Abstract
Stroke often results in both motor and sensory deficits, which may interact in the manifested functional impairment. Proprioception is known to play important roles in the planning and control of limb posture and movement; however, the impact of proprioceptive deficits on motor function has been difficult to elucidate due in part to the qualitative nature of available clinical tests. We present a quantitative and standardized method for evaluating proprioception in tasks directly relevant to those used to assess motor function. Using a robotic manipulandum that exerted controlled displacements of the hand, stroke participants were evaluated, and compared with a control group, in their ability to detect such displacements in a 2-alternative, forced-choice paradigm. A psychometric function parameterized the decision process underlying the detection of the hand displacements. The shape of this function was determined by a signal detection threshold and by the variability of the response about this threshold. Our automatic procedure differentiates between participants with and without proprioceptive deficits and quantifies functional proprioceptive sensation on a magnitude scale that is meaningful for ongoing studies of degraded motor function in comparable horizontal movements.
I. Introduction
Over 50 % of stroke patients present somatosensory impairments that are considered to have an important impact in their quality of the life and rehabilitation outcome [1], [2]. However, clinical testing procedures to evaluate somatosensory impairments have not received much attention and as a result, these tests lack standardized measures and suffer from poor reliability [3], [4]. Thus, in recent years, an effort is being made to design standardized tests [5], [6] as well as automated procedures [7], [8] to measure somatosensory deficits. Proprioception is known to play important roles in the planning and control of limb posture and movement. It has been proposed that while visual information is used primarily to plan the direction of movement relative to the initial position of the limb [9], proprioception is important for forming feedforward motor commands to control the complex inertial limb dynamics of the multiarticular limb [10], [12]. Recently, we have shown that stroke participants with proprioceptive impairment manifested deficits in trial by trial updating of motor commands for movement direction and final positions of their affected arm suggesting that proprioceptive deficits differentially affect the control of movement and stabilized limb postures [13]. This is interesting because it has been hypothesized that limb movement and position may be controlled by separate neural systems [14], [15].
As part of our studies on the control of arm posture and movement post-stroke, we developed an automated quantitative and standardized method of evaluating proprioception in tasks directly relevant to those used to assess motor function. As musculoskeletal motion stimulates muscle and joint receptors, we produced arm displacements (of differing magnitudes) by means of a robotic manipulandum to stimulate proprioception in stroke survivors with deficits in upper extremity function and in neurologically intact individuals.
II. METHODS
A. Subjects
Twelve unilateral, hemiparetic stroke survivors (SS; aged 36–69 years; Table 1A) and eleven age-range-matched neurologically intact control subjects (NI; 32–66 years; Table 1) gave written informed consent to participate in this study in compliance with policies established by Northwestern and Marquette University Institutional Review Boards. All SS were in the chronic stage of recovery (> 6 mo. post-stroke); they were recruited from a database of hemiparetic stroke outpatients maintained by the Rehabilitation Institute of Chicago. All SS also provided written consent allowing medical record review. Exclusion criteria for SS included: inability to give informed consent, inability to follow 2-step directions, history of tendon transfer in the involved limb, neurological or muscular disorder that might interfere with neuromuscular function, recent use (within the previous 8 months) of curare-like agents or other agents that may interfere with neuromuscular function, and/or shoulder pain in the test position of 75° to 90° abduction. The presence of contracture or shoulder subluxation did not exclude subjects from participating, unless it limited their ability to perform the task comfortably. NI control subjects had no history of neurological disorder and were able to achieve the test position without discomfort. All NI subjects were right handed. All subjects participated in two experimental sessions, each lasting ~2.0 h (including setup time).
TABLE 1.
Clinical assessments for stroke survivors
| Age/Sex | FM | MAS | Grip* | Touch | Proprioception |
|---|---|---|---|---|---|
| 59/M | 45 | 0.16 | 9 | N | N |
| 36/M | 43 | 0.66 | 24.7 | N | N |
| 56/M | 28 | 1.33 | 8 | N | N |
| 58/M | 48 | 0 | 18.3 | I:F | I:MCP |
| 50/M | 17 | 0.33 | 6.7 | I:F | I:MCP,W |
| 51/F | 21 | 1.33 | 1.7 | A:F,H,FA I:U |
A:MCP I:W,E,S |
| 54/M | 48 | 0.33 | 31 | N | N |
| 58/M | 30 | 0.5 | 12 | I:F,H | I:MCP |
| 54/F | 21 | 0.33 | 3.3 | N | N |
| 65/F | 41 | 0 | 3.7 | I:F,H | N |
| 53/F | 25 | 1.5 | 7 | A:F,H I:FA |
I:MCP |
| 69/F | 23 | 1.66 | 3.7 | N | N |
Grip force units are in Kilograms
Abbreviations: FM: Fugl-Meyer; MAS: Modified Ashworth Score; N: not impaired; I: impaired; A: absent; F: finger; H: hand; FA: forearm; U: upper arm; MCP: metacarpophalangeal; W: wrist; E: elbow; S: shoulder.
B. Clinical Assessments
All SS participated in a third consenting/evaluation session prior to experimentation. During this session, motor function and impairment level were assessed by the same clinician while the subject was seated in an armless chair. Clinical assessments included: 1) visual field evaluation and visual search task; 2) the upper extremity portion of the Fugl-Meyer (FM) Assessment of physical Performance to assess motor control [16]; 2) the Modified Ashworth Scale (MAS) to assess spasticity at the shoulder, elbow, and wrist; 3) grip strength; and 4) clinical evaluation of tactile and proprioceptive discrimination deficits. Touch was evaluated using a two-point discrimination test [17] in which the subject was to indicate whether he/she felt one or two points of contact as the clinician applied an aesthesiometer to the finger tips, hand, forearm and upper arm (10 mm, 20 mm, 100 mm and 100 mm separations, respectively). Six repetitions were performed at each location; if the response was brisk and accurate for every trial tactile discrimination was rated as “intact” (not impaired); if the subject was unable to respond with any confidence, or if he/she made errors, it was rated as “impaired”; and if the subject was unable to discriminate between one and two points, tactile discrimination was rated as “absent”. Proprioception was assessed similarly: the subject was instructed to keep his/her eyes closed while the clinician randomly moved the tested joint “up” or “down.” When the joint stopped moving, the subject was to indicate joint position. Six repetitions were performed at each joint. If the response was brisk and accurate for every trial, proprioception was rated as “intact.” If the subject was unable to respond with confidence, or if he/she made errors, proprioception was rated as “impaired.” If the subject was unable to determine position at all, proprioception was rated as “absent”. Grip strength measurement was obtained with a hydraulic hand dynamometer; the average of 3 consecutive measurements for the impaired hand is shown in Table 1. To obtain an overall estimate of spasticity of the upper extremity, the MAS scores were averaged across the joints tested [18].
C. Experimental Setup and Procedures
Subjects were seated in a high-backed chair fixed in front of a horizontal planar robot (Fig 1A) [19]. The robot monitored instantaneous hand position, reaction forces and torques at the handle. The robot generated stiff PID control of hand position at a rate of 1000 samples/s. A chest harness was strapped across the subject’s shoulders to minimize trunk motion. The upper arm was supported against gravity (between 75° and 90° abduction; ~45° horizontal flexion) using a sling suspended from the ceiling. The wrist (SS: paretic side; NI: right side) was splinted at 0° flexion and fixed to the robot’s hemi-spherical handle with Velcro® straps. The robot maintained its handle at a nominal position such that the elbow was maintained at a comfortable angle of ~90°. Direct view of the arm, hand, and robot was occluded by an opaque horizontal screen mounted 1 cm above the plane of hand motion. An adjustable vertical shield blocked the view of the shoulder and sling. During the experiments, textual instructions were displayed on the horizontal screen to reinforce verbal instructions.
Fig. 1.
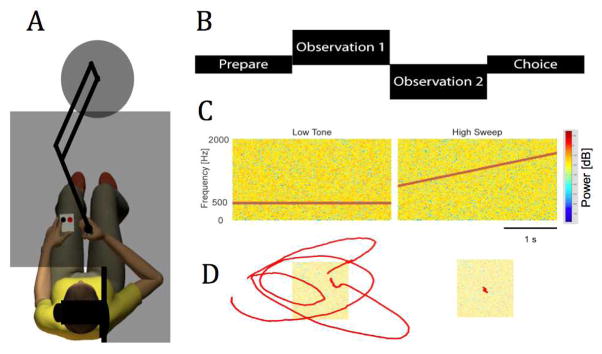
A: Experimental setup. B: Sequence of events in both the auditory discrimination task and the motion detection task. C: Auditory stimuli. D: Displacement stimuli (left: 1 cm motion; right: no motion).
D. Proprioceptive Sensitivity to Limb Displacement
In a series of 120 trials we tested the ability to detect displacements of the hand of different magnitudes at a single, comfortable, spatial location. Prior to each trial, the robot brought the handle to the origin and maintained it in place for 1.0 s using stiff positional control. Each trial consisted of two observation intervals delimited by white noise and a silence between the intervals (Fig 1B). One interval included a perturbation of magnitude wi and the other did not (the stationary condition). The subject’s task was to indicate which observation interval included the perturbation via a 2-button response box. A fixed set of 9 w’s spanned the range of perturbation magnitudes including 0.0 cm (necessary to determine if response bias is present) and wMAX (Fig 2A). Each perturbation was compared to the stationary condition 10 to 20 times in pseudo-random order (eg. Fig 1D). Instructions to the subject were “press the left button if the hand moved during the first interval or press the right button if the hand moved during the second interval”.
Fig. 2.
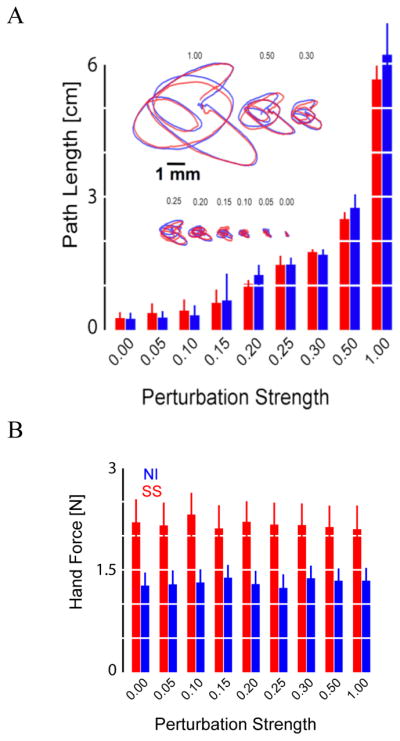
A: Hand path vs. perturbation revealed no variation across groups (error bars: 95% CI). Insert shows single-trial displacements for representative control (blue) and stroke (red) subjects; scale bar: 1 mm. B: Hand force was systematically higher after stroke (error bars: 95% CI).
E. Control Task
A tone discrimination task was performed before the displacement detection task. This task tested the subject’s ability to concentrate and understand instructions. At the same time, it familiarized subjects with the overall structure of the displacement detection task that followed. The tone discrimination task consisted of a series of 24 trials, each trial consisting of two observation intervals, one with a low tone embedded in auditory white noise and the other with a rising pitch embedded in the noise (Fig 1B, C). The subject’s task was to identify the interval with the rising pitch.
F. Data Analysis
Responses were fit using standard logistic regression techniques: Pr(w) = 0.5 + exp(a+b*w)/(1+exp(a+b*w) Detection threshold (DT) was defined as the perturbation magnitude at which the fitted curve passes through the 75% probability of a correct response (Fig 3A). Choice uncertainty (CU) was the perturbation range over which the subject demonstrated variable responses (i.e. the difference in perturbations yielding likelihoods of 63.5% and 85.5%; Fig 3A, shaded regions). CU values are low when the slope of the psychometric function is steep whereas CU is high when the slope is shallow. One-way analysis of variance (ANOVA) for independent samples was used to compare these performance measures between subject groups. Linear regression analysis was used to evaluate correlation between performance indices (threshold and choice uncertainty) and impairment (FM score), spasticity (MAS) and grip strength.
Fig. 3.
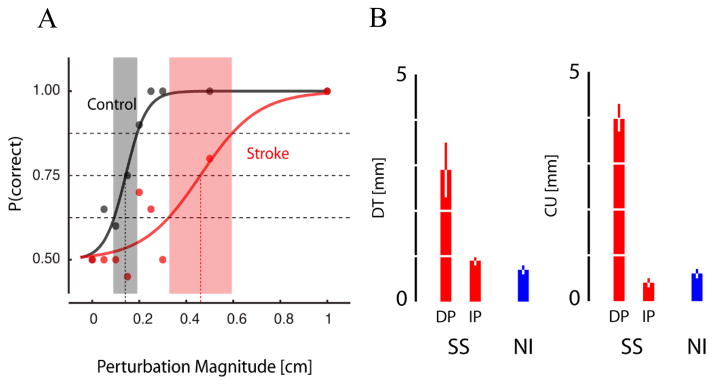
A: Detection curves for a representative control and stroke subject. B: Population statistics for DT (left) and CU (right).
III. RESULTS
The clinical tests showed that of the twelve stroke participants, five had impaired proprioception. Therefore SSs were further subdivided into one group of five participants who exhibited proprioceptive deficits (DP-SS) and another group of seven participants with intact proprioception (IP-SS). All subjects in the DP-SS group had tactile deficits whereas only one subject in the IP-SSs had tactile deficits. Thus none of the stroke survivors that we tested had impaired proprioception without tactile deficits.
Stroke survivors and control subjects performed very well and similarly in the tone discrimination task (t (11) = 1.24, P<0.24), indicating that both groups were able to maintain attention adequately for the proprioceptive tests.
Hand path length varied with perturbation magnitude to the same degree across NI and SS groups (Fig 2A), indicating that the position servo overcame any differences in muscle tone due to spasticity. However, the presence of spasticity in the SS group led to systematically higher hand forces recorded after stroke at all perturbation amplitudes (Fig 2B).
ANOVA disclosed a significant effect of group {NI, IP-SS, DP-SS} on both detection threshold [F(2,20) = 19.25, p <.0001] and choice uncertainty [F(2,20) = 42.97, p <.0001]. Detection threshold (2.9±0.6 cm) and choice uncertainty (4.0±0.4 cm) of DP-SS significantly exceeded those in both IP-SS (DT: 0.9±0.1 cm; CU: 0.4±0.1 cm) and control subjects (DT: 0.7±0.1 cm; CU: 0.6±0.1 cm) (p <.01 in all cases; Tukey’s HSD test) (Fig 3B). Detection threshold and choice uncertainty did not differ between IP-SS and controls.
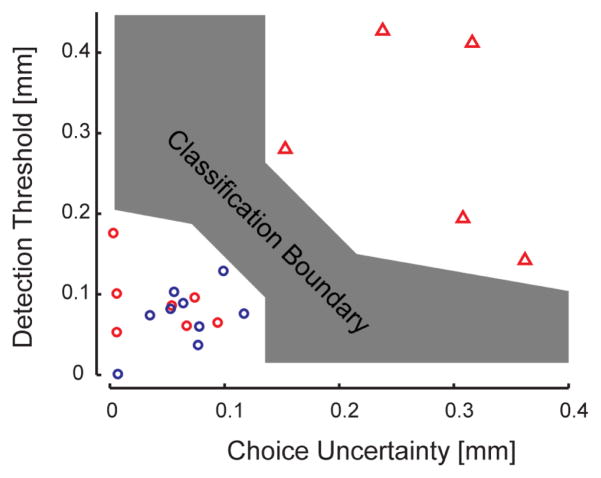
By considering both detection threshold and choice uncertainty, we found that a simple linear classifier with a broad range of slopes could separate subjects with proprioceptive deficits from those without proprioceptive deficits (Fig 4).
Fig. 4.
Linear classifier. Red triangles: SS with proprioceptive deificits; Red circles: SS without proprioceptive deficits; Blue circles: NI subjects.
Finally, linear regression analyses found no correlation between either DT or CU and upper extremity FM scores, MAS, or grip strength (Fig 5).
Fig. 5.
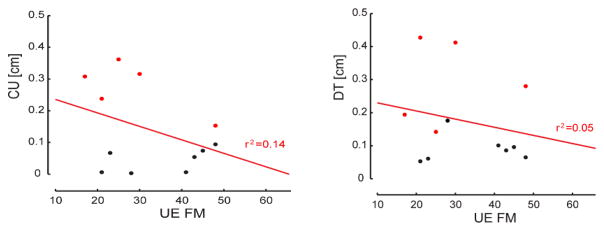
Regression results for DT vs. FM (Right) and CU vs. FM (Left).
IV. DISCUSSION
We aimed at evaluating a new robotic technique and signal detection methodology to quantify proprioceptive deficits following stroke. The present automated procedure differentiated very well between participants with (stroke) and without (stroke and control) clinically observed proprioceptive deficits while controlling for ability to understand and attend to instructions.
In addition to increased detection threshold, patients with proprioceptive deficits show increased levels of uncertainty during forced choice performance. In one of our recent studies on reach adaptation and final position control, stroke subjects with impaired proprioception also exhibited greater spatial variability in reaching final positions with the contralesional arm than stroke subjects with intact proprioception [14]. This further supports the idea that proprioception contributes importantly to the specification of final, stabilized limb position at the end of movement [20]. In a quantitative study of post-stroke arm proprioception using a robotic matching task Dukelow et al. [8] found that stroke patients exhibited greater variability matching with their unaffected arm than control participants matching with their nondominant hand. Leibowitz et al. [7] also noticed in their study that SS not only made more errors but “[they] show a significant increment in variance with repeated trials, compared with the much more stable and predictable performance of healthy individuals.” Anderson et al. [21] studied neglect patients and observed increased variability as a function of spatial location; interestingly, these authors made a distinction between the inability to reach a certain level of performance (i.e. constant error) and performance inconsistency (i.e. variable error) and suggested that there could be independent mechanisms for each of these aspects of performance. We also observed that for SS having similar detection thresholds but different choice uncertainty values; those with higher CU values had proprioceptive deficits (Fig 4). Altogether, our results and those of the studies just reviewed suggest that treatment should also address the issue of performance variability [7], [21].
Acknowledgments
This work was supported by NIH RO1HD053727.
Contributor Information
Lucia S. Simo, Email: l-simo@northwestern.edu, Northwestern University, Chicago, IL 60601 USA
Claude Ghez, Email: cpg1@columbia.edu, Columbia University Medical Center, Ney York NY 10032 USA.
Lior Botzer, Marquette University, Milwaukee WI 53233 USA.
Robert A. Scheidt, Northwestern University, Chicago, IL 60601 USA. Marquette University, Milwaukee WI 53233 USA.
References
- 1.Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil. 2008;22:758–767. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- 2.Carey LM. Somatosensory loss after stroke. Crit Reviews in Phys and Rehab Med. 1995;7:51–91. [Google Scholar]
- 3.Dellon AL, Mackinon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg. 1987;12:693–696. doi: 10.1016/s0363-5023(87)80049-7. [DOI] [PubMed] [Google Scholar]
- 4.Lincoln NB, Crow JL, Jackson JM, Waters GR, Adams SA, Hodgson P. The unreliability of sensory assessments. Clin Rehabil. 1991;5:272–282. [Google Scholar]
- 5.Lincoln NB, Jackson JM, Adams SA. Reliability and revision of the Nottingham Sensory Assessment for stroke patients. Physiotherapy. 1998;84:358–365. [Google Scholar]
- 6.Winward CE, Halligan PW, Wade DT. The Rivermead Assessment of Somatosensory Performance (RASP): standardization and reliability data. Clin Rehabil. 2002;16:523–533. doi: 10.1191/0269215502cr522oa. [DOI] [PubMed] [Google Scholar]
- 7.Leibowitz N, Levy N, Weingarten S, Grinberg Y, Karniel A, Sacher Y, Serfaty C, Soroker N. Automated measurement of proprioception following stroke. Disability and Rehabilitation. 2008;30:1829–1836. doi: 10.1080/09638280701640145. [DOI] [PubMed] [Google Scholar]
- 8.Dukelow SP, Herter TM, Moore KD, Demers MJ, Glasgow JI, Bagg SD, Norman KE, Scott SH. Quantitative assessment of limb position sense following stroke. Neurorehabil Neural Repair. 24:178–187. doi: 10.1177/1545968309345267. [DOI] [PubMed] [Google Scholar]
- 9.Ghez C, Krakauer JW, Sainburg R, Ghilardi MF. Spatial Representations and Internal Models of Limb Dynamics in Motor Learning. In: Gazzaniga M, editor. The New Cognitive Neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 501–514. [Google Scholar]
- 10.Sainburg RL, Ghilardi MF, Poizner H, Ghez C. The Control of Limb Dynamics in Normal Subjects and Patients without Proprioception. Journal of Neurophysiology. 1995;73:820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sober S, Sabes P. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarlegna FR, Sainburg RL. The roles of vision and proprioception in the planning of reaching movements. In: Sternad D, editor. Progress in Motor Control. New York: Springer Science; 2009. pp. 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheidt RA, Stoeckmann T. Reach and final position control amid environmental uncertainty after stroke. J Neurophysiol. 97:2824–2836. doi: 10.1152/jn.00870.2006. [DOI] [PubMed] [Google Scholar]
- 14.Scheidt RA, Ghez C. Separate adaptive mechanisms for controlling trajectory and final position in reaching. J Neurophysiol. 2007;93:3200–3213. doi: 10.1152/jn.00121.2007. [DOI] [PubMed] [Google Scholar]
- 15.Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggest distinct control of posture and movement. Nature Neurosci. 2005;8:498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- 16.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehab Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 17.Jukunen L, Tenovou O, Jaaskelainen SK, Hamalainen H. Recovery of somatosensory deficits in acute stroke. Acta Neurologica. 2005;111:366–372. doi: 10.1111/j.1600-0404.2005.00393.x. [DOI] [PubMed] [Google Scholar]
- 18.Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 19.Scheidt RA, Reinkensmeyer DJ, Conditt DJ, Rymer WZ, Mussa-Ivaldi A. Persistence of motor adaptationduring constrained, multi-joint, arm movements. J Neurophysiol. 84:853–862. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- 20.Ghez C, Scheidt RA, Heijink H. Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol. 2007;98:3614–3626. doi: 10.1152/jn.00652.2007. [DOI] [PubMed] [Google Scholar]
- 21.Anderson B, Mennemeier M, Chatterjee A. Variability not ability: another basis for performance decrements in neglect. Neuropsychologia. 2000;38:785–767. doi: 10.1016/s0028-3932(99)00137-2. [DOI] [PubMed] [Google Scholar]