Abstract
Background:
According to official data, 60–70% of clinical decisions about hospitalization and discharge are based on laboratory results.
Aims:
The objective of this study is to examine the frequency of errors before, during, and after analysis in a major medical laboratory.
Materials and Methods:
This descriptive, cross-sectional study was conducted throughout 2012 (January–December 2012). Errors are recorded by the Quality Control Committee in a specially designed record.
Results:
A total of 303,866 samples, 2,430,928 tests were received for analysis. The total number of errors was 153,148 (6.3%) (116,392 for inpatients and 36,756 for outpatients). Analysis of the results revealed that about 65.09% of the errors occur across preanalytical phase, whereas 23.2% and 11.68% are related to analytical and postanalytical phase, respectively.
Conclusion:
More than half of the laboratory errors are related to preanalytical phase; therefore, proper training and knowledge of intervening factors are essential for reducing errors and optimizing the quality.
Keywords: Different Pphases of Ttesting, errors, laboratory medicine
Introduction
Today, medical laboratories play a significant role in the healthcare system and the decision-making of clinical doctors about their patients. According to official data, 60–70% of clinical decisions about hospitalization, discharge, and prescription are based on laboratory results.[1,2,3,4] Clinical tests enjoy a high status in screening, treatment follow-up, and assessment of response to treatment.[1,2,3,4] Given this high percentage and the significant role, the quality of laboratory tests is of high importance.[1,2,3,4] In other words, quality is the cornerstone of management in a laboratory[5,6] and it needs to conform to the highest standards.[5,6] Laboratory services need to be accurate, precise, and quick to prove effective. Errors might happen throughout the process. The errors are categorized into three stages depending on whether they are done before, during, or after analysis: the preanalytic, analytic and postanalytic phase errors.[6,7]
Since there are no definite methods of sampling, most errors take place before the test, just before the sample is being prepared (it includes specimen collection, handling and processing variables, physiological variables, and endogenous variables).[6,7,8] Some of the preanalytical variables such as specimen collection can be controlled, while knowledge of uncontrollable variables needs to be well understood to be able to separate their effects from disease-related changes affecting the laboratory results.[7,8]
Preanalytical errors are reported to be up to 70% in various studies.[6,7,8,9] Since the quality is interconnected, precision and accuracy are not the only guarantors of the quality. From the very beginning, all three stages need to be under monitoring and quality control with precision and accuracy. The objective behind quality control is to minimize laboratory errors.
The objective of this study is to examine the frequency of errors before, during, and after analysis in a referral laboratory so that proper approaches could be developed for minimum errors.
Materials and Methods
This descriptive, cross-sectional study was conducted at Imam Teaching Hospital throughout 2012 (January–December 2012). The hospital, affiliated to Tehran University of Medical Sciences, is a referral hospital for the entire country. It has a department for every medical specialty (Surgery, Internal Medicine, Pediatrics, Intensive and coronary care units, etc.). Ethics and Research Committee of the university has endorsed this study.
The sections in the hospital's laboratory include hematology, urine biochemistry, parasitology and microbiology, general and specialized biochemistry, hormonology, serology, coagulation, and flow cytometry (FCM). Two associate professors and an assistant professor, as well as 106 employees are working in the laboratory, which provides laboratory testing for inpatients and outpatients round the clock.
The laboratory testing process starts either through a requisition from the hospital's departments or by physicians in private practice. For inpatients, the laboratory clerks process the patients’ identification data including their name, age, sex, file number, and physician's name and register them in the hospital information system (HIS). If the requisition is not stat order or emergent, the following morning a trained laboratory staff collects blood samples from all hospital departments. In stat and emergent occasions, nurses or medical students collect the blood samples, which are then transported by couriers to the laboratory department. For outpatients, the physicians in private practice fill out the appropriate requisition form, which serves as a referral to the laboratory department. Then, the laboratory clerk registers the patient's identification data into the laboratory information system (HIS). Sampling and blood collection is done by the laboratory staff. All samples were collected in 2012 and there was no exclusion criterion.
After transporting specimens to the laboratory department, a laboratory technologist evaluates the samples for misidentification errors, inappropriate container, improper labeling, inadequacy of sample collection, and inadequacy of sample/anticoagulant ratio volume. Any probable errors are reported to the ordering department and request is sent for a new proper sample. Also, the errors are recorded in a roster and a copy is sent to the laboratory's quality control committee (the quality control committee comprises of a laboratory director, who is a professor of pathology; a supervisor, who is a graduate of laboratory sciences with at least 15 years of experience; and two laboratory technologists with at least 10 years of experience in quality control). The specimen preparation, including preparing suitable samples for analytic procedures like centrifugation, aliquoting, and sorting specimens are performed. Then, in a different laboratory section, tests are done. The test results data are entered to HIS both manually and automatically, depending on the test type. After monitoring and checking by a quality manager who is a technologist or a laboratory physician, the test results are allowed to be observed by medical staff for evaluation, interpretation, and appropriate action.
The physicians and nurses of hospital's departments are trained to be observant of test results and are asked to report any questionable result to the quality control committee of the laboratory. If any result is labeled clinically questionable, the quality manger checks the testing process and usually the specimen is retrieved or in some circumstances asks for a new sample. Errors are recorded by quality manager on a special roster and the date, time, source of the test, type and detail, and signature are recorded.
The reported case was discussed in the committee based on Clinical and Laboratory Standards Institute (CLSI) instruction[9] and classified in database. The quality control committee comprised the laboratory director, faculty member, QC manager as well as supervisor and sub-directors.
The laboratory error definition that has been incorporated in the International Organization for Standardization (ISO) Technical Report 22367 is defined as a defect occurring at any part of the laboratory cycle, from ordering test to reporting, interpreting, and reacting to results.[10] Since studies on laboratory errors are diverse and there is no general acceptance on definitions used especially on the types of errors, it is an arduous task to categorize variable types of errors. However, in this study it is tried to utilize more general and practical categorization as Da Rin used in his article.[11]
The laboratory testing process is divided into three phases:
1. Preanalytical Errors:
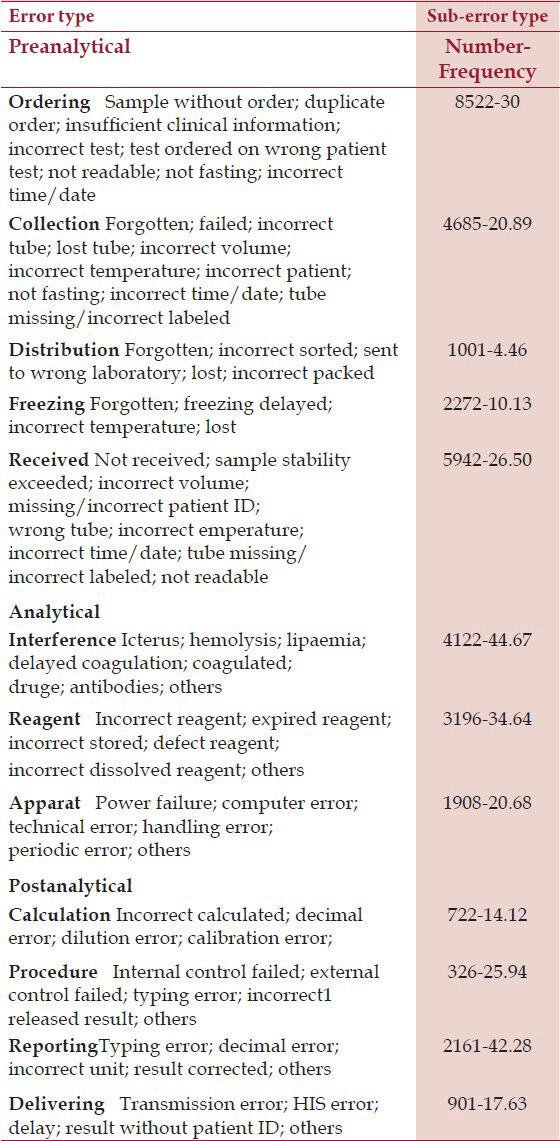
These errors are defined as the errors occurring from physician order to analytic phase, including test request, patient identification, collection, transportation, and preparation for analysis. For better classification and monitoring of preanalytical phase, it is subdivided to errors that occur outside the laboratory and errors that occur under the control of laboratory as Da Rin has mentioned in his study.[11] Outside Laboratory: inappropriate test request, order entry errors, misidentification of patient, inappropriate container, improperly labeled container, inadequate sample collection and transportation, specimen collected from infusion route, inadequate sample/anticoagulant volume ratio, and insufficient sample volume. Within laboratory: sorting and routing errors, pour-off errors, and labeling errors.
2. Analytical Errors:
These errors occur during the test, and include equipment malfunction, sample mix-ups, interference, undetected failure in quality control, and procedure not followed.
3. Postanalytical Errors:
These errors happen after the test is conducted, and include failure in reporting, erroneous validation of analytical data, improper data entry, and excessive turnaround time.
Statistical analysis
The sum of errors was calculated. Their relative frequencies when compared with the total specimens were also calculated and presented as percentage. The statistical significance level accepted in this study was set at P value equal to or less than 0.05. The difference between relative frequencies of mistakes observed in the departments considered was tested by proportional Z test.
Results
A total of 303,866 samples, 2,430,928 tests were received for analysis (230,938 samples, 1,847,504 tests were from inpatients and 72,928 samples, 583,424 tests were from outpatients). Total number of errors was 153,148 (6.3% – 62999.8 parts per million, ppm) (116392–62999.5 ppm for inpatients and 36756–63000 ppm for outpatients). Number and frequency of three main groups of errors in inpatients and outpatients are shown in Table 1, and type and subtype of three main groups of errors in inpatients and outpatients are shown in Tables 2 and 3, respectively.
Table 1.
Number, Frequency and 95% CI of Errors in Preanalytical, Analytical, Postanalytical Phases
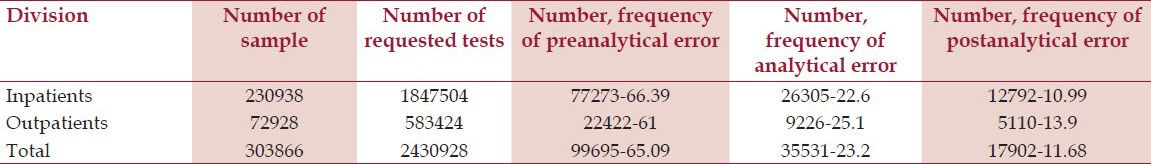
Table 2.
Error and Sub-error Type Tests in Inpatients
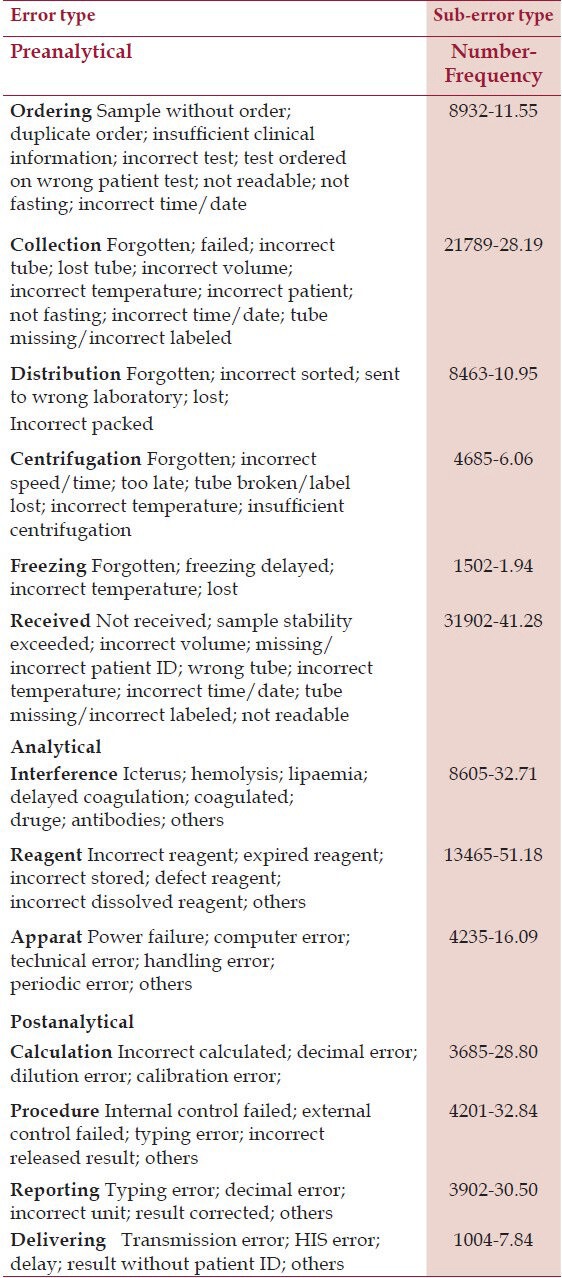
Table 3.
Error and Sub-error Type Tests in outpatients
Discussion
Medical laboratory test results are instrumental in the decision-making by clinical doctors. Some studies have found that about 70% of clinicians’ decisions are based on laboratory results. Therefore, the quality, accuracy, and precision of laboratory results are indispensable in clinical care.
According to a definition acknowledged by international organization for standardization, laboratory error is defined as “failure of planned action to be completed as intended, or use a wrong plan to achieve an aim, occurring at any part of the laboratory cycle, from ordering examinations to reporting results and appropriately interpreting and reacting to them.”[10] The aim of this descriptive study is to evaluate type and frequencies of errors in three phases of laboratory tests to reduce preventable errors. The analysis of results of previous studies shows that despite heterogeneity in methods, the error distribution across the different phases of testing process appears to be similar with most errors occurring in the preanalytical phase.[11]
In one review article written by Howkins in 2012,[12] he mentioned that the proportion of errors associated with pre- and postanalytical phases of testing is 4–5 times higher than that seen in analytical phase with preanalytical phase representing over half of the errors in published studies. In another study, preanalytical factor consists of 46–68.2% of total errors while a high error rate 18.5–47% of total errors has been found in postanalytical phase.[13]
In a retrospective study performed by Plebani et al.,[14] an Italian stat laboratory was assessed once in 1996 and then in 2006. They found out that despite a 34% reduction in error rate, the pattern of 62% preanalytical, 15% analytical, and 23% postanalytical phase errors remained basically unchanged. Analysis of results of this study shows that about 65.09% of errors occur in preanalytical phase, while about 23.2% and 11.68% occur across analytical and postanalytical phases, respectively.
According to different studies, there is a considerable difference between in- and outpatients with the 0.60% versus 0.039% for the two categories, respectively.[11] It seems that most of these differences are related to human factors including personal skills in venipuncture (drawing blood) and the sheer volume of laboratory tests carried out for inpatients.[15,16] The results of this study also revealed that errors in hospitalized patients were more numerous than in outpatients. That might be due to the grave conditions of hospitalized patients and variety of staff involved in the total testing processes. For instance, the samples may be taken by nonexpert staffs. Ambiguities about the standard methods and appropriate transporting times for different laboratory tests are also of great importance in decreasing preventable errors. Therefore, nurses and other laboratory workers need to be more specialized in taking blood samples and other interventions. The samples need to be carried in the shortest possible time by experienced individuals. The vacutainers (glass or plastic tubes) could be prevented from being broken if they are carried in special vessels.
Although the importance of preanalytical phase has been acknowledged for many years, laboratories have often overlooked this area in their quality management programs, focusing instead on analytical quality and the associated activities within their direct control.[12,17] In the analytical stage of this study, errors for inpatients and outpatients did not differ meaningfully and were mainly due to factors that could be prevented by an accurate and precise quality control procedure. Training special staff to conduct quality control or even offering a specialized university course is recommended for this purpose. In the postanalytical stage, the errors did not show any meaningful difference between these two groups and they resulted mainly from human errors in reporting procedures. To that effect, laboratories with high volume of work are recommended to be equipped with HIS system. In this way, the staff could be undercut and errors would be minimized. Postanalytical errors are indicative of the significance of thorough control on the results of the tests by the laboratory's chief technician before being confirmed by the laboratory director.
When compared with other studies,[9,11,15] this study shows more analytic errors, most likely because the aging equipment's state-of-the-art technology is not used for financial restrictions, or possibly, inadequate training of laboratory workers.
The limitations in this study were that registration of the errors during the evening, night-shifts, and holidays were not completely performed. More studies are recommended to be carried out to examine differences between day and night shifts separately and at multi centers. Hospital and laboratory directors can minimize errors and boost the quality of healthcare services based on the results of these studies.
Conclusion
Since more than half of the laboratory errors occur during preanalytical phase, proper training and knowledge of the intervening factors that can influence laboratory results are essential to minimize laboratory errors. It should be reminded that all three stages of laboratory tests need to be under thorough monitoring to improve the quality of results.
Acknowledgment
The authors thank all the staff at the laboratory who assisted and contributed to this study. The authors declare that there is no conflict of interests.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Carraro P, Plebani M. Errors in a stat laboratory:types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 2.Plebani M, Carraro P. Mistakes in a stat laboratory: Types and frequency. Clin Chem. 1997;43:1348–51. [PubMed] [Google Scholar]
- 3.Hooijberg E, Leidinger E, Freeman KP. An error management system in a veterinary clinical laboratory. J Vet Diagn Invest. 2012;24:458–68. doi: 10.1177/1040638712441782. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim F, Dosoo D, Kronmann KC, Ouedraogo I, Anyorigiya T, Abdul H, et al. Good clinical laboratory practices improved proficiency testing performance at clinical trials centers in Ghana and Burkina Faso. PLoS One. 2012;7:e39098. doi: 10.1371/journal.pone.0039098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza LG, Stagi L. Non-parametric error distribution analysis from the laboratory calibration of various rainfall intensity gauges. Water Sci Technol. 2012;65:1745–52. doi: 10.2166/wst.2012.075. [DOI] [PubMed] [Google Scholar]
- 6.Aakre KM, Langlois MR, Watine J, Barth JH, Baum H, Collinson P, et al. Critical review of laboratory investigations in clinical practice guidelines: Proposals for the description of investigation. Clin Chem Lab Med. 2013;51:1217–26. doi: 10.1515/cclm-2012-0574. [DOI] [PubMed] [Google Scholar]
- 7.Chhillar N, Khurana S, Agarwal R, Singh NK. Effect of pre-analytical errors on quality of laboratory medicine at a neuropsychiatry institute in north India. Indian J Clin Biochem. 2011;26:46–9. doi: 10.1007/s12291-010-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero A, Cobos A, Gómez J, Muñoz M. Role of training activities for the reduction of pre-analytical errors in laboratory samples from primary care. Clin Chim Acta. 2012;413:166–9. doi: 10.1016/j.cca.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Wayne, PA: NCCLS; 1997. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A4. [Google Scholar]
- 10.Geneva: International organization for standardization; 2008. ISO/WD TS 22367. Medical laboratories-reduction of error through risk management and continual improvement. [Google Scholar]
- 11.Da Rin G. Pre-analytical workstations: A tool for reducing laboratory errors. Clinl Chim Acta. 2009;404:68–74. doi: 10.1016/j.cca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Guidi GC, Mattiuzi C, Plebani M. Preanalyticalvariability: The dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins R. Managing the pre and postanalytical phases of the total testing process. Ann Lab Med. 2012;32:5–16. doi: 10.3343/alm.2012.32.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plebani M. Errors in clinical laboratories or error in laboratory medicine.? Clin Chem Lab Med. 2006;44:750–9. doi: 10.1515/CCLM.2006.123. [DOI] [PubMed] [Google Scholar]
- 15.Carraro P, Plebani M. Eroors in a stat laboratory: Types and frequencies 10 years later. Clin Chem. 2007;53:1338–42. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 16.Bonini PA, Plebani M, Ceriotti F, Rubolli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–8. [PubMed] [Google Scholar]
- 17.Karla J. Medical errors: Impact on clinical laboratories and other critical areas. Clin Biochem. 2004;37:1052–62. doi: 10.1016/j.clinbiochem.2004.08.009. [DOI] [PubMed] [Google Scholar]


