Abstract
Ascariasis mainly contributes to the global helminthic burden by infesting a large number of children in the tropical countries. Hepato-biliary ascariasis (HBA) is becoming a common entity now than in the past owing to the frequent usage of ultrasonograms and endoscopic diagnostic procedures in the clinical practice. There are a variety of manifestations in HBA and diagnosis depends on a high index of suspicion in endemic areas coupled with subsequent confirmation by sonographic or endoscopic demonstration of the worm. Most of them present with acute abdomen and jaundice. Oriental or recurrent pyogenic cholangiopathy is possibly the result of HBA, commonly encountered in South-East Asian countries. Conservative treatment with anthelminthic agents is used in the majority. Failure to respond to medical therapy usually indicates the need for endoscopic or surgical interventions. Overall, mortality is low and prognosis is good, but many epidemiological and immunological aspects of Ascaris infection are unclear, meaning our understanding the disease and infection still remains incomplete. Therefore, it is difficult to definitely put down a fixed modality of treatment for HBA. This underscores the need for further studies as ascariasis has the potential to adversely affect the national socio-economy by compromising the health of children and adults alike with its sheer number.
Keywords: Ascaris lumbricoides, Biliary ductal ascariasis, Clinical manifestations, Complications and principles of treatment Hepatobiliary ascariasis, Pathophysiology, Recurrent pyogenic cholangitis, Review of pathogenesis, Roundworm infestation
INTRODUCTION
Ascaris lumbricoides is the largest common nematode causing human ascariasis[1] and 33% of the world population are estimated to be infested with it.[2] It is mentioned in ancient Greko-Roman and Chinese texts, making it probably the earliest record of helminthic infection of mankind. The first scientific description of the genus Ascaris was given by Linnaeus in 1758, followed a century later by Epstan and Grassi who showed that the infection is preceded by ingestion of eggs.[3]
Ascarias is common in tropical countries with low standards of hygiene, malnutrition, heavy rainfall and where untreated sewage is discharged into rivers, lakes and agricultural land or is used as fertilizer. The clinical disease spectrum comprise of pulmonary, intestinal (including intestinal obstruction), appendicular, hepatobiliary and pancreatic ascariasis. However human ascariasis is silent in the majority of infected persons or only associated with vague abdominal symptoms. In children, it can lead to stunted growth, impaired learning, protein-energy and vitamin deficiencies.[4,5]
It is estimated that about 60 million of those infected are at risk of developing some form of morbid disease.[6] However, clinical disease occurs with heavy worm loads (13-40 worms) and around 10,000-20,000 deaths occur annually globally due to severe disease.[6,7,8] Epidemics of ascariasis can occur.[9] It is important to separate “infection” from “disease” and recognize the magnitude of the problem especially while formulating control and eradication strategies.
LIFE CYCLE
Man is infected by ingesting food, raw vegetables or water contaminated by mature ova. Children are mostly infected by contaminated fingers, toys and soil. To complete their life cycle, the worms leave the human body as eggs and re-infect it as larvae. The eggs hatch in the duodenum after being stimulated by gastric juice and the resultant rhabditiform larvae migrate to the cecum. They penetrate the epithelium to reach the portal vein and then the liver. Some will migrate through the hepatic veins or the lymphatics to be carried to heart and lungs. There they cross the capillary wall into the alveolar space and reach the bronchial tree. They molt twice during this journey and ascend to the larynx and hypopharynx before being swallowed.[10,11] In the upper gastrointestinal tract, they attain sexual maturity by 2-3 months and molt again to become adult worms. The adult worm resides in the jejunum as a facultative anaerobic organism, with a life-span of 6-18 months. Since adult worms do not multiply inside their host, manifestation of clinical disease depends on their absolute number. Female worms are bigger; approximately 20-40 cm and can produce a large number of eggs daily (~240,000/female) which pass out in the feces.[3] In the soil the fertilized eggs require 10-15 days to become infectious. Clay soil favors their survival. The eggs are resistant to cold weather, chemical water purifiers, disinfectants and can remain viable and infectious for up to 10 years[12] making eradication difficult.
EPIDEMIOLOGICAL CONSIDERATIONS
The world-wide distribution of A. lumbricoides has resulted in 1.4 billion people being infected with it[13] and most of them belong to South East Asia. However, it has acquired the character of a global disease due to increases in international travel.[6] Still the major burden is felt by the tropical countries which have moist soil and good rain. In the tropics, up to 70% of the children are found to be infected.[14] South-East Asian countries and China show prevalence rates of 41-92%[15] while in parts of Africa, it is about 95%.[16] Bangladesh is also highly endemic with a prevalence rate of 82%. In India, high prevalence rates are found in Tamil Nadu (85%)[17] and Kashmir (70%).[12] Table 1 shows the prevalence of roundworm infection as reported by some workers from different parts of India.[18,19,20,21,22,23,24,25,26] According to a World Health Organization report, advanced countries have the lowest rate of infection, and immigrants from endemic countries contribute to the bulk of infection there.[27] Still in some rural parts of Europe, the prevalence may reach as high as 52%.[12] This may be due to the so called “stratified” distribution[28] because the infection rates are found to be highly segregated in certain population within the same region.
Table 1.
Prevalence of Ascariasis in different Indian Studies
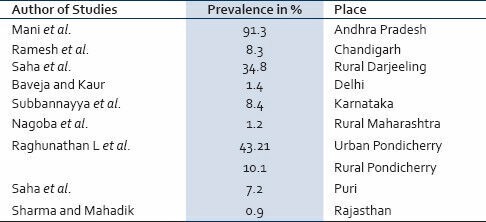
PREVALENCE AND PROPAGATION OF INFECTION
Prevalence of ascariasis is directly proportional to the population density in a region (overcrowding), sanitation status, educational level, use of untreated sewage/human excreta as manure for vegetable production, dietary and personal hygiene (e.g., eating unwashed food). 30% of adults and 60-70% of children harbor the adult worm in high endemic areas. In general, socio-economic improvement is associated with a falling prevalence. For example, Japan had prevalence of 80% after World War II which drastically fell to 0.04% in 1992; whereas the high endemicity in lesser developed Latin American countries over the same period remained almost unchanged.[29] This indicates that improvement in living standards, which is inversely proportional to the prevalence and infection rates in the society, is an important factor in control strategy. One way of propagating the infection is by “seeding” of the soil through eggs present in the feces of small children, who are re-infected by the eggs while playing in the contaminated soil.[3,13] There seems to be an age related change in the intensity of infection in the same individual.[30] In endemic areas, the prevalence rises at 2-3 years of age becoming maximum by the age of 8-14 years. Then it declines to a much lower level in the adult.[31]
Intensity of infection and reinfection
The observation, that clinically manifest diseases are restricted to a relatively small segment of those infected (1.2-2 million per annum in endemic zones worldwide)[12,32] with only a heavy worm load, means that there exists an uneven, “stratified” distribution of the infection demonstrating a negative binomial distribution.[33] This may be partly due to an increased susceptibility (genetic, behavioral or spatial) to acquire heavy infection in some than in others.[33,34,35,36] However, the reasons for such individual susceptibility is not definitely known.[30] Immunity is an important determinant of heavy infection serving as a regulatory mechanism in natural infection[16] although protective immunity is incomplete.[37] Studies are exploring the possible contribution of the host immunological reactions in influencing the global helminth burden.[38,39] Continuous exposure to infective eggs leading to progressive accumulation of worms over years also plays a major part in producing heavy infection. In endemic areas children are more likely to show heavy infection than adults (70% vs. 49%).[12] Of these, 80% get re-infected within 6 months of eradication therapy. This is found to be commoner in those who had prior heavy infection, implying individual susceptibility.[36]
HEPATO-BILIARY ASCARIASIS (HBA)
This is one type of human ascariasis, which is seen more commonly now in the endemic zones and in the earlier days the diagnosis was made either at autopsy or at laparotomy. Even then, the magnitude of the problem was probably underestimated because the worms move in and out of the bile ducts actively from the duodenum and therefore many would have been absent from the biliary tree at the time of surgery.[12] Since the late 80's and early 90's increasing number of reports from several parts of the world has drawn attention to this entity[40,41,42,43] especially as a cause for common bile duct (CBD) obstruction and stricture.[44,45] In Indian studies from Kashmir, at highly endemic area, ascariasis was found to be the cause in 36.7% cases of 109 patients with proven biliary and pancreatic disorders.[46,47] HBA is quite frequently seen in children in South Africa while in Philippines, 20% of all biliary diseases are reported to be due to dead or live worms.[48] However in one series from Middle-East, there were only two cases of biliary ascariasis found in 668 Jordanian patients evaluated by endoscopic retrograde cholangiopancreatography (ERCP) for biliary/pancreatic disease and unexplained upper abdominal pain.[49]
A. lumbricoides has a natural inclination to migrate and seek small orifices.[50] Heavy worm infestation or other intestinal infections of viral, bacterial or parasitic origin (leading to altered gut motility) are the usual pre-requisites to reach the duodenum from their natural habitat-the jejunum. However, host reaction to an adult worm can by itself alter the vasomotor reflexes and secretory responses which in turn affect the intestinal tone and motility.[51] From the duodenum, it can enter the ampulla of Vater to lodge: (a) In the ampulla itself (b) the CBD or (c) the hepatic ducts or anywhere in the biliary tree. It can also enter the orifice of the cystic duct and block it while traversing the CBD, but relatively rarely enters the gall-bladder or the pancreatic duct.
CLINICAL FEATURES
There is a female preponderance (F:M ratio of 3:1) in HBA as studies have shown higher prevalence of roundworm infestation in females.[21,52] HBA is commonly seen in the mid-thirties with a range of 4-70 years.[53] It is less common in children because they tend to present more with intestinal rather than biliary obstruction. This may partly be due to very small caliber of the biliary system in children.[54] Persons who may be at a greater risk of developing HBA include:
-
a)
Those who had prior biliary surgery (cholecystectomy, choledocholithotomy, sphincteroplasty, endoscopic sphincterotomy.[44,45,55]
-
b)
Pregnant when compared with non-pregnant women[56] probably owing to hormonal effects on the ampula during the pregnancy.
-
c)
Disturbance of the environment around the worm e.g., fever, anesthetics and tetrachlorethylene.[16]
Modes of presentation
Biliary colic in HBA presents as acute onset right hypochondrial pain which may be recurrent or continuous lasting a few days. It occurs due to entry of the worm into the ampullary orifice from the duodenum. Cholangitic features such as shaking chills, fever and mild jaundice are seen only occasionally.
Acute cholangitis in HBA is an emergency[53] , presenting with high grade fever, chill, icterus and upper abdominal pain. On examination, there is hypotension, tender hepatomegaly, leucocytosis, raised bilirubin (mostly conjugated) and raised liver enzymes - especially serum alanine aminotransferase and alkaline phosphatase. Those going on to develop pyogenic cholangitis, pus forms, which may be seen at the ampullary orifice or can be aspirated by ERCP.
Acute cholecystitis is suspected by right hypochondrial pain and guarding, vomiting and fever. The pain may be referred to the interscapular area or the tip of the right shoulder. Tenderness and a palpable mass in the right hypochondrium may be present. The temperature is usually of low grade and there is no shock. The gall-bladder reveals thickened wall with distension and biliary sludge is usually found.[57]
Hepatic abscess may be solitary or multiple and contains pus. There is tender hepatomegaly, high fever, intercostal tenderness and edema along with right hypochondrial pain. These abscesses may result from dead ova released by female worms migrating up the CBD, producing a granulomatous inflammatory reaction with subsequent breakdown with eosinophil infiltration. It may be commoner in children.[16]
Hemobilia can very rarely occur as a result of biliary ascariasis.[58]
DIAGNOSIS
The diagnosis depends upon demonstrating the worm in the biliary tree in a clinical set-up compatible with the conditions described above, especially in an endemic zone. This is not always easy because frequently most of the worms move in and out of the ducts within 7 days.[12] Ultrasonography is a highly sensitive and specific in visualizing a worm in the biliary system, as well as monitoring its mobility to and from the ducts over time.[12,45,59,60] A worm, which has not changed its position after 10 days in the duct system, is usually a dead and macerated one. The drawback of ultrasonography is in not being able to detect worms in the duodenum or the ampullary orifice and thereby has been reported to miss up to 50% cases of HBA.[53] ERCP is helpful in these situations both for diagnostic and therapeutic aspects.[42,61,62] The worms appear commonly as linear, smooth filling defects with or without characteristic movements but without distal acoustic shadowing, and may also be seen as parallel filling defects “Railway tract” sign,[63] curved defects or transverse loops across the ducts.[40,64] Worms in the gall-bladder appear as long tubular coiled echogenic structure which may be rapidly mobile and is easier to diagnose than biliary ductal ascariasis.[56] Computed tomography (CT) will reveal the worms as cylindrical structures.[62] Sometimes CT may be used for better visualization of the dilated ductal system.[59]
Stool examination may show Ascaris eggs in stool. Many a times the patient passes an adult worm with vomitus or with stool.
Peripheral eosinophilia, due to larval invasion of the blood, is very common.[1,10,11] Aspiration of the pus from hepatic abscesses may reveal Ascaris ova[53,65] because larval stages or the ovas are more likely to produce inflammation leading to granulomatous necrosis than adult worms.[51]
Although antibodies against ascariasis develop in infected persons, they are not of much help in the immuno-diagnosis, owing to extensive cross-reactivity with other helminthic antigen.[16]
RELATIONSHIP OF BILIARY LITHIASIS AND ASCARIASIS
In general, only brown pigment stones are associated to some extent with infections of the gall-bladder.[66] In the tropics gall-stones were considered to be relatively rare due to higher dietary fiber, compared to the western diet.[67] However, in Asian population, gall-stones are now increasingly being found to be of either the cholesterol or the black (rather than brown) pigment variety, a pattern similar to that in the West.[68] It is well-known that any biliary obstruction (complete, incomplete or recurrent) leads to impaired bile drainage with secondary bacterial infection and bacterial infection has been reported in about 66% cases of CBD stones.[69] The common offending organism is usually Escherichia coli which (and also other bacteria) produce beta-glucoronidase and it deconjugates the bilirubin glucoronides in bile.[70,71] The resultant unconjugated bilirubin precipitates with calcium to form calcium bilirubinate stones in the future. In addition, it also serves as a nidus for cholesterol stones.[70] As already mentioned, biliary sludge is often seen in HBA presenting with acute acalculous cholecystitis. The biliary sludge is composed of cholesterol crystals, mucin and calcium bilirubinate granules.[72] This provides an appropriate milieu for future gall-stone formation[73] and adult worm, ova and larvae can all initiate bile duct stones.[74] Indeed, part of macerated dead worms were found to form nidus of such stones in patients with HBA when followed-up for several years, and these stones usually were composed of calcium bilirubinate layers[53,55] although the eggs of the worm can also serve as a nidus.[43] But gall-bladder and CBD stones were rarely found in patients with previous HBA and instead tended to occur in the intrahepatic biliary ducts, as reported in one large study from India involving 500 patients of HBA.[12] This may in part be due to the relative lack of propensity of the worm to enter the gall-bladder as mentioned earlier. In contrast, in the far east, primary CBD stone are seen quite frequently to follow bacterial infection secondary to biliary ascariasis.[75] Strong epidemiologic correlations between ascariasis and the entity of recurrent pyogenic cholangitis (RPC) exist.[43]
Relationship with RPC
This condition was first described in 1954 in Hong Kong and is also called “Asiatic or Oriental cholangio-hepatitis” and is seen commonly in Hong Kong, Taiwan, South China, Korea and South East Asia.[75] It is also increasingly being seen in the west, and is probably a result of the migration of Oriental Nationals to these countries.[76] It is characterized by biliary sludge, intrahepatic bile duct stones and chronic secondary bacterial infection. The subjects are thin, young and occasionally malnourished. Recurrent upper abdominal pain, cholestatic jaundice and fever with chill are characteristic and the frequency of such recurrent attacks increase over time. Cholangiograms will demonstrate both the intra and extra hepatic biliary tree to be filled with soft “biliary mud”. The biliary radicles are dilated with excessive branching and single or multiple biliary strictures of variable length.[12] In many patients with severe distortion of biliary ducts, recurrent episodes of cholangitis appear to be self-perpetuating in absence of active ascariasis. In longstanding cases, hepatic abscess and scarring may result.[75] This is exacerbated by recurrent biliary sepsis as a result of papillitis and sphincter of Oddi motor dysfunction which is probably related to the mechanical injury of the papilla caused by the worm invading the orifice.[77] In addition, there is a breakdown of normal defense mechanisms and the enteric bacterial flora may reach the intrahepatic bile tree via the portal system.[76] RPC and ascariasis shows similar geographical distribution[78,79] and over 5% of HBA develop RPC after 2 or more years. Stones are common in RPC (90% cases) and 50% occur in CBD or common hepatic ducts whereas 15% of them have stones in the gall-bladder.[76] Conversely 10% of RPC have a definite evidence of ascariasis.[43]
The stones in RPC are pigment stones with layers of bilirubinates deposited on top of a nidus. More importantly, the nidus of biliary stones in 72% of RPC is formed by part or whole of Ascaris worm which confirms the significant role of this helminth in cholelithiasis.[43,80] All these strongly imply that hepatic duct stones found in RPC may be an aftermath of biliary damage by biliary ascariasis especially recurrent, frequent in endemic zones.
TREATMENT OF HBA
The patients with HBA are to be hospitalized without delay because in them the worm load is usually high. In addition, co-existing mechanical intestinal obstruction are common (especially in young children) but may also follow deworming during or after institution of treatment. It must be remembered that excretion products of the worms can cause marked bowel contraction.[81] Similarly, associated acute pancreatitis may complicate the clinical course and there is a definite mortality risk in those with hemorrhagic pancreatitis.[41]
Pure biliary ascariasis have a negligible mortality <2%.[82] The principles of treatment of biliary ascariasis are:[83]
Treatment of cholangitis or cholecystitis by conservative means.
Oral administration of anthelminthics, which allows the paralyzed worms to be expelled by normal intestinal activity.
Endoscopic and Surgical treatment.
Coexistent obstructive jaundice and intestinal obstruction in documented cases of HBA is usually an indication for surgery.[4]
Conservative treatment includes broad-spectrum antibiotics, analgesics, intravenous fluid and electrolytes and most of the acute acalculous cholecystitis patients recover without any complications.[47,53,61] However in acute pyogenic cholangitis, more specific antibiotics are indicated depending on the biliary pus culture and sensitivity results. The pus is obtained by duodenoscopy or ERCP from the pus points in the papillary orifice or bile aspiration respectively. Other common therapeutic measures to treat endotoxic shock are also to be instituted including correction of metabolic acidosis. However most of them require some form of interventional treatment to improve morbidity and mortality.
Chemotherapy: An ideal antihelminth should be:[30]
Safe at high therapeutic dosage,
Inexpensive, easily available and easy to administer orally,
Stable and effective for a long time in different climatic conditions.
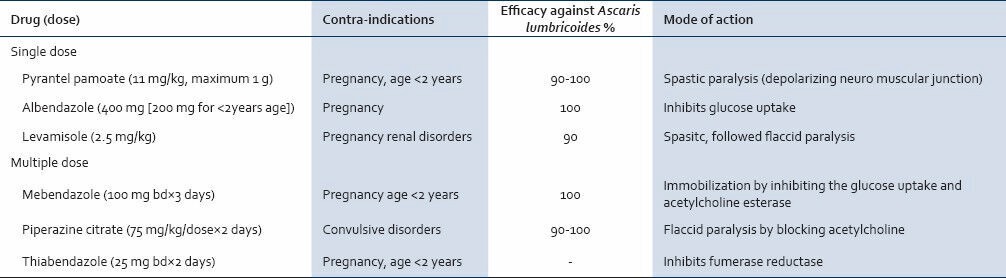
Oral anthelminths act by paralyzing the adult worm but none can affect the larval stage. They are administered only if the patient has passed flatus or feces. The worm clearance is usually completed by 3 days in most cases depending on the gut transit time, pre-existing diarrhea and worm load.[84] Preferably a soluble preparation is given.[85] Direct instillation of anthelminthic e.g., piperazine citrate in the biliary tree surgico-endoscopically is not helpful and is not recommended.[12] Treatment failure may occasionally occur and persistent eosinophilia should alert one to this possibility.[86] The names, dosage and important contraindications are mentioned in Table 2.[87,88,89]
Table 2.
Efficacy of anti-ascarial drugs with their mode of action
Recently, an oriental study involving 50 cases of infected biliary ascariasis has claimed 96% efficacy using Chinese herbal medicine and acupuncture.[90]
Endoscopic and surgical interventions are indicated when patients do not respond to energetic conservative treatment within few days after hospitalization or when the worm is not expelled from the biliary tree after 3 weeks despite vermifuge.[12,91] Acute pyogenic cholangitis needs biliary decompression or drainage in most cases.[47,61,77,92,93] According to some, cholangitis with biliary strictures or with worms in the gall-bladder are also indications for surgery.[94] Endoscopic worm extraction from ampullary orifice rapidly relieves the symptoms in biliary colic.[47,53,61] This may also be necessary in acute pyogenic cholangitis as an urgent measure. In almost 100% cases, endoscopic worm extraction from the ampulla is successful and from the bile ducts in 90% cases by using the endoscopic basket. The complications of endoscopic procedures in such cases are low (6%) consisting mainly of hypotension and cholangitis.[61] Therefore endoscopic extraction of the worms by snares, dormia basket or biopsy forceps is becoming the treatment of choice in biliary ascariasis.[94]
Percutaneous needle drainage under ultrasound guidance or rarely surgically is necessary in hepatic abscesses which are large.
Gall-bladder ascariasis usually requires cholecystectomy; but as a whole it is encountered less frequently than bile duct ascariasis.[95] Laparotomy is indicated if ERCP is not available for worm extraction in the patients who deteriorate during hospitalization. It must be remembered that acute pancreatitis, intestinal obstruction with complications (like volvulus, gangrene or perforation) may be present alongwith HBA which can be identified on hospitalization by ultrasonographic and biochemical studies.
In RPC, recurrent cholangitis with obstructing stones may be managed by placing a Roux-en-Y jejunal conduit for biliary access.[96]
CONCLUSION
HBA common in endemic zone and mostly presents with acute pain abdomen and they are diagnosed by ultrasonography or ERCP, but many cases are probably missed because of active migration of the worm to and from the biliary tree. They carry a good prognosis and respond to conservative therapy with oral anthelminthics. In non-responders (acute pyogenic cholangitis, worm in gall-bladder), endoscopic and surgical removal of the worm is necessary. Associated intestinal obstruction and acute pancreatitis should be looked for especially in children. Long-term effects of HBA include RPC and in some cases liver abscesses. There may be an association with biliary lithiasis/gall-stones with HBA in our region but it needs confirmation.
There is still lack in understanding of some epidemiological aspects of ascariasis namely a non-uniform mode of infection in the same community and the unknown genetic or environmental factors which make an individual more susceptible to heavier infection than others. Questions regarding the universal mode of management of certain sub-groups of HBA (e.g., in acalculous cholecystitis) remain to be answered whether urgent surgical intervention should be resorted to or not, or how long should one wait in such a patient who does not improve with conservative management. In our country (high endemic zone), finding a patient with g all-bladder sludge, ascariasis and/or symptoms suggestive of HBA pose another problem regarding their future mode of management should cholecystectomy be done or is vermifuge only needed if the patient responds to medical treatment? If cholecystectomy is contemplated in view of the possibility of future development RPC, then should it be an elective procedure? How often should they be followed-up? Cost factors, the degree of potential disability, risks and benefit of surgical interventions in these situations need further probing. More pointed indications of conservative versus operative procedures should be formulated and works in this direction needs to be carried out in well controlled studies in future.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gutierrez Y. Ascaridida - Ascaris, Lagochilascaris, Anisakis, Pseudoterranova and Baylisascaris. In: Gutierrez Y, editor. Diagnostic Pathology of Parasitic Infections with Clinical Correlations. Philadelphia: Lea & Febiger; 1990. pp. 236–47. [Google Scholar]
- 2.Bundy DA, Cooper ES, Thompson DE, Anderson RM, Didier JM. Age-related prevalence and intensity of Trichuris trichiura infection in a St. Lucian community. Trans R Soc Trop Med Hyg. 1987;81:85–94. doi: 10.1016/0035-9203(87)90293-8. [DOI] [PubMed] [Google Scholar]
- 3.Ascariasis. Lancet. 1989;1:997–8. [PubMed] [Google Scholar]
- 4.Stephenson LS, Latham MC, Kinoti SN, Kurz KM, Brigham H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Trans R Soc Trop Med Hyg. 1990;84:277–82. doi: 10.1016/0035-9203(90)90286-n. [DOI] [PubMed] [Google Scholar]
- 5.Nokes C, Bundy DA. Does helminth infection affect mental processing and educational achievement? Parasitol Today. 1994;10:14–8. doi: 10.1016/0169-4758(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 6.de Silva NR, Chan MS, Bundy DA. Morbidity and mortality due to ascariasis: Re-estimation and sensitivity analysis of global numbers at risk. Trop Med Int Health. 1997;2:519–28. doi: 10.1046/j.1365-3156.1997.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 7.Crompton DW, Nesheim MC, Pawlowski ZS, editors. Ascariasis and its Public Health Significance. London: Taylor & Francis; 1985. [Google Scholar]
- 8.Pawlowski ZS, Davies A. Morbidity and mortality in ascariasis. In: Crompton DW, Neisheim MC, Pawlowski ZS, editors. Ascariasis and its Prevention and Control. London: Taylor & Francis; 1989. pp. 45–69. [Google Scholar]
- 9.Shuval HT, Yekutial P, Fattal B. Epidemiological evidence for helminth and cholera transmission by vegetables irrigated with waste water: Jerusalem - A case study. Water Sci Technol. 1984;17:433–42. [Google Scholar]
- 10.Pawlowski ZS. Ascariasis. In: Warran KS, Mahmoud AA, editors. Tropical and Geographical Medicine. 2nd ed. New York: McGraw-Hillx; 1990. p. 369. [Google Scholar]
- 11.Sun T. Ascariasis. In: Sun T, editor. Pathology and Clinical Features of Parasitic Diseases. New York: Masson; 1980. pp. 115–20. [Google Scholar]
- 12.Khuroo MS. Ascariasis. In: Weinstock JV, editor. Gastroenterology Clinics of North America: Parasitic Diseases of the Liver and Intestines. No. 3. Vol. 25. Philadelphia: WB Saunders; 1996. pp. 553–77. [DOI] [PubMed] [Google Scholar]
- 13.Crompton DW. The prevalence of ascariasis. Parasitol Today. 1988;4:162–9. doi: 10.1016/0169-4758(88)90152-4. [DOI] [PubMed] [Google Scholar]
- 14.Louw JH. Abdominal complications of Ascaris lumbricoides infestation in children. Br J Surg. 1966;53:510–21. doi: 10.1002/bjs.1800530606. [DOI] [PubMed] [Google Scholar]
- 15.Hlaing T. A profile of ascariasis morbidity in Rangoon Children's Hospital, Burma. J Trop Med Hyg. 1987;90:165–9. [PubMed] [Google Scholar]
- 16.Gilles HM. Soil-transmitted helminths (geohelminths) In: Cook G, editor. Manson's Tropical Diseases. 20th ed. Vol. 71. 1996. pp. 1369–412. [Google Scholar]
- 17.Elkins DB, Haswell-Elkins M, Anderson RM. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg. 1986;80:774–92. doi: 10.1016/0035-9203(86)90384-6. [DOI] [PubMed] [Google Scholar]
- 18.Mani GG, Rao ST, Madhavi R. Estimation of hookworm intensity by anthelmintic expulsion in primary schoolchildren in south India. Trans R Soc Trop Med Hyg. 1993;87:634–5. doi: 10.1016/0035-9203(93)90268-u. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh GN, Malla N, Raju GS, Sehgal R, Ganguly NK, Mahajan RC, et al. Epidemiological study of parasitic infestations in lower socio-economic group in Chandigarh (north India) Indian J Med Res. 1991;93:47–50. [PubMed] [Google Scholar]
- 20.Saha SS, Behl JP, Sharma JK, Kumar A. Distribution of intestinal parasitic infection in rural area of District Darjeeling, West Bengal. J Commun Dis. 1993;25:43–4. [PubMed] [Google Scholar]
- 21.Baveja UK, Kaur M. Prevalence of intestinal parasitic infections in Delhi. J Commun Dis. 1987;19:362–7. [PubMed] [Google Scholar]
- 22.Subbannayya K, Babu MH, Kumar A, Rao TS, Shivananda PG. Entamoeba histolytica and other parasitic infections in south Kanara district, Karnataka. J Commun Dis. 1989;21:207–13. [PubMed] [Google Scholar]
- 23.Nagoba BS, Basutkar SH, Bhat SD. Status of intestinal parasitic infections in Loni - A rural area of Ahmednagar District of Maharashtra. J Commun Dis. 1992;24:58–9. [PubMed] [Google Scholar]
- 24.Ragunathan L, Kalivaradhan SK, Ramadass S, Nagaraj M, Ramesh K. Helminthic infections in school children in Puducherry, South India. J Microbiol Immunol Infect. 2010;43:228–32. doi: 10.1016/S1684-1182(10)60036-9. [DOI] [PubMed] [Google Scholar]
- 25.Saha SS, Behl JP, Sharma JK, Kumar A. Distribution of intestinal parasitic infections in selected areas in District Puri, Orissa (India) J Commun Dis. 1993;25:86–7. [PubMed] [Google Scholar]
- 26.Sharma RS, Mahadik VJ. Prevalence of intestinal parasites in a rural area of Rajasthan. J Commun Dis. 1988;20:312–5. [PubMed] [Google Scholar]
- 27.WHO Technical Report Series 666. Geneva: World Health Organization; 1981. Intestinal Protozoan and Helminthic Infections: Report of a WHO Scientific Group. [PubMed] [Google Scholar]
- 28.Crompton DW, Pawlowski ZS. Life history and development of Ascaris lumbricoides and the persistence of human ascariasis. In: Crompton DW, Neisheim MC, Pawlowski ZS, editors. Ascariasis and its Public Health Significance. London: Taylor Francis; 1985. pp. 9–23. [Google Scholar]
- 29.Botero D. Epidemiology and public health importance of intestinal nematode infections in Latin America. Prog Drug Res. 1975;19:28–43. doi: 10.1007/978-3-0348-7090-0_5. [DOI] [PubMed] [Google Scholar]
- 30.Ananthakrishnan S, Nalini P, Pani SP. Intestinal geohelminthiasis in the developing world. Natl Med J India. 1997;10:67–71. [PubMed] [Google Scholar]
- 31.Bradley M, Chandiwana SK, Bundy DA, Medley GF. The epidemiology and population biology of Necator americanus infection in a rural community in Zimbabwe. Trans R Soc Trop Med Hyg. 1992;86:73–6. doi: 10.1016/0035-9203(92)90448-l. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RM, Schad GA. Hookworm burdens and faecal egg counts: An analysis of the biological basis of variation. Trans R Soc Trop Med Hyg. 1985;79:812–25. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 33.Croll NA, Ghadirian E. Wormy persons: Contributions to the nature and patterns of overdispersion with Ascaris lumbricoides, Ancylosotma duodenale, Necator americanus and Trichuris trichiura. Trop Geogr Med. 1981;33:241–8. [PubMed] [Google Scholar]
- 34.Bundy DA. Immunoepidemiology of intestinal helminthic infections. 1. The global burden of intestinal nematode disease. Trans R Soc Trop Med Hyg. 1994;88:259–61. doi: 10.1016/0035-9203(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 35.Croll NA, Anderson RM, Gyorkos TW, Ghadirian E. The population biology and control of Ascaris lumbricoides in a rural community in Iran. Trans R Soc Trop Med Hyg. 1982;76:187–97. doi: 10.1016/0035-9203(82)90272-3. [DOI] [PubMed] [Google Scholar]
- 36.Hall A, Anwar KS, Tomkins AM. Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. Lancet. 1992;339:1253–7. doi: 10.1016/0140-6736(92)91593-w. [DOI] [PubMed] [Google Scholar]
- 37.Stities DP, Terr AI, Parslow TG. Basic and Clinical Immunology. Connecticut: Appleton and Lange; 1994. pp. 673–8. [Google Scholar]
- 38.Bundy DA, Medley GF. Immuno-epidemiology of human geohelminthiasis: Ecological and immunological determinants of worm burden. Parasitology. 1992;104(Suppl):S105–19. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- 39.Needham CS, Lillywhite JE. Immunoepidemiology of intestinal helminthic infections. 2. Immunological correlates with patterns of Trichuris infection. Trans R Soc Trop Med Hyg. 1994;88:262–4. doi: 10.1016/0035-9203(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 40.Desai S, Tobin K. Biliary ascariasis: Sonographic findings. AJR Am J Roentgenol. 1995;164:767–8. doi: 10.2214/ajr.164.3.7863917. [DOI] [PubMed] [Google Scholar]
- 41.Maddern GJ, Dennison AR, Blumgart LH. Fatal ascaris pancreatitis: An uncommon problem in the west. Gut. 1992;33:402–3. doi: 10.1136/gut.33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraswat VA, Gupta R, Dhiman RK, Gujral RB. Biliary ascariasis: Endoscopic extraction of a living worm from the bile duct. Endoscopy. 1993;25:552–3. doi: 10.1055/s-2007-1010403. [DOI] [PubMed] [Google Scholar]
- 43.Schulman A. Intrahepatic biliary stones: Imaging features and a possible relationship with Ascaris lumbricoides. Clin Radiol. 1993;47:325–32. doi: 10.1016/s0009-9260(05)81448-5. [DOI] [PubMed] [Google Scholar]
- 44.Leung JW, Chung SC. Endoscopic management of biliary ascariasis. Gastrointest Endosc. 1988;34:318–20. doi: 10.1016/s0016-5107(88)71364-4. [DOI] [PubMed] [Google Scholar]
- 45.van Severen M, Lengele B, Dureuil J, Shapira M, Dive C. Hepatic ascaridiasis. Endoscopy. 1987;19:140–2. doi: 10.1055/s-2007-1018261. [DOI] [PubMed] [Google Scholar]
- 46.Khuroo MS, Mahajan R, Zargar SA, Javid G, Sapru S. Prevalence of biliary tract disease in India: A sonographic study in adult population in Kashmir. Gut. 1989;30:201–5. doi: 10.1136/gut.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khuroo MS, Zargar SA. Biliary ascariasis. A common cause of biliary and pancreatic disease in an endemic area. Gastroenterology. 1985;88:418–23. [PubMed] [Google Scholar]
- 48.Louw JH. Biliary ascariasis in childhood. S Afr J Surg. 1974;12:219–25. [PubMed] [Google Scholar]
- 49.Shennak MM. Endoscopic retrograde cholangiopancreatography (ERCP) in the diagnosis of biliary and pancreatic duct disease: A prospective study on 668 Jordanian patients. Ann Saudi Med. 1994;14:409–14. doi: 10.5144/0256-4947.1994.409. [DOI] [PubMed] [Google Scholar]
- 50.Chatterjee KD. Parasitology. Calcutta: Chatterjee Medical Publishers; 1980. pp. 158–207. [Google Scholar]
- 51.Pawłowski ZS. Ascariasis. Clin Gastroenterol. 1978;7:157–78. [PubMed] [Google Scholar]
- 52.Singh S, Raju GV, Samantaray JC. Parasitic gut flora in a north Indian population with gastrointestinal symptoms. Trop Gastroenterol. 1993;14:104–8. [PubMed] [Google Scholar]
- 53.Khuroo MS, Zargar SA, Mahajan R. Hepatobiliary and pancreatic ascariasis in India. Lancet. 1990;335:1503–6. doi: 10.1016/0140-6736(90)93037-p. [DOI] [PubMed] [Google Scholar]
- 54.Zargar SA, Khuroo MS. Management of biliary ascariasis in children. Indian J Gastroenterol. 1990;9:321. [PubMed] [Google Scholar]
- 55.Khuroo MS, Mahajan R, Zargar SA. Biliary and pancreatic ascariasis: A long term follow-up. Natl Med J India. 1989;2:4. [Google Scholar]
- 56.Khuroo MS, Zargar SA, Yattoo GN, Dar MY, Javid G, Khan BA, et al. Sonographic findings in gallbladder ascariasis. J Clin Ultrasound. 1992;20:587–91. doi: 10.1002/jcu.1870200904. [DOI] [PubMed] [Google Scholar]
- 57.Kirei B. Ascariasis. Surgery (Add On) 1989;1:1540–2. [Google Scholar]
- 58.Corr P, Smit J, Hadley GL. An unusual cause of haemobilia: Biliary ascariasis. Pediatr Radiol. 1997;27:348–9. doi: 10.1007/s002470050148. [DOI] [PubMed] [Google Scholar]
- 59.Manialawi MS, Khattar NY, Helmy MM, Burcharth F. Endoscopic diagnosis and extraction of biliary ascaris. Endoscopy. 1986;18:204–5. doi: 10.1055/s-2007-1018375. [DOI] [PubMed] [Google Scholar]
- 60.Larrubia JR, Ladero JM, Mendoza JL, Morillas JD, Diaz-Rubio M. The role of sonography in the early diagnosis of biliopancreatic ascaris infestation. J Clin Gastroenterol. 1996;22:48–50. doi: 10.1097/00004836-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 61.Khuroo MS, Zargar SA, Yattoo GN, Javid G, Dar MY, Boda MI, et al. Worm extraction and biliary drainage in hepatobiliary and pancreatic ascariasis. Gastrointest Endosc. 1993;39:680–5. doi: 10.1016/s0016-5107(93)70222-9. [DOI] [PubMed] [Google Scholar]
- 62.Mani S, Merchant H, Sachdev R, Rananavare R, Cunha N. Sonographic evaluation of biliary ascariasis. Australas Radiol. 1997;41:204–6. doi: 10.1111/j.1440-1673.1997.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 63.Wasadikar PP, Kulkarni AB. Intestinal obstruction due to ascariasis. Br J Surg. 1997;84:410–2. [PubMed] [Google Scholar]
- 64.Khuroo MS, Zargar SA, Mahajan R, Bhat RL, Javid G. Sonographic appearances in biliary ascariasis. Gastroenterology. 1987;93:267–72. doi: 10.1016/0016-5085(87)91013-4. [DOI] [PubMed] [Google Scholar]
- 65.Chugh TD, Khurana S, Kaur H, Chitkara NL. Hepatic ascariasis. Indian J Pathol Bacteriol. 1970;13:38–40. [PubMed] [Google Scholar]
- 66.Cetta FM. Bile infection documented as initial event in the pathogenesis of brown pigment biliary stones. Hepatology. 1986;6:482–9. doi: 10.1002/hep.1840060327. [DOI] [PubMed] [Google Scholar]
- 67.Burkitt DP, Walker AR. Saint's triad: Confirmation and explanation. S Afr Med J. 1976;50:2136–8. [PubMed] [Google Scholar]
- 68.Hikasa Y, Nagase M, Tanimura H, Shioda R, Setoyama M, Kobayashi N, et al. Epidemiology and etiólogy of gallstones. Nihon Geka Hokan. 1980;49:555–71. [PubMed] [Google Scholar]
- 69.Chetlin SH, Elliott DW. Biliary bacteremia. Arch Surg. 1971;102:303–7. doi: 10.1001/archsurg.1971.01350040065012. [DOI] [PubMed] [Google Scholar]
- 70.Maki T. Pathogenesis of calcium bilirubinate gallstone: Role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164:90–100. doi: 10.1097/00000658-196607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabata M, Nakayama F. Bacteria and gallstones. Etiological significance. Dig Dis Sci. 1981;26:218–24. doi: 10.1007/BF01391633. [DOI] [PubMed] [Google Scholar]
- 72.Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology. 1986;90:677–86. doi: 10.1016/0016-5085(86)91123-6. [DOI] [PubMed] [Google Scholar]
- 73.Schoenfield LJ. Developments in cholelithiasis during the 20th century. In: Kirsner JB, editor. The Growth of Gastroenterologic Knowledge during the Twentieth Century. Vol. 22. New Delhi: BI Waverly Pvt. Ltd; 1995. pp. 373–90. [Google Scholar]
- 74.Batu AT, Myint S, Myint A, Oo M, Aye T. Letter: Ascaris larvae and ova in the core of bile-duct stones in Rangoon. Trans R Soc Trop Med Hyg. 1975;69:167. doi: 10.1016/0035-9203(75)90033-4. [DOI] [PubMed] [Google Scholar]
- 75.Finlayson ND, Bouchier IA. Diseases of the liver and biliary system. In: Edwards CR, Bouchier IA, Haslett C, Chilvers E, editors. Principles and Practice of Medicine. London: Churchill Livingstone; 1995. p. 543. [Google Scholar]
- 76.Fan ST, Wong J. Recurrent pyogenic cholangitis. In: Zinner MJ, Schwartz SJ, Ellis H, editors. Maingot's Abdominal Operations. 63. Vol. I. London: Prentice Hall Int. (UK) Ltd; 1997. p. 1771. [Google Scholar]
- 77.Khuroo MS, Zargar SA, Yattoo GN, Allai MS, Khan BA, Dar MY, et al. Oddi's sphincter motor activity in patients with recurrent pyogenic cholangitis. Hepatology. 1993;17:53–8. [PubMed] [Google Scholar]
- 78.Lim JH. Oriental cholangiohepatitis: Pathologic, clinical, and radiologic features. AJR Am J Roentgenol. 1991;157:1–8. doi: 10.2214/ajr.157.1.2048504. [DOI] [PubMed] [Google Scholar]
- 79.Sperling RM, Koch J, Sandhu JS, Cello JP. Recurrent pyogenic cholangitis in Asian immigrants to the United States: Natural history and role of therapeutic ERCP. Dig Dis Sci. 1997;42:865–71. [PubMed] [Google Scholar]
- 80.Schulman A. Non-western patterns of biliary stones and the role of ascariasis. Radiology. 1987;162:425–30. doi: 10.1148/radiology.162.2.3541030. [DOI] [PubMed] [Google Scholar]
- 81.Holocombe C. Surgery in the tropics. In: Cook G, editor. Manson's Tropical Diseases. 20th ed. Vol. 16. London: WB Saunders; 1996. p. 382. [Google Scholar]
- 82.Khuroo MS, Zargar SA, Yattoo GN, Koul P, Khan BA, Dar MY, et al. Ascaris-induced acute pancreatitis. Br J Surg. 1992;79:1335–8. doi: 10.1002/bjs.1800791231. [DOI] [PubMed] [Google Scholar]
- 83.Zargar SA, Khuroo MS. Treatment of biliary ascariasis and its rationale. Gastroenterology. 1987;93:668–9. doi: 10.1016/0016-5085(87)90952-8. [DOI] [PubMed] [Google Scholar]
- 84.Davis A. Drug Treatment in Intestinal Helminthiasis. Geneva: World Health Organization; 1973. [Google Scholar]
- 85.Pawlowski ZS. Ascaris. In: Pawlowski ZS, editor. Intestinal Helminthic Infections. No. 3. Vol. 12. London: Bailliere Tindal; 1987. [Google Scholar]
- 86.Dunn MA. Schiff's Diseases of the Liver. 8th ed. Vol. 62. Philadelphia: Lippincott-Raven; 1999. Parasitic diseases; p. 1536. [Google Scholar]
- 87.Das P, Bhattacharya SK, Sen P. Pharmacology. New Delhi: BI Churchill Livingstone; 1995. pp. 417–22. [Google Scholar]
- 88.Bennet DR. Drug Evaluations 1994. New York: American Medical Association; 1994. Anthelminthics; pp. 1729–60. [Google Scholar]
- 89.Gulhati CM. Anthelminthics and other anti-infestive drugs. Monthly Index of Medical Specialities India. 1995;15:175–7. [Google Scholar]
- 90.Liangmin L. Clinical observation on combined use of herbal medicine and acupuncture for treatment of 50 cases of biliary ascariasis complicated by infection. J Tradit Chin Med. 1996;16:194–7. [PubMed] [Google Scholar]
- 91.Holocombe C. Surgical emergencies in tropical gastroenterology. In: Cook GC, editor. Gastroenterological Problems from the Tropics. 1st ed. Vol. 13. London: BMJ Publishing Group; 1995. p. 133. [Google Scholar]
- 92.Baldwin M, Eisenman RE, Prelipp AM, Breuer RI. Ascaris lumbricoides resulting in acute cholecystitis and pancreatitis in the Midwest. Am J Gastroenterol. 1993;88:2119–21. [PubMed] [Google Scholar]
- 93.Khuroo MS, Mahajan R, Zargar SA, Javid G, Banday M. Endoscopic vs surgical drainage of biliary tract in acute pyogenic cholangitis: A controlled study. Indian J Gastroenterol. 1989;8:119. [PubMed] [Google Scholar]
- 94.Beckingham IJ, Cullis SN, Krige JE, Bornman PC, Terblanche J. Management of hepatobiliary and pancreatic Ascaris infestation in adults after failed medical treatment. Br J Surg. 1998;85:907–10. doi: 10.1046/j.1365-2168.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 95.Javid G, Wani N, Gulzar GM, Javid O, Khan B, Shah A. Gallbladder ascariasis: Presentation and management. Br J Surg. 1999;86:1526–7. doi: 10.1046/j.1365-2168.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 96.Gott PE, Tieva MH, Barcia PJ, Laberge JM. Biliary access procedure in the management of oriental cholangiohepatitis. Am Surg. 1996;62:930–4. [PubMed] [Google Scholar]


