Abstract
Instructive and permissive models of commitment have been proposed for hematopoietic cytokines. In recent issues of Science and Cell, Rieger et al. (2009) and Sarrazin et al. (2009) together show that cytokines can instruct lineage choice.
Multipotent hematopoietic stem cells (HSCs) with the ability to reconstitute the blood and lymphoid lineages long term and to self-renew are predominantly quiescent, dividing only in response to demand for blood cells (Wilson et al., 2008). Both multilineage and lineagespecific cytokines synergistically regulate the proliferation and differentiation of these long term (LT)-HSCs that express receptors for several of the lineage-specific cytokines. A major area of conjecture in the HSC field has been whether cytokines can “instruct” lineage commitment (Metcalf, 1998) or whether their actions are simply “permissive” (Enver et al., 1998), allowing the survival and expansion of already committed cells. Although both cell-autonomous transcription factors (Laiosa et al., 2006) and the activation of ectopically expressed cytokine receptors (Kondo et al., 2000) have been shown to instruct lineage choice, it has been technically difficult to demonstrate an instructive action of cytokines in nonengineered cells. Two recent papers that used novel approaches (Rieger et al., 2009; Sarrazin et al., 2009) convincingly demonstrate that cytokines can instruct.
Macrophage CSF (M-CSF), also known as colony stimulating factor-1 (CSF-1) and granulocyte CSF (G-CSF), generate clones from cultured hematopoietic cells that are composed almost exclusively of macrophages (Ms) or granulocytes (Gs), respectively. Using novel bioimaging approaches that permit continuous long-term observation at the single-cell level (Eilken et al., 2009), Rieger et al. (2009) used time-lapse movies of cultures of purified bipotent granulocyte/monocyte progenitors (GMPs) to follow the fate of unselected progenitors as they differentiate to either Ms or Gs. GMPs, which do not express lysozyme, were obtained from Lys∷GFP mice in which enhanced green fluorescent protein expression is driven from the lysozymeM gene locus. Thus, upon initial commitment to either the M or G lineage, the differentiating GMPs could be detected on the basis of the onset of GFP expression. Ms, which are adherent, spindle shaped, and F4/80+, were easily distinguished from Gs, which smaller, non-adherent, and Ly6G+. After short-term culture of GMPs in G-CSF or CSF-1, they were switched to medium containing other cytokines that maintained the cells. Importantly, this system allows visualization of cell death and unilineage commitment of all GMPs in the field. After culture of GMPs and their progeny in G-CSF or CSF-1 for 2 days, almost all the resulting pedigrees were pure Gs or pure Ms, respectively, and cell death occurred in less than 13% of these pedigrees. In the absence of cell death, colonies could have resulted from “instructed” differentiation of bipotent GMPs or by “permissive” expansion of any unilineage-restricted cells that contaminated the pre-existing GMP pool. However, the maximum percentages of potentially unilineage-restricted cells in the GMP population, simultaneously determined by scoring colonies that arose in cultures with a mixture of multilineage cytokines, were much lower than the high percentage of unilineage colonies induced by CSF-1 or G-CSF. Furthermore, other experiments indicated that ~90% of the GMPs in the starting population were bipotent. Thus, the authors have clearly demonstrated that CSF-1 and G-CSF can instruct most GMPs into a specific lineage.
Focusing on a different multipotent cell, the HSC, and the role of a transcription factor, which they show regulates responsiveness to CSF-1, Sarrazin et al. (2009) independently conclude that cytokines can instruct progenitor fates. Highly expressed in mature monocytes and macrophages, MafB is a bZip-type transcription factor that decreases proliferation and accelerates macrophage differentiation when ectopically expressed in committed myeloid progenitors (Kelly et al., 2000). Comparing LT-HSC-enriched populations from WT and MafB−/− mice, the authors showed that the primitive MafB−/− cells exhibited higher proliferation rates than WT cells. Furthermore, in a competitive reconstitution assay, MafB−/− HSCs competed better than WT HSCs, resulting in an increased contribution of the MafB−/− cells to the HSC fraction. However, the competitive advantage of the MafB−/− HSCs was not observed in the lymphoid or erythroid lineages. Rather, it was restricted to a myeloid-specific repopulation. Interestingly, despite this myeloid-based repopulation advantage, there was no increase in the size of the myeloid cell compartment. Furthermore, after serial transplantation, MafB−/− HSCs did not differ from WT HSCs, either in their self-renewal capacity or their ability to contribute nonmyeloid lineages, but did maintain a progressively increasing myeloid repopulation advantage on serial transfer in irradiated hosts. In vitro experiments demonstrated that MafB−/− HSCs had the intrinsic capacity to generate more myeloid progeny than WT HSC and that MafB selectively attenuates CSF-1R signaling. Additional transplantation experiments with fetal liver cells in which the CSF-1R was knocked down showed that MafB−/− HSCs are specifically sensitized to CSF-1-driven myeloid repopulation in vivo.
Concentrating on the effect of MafB on CSF-1-regulated differentiation, Sarrazin et al. next showed that loss of MafB enhanced CSF-1-driven commitment to the myeloid lineage. The transcription factor PU.1 is a myeloid master regulator that is both necessary and sufficient to drive myeloid fate (Iwasaki and Akashi, 2007), and its increased expression is an important early step in myeloid differentiation. Thus, to detect myeloid commitment, Sarrazin et al. used purified HSCs from PU.1-GFP reporter mice. Incubation of these cells with CSF-1 for only 16 hr increased PU.1 expression significantly in wild-type cells and dramatically in MafB−/− cells, indicating that MafB attenuates CSF-1-induced activation of PU.1. Interestingly, MafB was highly expressed in LT-HSC-enriched populations and in the PU.1− HSC-enriched fraction, but not in the downstream PU.1+ multipotent progenitor (MPP) or committed myeloid progenitor fractions. This observation, together with the increased PU.1 expression of MafB-deficient HSCs compared with WT HSCs after short-term incubation with CSF-1, suggested that MPP development requires MafB downregulation. To directly examine CSF-1-induced commitment and its regulation by MafB, the authors cultured single cells of the PU.1− HSC-enriched fraction of PU.1-GFP reporter mice until their first division. By following GFP expression, they observed proliferative, differentiative, and asymmetric divisions, but found that MafB deficiency, consistent with its failure to affect self-renewal or commitment to other lineages, specifically enhanced CSF-1-driven asymmetric myeloid commitment divisions.
The studies of Sarrazin et al., with their focus on MafB, do not as directly address the question of whether CSF-1 induces commitment as those of Riegler et al. However, their results indicate an instructive role revealed by MafB deficiency and provide us with a fascinating paradigm for cytokine/transcription factor interactions. Together, these studies demonstrate the direct effect of cytokines on lineage choice in nonengineered cells and how transcription factor expression can limit this instructive cytokine effect (Figure 1). The results set the scene for future investigations. The novel imaging technology will be useful in determining which receptor-based signaling pathways are responsible for commitment. Other important questions are: How do transcription factors, such as MafB, attenuate cytokine signaling? Are the factors down-regulating MafB synthesis cell intrinsic (stochastic events or epigenetic changes), or are external cues involved? As far as CSF-1 receptor signaling is concerned, are the three functionally distinct CSF-1 isoforms (Pixley and Stanley, 2004) and the novel CSF-1 receptor ligand, interleukin-34, involved in differential regulation in the stem cell niche? Finally, in view of the interesting interaction between MafB and CSF-1 receptor signaling and the deterministic effect of CSF-1, it will be of interest to determine how CSF-1 receptor deficiency affects HSC self-renewal and differentiation in vivo.
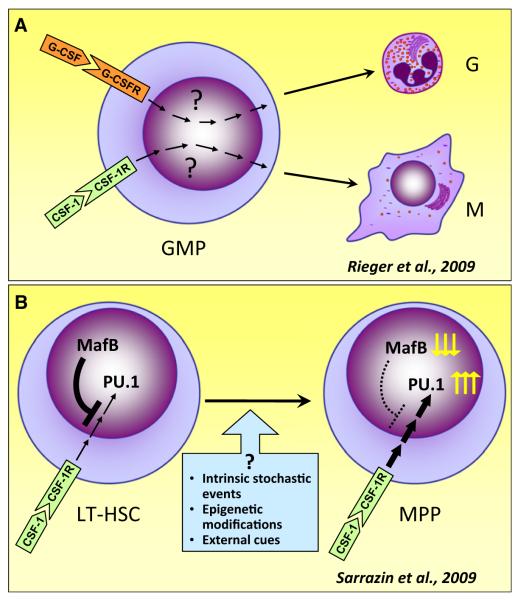
Figure 1. Cytokines Instruct Lineage Commitment.
(A) Approximately 90% of purified granulocyte-monocyte progenitors (GMPs) differentiate to pure granulocyte (G) colonies with G-CSF or pure macrophage (M) colonies with CSF-1.
(B) The transcription factor MafB selectively limits CSF-1-instructed myeloid commitment in hematopoietic stem cells. In populations enriched for long-term repopulating hematopoietic stem cells (LT-HSC), CSF-1-induced expression of the myeloid transcription factor PU.1 is repressed. Decreased expression of MafB in multipotent progenitors (MPPs) leads to increased PU.1 expression and their subsequent differentiation into the myeloid lineage. Not shown is that MafB deficiency in LT-HSC specifically increases asymmetric cell divisions, in which one daughter cell is PU.1+ and the other PU.1−, thereby maintaining stem cell numbers for differentiation to nonmyeloid lineages.
REFERENCES
- Eilken HM, Nishikawa S, Schroeder T. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Enver T, Heyworth CM, Dexter TM. Blood. 1998;92:348–352. [PubMed] [Google Scholar]
- Iwasaki H, Akashi K. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. EMBO J. 2000;19:1987–1997. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- Laiosa CV, Stadtfeld M, Graf T. Annu. Rev. Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Blood. 1998;92:345–352. [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitel-huber AC, Schroeder T. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, et al. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]