Abstract
Background:
There was an outbreak of acute hepatitis in Mylapore village, Kollam district, Kerala, southern India during February to June 2013. An outbreak investigation was initiated with the objective of describing the epidemiological features of the hepatitis outbreak.
Materials and Methods:
House-to-house visits were undertaken to identify symptomatic cases. The outbreak was described in terms of person, place and time. Hypothesis was generated based on findings from descriptive study, laboratory investigation of water samples, and environmental observations. A case-control study was designed to test the hypothesis. Chi-square test, univariate analysis, and logistic regression to identify the risk factors associated with hepatitis A infection were done.
Results:
Line list generated consisted of 45 cases. Attack rate was the highest among the age group 15-24 years (4.6%) followed by 5-14 years (3.1%). The geographical distribution of the cases suggested a clustering around the water supply through the pipeline and epidemic curve showed a sharp rise in cases suggestive of a common source outbreak. Water samples collected form pipeline showed evidence of fecal contamination and absence of residual chlorine. In the case-control study, having consumed water from the pipeline (odds ratio: 9.01 [95% confidence interval: 2.16-37.61]) was associated with the hepatitis A cases.
Conclusion:
The time frame of disease occurrence, environmental observations, anecdotal evidences, laboratory results and results of the analytical study indicated the possibility of occurrence of hepatitis A outbreak as a result of pipe water contamination supplied from a bore well. The study warrants establishment of an efficient water quality surveillance system.
Keywords: Hepatitis A, Outbreak investigation, Vaccination, Water contamination, Water supply
INTRODUCTION
Hepatitis A, a self-limiting viral disease, is the most common form of acute viral hepatitis worldwide. Hepatitis A virus (HAV) infection occurs sporadically and epidemically and every year there are about 1.4 million cases of hepatitis A occurring worldwide. The disease is closely associated with a lack of safe water, inadequate sanitation and poor personal hygiene.[1] Even though a significant proportion remains asymptomatic and most of the infected persons recover completely, HAV infection causes significant morbidity. People affected with HAV may take few months to return to work, school, or daily life and so itself HAV infections can lead to economic losses and social consequences in the community.[1,2,3]
The HAV is transmitted through ingestion of contaminated food and water or through direct contact with an infectious person.[2,3,4,5] The virus is shed in the feces of persons with both asymptomatic and symptomatic infection. Under favorable conditions HAV may survive in the environment for months.[2,5] Presentation of disease is determined by the age of exposure, which tends to be asymptomatic or subclinical during childhood and symptomatic usually among adults. It has been reported that 70% of children <6 years of age are asymptomatically infected or develop a mild self-limiting illness.[5,6]
Viral hepatitis continues to be a major public health problem in India. Several large outbreaks of hepatitis A in various parts of the country have been recorded in the past decade.[7,8,9,10] Most hepatitis A outbreaks were due to fecal-oral route of transmission because of contamination of water with sewage.
On March 17, 2013, health workers reported to District Surveillance Unit, an unusual occurrence of 20 cases of acute jaundice at Mylapore village, Kollam district, Kerala. An outbreak investigation was initiated with the objective of describing the epidemiological features of the hepatitis outbreak and to make recommendations to further prevent the spread of the disease.
MATERIALS AND METHODS
Mylapore is a village with a total population of 3103, located in Kollam district, Kerala. Majority of the population belonged to Muslim community. The area is marked with overcrowding, open but cemented drains and closely clustered houses. Residents of the village mainly depend on well water and public taps, but due to severe scarcity of drinking water since March 2013, many had to depend on water tanker supply, which collected water from a distant bore well, also.
Current incidence of hepatitis was compared with the background rates reported to Integrated Disease Surveillance Project from the area.[11] Possibility of an artifact in the form of improved surveillance or mass population movements was excluded, based on discussions with the primary health care team and the local leaders respectively.[12] Eight cases were visited initially to describe the clinical picture and a shortlist of possible diagnosis was prepared. Eight blood samples were sent to State Public Health Laboratory for confirming the diagnosis.
A probable case of hepatitis A was defined as an acute illness with fever or loss of appetite followed by yellowish discoloration of sclera or urine after January 1, 2013 in a resident of Mylapore. Case definition was framed based on opinions from treating clinicians and group consensus, and it has been made simple to pick up the cases from the community by the field level health workers. A line list of cases was developed based on house to house visit over the entire area over 3 days by eight health workers. Information regarding the date of onset, age, sex, place of residence, treatment, and laboratory investigation were collected. The catchment area hospitals and traditional healers were visited to finalize the line list. Surveillance was strengthened in nearby areas.
Attack rates of acute hepatitis by age and sex were calculated. An epidemic curve was drawn and a spot map was generated with cases marked along with the water sources.
Key informant interview was conducted with eight people who acquired the disease recently, leader of the local self-government, health workers in that area and the local pump operator. Information regarding the source of drinking water, drainage system, important events before the onset of illness, public gatherings, exposure to outside food and local food vendors were collected.
Sanitary inspection was done by visiting the pump house, water lines, source from which the tanker lorries are collecting water and a few household wells. Log book for chlorination was verified to identify the frequency of chlorination and supply of public tap water. Six water samples; two from public tap and three from different household wells and one from well water source of tanker lorry had been collected for microbiological analysis and residual chlorine.
Hypothesis was generated and a case-control study was planned to test the hypotheses. Cases were selected based on the line list prepared. Controls were age and gender matched neighborhood individuals with no history of jaundice. For each case, one age (±5) and gender matched control living in the house with the next number was included. If there was no eligible control in the neighborhood house, the houses were visited serially until an eligible control was obtained. All the cases and controls were interviewed at their home using a structured questionnaire which included information regarding the demographic characteristics, source of water, personal hygiene, and treatment of water before consumption, habit of consuming food from outside home and past history of hepatitis.
Data were analyzed using Statistical Package for Social Sciences version 12 (SPSS Inc., Chicago, IL, USA), for Microsoft Windows. Chi-square test for any associations and odds ratios (ORs) with confidence intervals (CIs) were estimated to identify the risk factors associated with HAV infection. Selected variables were then entered into a backward conditional logistic regression model and adjusted ORs with 95% CIs were calculated.
RESULTS
The comparison with the background data confirmed the existence of an outbreak of hepatitis and all eight blood samples tested were positive for IgM HAV. Thus, an outbreak of hepatitis A was confirmed at Mylapore village, Kollam district.
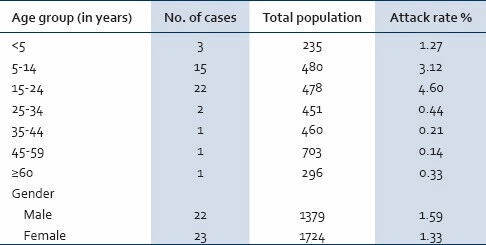
The line list developed consisted of 45 cases. Attack rate was highest among the age group 15-24 years (4.6%) followed by 5-14 years (3.1%) and 0-5 years (1.2%) [Table 1]. Epidemic curve drawn based on date of onset of jaundice depicted a sharp increase in the number of cases during March 4-10th (12 cases) and 11-17th (21 cases), suggestive of a common source outbreak. Neither deaths nor fatal complications were reported.
Table 1.
Incidence of acute hepatitis A cases by age and sex (N = 45)
There were clustering of cases in four streets; it was noticed that all the four streets received public water supply from a common pipeline. The geographical distribution of the cases suggested a clustering around the water supply through the pipeline. There was one adjacent street free of hepatitis cases, and the residents reported that the public water supply had been damaged in the street for past 4 months. Furthermore, noteworthy was a housing complex free of cases in the midst of clustering, which had not received public water supply yet.
Water supply was intermittent in the pipeline. Water was pumped directly from a bore well and there was no overhead tank. It was noticed that chlorination was stopped since last week of January, due to complaints from the general public regarding distaste of water. Residents reported absence of smell of chlorine in pipeline water for past few months. The valve of the pump was found broken and there was water stagnated around. Residents observed small breaks in the pipeline in prior months. There were open drains found running parallel to water pipes. The septic tanks of a nearby big 700 bedded hospital were constructed on a marshy land, which is alleged to have badly constructed percolation systems sometimes allowing waste water to escape without proper treatment and pushing leaks to the open drains in the village.
None of the other hypothesis-generating interviews led to suspect any other event or factors that could explain the outbreak. Microbiological analysis of the water samples collected during the outbreak showed that all the pipe water samples and one well water sample had shown presence of Escherichia coli in pure culture. None of the samples tested had residual chlorine. The possibility of occurrence of hepatitis A outbreak as a result of pipe water contamination supplied from the bore well was considered to be the hypothesis.
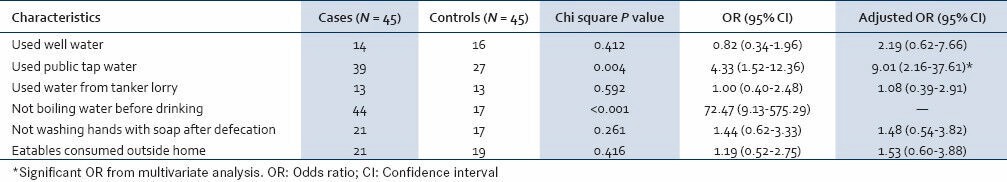
The results of analysis of case-control studies are shown in Table 2. Only one among the cases had the habit of drinking boiled water, while 72.6% of the controls used to drink boiled water (P < 0.001). In the study, 86.6% of the cases and the 60% of the controls used pipe water (OR: 4.33 [95% CI: 1.52-12.36]). Using water from own well and from tanker lorries were not associated with hepatitis A cases. For exposure to pipeline water supply, there were 3 (16.6%) pairs where the control was exposed to the risk factor but the case was not, and 15 (83.3%) pairs where the case was exposed to the risk factor but the control was not (OR: 5 [95% CI: 1.41-26.94]). Logistic regression was done by entering age, gender, water consumption from well, pipeline, tanker lorry, not washing hands after defecation and eatables consumed outside in a backward conditional logistic regression model. As not boiling water was very strongly associated with hepatitis A cases, it was not entered in to the regression model. In the final model, having consumed water from pipeline was significantly associated with HAV disease (adjusted OR: 9.01 (95% CI: 2.16-37.61)).
Table 2.
Factors associated with hepatitis A cases
DISCUSSION
India was considered as hyperendemic region for HAV infection with very high infection rates in the early years of life.[13,14,15] An epidemiological transition has been observed about the HAV infections transmission in India, from hyperendemicity to intermediate endemicity with a decline in HAV infection rate in children and increase in the number of susceptible adults.[16,17] A few recent hospital-based studies from India suggest that the prevalence of anti-HAV antibodies among Indian adults has declined to <70%, possibly due to improved sanitation and urbanization.[18,19,20]
Kerala is one state where early and rapid socioeconomic development and urbanization happened. The HAV antibody sero prevalence rates reported from Kerala was <10% in children below 5 years when compared to 60-80% from many other parts of the country.[18,21,22,23,24] Improvement in hygienic and socio-economic conditions in the state might have resulted in a decrease in the number of natural childhood infections. An epidemic of hepatitis A in the age range of 2-75 year was reported from central Kerala in 1998. Out of 399 cases of acute hepatitis A during that outbreak, majority (65%) were in the age range of 15-33 year.[25] In 2004, an epidemic of hepatitis A occurred in Kottayam district of Kerala, which also mainly involved young adults.[7] The age group affected in the current outbreak was same as in the previous two huge hepatitis A outbreaks reported from the state, adding evidence to the fact that a substantial proportion of individuals were not exposed to HAV until adulthood. These outbreaks of hepatitis A in young adults from Kerala are suggestive of a region with intermediate HAV endemicity. These findings reiterate the fact that huge outbreaks of hepatitis A have to be expected in the state in coming years.
Millennium Development Goal had its 7th goal with one of its objectives to halve, by 2015 the proportion of the population without sustainable access to safe drinking water. The key to providing microbiologically safe drinking water lies in understanding the various mechanisms by which water gets contaminated, and formulating interventions at critical points to decrease and prevent contamination of drinking water. In the present study, the time frame of disease occurrence, environmental observations, anecdotal evidences, laboratory results and results of the analytical study indicated the probability of occurrence of hepatitis A outbreak as a result of pipe water contamination supplied from a bore well. Similar cases of pipe line contamination and abstinence from chlorination of water, which led to hepatitis E had been reported in the past from different parts of India.[26,27,28] A model guideline regarding proper planning and execution of water supply and drainage systems is needed. Periodic monitoring and stricter implementation of these guidelines have to be ensured.
In a country like India with an extensive variations and heterogeneity in the determinants of acquiring anti-HAV antibodies, a unified approach for vaccination would appear epidemiologically inappropriate.[17] Routine vaccination is recommended in populations who remain unexposed to the HAV infection during early childhood. Small localized or large outbreaks of HAV infection will remain a threat in areas like Kerala where an obvious epidemiological transition is happening. Universalizing HAV vaccination could prevent the disease incidence in community, but the cost of vaccine would be a limiting factor. In Kerala, families who can afford should be advised to consider immunizing their children with hepatitis A vaccine. The situation demands capturing epidemiological data regarding HAV systematically and economic analysis of initiating universal HAV vaccination in the state.
Community-wide outbreaks of HAV infection are often prolonged and difficult to control. Usually they persist for 6-18 months, until the pool of susceptible persons is exhausted.[29,30,31] Control measures were intensified from the next day of reporting the current outbreak which included daily chlorination of wells for a week followed by weekly chlorination. The chlorination in pipeline was restarted on March 23rd after interference from District Collector. The decline in cases was noticed since April 1st week. The current outbreak at Mylapore is at its tail end with a total of 129 cases until July 30th . The epidemic curve depicted that person-to-person transmission might be happening after April 15th with a small peak at regular interval of 3 weeks [Figure 1]. Intensive health education campaign, focusing on water treatment at household level and personal hygiene, was launched.
Figure 1.
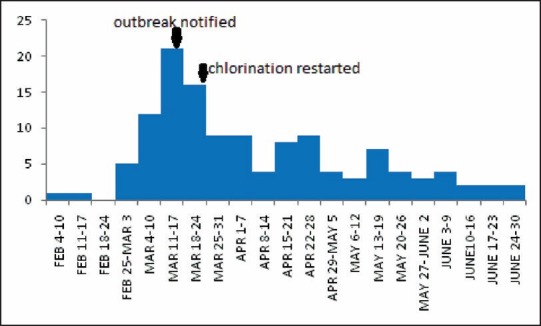
Epidemic curve showing number of cases of hepatitis A by week of onset, Mylapur, Kollam (February 4 to June 30, 2013)
The outbreak was investigated as soon as the intimation regarding the outbreak was obtained. There was an obvious failure in the surveillance system to pick up the outbreak earlier. Furthermore, nobody identified the problem in time to do anything about the event that led to the exposure. This investigation was helpful in documenting the missed opportunities for prevention of this outbreak. The investigation helped in eliminating the common source of outbreak, convincing the health workers regarding the need for proper surveillance, the water authority regarding their role in preventing outbreaks, the local self-government regarding the importance of sanitary regulations and proper licensing mechanisms.
The study had several limitations. Attempts for isolating HAV from water samples were not done for want of laboratory infrastructure. Case definition was narrow, and so many mild cases would have been missed. Selection of controls proved tough with age and gender matched neighborhood controls, as there were chances of asymptomatic or subclinical presentation of infection. Ideally, serology should have been done to exclude infections among controls. No controls selected were affected with the disease until July 30th . Further investigation has not been conducted to prove the person-to-person transmission. Despite these limitations, the study has public health implications and will add on evidences to the epidemiology of hepatitis A in the state. Well-structured and uniform protocols to monitor the HAV sero prevalence and disease burden are necessary to capture the changing exposure profiles of the population.
To conclude, there was an outbreak of hepatitis A in Mylapore village, Kollam district, Kerala affecting mainly youths. The outbreak was probably due to contamination of pipeline water supply in the area and the study warrants establishment of an efficient water quality surveillance system and a model guideline regarding proper planning and execution of water supply and drainage systems.
ACKNOWLEDGMENTS
Primary Health Care Team, Thrikkovivattom PHC, Data Management team IDSP, Kollam, State Public Health Laboratory, Kerala, India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Hepatitis A. Fact sheet 328. Geneva: World Health Organization; 2012. [Last accessed on 2013 Jul 30]. Available from: http://www.who.int/mediacentre/factsheets/fs328/en/ [Google Scholar]
- 2.Hollinger FB, Ticehurst JR. Hepatitis A virus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3rd ed. Philadelphia: Lippincott-Raven; 1996. pp. 735–82. [Google Scholar]
- 3.Stapleton JT, Lemon SM. Hepatitis A and hepatities E. In: Hoeprich PD, Jordan MC, Ronald AR, editors. Infectious Diseases. 5th ed. Philadelphia: Lippincott Co; 1994. pp. 790–7. [Google Scholar]
- 4.Lemon SM. Type A viral hepatitis: Epidemiology, diagnosis, and prevention. Clin Chem. 1997;43:1494–9. [PubMed] [Google Scholar]
- 5.Lemon SM. Hepatitis A virus. In: Webster RG, Granoff A, editors. Encyclopedia of Virology. London: Academic Press Ltd; 1994. pp. 546–54. [Google Scholar]
- 6.Gust ID. Epidemiological patterns of hepatitis A in different parts of the world. Vaccine. 1992;10(Suppl 1):S56–8. doi: 10.1016/0264-410x(92)90544-t. [DOI] [PubMed] [Google Scholar]
- 7.Arankalle VA, Sarada Devi KL, Lole KS, Shenoy KT, Verma V, Haneephabi M. Molecular characterization of hepatitis A virus from a large outbreak from Kerala, India. Indian J Med Res. 2006;123:760–9. [PubMed] [Google Scholar]
- 8.Chobe LP, Arankalle VA. Investigation of a hepatitis A outbreak from Shimla Himachal Pradesh. Indian J Med Res. 2009;130:179–84. [PubMed] [Google Scholar]
- 9.Sowmyanarayanan TV, Mukhopadhya A, Gladstone BP, Sarkar R, Kang G. Investigation of a hepatitis A outbreak in children in an urban slum in Vellore, Tamil Nadu, using geographic information systems. Indian J Med Res. 2008;128:32–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Chadha MS, Lole KS, Bora MH, Arankalle VA. Outbreaks of hepatitis A among children in western India. Trans R Soc Trop Med Hyg. 2009;103:911–6. doi: 10.1016/j.trstmh.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Integrated Disease Surveillance Project. New Delhi: Ministry of Health and Family Welfare; [Last accessed on 2013 Jun 30]. Government of India. Available from: http://www.idsp.nic.in/ [Google Scholar]
- 12.Murhekar M, Moolenaar R, Hutin Y, Broome C. Investigating outbreaks: Practical guidance in the Indian scenario. Natl Med J India. 2009;22:252–6. [PubMed] [Google Scholar]
- 13.Batra Y, Bhatkal B, Ojha B, Kaur K, Saraya A, Panda SK, et al. Vaccination against hepatitis A virus may not be required for schoolchildren in northern India: Results of a seroepidemiological survey. Bull World Health Organ. 2002;80:728–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–50. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Jindal M, Rana SS, Gupta RK, Das K, Kar P. Serological study of hepatitis A virus infection amongst the students of a medical college in Delhi & evaluation of the need of vaccination. Indian J Med Res. 2002;115:1–4. [PubMed] [Google Scholar]
- 16.Kar P. Is there a change in seroepidemiology of hepatitis A infection in India? Indian J Med Res. 2006;123:727–9. [PubMed] [Google Scholar]
- 17.Mathur P, Arora NK. Epidemiological transition of hepatitis A in India: Issues for vaccination in developing countries. Indian J Med Res. 2008;128:699–704. [PubMed] [Google Scholar]
- 18.Mall ML, Rai RR, Philip M, Naik G, Parekh P, Bhawnani SC, et al. Seroepidemiology of hepatitis A infection in India: Changing pattern. Indian J Gastroenterol. 2001;20:132–5. [PubMed] [Google Scholar]
- 19.Das K, Jain A, Gupta S, Kapoor S, Gupta RK, Chakravorty A, et al. The changing epidemiological pattern of hepatitis A in an urban population of India: Emergence of a trend similar to the European countries. Eur J Epidemiol. 2000;16:507–10. doi: 10.1023/a:1007628021661. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan PS, Shah SS, Alvares JF, Kher A, Shankaran, Kandoth PW, et al. Seroprevalence of hepatitis A virus in Mumbai, and immunogenicity and safety of hepatitis A vaccine. Indian J Gastroenterol. 1998;17:16–8. [PubMed] [Google Scholar]
- 21.Mathew P, Bobba R, Zacharias P. Hepatitis A seroprevalence in Kerala. Indian J Gastroenterol. 1998;17:71–2. [Google Scholar]
- 22.Tandon BN, Gandhi BM, Joshi YK. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B virus infection in North India. Bull World Health Organ. 1984;62:67–73. [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal SK, Rastogi A, Rastogi A, Kumar N, Talukdar B, Kar P. Seroprevalence of hepatitis A in children - Implications for hepatitis A vaccine. Trop Gastroenterol. 1998;19:120–1. [PubMed] [Google Scholar]
- 24.Dutta AK, Aggarwal A, Kapoor AK, Ray GN, Batra S. Seroepidemiology of hepatitis A in Delhi. Indian J Pediatr. 2000;67:77–9. doi: 10.1007/BF02726169. [DOI] [PubMed] [Google Scholar]
- 25.Sebastian B, Mathai S, Mathew G, Ouseph M, Balakrishnan P. An outbreak of hepatitis A in central Kerala-Clinical profile. Indian J Gastroenterol. 1998;17:10. [Google Scholar]
- 26.Das P, Adhikary KK, Gupta PK. An outbreak investigation of viral hepatitis E in South Dumdum municipality of Kolkata. Indian J Community Med. 2007;32:84–5. [Google Scholar]
- 27.Swain SK, Baral P, Hutin YJ, Rao TV, Murhekar M, Gupte MD. A hepatitis E outbreak caused by a temporary interruption in a municipal water treatment system, Baripada, Orissa, India, 2004. Trans R Soc Trop Med Hyg. 2010;104:66–9. doi: 10.1016/j.trstmh.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Martolia HC, Hutin Y, Ramachandran V, Manickam P, Murhekar M, Gupte M. An outbreak of hepatitis E tracked to a spring in the foothills of the Himalayas, India, 2005. Indian J Gastroenterol. 2009;28:99–101. doi: 10.1007/s12664-009-0036-x. [DOI] [PubMed] [Google Scholar]
- 29.Gildon B, Makintubee S, Istre GR. Community-wide outbreak of hepatitis A among an Indian population in Oklahoma. South Med J. 1992;85:9–13. doi: 10.1097/00007611-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Majeed FA, Stuart JM, Cartwright KA, Room R, Gilkes JR, Smith MC, et al. An outbreak of hepatitis A in Gloucester, UK. Epidemiol Infect. 1992;109:167–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Pavia AT, Nielsen L, Armington L, Thurman DJ, Tierney E, Nichols CR. A community-wide outbreak of hepatitis A in a religious community: Impact of mass administration of immune globulin. Am J Epidemiol. 1990;131:1085–93. doi: 10.1093/oxfordjournals.aje.a115601. [DOI] [PubMed] [Google Scholar]


