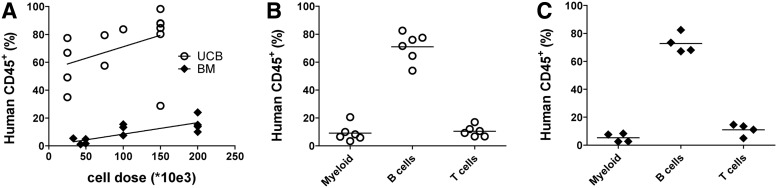
We tested whether our optimized protocol gave good engraftment of HSPCs from cryopreserved BM and development of different lymphoid lineages. Different cell doses of overnight cultured HSPCs from UCB and BM were transplanted in NSG mice. For both HSPC sources it was observed that there was a higher engraftment when more cells were transplanted (Fig. 4A). Although engraftment was consistently higher in mice transplanted with cryopreserved UCB, we observed good repopulation in mice transplanted with cells obtained from cryopreserved BM but higher cell doses were needed for robust engraftment compared to UCB. There was no difference observed in lineage contribution in peripheral blood between the two cell sources (Fig. 4B and 4C). These data show that with our optimized protocol it is possible to get good engraftment and development of all lineages in NSG mice transplanted with HSPCs isolated from cryopreserved human BM samples.
FIG. 4.
Transplantation of overnight cultured HSCs from both cryopreserved UCB and cryopreserved BM resulted in the development of all lymphoid lineages and high human chimerism. (A) Engraftment in peripheral blood of human CD45+ (hCD45+) cells in mice transplanted with different cell doses of HSCs isolated from UCB or human BM. Contribution of different lineages in peripheral blood of NSG mice transplanted with 150,000 HSCs obtained from UCB (B) or 200,000 HSCs obtained from BM (C). Gated on human CD45+ cells. UCB: three different pools of at least two donors in a total of 13 recipients; BM: three different donors in a total of 13 recipients.
Discussion
Here we report on adaptations to the NSG mouse model to achieve high and robust engraftment even with HSPCs isolated from cryopreserved human BM samples. An overnight culture of HSPCs was introduced because this was needed to allow for genetic modification of HSPCs. Using the described protocol, cryopreserved patient BM material could be used to test the efficacy of gene therapy in an in vivo setting. With culturing of HSPCs for longer periods, the repopulation capacity can be lost.15 However, reports have shown that culturing in defined serum-free medium supplemented with different cytokines can enhance engraftment,16 even of HSPCs in an allogeneic setting.17
We demonstrated reproducibly high engraftment in the NSG mouse model using a simple protocol of intravenous transplantation in the tail vein of young NSG mice. Here we obtained high engraftment of cultured HSPCs transplanted in young adult NSG mice even when low cell numbers (25,000 cells) were transplanted. Using this protocol we observed T-cell development similar to fresh human thymi,18 successful immunization leading to human antibody production, and successful engraftment into secondary recipients.
The humanized mouse model is frequently used when the immune system, HIV biology, or stem cell expansion protocols are studied. Although myeloid and lymphoid cell lineages do develop and specific immune responses can be measured, it remains unclear how T-cell progenitors are selected in the thymus of humanized mice due to MHC differences between species. However, it has been reported that a diverse repertoire is generated in NSG mice, and HLA-specific immune responses can be detected, although weak.19 Furthermore, it has been demonstrated that cryopreservation of UCB does not compromise the production of immunoglobulins in the NSG mouse model.20 Here, we have demonstrated that both T cells and B cells were functional by immunization with a T cell–dependent B-cell antigen. Plasma cells and germinal centers were found in the spleen of immunized mice, as also shown by others.21,22 Therefore, we think the NSG mouse is a good model to study human lymphoid cell development in an in vivo setting. Furthermore, LT-HSCs were found in the BM of primary recipients, and these were able to repopulate secondary recipients with development of myeloid and lymphoid lineages. This shows that the transplanted stem cells were not exhausted in the primary recipient and even expansion of HSCs was observed. The number of primitive HSCs tended to be increased in BM of primary recipients that were transplanted with 0.5×105 CD34+ cells. This is in line with work by Zheng et al.,17 who used a similar cytokine cocktail and showed that murine HSCs can be transplanted over major MHC barriers because of up-regulation of the CD47 molecule that gives an anti-endocytosis signal to macrophages. Extrapolating these findings to the current xenotransplantation data, we also found CD47 up-regulation on human CD34+ cells after culturing (Supplementary Fig. S3). No increase in number of primitive HSCs was observed when a higher dose of 1.5×105 CD34+ cells were transplanted, probably due to the already high engraftment observed with 0.5×105 CD34+ cells.
CD34+ cells can be readily isolated from mobilized peripheral blood, UCB, or BM. It has been demonstrated using the NOD-scid model that there are differences in engraftment efficiency between the different sources, with HSPCs from UCB being superior to HSPCs obtained from mobilized peripheral blood and BM.23,24 Furthermore, HSPCs isolated from UCB have a higher cloning efficiency25 and UCB contains more primitive HSCs.26 As NOD-scid mice do not allow proper T-cell development and still have residual NK cell activity, causing much lower engraftment, it is not easy to compare this strain to the NSG mouse side by side. However, anecdotal information from various researchers also indicated that engraftment and T-cell development from cryopreserved BM material was highly problematic in NSG mice. Here, we show that with the described protocol, good engraftment was achieved when HSPCs from cryopreserved human BM samples were transplanted. As we did not transplant uncultured BM-derived HSPCs, it is hard to determine the effect of culturing on their engraftment potential. On the other hand, for HSPCs isolated from UCB we did not observe a difference in repopulation between fresh and cultured HSPC. Furthermore, we do not know what the effect of cryopreservation is on engraftment potential of BM-derived HSPC. HSPCs isolated from cryopreserved and fresh UCB have been reported to perform equally well in engrafting NSG mice.20 Therefore, it seems that HSPC quality is not affected by cryopreservation.
In conclusion, the optimization of the NSG humanized mouse model as described here facilitates the use of human HSCs obtained from different sources with robust engraftment. The described protocol does allow transplantation of HSPCs derived from cryopreserved BM with good engraftment and robust T-cell development. This might be very relevant for material present in bio-banks that could now be used for fundamental studies or preclinical evaluations of transplantation protocols regarding stem cell expansion and gene therapy approaches.
Supplementary Material
Abbreviations Used
- BM
bone marrow
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorter
- FCS
fetal calf serum
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- IMDM
Iscove's modified Dulbecco's medium
- NK
natural killer
- NOD
nonobese diabetic
- PBS
phosphate-buffered saline
- SCID
severe combined immunodeficiency
- TNP-KLH
trinitrophenyl-keyhole limpet hemocyanin
Acknowledgments
The authors gratefully acknowledge Dr. Arend Mulder (Leiden University Medical Center, Leiden, The Netherlands) for providing goat anti-human IgG biotin. This work was supported by funding obtained from KIKA (Children Cancer Free, grant no. 36) and The Netherlands Institute for Regenerative Medicine (NIRM).
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Eapen M, Rocha V, Sanz G, et al. . Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gennery AR, Slatter MA, Grandin L, et al. . Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126:602–610.e601–611. [DOI] [PubMed] [Google Scholar]
- 3.Servais S, Porcher R, Xhaard A, et al. . Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica. 2014;99:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaspar HB, Qasim W, Davies EG, et al. . How I treat severe combined immunodeficiency. Blood. 2013;122:3749–3758 [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Pflumio F, Doedens M, et al. . Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in scid mice. Science. 1992;255:1137–1141 [DOI] [PubMed] [Google Scholar]
- 6.Prochazka M, Gaskins HR, Shultz LD, et al. . The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U S A. 1992;89:3290–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman JP, Blundell MP, Lopes L, et al. . Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–342 [DOI] [PubMed] [Google Scholar]
- 8.Mazurier F, Fontanellas A, Salesse S, et al. . A novel immunodeficient mouse model—RAG2×common cytokine receptor gamma chain double mutants—requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res. 1999;19:533–541 [DOI] [PubMed] [Google Scholar]
- 9.Traggiai E, Chicha L, Mazzucchelli L, et al. . Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107 [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Hiramatsu H, Kobayashi K, et al. . NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182 [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa F, Yasukawa M, Lyons B, et al. . Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shultz LD, Lyons BL, Burzenski LM, et al. . Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489 [DOI] [PubMed] [Google Scholar]
- 13.McDermott SP, Eppert K, Lechman ER, et al. . Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200 [DOI] [PubMed] [Google Scholar]
- 14.Notta F, Doulatov S, Laurenti E, et al. . Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221 [DOI] [PubMed] [Google Scholar]
- 15.Bhatia M, Bonnet D, Kapp U, et al. . Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105:4314–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J, Umikawa M, Zhang S, et al. . Ex vivo expanded hematopoietic stem cells overcome the MHC barrier in allogeneic transplantation. Cell Stem Cell. 2011;9:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerkamp F, de Haas EF, Naber BA, et al. . Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol. 2005;115:834–840 [DOI] [PubMed] [Google Scholar]
- 19.Marodon G, Desjardins D, Mercey L, et al. . High diversity of the immune repertoire in humanized NOD.SCID.gamma c-/- mice. Eur J Immunol. 2009;39:2136–2145 [DOI] [PubMed] [Google Scholar]
- 20.Scholbach J, Schulz A, Westphal F, et al. . Comparison of hematopoietic stem cells derived from fresh and cryopreserved whole cord blood in the generation of humanized mice. PLoS One 2012; 7:e46772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker PD, Legrand N, van Geelen CM, et al. . Generation of human antigen-specific monoclonal igm antibodies using vaccinated “human immune system” mice. PLoS One 2010;5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi B, Chun E, Kim M, et al. . Human B cell development and antibody production in humanized NOD/SCID/IL-2Rγ(null) (NSG) mice conditioned by busulfan. J Clin Immunol. 2011;31:253–264 [DOI] [PubMed] [Google Scholar]
- 23.Ng YY, van Kessel B, Lokhorst HM, et al. . Gene-expression profiling of CD34+cells from various hematopoietic stem-cell sources reveals functional differences in stem-cell activity. J Leukoc Biol. 2004;75:314–323 [DOI] [PubMed] [Google Scholar]
- 24.Noort WA, Wilpshaar J, Hertogh CD, et al. . Similar myeloid recovery despite superior overall engraftment in nod/scid mice after transplantation of human CD34(+) cells from umbilical cord blood as compared to adult sources. Bone Marrow Transplant. 2001;28:163–171 [DOI] [PubMed] [Google Scholar]
- 25.Hao QL, Shah AJ, Thiemann FT, et al. . A functional comparison of CD34+CD38- cells in cord blood and bone marrow. Blood. 1995;86:3745–3753 [PubMed] [Google Scholar]
- 26.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.