Abstract
Background
We investigated the association between the newly proposed International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification and 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET), and whether the combination of these radiologic and pathologic factors can further prognostically stratify patients with stage I lung adenocarcinoma.
Methods
We retrospectively evaluated 222 patients with pathologic stage I lung adenocarcinoma who underwent FDG-PET scanning before undergoing surgical resection between 1999 and 2005. Patients were classified by histologic grade according to the IASLC/ATS/ERS classification (low, intermediate, or high grade) and by maximum standard uptake value (SUVmax) (low <3.0, high ≥3.0). The cumulative incidence of recurrence (CIR) was used to estimate recurrence probabilities.
Results
Patients with high-grade histology had higher risk of recurrence (5-year CIR, 29 % [n = 25]) than those with intermediate-grade (13 % [n = 181]) or low-grade (11 % [n = 16]) histology (p = 0.046). High SUVmax was associated with high-grade histology (p < 0.001) and with increased risk of recurrence compared to low SUVmax (5-year CIR, 21 % [n = 113] vs. 8 % [n = 109]; p = 0.013). Among patients with intermediate-grade histology, those with high SUVmax had higher risk of recurrence than those with low SUVmax (5-year CIR, 19 % [n = 87] vs. 7 % [n = 94]; p = 0.033). SUVmax was associated with recurrence even after adjusting for pathologic stage (p = 0.037).
Conclusions
SUVmax on FDG-PET correlates with the IASLC/ATS/ERS classification and can be used to stratify patients with intermediate-grade histology, the predominant histologic subtype, into two prognostic subsets.
For patients with stage I lung cancer, surgical resection alone remains the standard of care. However, survival outcomes remain variable, with 5-year overall survival rates of 73 and 58 % for patients with stage IA and IB lung cancer, respectively, which underscores the biological heterogeneity of early-stage lung cancer.1 Clearly, there is a need to better stratify stage I lung cancer patients by prognosis.
To address this need, the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) developed the new classification of lung adenocarcinoma.2 Histologic subtyping according to the IASLC/ATS/ERS classification has been shown to have prognostic value, a finding which has been reproduced in independent cohorts.3,4 Although histologic subtyping has been shown to be a powerful tool for stratifying stage I lung adenocarcinoma patients into three prognostic groups (low-, intermediate-, and high-grade) with the 5-year recurrence-free probability of 96, 85, and 65 %, respectively, it is limited by the fact that the majority of these patients (75 %) have intermediate-grade histology.5 Thus, additional biological factors are needed to better prognostically stratify lung adenocarcinoma patients with intermediate-grade histology.
18F-fluorodeoxyglucose (FDG)–positron emission tomography (PET) is a standard imaging modality used in clinical practice. FDG-PET measures the metabolic activity of tumors, and the maximum standardized uptake value (SUVmax) on FDG-PET has been shown to correlate with prognosis in lung cancer.6–17 Furthermore, SUVmax has been shown to be associated with histology in lung cancer. For example, it has been reported that SUVmax is lower in adenocarcinoma than in squamous cell carcinoma, and in adenocarcinoma with bronchioloalveolar carcinoma features than those without bronchioloalveolar carcinoma.12–14,18,19 However, to our knowledge, no study has specifically investigated the association between SUVmax on FDG-PET and histologic subtypes by the IASLC/ATS/ERS classification and whether these radiologic and pathologic factors can be combined to better prognostically stratify stage I lung adenocarcinoma patients.
Thus, in this study, we determined (1) whether SUVmax on preoperative FDG-PET correlates with the histologic subtype, and (2) whether the combination of radiographic (FDG-PET SUVmax) and pathologic (histologic subtype) factors can result in better clinical stratification of stage I lung adenocarcinoma patients.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the institutional review board (WA0269-08). We reviewed all patients with pathologic stage I solitary lung adenocarcinoma who underwent surgical resection at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1999 and 2005. A total of 414 patients had tumor slides available for histologic evaluation. Patients with invasive mucinous (n = 9) and colloid adenocarcinoma (n = 9) were excluded because of the limited ability of FDG-PET to detect these tumors.20,21 Of the remaining 396 patients, 222 underwent FDG-PET scanning before surgical resection. Clinical data were collected from the prospectively maintained Thoracic Service lung adenocarcinoma database. Disease stage was based on the 7th edition of the American Joint Committee on Cancer tumor, node, metastasis (TNM) staging manual.22 Of the 222 patients, 207 (93 %) underwent mediastinal lymph node dissection or sampling to exclude N2 disease. None of the patients received adjuvant or neoadjuvant chemotherapy. The cases reported in this study are a subset of stage I cases reported previously.4,5
FDG-PET
The PET scans were performed at multiple locations. Ninety-six (43 %) of 222 patients underwent PET scans at MSKCC. Thirty percent of the PET scans (n = 66) were performed after the diagnosis of lung cancer was made. For PET scans performed at MSKCC, the following technique was used. Patients received 10–15 mCi (370–555 MBq) FDG intravenously after fasting a minimum of 6 h. Before injection, plasma glucose was measured and was less than 200 mg/dL in all patients. Approximately 60–90 min after injection, PET torso images were acquired with GE Advance (GE Medical Systems, Waukesha, WI) or the Exact HR plus (Siemens/CTI, Knoxville, TN) scanners. Since November 2001, images were also acquired with a hybrid PET/computed tomography (CT) imaging system (either the Discovery LS [GE Medical Systems] or the Biograph [Siemens/CTI]). The Discovery LS system incorporates an Advance PET tomograph, and the Biograph incorporates an HR plus PET tomograph. For PET-CT, a low-dose CT scan was acquired before PET for attenuation correction of PET images and anatomic localization of PET abnormalities. Each PET image was reconstructed by using iterative algorithms, both with and without attenuation correction. All PET images were reviewed by experienced nuclear medicine physicians. FDG uptake was quantified by calculating the SUVmax using PET region-of-interest analysis:
SUV = {(decay − corrected activity [kBq]/tissue volume [ml])/(injected − FDG activity [kBq]/body weight [g])}
Internal Separate Cohort for FDG-PET
In order to validate our finding with a more uniform group of patients, we established an internal separate cohort consisting of 55 patients with pathologic stage I solitary lung adenocarcinoma who underwent surgical resection and FDG-PET scanning before surgical resection at MSKCC between 2008 and 2009. This cohort had complete data of time from FDG injection to image acquisition, plasma glucose level before injection, and PET-CT scanners. All FDG-PET studies were acquired on PET-CT scanners, including Discovery STE, Discovery LS, Discovery ST (all GE Medical Systems), or Biograph (Siemens/CTI).
Histologic Evaluation
All available hematoxylin and eosin–stained slides (mean 5 per case; range 1–12 per case) were reviewed by a pathologist (KK) for the purpose of this study, and problem cases were reviewed by two pathologists (KK andWDT) who were blinded to the patients’ clinical outcome. Assessment was made with an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard eyepiece 22 mm in diameter. Tumors were classified according to the IASLC/ATS/ERS classification as: (1) adenocarcinoma-in-situ; (2) minimally invasive adenocarcinoma; and invasive adenocarcinoma, which was further subdivided into (3) lepidic predominant, (4) acinar predominant, (5) papillary predominant, (6) micropapillary predominant, and (7) solid predominant.2,4 Tumors were graded according to the histologic grading based on predominant subtype as: (1) low grade (adenocarcinoma-in-situ, minimally invasive adenocarcinoma, or lepidic predominant invasive adenocarcinoma); (2) intermediate grade (papillary or acinar predominant); and (3) high grade (micropapillary or solid predominant).4,5
Nuclear features were examined with a high-power field (HPF) at × 400 magnification (0.237 mm2). Nuclear atypia was graded as (1) mild—nuclei were uniform in size and shape; (2) moderate—nuclei were of intermediate size and had slight irregularity; and (3) severe—nuclei were enlarged to varying degrees, with some nuclei at least twice as large as others.23–25 Mitoses were evaluated in 50 HPFs in areas with the highest mitotic activity, and counted as an average of mitotic figures per 10 HPF.25–27 According to the mitotic count, tumors were classified as (1) low—0 to 1/10 HPF; (2) intermediate—2 to 4/10 HPF; or (3) high— ≥ 5/10 HPF.28
The following histologic factors were also investigated: (1) visceral pleural invasion, which was classified as absent (PL0) or present (PL1 and PL2); (2) lymphatic and vascular invasion; and (3) necrosis.22
Statistical Analysis
For all patients, associations between SUVmax and clinicopathologic variables, histologic grade, and nuclear features were analyzed by the Wilcoxon test. The distribution of SUVmax by histologic grade was examined by box plots. Because traditional Kaplan-Meier estimates of survival probabilities can be biased when a large number of patients die without recurrence and are censored, especially if the rate of death is differential across groups, in this analysis, we considered patients who died without recurrence as competing events. The cumulative incidence of recurrence (CIR) was used to estimate recurrence probabilities. Follow-up time was defined as the date from surgery to the date of first recurrence, death from any cause, or last follow-up, whichever came first. Differences in CIR between groups were examined by Gray’s methods.29 Competing risks regression analysis was used to examine associations between SUVmax and recurrence after adjusting for important potential confounders. All p values were based on two-tailed statistical tests, and a p value of <0.05 was considered to indicate statistical significance. All statistical analyses were conducted by R software (R Development Core Team, 2010), with the “survival” and “cmprsk” packages.
RESULTS
Association Between Clinicopathologic Factors and Recurrence
The clinicopathologic factors and their CIR for all 222 patients are shown in Table 1. The median age was 70 years (range 37–89 years). Most patients were female (65 % [n = 145]), and most had stage IA disease (68 % [n = 150]). Of all patients, 87 % (n = 192) underwent lobectomy, 6 % (n = 14) segmentectomy, and 7 % (n = 16) wedge resection. Visceral pleural invasion was observed in 19 % (n = 42) of patients, lymphatic invasion in 22 % (n = 48), vascular invasion in 30 % (n = 66), and necrosis in 14 % (n = 32).
TABLE 1.
Patient clinicopathologic characteristics and association with CIR
| Variable | n | % |
n for recurrence |
5-Year CIR (%) |
p |
|---|---|---|---|---|---|
| All patients | 222 | 100 | 28 | ||
| Age (y) | 0.955 | ||||
| ≤65 | 71 | 32 | 9 | 14 | |
| >65 | 151 | 68 | 19 | 14 | |
| Sex | 0.074 | ||||
| Female | 145 | 65 | 14 | 10 | |
| Male | 77 | 35 | 14 | 22 | |
| Smoking status | 0.201 | ||||
| Never | 42 | 19 | 3 | 8 | |
| Former/current | 180 | 81 | 25 | 16 | |
| Pathologic TNM stage | 0.040a | ||||
| IA | 150 | 68 | 14 | 11 | |
| IB | 72 | 32 | 14 | 22 | |
| Visceral pleural invasion | 0.064 | ||||
| Absent | 180 | 81 | 19 | 13 | |
| Present | 42 | 19 | 9 | 22 | |
| Lymphatic invasion | 0.174 | ||||
| Absent | 174 | 78 | 19 | 13 | |
| Present | 48 | 22 | 9 | 19 | |
| Vascular invasion | 0.020a | ||||
| Absent | 156 | 70 | 14 | 11 | |
| Present | 66 | 30 | 14 | 23 | |
| Necrosis | 0.005a | ||||
| Absent | 190 | 86 | 19 | 12 | |
| Present | 32 | 14 | 9 | 30 | |
| Histologic grade | 0.046a | ||||
| Low | 16 | 7 | 1 | 11 | |
| Intermediate | 181 | 82 | 20 | 13 | |
| High | 25 | 11 | 7 | 29 | |
| SUVmax | 0.013a | ||||
| Low (<3) | 109 | 49 | 8 | 8 | |
| High (≥3) | 113 | 51 | 20 | 21 | |
CIR cumulative incidence of recurrence, TNM tumor, node, metastasis staging system, SUVmax maximum standard uptake value
Statistically significant p value
According to histologic subtyping, 2 (1 %) tumors were minimally invasive adenocarcinoma, 14 (6 %) lepidic predominant, 118 (53 %) acinar predominant, 63 (28 %) papillary predominant, 3 (1 %) micropapillary predominant, and 22 (10 %) solid predominant. By histologic grading, 16 (7 %) tumors were low grade, 181 (82 %) intermediate grade, and 25 (11 %) high grade.
Of the 222 patients, 28 (13 %) experienced recurrence, and 36 (16 %) died from any cause without recurrence. In the 158 patients who were alive without recurrence, the median follow-up time was 47.9 months (range 0.3–105.3 months). On univariate analysis (Table 1), higher stage (stage IB; p = 0.040), vascular invasion (p = 0.020), and necrosis (p = 0.005) were associated with increased CIR. Patients with high-grade histology had higher risk of recurrence (5-year CIR, 29 % [n = 25]) than those with intermediate-grade (13 %[n = 181]) or low-grade (11 %[n = 16]) (p = 0.046) histology.
Association Between SUVmax and Recurrence
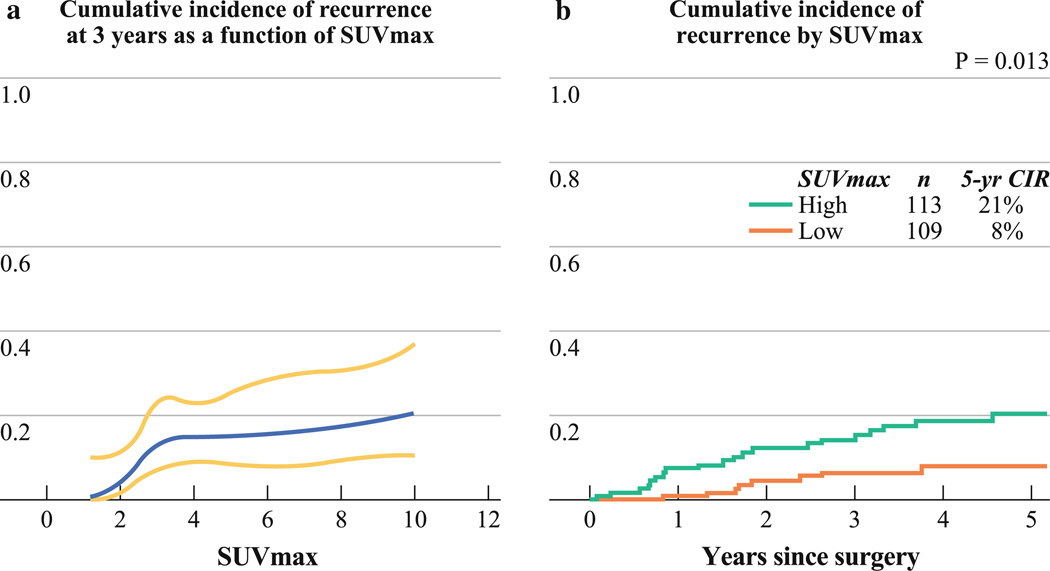
SUVmax ranged from 0.7 to 14.9 (median 3.0; mean ± SD, 3.9 ± 2.7). Within tumors with 1.0 cm in size or less (n = 18), the median SUVmax was 2.5 (mean ± SD, 2.7 ± 1.1). Figure 1a presents the CIR at 3 years as a function of SUVmax. As shown, as SUVmax increases from 1, CIR at 3 years also increases. However, when SUVmax reaches approximately 3, CIR at 3 years levels off. On the basis of these results, we classified tumors into two groups by the median SUVmax: low (< 3.0) and high (≥ 3.0). High SUVmax was associated with higher risk of recurrence than low SUVmax (5-year CIR, 21 % [n = 113] vs. 8 % [n = 109]; p = 0.013) (Fig. 1b).
FIG. 1.
Cumulative incidence of recurrence (CIR) by maximum standardized uptake value (SUVmax). a CIR at 3 years (blue line) with corresponding 95 % confidence intervals (yellow lines), as a function of continuous SUVmax. As SUVmax increases from 1, CIR at 3 years also increases. When SUVmax reaches approximately 3, CIR at 3 years levels off. b Patients with high SUVmax have higher risk of recurrence than those with low SUVmax (5-year CIR, 21 % [n = 113] vs. 8 % [n = 109]; p = 0.013)
Association Between SUVmax, Histology, and Recurrence
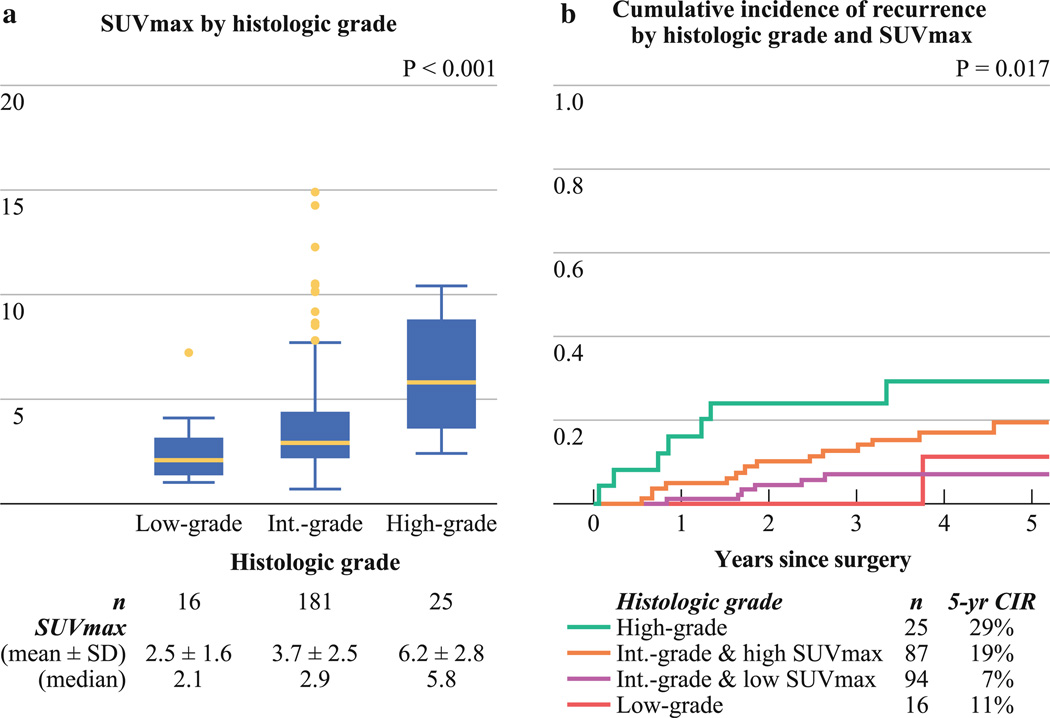
Solid-predominant invasive adenocarcinoma had the highest SUVmax (mean ± SD, 6.4 ± 2.9), followed by micropapillary-predominant (4.8 ± 2.5), acinar-predominant (4.0 ± 2.8), papillary-predominant (3.2 ± 1.9), lepidic-predominant (2.5 ± 1.7), and minimally invasive adenocarcinoma (2.2 ± 0.4). Tumors with high-grade histology had a higher SUVmax (6.2 ± 2.8) than those with intermediate-grade (3.7 ± 2.5) or low-grade (2.5 ± 1.6) histology (p < 0.001; Fig. 2a). A subanalysis of the 18 patients with intermediate-grade histology who had higher SUVmax (>1.5 × interquartile range) revealed that they had either high-grade histology as the second predominant pattern (61 % [n = 11]) or high mitotic count (72 % [n = 13]).
FIG. 2.
Maximum standardized uptake value (SUVmax), histology, and cumulative incidence of recurrence (CIR). a Tumors with high-grade histology have the highest SUVmax (mean ± SD, 6.2 ± 2.8), followed by those with intermediate-grade (3.7 ± 2.5) and low-grade (2.5 ± 1.6) histology. b Patients with high-grade histology have the highest risk of recurrence (5-year CIR, 29 % [n = 25]), followed by those with intermediate-grade histology/high SUVmax (5-year CIR, 19 % [n = 87]). Patients with intermediate-grade histology/high SUVmax have a higher risk of recurrence than those with intermediate-grade histology/low SUVmax (5-year CIR, 7 % [n = 94]). Patients with intermediate-grade histology/low SUVmax have a risk of recurrence similar to that of patients with low-grade histology (5-year CIR, 11 % [n = 16])
Figure 2b presents the CIR by histologic grade, with patients with intermediate-grade histology stratified by SUVmax. Patients with high-grade histology had the highest risk of recurrence (5-year CIR, 29 % [n = 25]), followed by those with intermediate-grade histology/high SUVmax (5-year CIR, 19 % [n = 87]). Patients with intermediate-grade histology/high SUVmax had higher risk of recurrence than those with intermediate-grade histology/low SUVmax (5-year CIR, 7 % [n = 94]) (p = 0.033). Patients with intermediate-grade histology/low SUVmax had a risk of recurrence similar to that of patients with low-grade histology (5-year CIR, 11 % [n = 16]) (p = 0.841). The small number of patients with low-grade and high-grade histology precluded their stratification by SUVmax.
Potential confounders were defined as variables that were associated with recurrence, and these included stage, vascular invasion, and necrosis. Given the small number of recurrence events (n = 28), it was not possible to incorporate all variables into a single multivariate model. To assess whether SUVmax was an independent predictor of recurrence, we adjusted for each confounder individually and found that SUVmax was still associated with recurrence even after adjusting for stage (p = 0.037) and was still marginally associated with recurrence after adjusting for vascular invasion (p = 0.064) and necrosis (p = 0.055).
Validation of the Association Between SUVmax and Histology
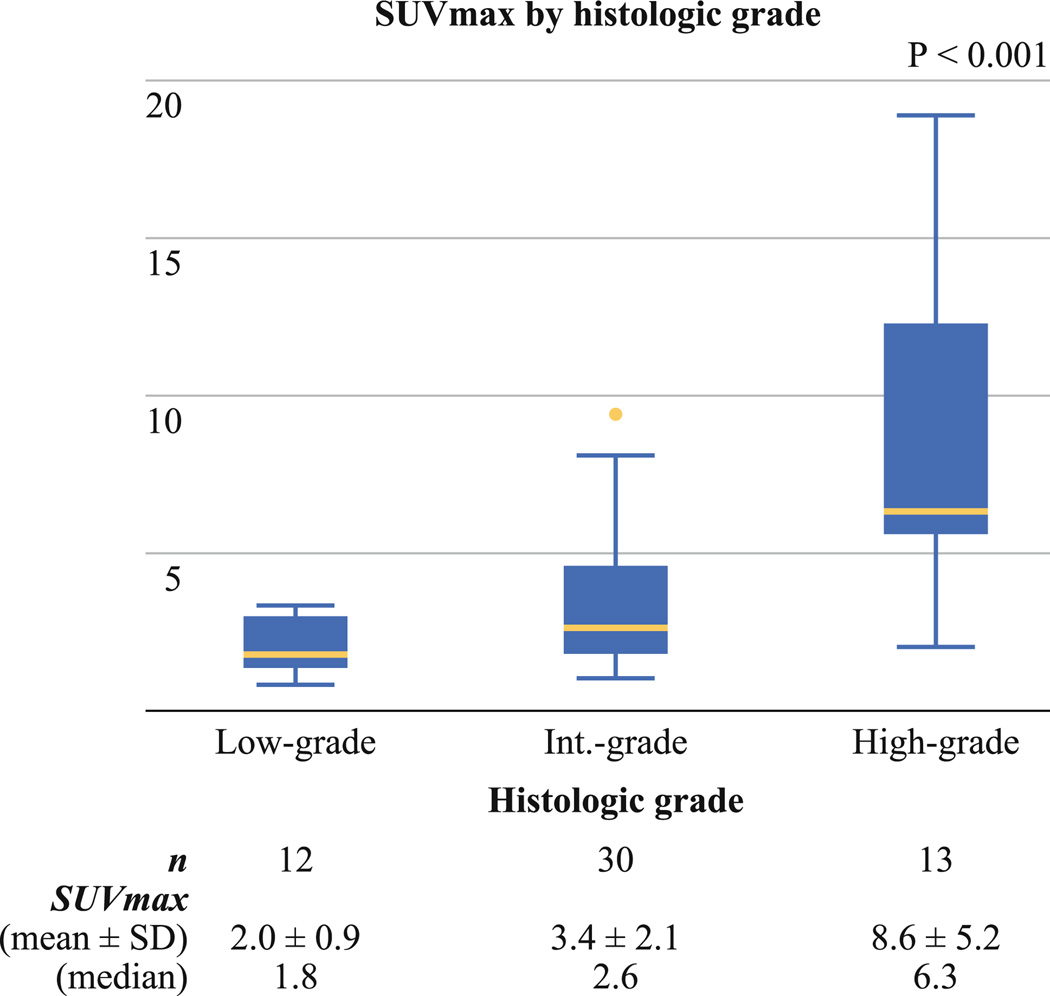
In a separate cohort consisting of 55 patients, median time from FDG injection to image acquisition was 65 minutes (range 60–89), and median plasma glucose level was 97 mg/dL (range 72–147). SUVmax ranged from 0.8 to 18.9 (median 2.9; mean ± SD, 4.3 ± 3.8). Twenty-three patients were imaged on Discovery STE, 13 on Discovery LS, 3 on Discovery ST, and 16 on Biograph machines. In all 55 patients, tumors with high-grade histology (n = 13) had a higher SUVmax (8.6 ± 5.2) than those with intermediate-grade (n = 30, 3.4 ± 2.1) or low-grade (n = 12, 2.0 ± 0.9) histology (p < 0.001; Fig. 3). In 23 patients imaged on the Discovery STE unit, which was the most frequently used scanner in this cohort, tumors with high-grade histology (n = 6) had a higher SUVmax (7.9 ± 3.0) than those with intermediate-grade (n = 13, 3.6 ± 1.9) or low-grade (n = 4, 2.1 ± 0.7) histology, although the small sample size did not allow us to perform statistical analysis.
FIG. 3.
Association between maximum standardized uptake value (SUVmax) and histology in a separate cohort. Tumors with high-grade histology had the highest SUVmax (mean ± SD, 8.6 ± 5.2), followed by those with intermediate-grade (3.4 ± 2.1) and low-grade (2.0 ± 0.9) histology
Association Between SUVmax and Clinicopathologic Factors
In addition to histologic grade, higher SUVmax was associated with smoking history (p = 0.004), higher T stage (p < 0.001), higher TNM stage (p < 0.001), visceral pleural invasion (p = 0.015), vascular invasion (p < 0.001), and necrosis (p < 0.001) (Table 2). Tumors with severe nuclear atypia had a higher SUVmax (5.1 ± 2.9) than those with moderate (4.6 ± 3.2) or mild (2.9 ± 1.5) (p < 0.001; Table 2). Tumors with high mitotic count had a higher SUVmax (5.5 ± 3.3) than those with intermediate (3.7 ± 2.3) or low (2.8 ± 1.4) (p < 0.001; Table 2).
TABLE 2.
Association between SUVmax and patient clinicopathologic characteristics
| Variable | SUVmax | p | |
|---|---|---|---|
| Mean ± SD | Median | ||
| Age (y) | 0.468 | ||
| ≤65 | 4.2 ± 2.8 | 3.2 | |
| >65 | 3.8 ± 2.6 | 3.0 | |
| Gender | 0.197 | ||
| Female | 3.8 ± 2.7 | 2.8 | |
| Male | 4.1 ± 2.6 | 3.4 | |
| Smoking status | 0.004a | ||
| Never | 2.9 ± 1.9 | 2.6 | |
| Former/current | 4.1 ± 2.8 | 3.4 | |
| Pathologic T stage | <0.001a | ||
| T1a | 3.1 ± 1.9 | 2.5 | |
| T1b | 4.1 ± 2.7 | 3.2 | |
| T2a | 4.9 ± 3.2 | 3.9 | |
| Pathologic TNM stage | <0.001a | ||
| IA | 3.4 ± 2.2 | 2.8 | |
| IB | 4.9 ± 3.2 | 3.9 | |
| Visceral pleural invasion | 0.015a | ||
| Absent | 3.8 ± 2.7 | 2.9 | |
| Present | 4.5 ± 2.4 | 3.8 | |
| Lymphatic invasion | 0.596 | ||
| Absent | 3.8 ± 2.6 | 3.0 | |
| Present | 4.1 ± 2.9 | 3.3 | |
| Vascular invasion | <0.001a | ||
| Absent | 3.4 ± 2.3 | 2.7 | |
| Present | 5.2 ± 3.1 | 4.1 | |
| Necrosis | <0.001a | ||
| Absent | 3.4 ± 2.1 | 2.8 | |
| Present | 6.8 ± 3.5 | 6.3 | |
| Nuclear atypia | <0.001a | ||
| Mild | 2.9 ± 1.5 | 2.6 | |
| Moderate | 4.6 ± 3.2 | 3.6 | |
| Severe | 5.1 ± 2.9 | 4.1 | |
| Mitotic count | <0.001a | ||
| Low | 2.8 ± 1.4 | 2.5 | |
| Intermediate | 3.7 ± 2.3 | 3.1 | |
| High | 5.5 ± 3.3 | 4.4 | |
SUVmax maximum standard uptake value, TNM tumor, node, metastasis staging system
Statistically significant p value
DISCUSSION
Stage I lung adenocarcinoma represents a biologically heterogenous disease. To enable the stratification of patients with stage I lung adenocarcinoma, the IASLC/ATS/ERS recently proposed a new classification.2 FDG-PET SUVmax reflects the metabolic activity of tumors and has been shown to correlate with prognosis and histology in lung cancer.9–17 In this study, we demonstrated that (1) SUVmax on preoperative FDG-PET correlates with histologic grade based on the IASLC/ATS/ERS classification, a finding that was validated in an independent cohort; and (2) SUVmax can be used to stratify patients with intermediate-grade histology by prognosis.
We found a positive association between SUVmax and histologic grade (Fig. 2a). Mean SUVmax was 2.5 in tumors with low-grade histology compared to 3.7 in tumors with intermediate-grade and 6.2 in those with high-grade histology. However, lung adenocarcinomas with a lepidic pattern are known to have lower SUVmax than those without a lepidic pattern.18,19 Our study extended the observation of an association between SUVmax and the IASLC/ATS/ERS histologic classification. Furthermore, we observed that among the 18 tumors with intermediate-grade histology that had an unusually high SUVmax, 11 had high-grade histology as the second predominant pattern. In addition, the association between SUVmax and the histologic classification was validated in an independent and more uniform cohort in which FDG-PET scans were performed at a single institution and by the same technique. These observations reflect the biological heterogeneity of lung adenocarcinoma, especially in tumors with intermediate-grade histology, and indicate that a combination of histology and FDG-PET better accounts for this heterogeneity than histology alone.
We also found a positive association between SUVmax and mitotic count, which further supports the ability of SUVmax to reflect the tumor biology of lung adenocarcinoma. Because FDG-PET uptake reflects tumor metabolism, SUVmax has been shown to correlate with tumor proliferation index and mitotic count.6–8,30 To our knowledge, this association between SUVmax and mitotic count has not been previously investigated in stage I lung adenocarcinoma patients.
Predominant histologic subtypes result in three prognostically distinct groups.3,4 In this study, 222 stage I lung adenocarcinoma patients were categorized into three histologic grades (low, intermediate, and high grade) and the 5-year CIR was 11, 13, and 29 %, respectively. One limitation of the histologic grade, however, is the predominance of patients with intermediate-grade histology, with 82 % of our study cohort belonging to this group. With the addition of SUVmax, we were able to stratify patients with intermediate-grade histology into two prognostically distinct subsets; the 5-year CIR was 7 and 19 %, respectively, for those with SUVmax below and above the median SUVmax (3.0). Because of the small number of tumors classified as having low-grade and high-grade histology, we were not able to evaluate the added prognostic value of FDG-PET in these groups. Another limitation of this study is that stage I lung adenocarcinoma patients may not always undergo FDG-PET scanning before surgical resection, although PET scanning is currently becoming more routine practice in the care of cancer patients. In our cohort, only 222 of 396 patients underwent FDG-PET scanning before surgical resection.
Of note, the stratification of SUVmax in our study was above and below 3.0. This cutoff value is considerably lower than that used in studies investigating lung cancer patients with stage I–IV disease (SUVmax cutoffs of 7.0–15.0) or with mixed histology of adenocarcinoma and squamous cell carcinomas (SUVmax cutoffs of 4.7–5.5).11–13,15–17 However, our cutoff value, which was based on a homogenous cohort of stage I lung adenocarcinoma patients, is similar to that used in studies of stage I adenocarcinoma patients (SUVmax cutoffs of 2.5–3.3).9,10
The limitation of our study is that the PET scan was performed with several different scanning machines. To obtain a large series of patients with PET scan performed on a single scanner is not possible because of our hospital patient referral pattern. To combat this deficiency, we investigated a separate cohort of patients in whom the PET scan was performed at MSKCC with uniform protocols, optimized pre-PET scanning plasma glucose values, and image acquisition times. The findings from this cohort of patients mirror the findings from the larger cohort.
The combination of a noninvasive imaging modality that measures tumor metabolism (FDG-PET) with histologic tumor characterization on microscopic examination (histologic subtype) better stratifies a homogeneous cohort of stage I lung adenocarcinoma patients by prognosis than either alone. Because the use of FDG-PET in the preoperative assessment and hematoxylin and eosin–stained slides in postoperative pathologic diagnosis of resected tumors is routine in the care of patients with lung adenocarcinoma, the prognostic stratification by SUVmax of patients classified as having intermediate-grade histology can be readily implemented without additional cost. We believe that this multimodal method is easily implementable at most institutions that care for lung adenocarcinoma patients and that it should be tested in future clinical trials to better stratify early-stage lung adenocarcinoma patients.
ACKNOWLEDGMENT
We thank Joe Dycoco for his help with the Thoracic Service lung adenocarcinoma database and Lionel Santibáñez for his editorial assistance. This work was supported in part by the International Association for the Study of Lung Cancer (IASLC)-Young Investigator Award; National Lung Cancer Partnership/LUNGevity Foundation Research grant; Stony Wold-Herbert Fund; American Association for Thoracic Surgery (AATS)-Third Edward D. Churchill Research Scholarship; Mesothelioma Applied Research Foundation (MARF) grant in memory of Lance S. Ruble; William H. Goodwin, and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center; New York State Empire Clinical Research Investigator Program (ECRIP); the National Cancer Institute (grants U54CA137788, U54CA132378); and the U.S. Department of Defense (grant PR101053).
Footnotes
DISCLOSURE The authors declare no conflicts of interest.
REFERENCES
- 1.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshizawa A, Sumiyoshi S, Moreira AL, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification and use of comprehensive histologic subtyping for architectural grading in 432 Japanese patients. Mod Pathol. 2011;24:429A. [Google Scholar]
- 4.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 5.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 6.Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–3844. [PubMed] [Google Scholar]
- 7.Takenaka T, Yano T, Ito K, et al. Biological significance of the maximum standardized uptake values on positron emission tomography in non–small cell lung cancer. J Surg Oncol. 2009;100:688–692. doi: 10.1002/jso.21386. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura H, Hirata T, Kitamura H, et al. Correlation of the standardized uptake value in FDG-PET with the expression level of cell-cycle-related molecular biomarkers in resected non–small cell lung cancers. Ann Thorac Cardiovasc Surg. 2009;15:304–310. [PubMed] [Google Scholar]
- 9.Ohtsuka T, Nomori H, Watanabe K, et al. Prognostic significance of [(18)F]fluorodeoxyglucose uptake on positron emission tomography in patients with pathologic stage I lung adenocarcinoma. Cancer. 2006;107:2468–2473. doi: 10.1002/cncr.22268. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer. 2010;116:3170–3177. doi: 10.1002/cncr.25244. [DOI] [PubMed] [Google Scholar]
- 11.Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol. 2011;6:43–47. doi: 10.1097/JTO.0b013e3181f9abca. [DOI] [PubMed] [Google Scholar]
- 12.Goodgame B, Pillot GA, Yang Z, et al. Prognostic value of preoperative positron emission tomography in resected stage I non–small cell lung cancer. J Thorac Oncol. 2008;3:130–134. doi: 10.1097/JTO.0b013e318160c122. [DOI] [PubMed] [Google Scholar]
- 13.Nair VS, Barnett PG, Ananth L, et al. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non–small cell lung cancer. Chest. 2010;137:1150–1156. doi: 10.1378/chest.09-2356. [DOI] [PubMed] [Google Scholar]
- 14.Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose–positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 15.Vesselle H, Freeman JD, Wiens L, et al. Fluorodeoxyglucose uptake of primary non–small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res. 2007;13:3255–3263. doi: 10.1158/1078-0432.CCR-06-1128. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sarraf N, Gately K, Lucey J, et al. Clinical implication and prognostic significance of standardised uptake value of primary non–small cell lung cancer on positron emission tomography: analysis of 176 cases. Eur J Cardiothorac Surg. 2008;34:892–897. doi: 10.1016/j.ejcts.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Cerfolio RJ, Bryant AS, Ohja B, et al. The maximum standardized uptake values on positron emission tomography of a non–small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005;130:151–159. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Vesselle H, Salskov A, Turcotte E, et al. Relationship between non–small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 19.Higashi K, Ueda Y, Yagishita M, et al. FDG PET measurement of the proliferative potential of non–small cell lung cancer. J Nucl Med. 2000;41:85–92. [PubMed] [Google Scholar]
- 20.Sawada E, Nambu A, Motosugi U, et al. Localized mucinous bronchioloalveolar carcinoma of the lung: thin-section computed tomography and fluorodeoxyglucose positron emission tomography findings. Jpn J Radiol. 2010;28:251–258. doi: 10.1007/s11604-009-0414-4. [DOI] [PubMed] [Google Scholar]
- 21.Shim SS, Han J. FDG-PET/CT imaging in assessing mucinproducing non–small cell lung cancer with pathologic correlation. Ann Nucl Med. 2010;24:357–362. doi: 10.1007/s12149-010-0358-x. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2009. pp. 253–270. [Google Scholar]
- 23.Thomas JS, Kerr GR, Jack WJ, et al. Histological grading of invasive breast carcinoma—a simplification of existing methods in a large conservation series with long-term follow-up. Histopathology. 2009;55:724–731. doi: 10.1111/j.1365-2559.2009.03429.x. [DOI] [PubMed] [Google Scholar]
- 24.Asamura H, Ando M, Matsuno Y, et al. Histopathologic prognostic factors in resected adenocarcinomas: is nuclear DNA content prognostic? Chest. 1999;115:1018–1024. doi: 10.1378/chest.115.4.1018. [DOI] [PubMed] [Google Scholar]
- 25.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer. 2010;116:659–669. doi: 10.1002/cncr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Baak JP. Mitosis counting in tumors. Hum Pathol. 1990;21:683–685. doi: 10.1016/0046-8177(90)90026-2. [DOI] [PubMed] [Google Scholar]
- 28.Kadota K, Suzuki K, Rusch VW, et al. Nuclear grading system predicts recurrence in stage I lung adenocarcinoma patients. Mod Pathol. 2011;24:413A. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 30.Ueda S, Tsuda H, Asakawa H, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–258. doi: 10.1093/jjco/hyn019. [DOI] [PubMed] [Google Scholar]