Abstract
Intravenous immunoglobulin (IVIG) has been utilized in patients with recurrent and refractory Clostridium difficile colitis. It is increasingly being used in patients with initial clinical presentation of severe colitis. Herein, we report a case of severe C. Difficile colitis successfully treated with IVIG with a review of the medical literature to identify the optimal timing and clinical characteristics for this treatment strategy.
Keywords: Clostridium difficile, Colitis, Intravenous immunoglobulin
INTRODUCTION
Clostridium difficile colitis is the most common cause of nosocomial-related diarrhea. In the United States, the incidence of hospital-acquired Clostridium difficile infection (CDI) increased by 2 to 2.5-fold from the late 1990s to the early 2000s. Even though, many attempts have been made to address this enormous burden on health care, CDI has been associated with increasing morbidity and mortality in the past decade.[1,2,3] This grave problem has been attributed to an increasing number of patients with co-morbidities, metronidazole failure, poor attention to infection control and the spread of the BI/NAP1 C. difficile strain.[1] Intravenous immunoglobulin (IVIG) is prepared from pooled human serum and has been documented to contain anti-toxin antibodies against C. difficile toxins. Some of the studies have documented low serum antibody response to C. difficile toxins in a subset of patients with recurrent disease.[2] For this reason, IVIG has been used in patients with recurrent CDI and is increasingly being used in patients with severe CDI. Herein, we report a case of severe CDI successfully treated with IVIG with a review of the medical literature to identify the ideal timing and clinical characteristics for this treatment strategy.
CASE REPORT
This was a case report of a 77-year-old African American male patient with a past medical history of hypertension, cerebrovascular accident, bladder cancer, chronic renal insufficiency, right nephrectomy and multiple hospitalizations for sepsis presented to the hospital with a change in mental status, worsening renal failure and leukocytosis. He complained of watery non-bloody diarrhea. His stool test for C. difficile toxin was positive. The laboratory findings on admission revealed white blood count of 35.7 K/uL, hemoglobin of 16.0 mg/dL, platelets of 258 K/uL, Albumin of 2.2 mg/dL and creatinine of 2.5 mg/dL.
The patient was initially treated with oral vancomycin. On day 4 of antibiotic treatment, the diarrhea persisted. IV metronidazole was added to the therapeutic regimen. On day 15, in light of poor response to dual therapy, a sigmoidoscopy was performed showing severe pseudomembranous colitis. The patient developed severe sepsis with worsening leukocytosis, hypoalbuminemia and renal failure. A second sigmoidoscopy was performed on day 21 showing worsening of the pseudomembranous colitis with no improvement. After 25 days of dual oral vancomycin and IV metronidazole antibiotic therapy, a single dose of IVIG 400 mg/kg was administered over 12 h. The number of bowel movements per day immediately started to decrease with subsequent resolution in the patient's diarrhea, fever and leukocytosis. There was no recurrence following the IVIG therapy.
DISCUSSION
The study done by Kyne et al. demonstrated that patients with IgG ≤3.00 units are 48 times more likely to get severe CDI.[3] Other contributing factors include age, the presence of immunodeficiency and the virulence of the organism responsible for disease. The first line of treatment of CDI is still metronidazole with the removal of the responsible antibiotic if possible. To prevent spread, infection control practices must be implemented. Other treatment options include oral and/or intracolonic vancomycin and fidaxomicin. Treatment with probiotic Saccharomyces boulardi is also another treatment option that can be considered for refractory cases. Administration of donor stool by colonoscopy or retention enemas to replace the colonic microflora is a well-described therapeutic option for the treatment of patients with recurrent CDI. Patients with severe CDI who either do not respond or who are not eligible for first line therapeutic treatment may benefit from adjunctive intracolonic vancomycin treatment administered by rectal enema or through long catheters. Surgical intervention, in the form of subtotal colectomy with ileostomy, is usually reserved for patients who present with peritoneal signs, severe ileus, or toxic megacolon.
Passive immunity against C. difficile toxins may protect against colitis. The primary hypothesis for the mechanism of action of IVIG is through binding and neutralization of toxin A by IgG anti-toxin A antibodies. The exact mechanism of IgG anti-toxin A antibody delivery to the lumen is not exactly known, however it is presumed to occur secondary to inflammation-induced mucosal damage.[3] The successful use of IVIG has mainly been studied in cases of chronic relapsing and refractory CDI.[4,5] There are only a handful of case reports in the medical literature reporting its use in the acute initial cases of severe CDI.[6,7,8,9] There are different definitions of severe CDI that have been described in these previous reports.
Salcedo et al. defined severe CDI as one causing pancolitis in one patient and thumb printing on CT scan in another.[6] In the study by Juang et al., disease severity was assessed using Rubin's modified criteria [Table 1].[7] Hassoun et al. defined severe disease on the basis of computed tomography (CT) scan findings of pancolitis either with or without megacolon.[8]
Table 1.
Rubin et al. (1995) Prediction tool for unfavorable outcomes clostridium difficile infection
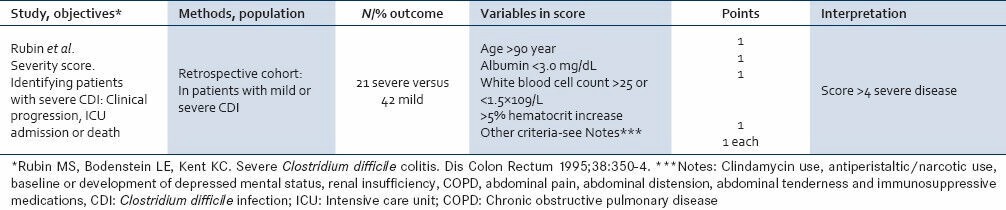
A study done by Abougergi et al., provided two scales for inclusion: One based on extent of colonic disease and the other based on the acute physiology and chronic health evaluation (APACHE) 2 score to assess severity of systemic involvement.[9]
A review of the severe cases of CDI published in the medical literature suggests that the earlier the administration of IVIG, the greater the likelihood of attaining therapeutic and survival benefit. It is essential to target CDI whereas it is still restricted to the colon (without other organ dysfunction or at least at an early stage of extracolonic organ failure and a low APACHE 2 score) but not for severe colonic disease with secondary multiple organ failure (high APACHE 2 score). Colonic disease is toxin-mediated whereas secondary systemic involvement is mediated through toxin-induced inflammatory mediators (interleukin-8, macrophage-inflammatory protein-2, substance P, tumor necrosis factor-alpha) released locally in the colon, triggering a systemic inflammatory response and hematogenous translocation of colonic bacteria, both of which are poorly responsive to immunoglobulin infusion. It is necessary to curb this potentially fatal cascade by early administration of IVIG prior to the development of signs and symptoms of multi-organ dysfunction e.g. renal failure.
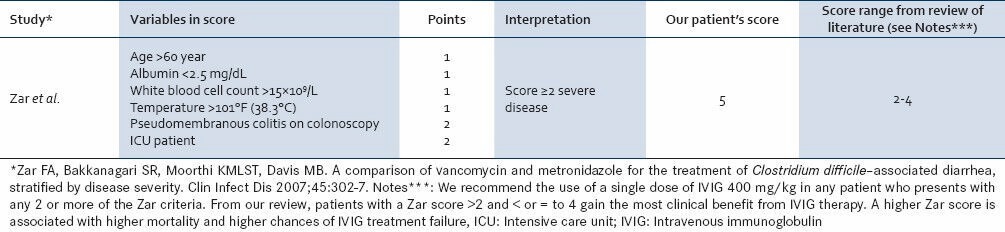
Based on the review of the cases, we are proposing the utilization of a severity index based on the Zar criteria [Table 2].[10] By utilizing this clinical criteria, we can identify a subset population of early severe CDI that has not developed toxin-mediated end-organ damage and hence would attain the survival benefit of IVIG therapy. Compared with the other definitions of severe CDI, the Zar score is less tedious and could be more reliable in identifying the ideal patient who would respond to IVIG. It is clear that further studies, especially randomized trials need to be carried out to evaluate importance of Zar Score in the treatment of severe CDI by IVIG.
Table 2.
Zar et al. (2007) prediction tool for severity of clostridium difficile infection
There is strong evidence to support the role of IVIG in intractable, relapsing and recurrent C. difficile colitis to avoid the serious complications of megacolon and perforation and hence ultimately, death.[6,7,8,9] However, a trial of IVIG in severe disease at the initial stages, as in our case, can halt the progress and intensity of CDI, thus preventing the need of such high risk, invasive management such as a total colectomy. CDI and especially recurrent infection consumes considerable medical resources. Hence, IVIG can still be a cost-effective treatment option for these cases, particularly given the absence of proven therapeutic alternatives.
Preexisting renal disease, sepsis, volume depletion, and old age are risk factors for renal failure in patients who receive IVIG. Although Chandrasekar et al.[5] and our patient demonstrated that severe CDI and chronic kidney disease can safely be treated with IVIG, it should probably be used early in the course of disease before the manifestation of end-organ damage like worsening renal failure. Slow infusion rate and good hydration may prevent renal failure. Avoiding the use of sucrose-based IVIG has reduced the incidence of renal failure since sucrose has been implicated in nephrotoxicity associated with the use of IVIG. Nevertheless, we recommend that it would still be ideal to safely administer IVIG prior to the development of CDI-related kidney injury to attain the maximum benefit of therapy.
Therefore, we propose that if a patient presents with an initial case of CDI, it is imperative to identify the Zar score. If the score is 2-4, IVIG should be considered as part of the therapeutic regimen to reduce morbidity and mortality. With increasing social and political interest in reducing the cost of health care delivery, tests or models, like the Zar score, that can predict clinical worsening or mortality in CDI may be useful for targeting interventions, like the administration of IVIG, aimed at preventing readmissions and ultimately mortality.
This review of the literature highlights how important timing and patient selection (i e., severity of illness, co-morbidities, renal failure etc.) are to consider prior to administration of IVIG. There is a need for randomized trials to evaluate the ideal dose and timing of IVIG administration. These trials would also assist in identifying the clinical characteristics of the patient population that would gain the most clinical benefit from IVIG.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Khanna S, Pardi DS. Clostridium difficile infection: New insights into management. Mayo Clin Proc. 2012;87:1106–17. doi: 10.1016/j.mayocp.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kink JA, Williams JA. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–25. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 4.McPherson S, Rees CJ, Ellis R, Soo S, Panter SJ. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49:640–5. doi: 10.1007/s10350-006-0511-8. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar T, Naqvi N, Waddington A, Cooke RP, Anijeet H, Gradden CW, et al. Intravenous immunoglobulin therapy for refractory Clostridium difficile toxin colitis in chronic kidney disease : c0 ase reports and literature review. NDT Plus. 2008;1:20–2. doi: 10.1093/ndtplus/sfm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcedo J, Keates S, Pothoulakis C, Warny M, Castagliuolo I, LaMont JT, et al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–70. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juang P, Skledar SJ, Zgheib NK, Paterson DL, Vergis EN, Shannon WD, et al. Clinical outcomes of intravenous immune globulin in severe clostridium difficile-associated diarrhea. Am J Infect Control. 2007;35:131–7. doi: 10.1016/j.ajic.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Hassoun A, Ibrahim F. Use of intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis. Am J Geriatr Pharmacother. 2007;5:48–51. doi: 10.1016/j.amjopharm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Abougergi MS, Broor A, Cui W, Jaar BG. Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: An observational study and review of the literature. J Hosp Med. 2010;5:E1–9. doi: 10.1002/jhm.542. [DOI] [PubMed] [Google Scholar]
- 10.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]


