1. Introduction
Across adulthood, the brain undergoes a variety of structural and cognitive changes that reflect healthy aging (Caserta et al., 2009). Typical changes in brain morphology include decreased volume, reduced synaptic density, and increased white matter abnormalities (Jernigan et al., 2001; Masliah, Mallory, Hansen, DeTeresa, & Terry, 1993; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003). Yet, the regional distribution and the cognitive correlates of this tissue degradation remain unclear. The structural deterioration of brain tissue in older adults is thought to be responsible for age-related cognitive decline; thus, studies have begun investigating the correlations between structural and functional changes (Bendlin et al., 2010; Caserta et al., 2009). This research has focused heavily on working memory, processing speed, and executive processes, while other aspects of cognition, such as language functioning have received less attention (Charlton et al., 2008; Gunning-Dixon & Raz, 2000; Gunning-Dixon & Raz, 2003). As life expectancy continues to increase, it is becoming more important to understand age-related neuropsychological changes and their structural underpinnings.
1.1. White Matter and Language
Early anatomical models of language focused on the superior longitudinal fasciculus (SLF), a white matter tract believed to connect temporo-parietal language regions to ipsilateral frontal and opercular areas. For many years, the SLF and arcuate fasciculus (AF) were viewed as synonymous. However, more recently, four components of the SLF have been described. These components connect frontal and opercular areas with the superior parietal lobe (SLF-I), the angular gyrus (SLF-II), the supramarginal gyrus (SLF-III), and the superior temporal gyrus (SLF-IV) (Dick & Tremblay, 2012; Makris et al., 2005). Attention has been given to SLF-IV, the partition viewed as the AF, given its apparent connections between cortical regions involved in language comprehension (Wernicke’s area) and language production (Broca’s area). The importance of the AF, originally documented by Wernicke (1874) and later supported by Geschwind (1965) has remained of theoretical interest. Over time, slightly different anatomical models of SLF have been proposed (Catani, Jones, & ffytche, 2005; Glasser & Rilling, 2008) each emphasizing the importance of pathways connecting frontal regions not only to temporal but also to parietal cortex, which is involved in semantic processing (Price, 2010).
Diffusion tensor imaging (DTI) is an MRI technique used to measure the diffusion of water molecules (Basser, Mattiello, & LeBihan, 1994; Pierpaoli & Basser, 1996; Pierpaoli, Jezzard, Basser, Barnett, & Di Chiro, 1996) in order to visualize white matter fibers and to assess white matter integrity. These studies have shown age related changes in fractional anisotropy (FA) and mean diffusivity (MD) (Barrick, Charlton, Clark, & Markus, 2010; Bennett, Madden, Vaidya, Howard, & Howard, 2010; Draganski et al., 2011) that are measurable much earlier than age-related white matter volume loss (Westlye et al., 2010).
There is a dearth of studies that assess the mediating effect of white matter integrity on the relationship between age and language performance. However, there is evidence that FA in language-related brain regions can predict performance on a variety of language-related tasks including grammar learning (Floel, de Vries, Scholz, Breitenstein, & Johansen-Berg, 2009), reading ability (Klingberg et al., 2000), lexical decision making speed (Gold, Powell, Xuan, Jiang, & Hardy, 2007), and verbal fluency (O’Sullivan et al., 2001). Evidence from recent studies investigating the relationship between white matter integrity and picture naming suggests that decreased FA contributes to impaired word retrieval in healthy aging (Stamatakis et al., 2011; Obler et al., 2010). These authors suggested that with advancing age, successful naming requires an intact system of white matter tracts as older adults recruit left hemisphere areas beyond the traditional perisylvian region as well as right hemisphere areas to compensate for increased difficulty performing the task. In both studies (Obler et al., 2010; Stamatakis et al., 2011), the authors examined correlations between naming performance and white matter integrity on a voxel-by-voxel basis rather than by investigating the contribution of specific white matter tracts; other aspects of language functioning beyond naming performance were not assessed.
1.2 Language and Aging
Language processing relies on the recruitment of specific cortical regions as well as proper organization of these regions into effective networks. For the majority of individuals, this network includes anterior (frontal) and posterior (temporo-parietal) regions of the left hemisphere; several right hemisphere regions participate in this process to a lesser extent (Donnelly, Allendorfer, & Szaflarski, 2011; Kim, Karunanayaka, Privitera, Holland, & Szaflarski, 2011; Price, 2000). Thus, one would expect age-related neuroanatomical changes to negatively impact the efficiency of this widespread language system. Yet, when compared to other neuropsychological skills, rather subtle changes in linguistic ability are noted with age. For example, word knowledge, or one’s understanding of vocabulary, has been shown to increase throughout middle adulthood, eventually declining in late adulthood (Schaie & Willis, 1993; Zelinski & Burnight, 1997). In addition, both the grammatical complexity and propositional content of spontaneous speech appear to decline during the mid-70s (Kemper, Marquis, & Thompson, 2001).
Older individuals frequently complain of naming difficulties, or an inability to retrieve the names of people and objects. Their perception is consistent with research showing that not only does confrontation naming ability decrease with age (Albert, Heller, & Milberg, 1988; Nicholas, Obler, Albert, & Goodglass, 1985; Zec, Burkett, Markwell, & Larsen, 2007), but older individuals are significantly slower than younger individuals in producing the name of common objects (Thomas, Fozard, & Waugh, 1977). Performance on the Boston Naming Test (BNT), a common test of confrontation naming ability (Kaplan, Goodglass, Weintraub, 1983), remains fairly stable until individuals are in their 70s, at which point a significant decline is noted (Albert et al., 1988).
Other age-related changes include the emergence of the tip-of-the tongue phenomenon (Burke & Shafto, 2004; Stamatakis et al., 2011) that has been proposed to occur when semantic and lexical representations of a given word are activated simultaneously, but activation of the phonological information about the word is incomplete (Burke & Shafto, 2004). Further, verbal fluency measures such as the Controlled Oral Word Association Test (COWAT) and the Semantic Fluency Test (SFT) are also known to decrease with age (Hultsch, Hertzog, Small, McDonald-Miszczak, & Dixon, 1992; McDowd et al., 2011). However, studies have shown that in contrast to the semantic measures, older adults’ comprehension of spoken language remains largely intact, with significant declines in performance occurring only with increased task demands, such as syntactic complexity and increased speech rate (Peelle, Troiani, Wingfield, & Grossman, 2010; Wingfield, Peelle, & Grossman, 2003). Taken together, the literature on language functioning in adults suggests that healthy aging affects the successful retrieval and production of language as well as certain aspects of language comprehension. However, the role of white matter integrity in the relationship between healthy aging and language is not clearly understood.
1.3 Aim
The aim of the current study was to examine the relationship between white matter microstructure and language functioning in a large group of healthy adults ranging in age from early to late adulthood. We hypothesized that fiber integrity of white matter tracts connecting cortical regions known to be involved in language processes will decrease significantly across the adult age range. Specifically, we hypothesized that increased age will be associated with decreased FA in the SLF of the left hemisphere and that it would be linked to poorer performance on neuropsychological measures of language including the Boston Naming Test (BNT), Semantic Fluency Test (SFT), Controlled Oral Word Association Test (COWAT), and the Peabody Picture Vocabulary Test (PPVT-4).
2. Results
There were no differences between males and females in age or mean test scores on the PPVT-4, BNT, COWAT, and SFT (Table 1).
Table 1.
Sample characteristics and mean comparisons between males and females
| Total (N = 112) | Male (N = 45) | Female (N = 67) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M | SD | M | SD | M | SD | t | p | |
| Age (years) | 42.55 | 15.14 | 43.56 | 16.02 | 41.88 | 14.60 | 0.57 | 0.57 |
| PPVT-4 | 215.30 | 7.40 | 216.40 | 7.40 | 214.60 | 7.30 | 1.29 | 0.20 |
| BNT | 57.40 | 2.70 | 57.60 | 3.10 | 57.20 | 2.40 | 0.85 | 0.40 |
| COWAT | 40.80 | 11.70 | 41.40 | 12.80 | 40.40 | 10.90 | 0.44 | 0.66 |
| SFT | 57.40 | 12.10 | 55.70 | 12.70 | 58.60 | 11.60 | −1.22 | 0.23 |
| SLFt | 0.46 | 0.05 | 0.48 | 0.05 | 0.46 | 0.05 | 2.12 | 0.04 |
| SLFp | 0.44 | 0.06 | 0.44 | 0.06 | 0.43 | 0.07 | 0.45 | 0.66 |
2.1. Effects of white matter microstructural integrity on language functioning
The relationship between FA and language performance was initially examined using multiple regression analyses. However, due to the non-normal distribution of BNT scores, robust regression analyses were also employed. Multiple regression and robust regression yielded similar results for PPVT-4, COWAT, and SFT scores. Therefore, only multiple regression results are reported for these test variables. Given that BNT scores were significantly negatively skewed, and the prediction of scores on this test was different depending on whether multiple regression or robust regression was employed, the use of robust regression was preferred for this measure.
As shown in Table 2, regression models predicting language performance using age and FA were not significant. Neither age nor FA from the SLFt and SLFp tracts were able to independently predict scores on the PPVT-4 or SFT. However, SLFt FA was a significant predictor of COWAT performance, and age was a significant predictor of BNT performance. Increased SLFt FA and increased age were positively associated with performance on the COWAT and BNT, respectively.
Table 2.
Regression analyses predicting language performance from age and FA for all subjects
| PPVT-4 | COWAT | SFT | BNTa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| R2 | p | R2 | p | R2 | p | R2 | ||||||
| Model | .03 | .36 | .05 | .14 | .01 | .83 | .04 | |||||
|
| ||||||||||||
| b | stan b | p | b | stan b | p | b | stan b | p | b | stan b | p | |
|
| ||||||||||||
| Age | .07 | .14 | .14 | −.06 | −.08 | .42 | .05 | .07 | .49 | .04 | .15 | .03 |
| SLFt FA | −6.22 | −.04 | .68 | 47.51 | .21 | .04 | −9.42 | −.04 | .70 | −3.18 | −.15 | .53 |
| SLFp FA | −4.96 | −.04 | .68 | −28.93 | −.16 | .12 | −3.95 | −.02 | .84 | 4.20 | .10 | .30 |
Multiple regression results are reported for PPVT-4, COWAT, and SFT. In the case of BNT, robust regression results are reported; in this one case, the standardized b weights are approximate, having been calculated by multiplying the robust b weight by the ratio of the ordinary standard deviations.
Next, the ability of age and FA to predict language performance was analyzed separately in males and females. For males, age and FA were once again not significant predictors of performance on the PPVT-4 and SFT. In the model predicting COWAT performance, SLFt FA was found to be a significant predictor. Once again, robust regression was preferred for the prediction of BNT performance, and both age and SLFp FA were found to be positively associated with BNT scores (Table 3). When female subjects were analyzed independently, age and FA were not significant predictors of scores on PPVT-4, SFT, and BNT. However, SLFp FA was found to be a negatively associated with COWAT performance (Table 4).
Table 3.
Regression analyses predicting language performance from age and FA for male subjects
| PPVT-4 | COWAT | SFT | BNTa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| R2 | p | R2 | p | R2 | p | R2 | ||||||
| Model | .03 | .74 | .14 | .10 | .05 | .52 | .11 | |||||
|
| ||||||||||||
| b | stan b | p | b | stan b | p | b | beta | p | b | stan b | p | |
|
| ||||||||||||
| Age | .05 | .11 | .50 | −.05 | −.06 | .70 | .16 | .20 | .22 | .06 | .15 | .01 |
| SLFt FA | 27.08 | .17 | .34 | 93.30 | .33 | .04 | 41.37 | .15 | .38 | −2.34 | −.15 | .77 |
| SLFp FA | −2.86 | −.02 | .89 | 9.97 | .05 | .77 | 15.06 | .07 | .67 | 13.36 | .26 | .03 |
Multiple regression results are reported for PPVT-4, COWAT, and SFT. In the case of BNT, robust regression results are reported; in this one case, the standardized b weights are approximate, having been calculated by multiplying the robust b weight by the ratio of the ordinary standard deviations.
Table 4.
Regression analyses predicting language performance from age and FA for female subjects
| PPVT-4 | COWAT | SFT | BNTa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| R2 | p | R2 | p | R2 | p | R2 | ||||||
| Model | .10 | .08 | .09 | .13 | .03 | .63 | .04 | |||||
|
| ||||||||||||
| b | beta | p | b | beta | p | b | beta | p | b | stan b | p | |
|
| ||||||||||||
| Age | .10 | .19 | .12 | −.03 | −.04 | .76 | .02 | .02 | .87 | .02 | .15 | .25 |
| SLFt FA | −28.69 | −.21 | .11 | 23.10 | .11 | .39 | −23.92 | −.12 | .41 | −6.86 | −.15 | .26 |
| SLFp FA | −7.53 | −.07 | .60 | −52.05 | −.31 | .02 | −15.39 | −.09 | .51 | 1.14 | .03 | .82 |
Multiple regression results are reported for PPVT-4, COWAT, and SFT. In the case of BNT, robust regression results are reported; in this one case, the standardized b weights are approximate, having been calculated by multiplying the robust b weight by the ratio of the ordinary standard deviations.
2.2. Effects of age on white matter microstructure
We also analyzed the effect of age on FA in the SLF. The overall model using age and sex to predict FA in the SLFt tract was significant, F (1, 111) = 3.25, p = 0.04, R2 = 0.06. A significant main effect of sex was observed, F (1, 111) = 4.84, p = 0.03. However, there was no main effect of age. To better understand the main effect of sex, the relationship between sex and FA was examined. Females had significantly lower FA values than males in the SLFt (Table 1). Regression analyses revealed that, with increasing age, males show a linear decrease in FA. In contrast to males, the relationship between age and FA in females was better explained by a quadratic curve (Figure 1). Males showed a gradual decline beginning in early adulthood while females showed an increase in FA until approximately age 40, followed by a decline.
Figure 1.
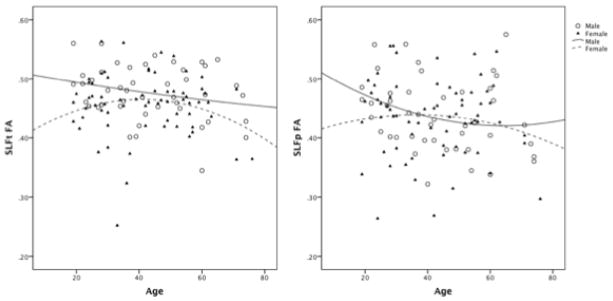
The relationship between age and FA in males and females.
The overall model using age and sex to predict FA in the SLFp tract was not significant, F (1, 111) = 1.38, p = 0.26, R2 = 0.03. There was no main effect observed for either age or sex. Given that the relationship between age and FA differed for males and females in the SLFt, this relationship was explored in the SLFp tract as well. Mean FA values from the SLFp did not significantly differ between males and females (Table 1). The relationship between age and FA in the SLFp tract was best described with a quadratic curve for both males and females. As in the SLFt tract, females showed a slight increase in FA until approximately age 40 before a gradual decline was noted. As age increased in males, FA values decreased across early adulthood before plateauing around age 50 (Figure 1).
3. Discussion
The results of the current study suggest that white matter integrity of the SLF follows a different pattern of decline in adulthood for males and females, and this decline differentially affects language functioning. Until recently, research investigating the neural correlates of aging has focused on macrostructural variables such as white and grey matter volume, as well as white matter hyperintensity burden. DTI has allowed for the investigation of axon-based microstructural properties of brain; it consistently shows widespread age-related declines in FA and increases in MD (Barrick et al., 2010; Bennett et al., 2010; Burgmans et al., 2011; Charlton et al., 2006; Draganski et al., 2011; Stamatakis et al., 2011; Ziegler et al., 2010) that usually precede white matter volume loss (Westlye et al., 2010), suggesting that a decline in white matter integrity may be an early sign of age-related brain degradation, and perhaps the onset of cognitive decline. In a sample of healthy participants aged 8 to 85 global FA showed an inverted U-shaped relationship with age, peaking around age 30 and slowly declining thereafter; FA in the SLF showed a similar pattern, peaking at approximately 30 years of age (Westlye et al., 2010). The results of the current study reveal a similar pattern for FA in the SLFt, although FA in the SLFp showed a gradual linear decline (Figure 2). Despite a similar pattern of results, the relationship between age and FA in the SLF was not significant for the current study; this discrepancy between studies is likely related to the much larger number of subjects and age range in the sample examined by Westlye et al.
Figure 2.
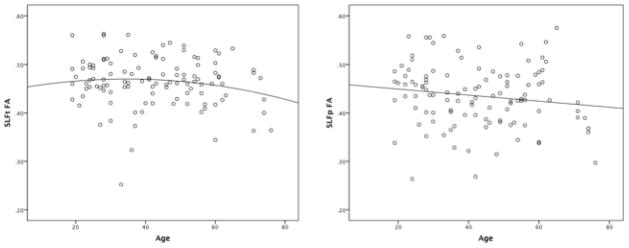
The relationship between age and FA in the SLF.
The current study highlights the importance of investigating the neural correlates of aging separately in males and females. Previous research indicates that males and females differ in total white matter volume and its patterns of age-related decline (Bendlin et al., 2010; Good et al., 2001). However, males and females show an equivalent pattern of age-related microstructural deterioration of white matter (Sullivan, Adalsteinsson, Hedehus, Ju, Moseley, Lim, & Pfefferbaum, 2001). For example, Sullivan et al. (2001) assessed age-related decline of regional white matter coherence in five regions (genu, splenium, bilateral frontal and bilateral parietal pericallosal white matter, and centrum semiovale) and showed similar patterns of decline for males and females. In contrast, the current study found different patterns of age-related decline in FA of the SLF for males and females. FA in females followed an inverted U-shaped relationship with age for both the SLFt and SLFp while males showed a gradual decline in SLFt FA with age, and in the SLFp, FA gradually declined until approximately age 50 before reaching a plateau. Interestingly, males showed significantly higher mean FA values than females in the SLFt. Therefore, despite showing an earlier decline in this tract, males do not show decreased white matter integrity beyond that of females (Figure 1).
While the results suggest a decrease in white matter integrity of the SLF in late adulthood, a significant relationship between age and FA was not observed. It is possible that only portions of the SLF are negatively impacted with increased age. For example, there is evidence that aging selectively impacts frontal white matter regions (Dempster, 1992; Greenwood, 2000; West, 1996). Several DTI studies support this “frontal aging hypothesis,” and show an anterior to posterior gradient in the decline of white matter integrity with reduced FA and increased MD of frontal lobe structures despite relative preservation of posterior brain regions (Abe et al., 2008; Lehmbeck et al., 2006; Pfefferbaum et al., 2005; Salat et al., 2005; Stamatakis et al., 2011). Further, there is a much steeper decline in FA of the genu compared to the splenium of the corpus callosum (Abe et al., 2008; Kochunov et al., 2007; Lehmbeck et al., 2006; Salat et al., 2005; Sullivan, Adalsteinsson, Hedehus, Ju, Moseley, Lim, & Pfefferbaum, 2001). Given our findings, it is possible that the SLF shows a similar pattern of decline with white matter integrity decreasing earlier in more anterior portions of the tract. Average FA values were extracted from two separate bundles of the SLF, extending from the frontal lobe to both temporal and parietal cortex. However, variations in FA between anterior and posterior regions of this tract were not assessed.
Although age-related differences in white matter microstructure are interesting, little is known about the impact of white matter integrity on cognitive functioning across the age span, and studies investigating the neuroanatomical correlates of cognitive performance have provided inconsistent results (Raz & Rodrigue, 2006). The claim of the frontal aging hypothesis is that selective deterioration of frontal brain regions drives age-related cognitive decline. However, the frontal aging hypothesis remains controversial, and some studies have failed to find preferential frontal involvement (Barrick et al., 2010; Draganski et al., 2011). Thus, Greenwood (2000) recommends moving away from a localizationist approach such as the frontal aging hypothesis and toward a network-based theory of cognitive aging, focusing on functional networks involving more than one lobe. This is because the age-related decline in white matter integrity may lead to decreased cognition through the process of “cortical disconnection” and disruption of the white matter pathways connecting widespread cortical networks (Geschwind, 1965; Mesulam, 1990; O’Sullivan et al., 2001).
The current study utilizes a network-based theory of cognitive aging to examine the effect of age-related changes in the connections of the SLF, which is known to play a large role in the language network of the frontal, temporal, and parietal brain regions. We hypothesized that performance on neuropsychological language measures would be associated with SLF FA. However, regression models using age, SLFt FA, and SLFp FA to predict performance on each of the language measures (PPVT-4, COWAT, SFT, BNT) were not significant (Table 2). In attempting to assess the interaction between sex and SLF FA, it became clear that the interaction terms were highly collinear in spite of centering. In order to reduce this collinearity, the relationship between FA and language performance was examined separately for males and females. Although the models were not significant in predicting language performance for either males or females, SLFt FA, SLFp FA, and age predicted language performance differently across the sexes. Overall, SLFt and SLFp FA values were not significant predictors of performance on the PPVT-4 and SFT. Increased SLFt FA was significantly related to improved performance on the COWAT. However, when males and females were analyzed separately this relationship remained significant only for males. The relationship between SLFt FA and COWAT performance is consistent with the current knowledge regarding verbal fluency tasks in that performance on such tasks draws heavily on semantic knowledge, but also requires speeded executive and retrieval processes; therefore, it relies on the transfer of information between temporal and frontal language regions. Similar results were found by O’Sullivan et al. (2001) who showed verbal fluency scores to correlate with FA of middle white matter. This relationship was independent of age, sex, and premorbid IQ. Although SLFt FA was not a significant predictor of COWAT performance in females, the results revealed that increased SLFp FA was associated with decreased performance on the COWAT. It is unclear why SLFp white matter integrity would be negatively related to language functioning. SLFp FA was positively associated with BNT performance in males, which is not surprising given the importance of parietal language regions to semantic retrieval and speech production. FA from the parietal portion of the SLF is perhaps related to BNT performance given its close proximity to visual cortex. BNT performance requires processing of visually presented stimuli as well as semantic retrieval.
Although there is some evidence that SLF FA is associated with phonemic verbal fluency performance and naming performance, a strong relationship between FA and language performance as measured by various neuropsychological measures was not observed in the current study. The lack of strong findings is likely a result of the limited number of adults over the age of 70 included in the study. There is evidence that cognitive decline occurs rapidly in this age group (Giambra, Arenberg, Kawas, Zonderman, & Costa, 1995). Longitudinal data from the Berlin Aging Study revealed a steeper decline in verbal fluency, episodic memory, processing speed, and word knowledge in individuals in their 80s and 90s compared to those at earlier stages in adulthood (Singer, Verhaeghen, Ghisletta, Lindenberger, & Baltes, 2003). Thus, the observed lack of association between age and language performance in our study is not entirely surprising. In order to determine whether age-related disruption in white matter integrity explains declines in language functions the sample needs to include an expanded age range.
The importance of including older individuals when assessing the relationship between age and cognition is highlighted in the cognitive aging literature. Research in this area has established that a variety of neuropsychological functions decline with healthy aging. However, there are some cognitive domains that seem to remain relatively preserved until late in adulthood, leading to a differentiation between “crystallized” abilities (e.g. acquired knowledge) and “fluid” abilities (e.g. reasoning, working memory, processing speed) (Caserta et al., 2009; Cattell, 1971; Horn, 1978). It is often assumed that fluid abilities show a decline with increasing age, while crystallized abilities remain relatively stable or even show a positive relationship with increasing age (Cattell, 1971; Horn, 1978; Singer et al., 2003). However, there is evidence that this pattern does not remain in very old age, at which time both crystallized and fluid abilities show a decline (Giambra et al., 1995; Lindenberger & Baltes, 1997). In the current study, age was positively associated with BNT performance, which is likely a reflection of the ability for crystallized abilities to improve with age. Given the differentiation between crystallized and fluid abilities, it is reasonable to speculate that fluid language abilities, such as phonemic (COWAT) and semantic (SFT) verbal fluency will decline at an earlier age than receptive vocabulary (PPVT-4) and naming ability (BNT). It is believed that with the inclusion of more subjects over the age of 70 this pattern of decline would be evident. This is comparable to a theory of language and aging put forth by Harley et al. (2011) who argued that aging is associated with a decline in deliberative processing skills rather than automatic language processes, which do not demand attention. Deliberative processing requires general executive resources and depends on the integrity of brain areas outside traditional language regions, most notably the frontal lobes. It involves the recruitment of more general cognitive processes, such as planning, strategy, and suppression for language use. Harley et al. (2011) proposed that age-related deterioration of the frontal lobes has profound effects on deliberative processing and is responsible for language difficulties experienced by older adults.
Proponents of the common-cause theory of cognitive aging would argue that age-related decline in language functioning, as well as in other cognitive domains is the result of a deficit in a single underlying principle (Span, Ridderinkhof, & van der Molen, 2004). The most prominent example of this theory is the notion that age-related decline in information processing speed is responsible for a variety of cognitive deficits in adulthood, including verbal fluency, executive functioning, spatial abilities, and memory (Bryan et al., 1997; Salthouse, 1993). Unfortunately, the current study did not assess performance in cognitive domains beyond language functioning, and therefore, it was not possible to examine whether variability in language performance can be attributed to efficiency of a separate cognitive operation such as processing speed.
A variety of imaging modalities have been used in the cognitive aging literature to evaluate structure-function relationships. Unfortunately, the strength of these relationships remains modest and difficult to replicate (Raz & Rodrigue, 2006). As a result, our current understanding of the relationship between white matter integrity and cognition across adulthood remains incomplete. The discrepancies found in the literature are likely a result of the varying methods of measurement utilized. While some studies employ whole brain voxel-wise analyses, others either evaluate white matter indices in large regions of interest or obtain localized, fiber-tract specific measures. In the current study, FA was analyzed in a single white matter tract. However, while there are individual differences in white matter integrity across different tracts, an overall global decrease in white matter integrity is observed (Penke et al., 2010; Wahl et al., 2010). Thus, some proposed the use of global rather than tract-specific measures to assess language functioning across ages (Penke et al., 2010).
It is important to look beyond the SLF when examining the relationship between white matter integrity and language functioning (Dick & Tremblay, 2012). For example, recent findings questioned the long-held belief that the structural symmetry of the AF reflects functional hemispheric lateralization for language (Vernooij et al., 2007). Further, anatomically the AF appears to project to the premotor area and not Broca’s area as previously believed (Bernal & Altman, 2010). Although Bernal and Altman (2010) are the first to report this discrepancy, they indicate that a closer look at the previous tractography studies of AF (Catani & Thiebaut de Schotten, 2008; Glasser & Rilling, 2008) supports their findings. While previous publications describe the AF connecting the posterior temporal lobe to the inferior frontal gyrus (Catani & Thiebaut de Schotten, 2008; Glasser & Rilling, 2008), Bernal and Altman (2010) point out that their images show anterior projections of the AF located in the premotor area (BA6), not Broca’s area (BA44) and speculate that earlier reports may have been affected by “knowledge bias.” These emerging findings may result in a revision of the language model. However, there is evidence that the premotor area is involved in producing and perceiving speech (Wilson, Saygin, Sereno, & Iacoboni, 2004). Thus, the importance of the AF will likely remain regardless of whether it terminates in premotor cortex or the inferior frontal gyrus as previously believed. Given the complexity of language, it is important to note that the current study provides limited information, and an investigation of the relationship between white matter integrity and language functioning should include white matter pathways beyond the SLF.
The cross sectional design is a limitation of the current study as previous research revealed discrepancies between cross-sectional and longitudinal data on aging populations (Singer et al., 2003). Comparing older and younger adults provides useful information regarding the relationship between structural and functional decline. However, longitudinal data is better in drawing the connection between the decline in white matter integrity, age, and decreased language functioning. Another weakness of the current study is that information regarding the participants’ education was not included. It would be helpful to control for one’s level of education as one’s previous knowledge may help to delay the decline associated with the deterioration of white matter integrity. Similarly, bilingualism has also been shown to improve the brain’s ability to cope with white matter decline in aging, minimizing its effects on cognition (Gold, Johnson, & Powell, 2013). Participants in the current study spoke English as a first language. However, it is unclear how many of these individuals were bilingual. Finally, an FA value averaged over the entire length of the SLF was utilized in the study. It has been shown that diffusion measurements vary along the tract trajectory (Yeatman et al., 2011; Yeatman, Dougherty, Myall, Wandell, & Feldman, 2012). This can occur as a result of anatomical features of the tract, such as curvature, as well as crossing fibers that lower FA where they intersect. In addition, axons do not run the entire length of a fascicle. Therefore, averaging along tracts may have masked potentially important information regarding the diffusion architecture of the SLF, and impaired the ability to make correlations between language functioning and white matter integrity. Yeatman et al. (2012) examined correlations at multiple locations along the trajectory of the SLF and found that the correlation between FA and basic reading skills was higher for a central portion of the tract compared to distal portions of the tract in a group of children ages 9 through 16. It would be interesting to apply a similar technique to determine whether FA in portions of the SLF are more strongly correlated with the neuropsychological measures of language functioning collected in the current study. Lastly, the current study did not assess the relationship between language functioning and FA in white matter tracts beyond the SLF, and it did not include cognitive domains other than language. Therefore, it is unclear whether the correlations between language performance and FA in the SLF are specific to language abilities or reflect a broader relationship between SLF FA and cognition in general. In future studies, it will be important to assess the relationship between language performance and FA values obtained from a white matter tract that is believed to be unrelated to language functioning.
In summary, white matter integrity of the SLF follows a different pattern of deterioration for males and females. While this deterioration was not strongly associated with performance on language related neuropsychological tests, the results suggest that a decline in whiter matter integrity is associated with impaired verbal fluency. Given that language ability is thought to remain relatively stable until late adulthood, it is believed that replication of this study with a larger number of older adults will significantly improve our understanding of the relationship between white matter integrity of the SLF and language functioning.
4. Experimental Procedure
4.1 Participants
Participants included 112 healthy, right-handed volunteers ranging in age from 19 to 76 years (M = 43, SD = 15.14) recruited through local advertisements and word of mouth. Of these participants, 67 were female and 45 were male (see Table 5). Handedness was determined by the Edinburgh Handedness Inventory (EHI), with a laterality quotient greater than 50 representing right-handed preference (Oldfield, 1971). For inclusion, participants were required to speak English as a first language and have no history of neurological or psychiatric disease. This research is part of a larger study, which was approved by the University of Cincinnati, Cincinnati Children’s Hospital, and University of Alabama at Birmingham Institutional Review Boards. Each participant provided written informed consent prior to participation in the study.
Table 5.
Males and females by age group
| 18–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | Total | |
|---|---|---|---|---|---|---|---|
| Female | 19 | 11 | 15 | 14 | 5 | 3 | 67 |
| Male | 11 | 10 | 7 | 6 | 7 | 4 | 45 |
| Total | 30 | 21 | 22 | 20 | 12 | 7 | 112 |
4.2 Magnetic resonance imaging
All MRI data were acquired on a 3.0 Tesla Phillips MRI system. First, a high-resolution T1-weighted three-dimensional anatomical scan was obtained (TR/TE = 8.1/3.7 ms, FOV 25.0 × 21.1 × 18.0 cm, matrix 252 × 211, flip angle 8°, slice thickness = 1 mm). Diffusion weighted data were acquired using echo planar imaging (TR/TE = 9513/69 ms, slice thickness = 2.38 mm, matrix = 76 × 67, FOV = 18.0 × 16.2 cm). Diffusion weighting was isotropically distributed along 32 directions by using a b value of 800 s/mm2.
4.3 DTI processing
Automated procedures in FreeSurfer (version 5.1.0; http://surfer.nmr.mgh.harvard.edu) were used to reconstruct each subject’s T1-weighted anatomical image. The procedure for surface construction with FreeSurfer has been described and validated previously (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Fischl & Dale, 2000). Briefly, the process involves intensity normalization, skull-stripping, segmentation of white and grey matter, tessellation and smoothing of the white matter/grey matter junction, and cortical parcellation. Next, white matter pathways were reconstructed with TRACULA (TRActs Constrained by UnderLying Anatomyhttp://surfer.nmr.mgh.harvard.edu/fswiki/Tracula), a tool available in FreeSurfer for automatically reconstructing 18 white matter pathways from diffusion weighted images using probabilistic tractography. TRACULA relies on prior knowledge of pathway anatomy from a set of manually labeled training subjects to reconstruct major white matter bundles. Thus, pathways are constrained based on anatomical knowledge of surrounding structures rather than to the exact spatial location of tracts (Yendiki et al., 2011).
For the purpose of this study, the focus was on the SLF of the left hemisphere. TRACULA differentiates between two components of the SLF based on those listed in Wakana et al. (2007): a superior portion (SLFp) that connects parietal (angular and supramarginal gyri) regions to ipsilateral frontal and opercular areas and an inferior portion (SLFt) that connects temporal (superior/middle temporal gyri) with ipsilateral frontal areas (Bernal & Altman, 2010; Wakana et al., 2007). Based on the subdivision of the SLF discussed above (Makris et al., 2005), the SLFt and SLFp correspond most closely to SLF III and the AF, respectively. An example of these reconstructed pathways is shown in Figure 3. Average FA values were extracted from each of these white matter tracts, resulting in two FA values for each subject: SLFp, and SLFt.
Figure 3.
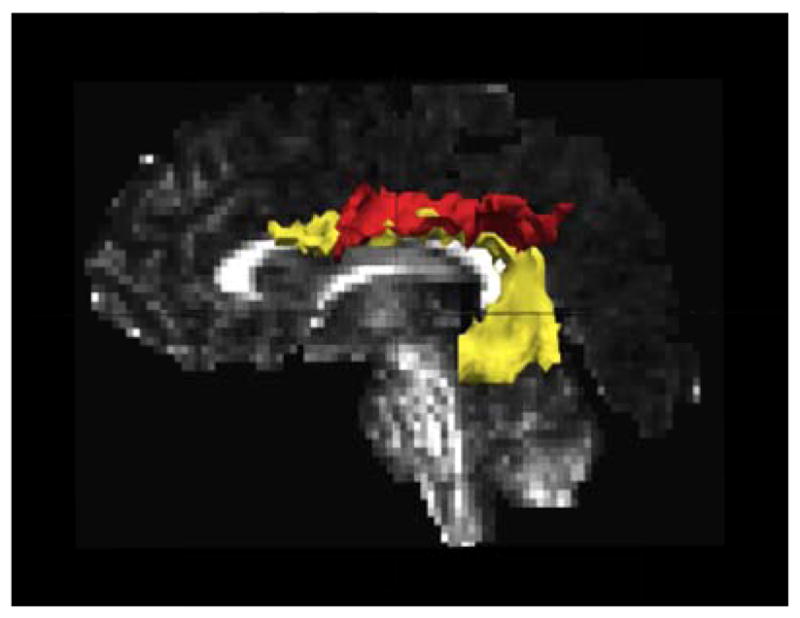
Example of SLFt (yellow) and SLFp (red) reconstructed in one study participant.
4.4 Neuropsychological Measures
The PPVT-4 is a measure of receptive vocabulary. The examinee is presented with a series of pages, each containing four pictures. For each page, they are read a word and asked to point to the picture that the word describes. The BNT is a tool used to measure confrontation naming ability. The BNT contains 60 line drawings and the examinee is asked to name each picture. The COWAT is a measure of one’s ability to spontaneously produce words beginning with a given letter. Subjects were presented with three letters and given 60 seconds for each letter. The total number of words produced across the three trials was used for this study. The SFT is a measure of one’s ability to say aloud as many names of items belonging to a certain category in a 60 second trial. Subjects were presented with three categories and the total number of items produced over the three trials was used. Raw scores for each of these measures were used for the purpose of this study.
4.5 Data Analysis
To assess the relationship between age and FA, data for each white matter tract were analyzed in a separate general linear model. In each model, age served as a continuous independent variable, sex was a categorical independent variable, and FA values from the tracts of interest (SLFt, SLFp) served as continuous dependent variables. The interaction between sex and age was explored and found to be not significant, and so it was excluded from the models. Each language measure (PPVT, BNT, COWAT, SFT) was then analyzed using a separate regression model with age and FA from SLFt and SLFp tracts as predictors. In addition to these models, robust regressions were conducted to evaluate whether influential data points were affecting the results. If the results of the two procedures were similar, then this offered assurance that the ordinary regression results were not misleading due to the influence of outliers or highly leveraged data points. In the few cases where the robust regressions did yield different results, we reported those. The decision was in no case based on which of the two results yielded significant findings. To reduce the effects of multicollinearity, all interaction terms were calculated by centering on the sample means of the constituent main effects.
Effects of sex
While males and females are reported to show an equivalent pattern of age-related microstructural deterioration of white matter (Sullivan, Adalsteinsson, Hedehus, Ju, Moseley, Lim, & Pfefferbaum, 2001), previous research indicates that males and females differ in total white matter volume and patterns of age-related decline in white matter volume (Bendlin et al., 2010; Good et al., 2001). Further, some differences in the patterns of cortical involvement in language production between sexes have been observed (Allendorfer et al., 2012). Thus, the possibility of structural differences in white matter underlying these cortical areas needed to be considered in the present study. The interactions between sex and both SLFt FA and SLFp FA were included in the models used to predict performance on each of the language measures. It was discovered that the interaction terms were significant in several preliminary models, but the significance was inconsistent and contingent upon whether one or both interaction terms were included simultaneously. These indistinct results in conjunction with the literature brought into question whether sex moderates the relationship between FA and language functioning. Therefore, the models described above were estimated separately for males and females.
Highlights.
Integrity of the left superior longitudinal fasciculus was assessed across adulthood.
Males and females show a different pattern of decline in fractional anisotropy.
White matter integrity affects language performance differently for males and females.
Acknowledgments
The necessary financial support for this research was provided by the National Institutes of Health (R01 NS048281; PI: Szaflarski).
Footnotes
The results of this study were presented in part at the Annual Meeting of the Organization for Human Brain Mapping in Seattle, WA in June of 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Ohtomo K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging. 2008;29(1):102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Albert MS, Heller HS, Milberg W. Changes in naming ability with age. Psychology and Aging. 1988;3(2):173–178. doi: 10.1037//0882-7974.3.2.173. [DOI] [PubMed] [Google Scholar]
- Allendorfer JB, Lindsell CJ, Siegel M, Banks CL, Vannest J, Holland SK, Szaflarski JP. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. 2012;48(9):1218–1233. doi: 10.1016/j.cortex.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: A prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51(2):565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Johnson SC. White matter in aging and cognition: A cross-sectional study of microstructure in adults aged eighteen to eighty-three. Developmental Neuropsychology. 2010;35(3):257–277. doi: 10.1080/87565641003696775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping. 2010;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Altman N. The connectivity of the superior longitudinal fasciculus: A tractography DTI study. Magnetic Resonance Imaging. 2010;28(2):217–225. doi: 10.1016/j.mri.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Bryan J, Luszcz MA, Crawford JR. Verbal knowledge and speed of information processing as mediators of age differences in verbal fluency performance among older adults. Psychology and Aging. 1997;12(3):473–478. doi: 10.1037//0882-7974.12.3.473. [DOI] [PubMed] [Google Scholar]
- Burgmans S, Gronenschild EH, Fandakova Y, Shing YL, van Boxtel MP, Vuurman EF, Raz N. Age differences in speed of processing are partially mediated by differences in axonal integrity. Neuroimage. 2011;55(3):1287–1297. doi: 10.1016/j.neuroimage.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Aging and language production. Current Directions in Psychological Science: A Journal of the American Psychological Society. 2004;13(1):21–24. doi: 10.1111/j.0963-7214.2004.01301006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Bannon Y, Fernandez F, Giunta B, Schoenberg MR, Tan J. Normal brain aging clinical, immunological, neuropsychological, and neuroimaging features. International Review of Neurobiology. 2009;84:1–19. doi: 10.1016/S0074-7742(09)00401-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29(10):1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–46. 75. [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain: A Journal of Neurology. 2012;135(Pt 12):3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Donnelly KM, Allendorfer JB, Szaflarski JP. Right hemispheric participation in semantic decision improves performance. Brain Research. 2011;1419:105–116. doi: 10.1016/j.brainres.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Ashburner J, Hutton C, Kherif F, Frackowiak RS, Helms G, Weiskopf N. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ) Neuroimage. 2011;55(4):1423–1434. doi: 10.1016/j.neuroimage.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of broca’s area predicts grammar learning success. Neuroimage. 2009;47(4):1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain: A Journal of Neurology. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PT., Jr Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10(1):123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathways. Cerebral Cortex (New York, NY : 1991) 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK. Lifelong bilingualism contributes to cognitive reserve against white matter integrity declines in aging. Neuropsychologia. 2013;51:2841–2846. doi: 10.1016/j.neuropsychologia.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society: JINS. 2000;6(6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Harley TA, Jessiman LJ, MacAndrew SBG. Decline and fall: A biological, developmental, and psycholinguistic account of deliberative language processes and ageing. Aphasiology. 2011;25(2):123–124. 153. [Google Scholar]
- Horn JL. Human ability systems. In: Baltes PB, editor. Lifespan development and behavior. New York: Academic Press; 1978. pp. 212pp. 213–257. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA. Short-term longitudinal change in cognitive performance in later life. Psychology and Aging. 1992;7(4):571–584. doi: 10.1037//0882-7974.7.4.571. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kemper S, Marquis J, Thompson M. Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psychology and Aging. 2001;16(4):600–614. doi: 10.1037//0882-7974.16.4.600. [DOI] [PubMed] [Google Scholar]
- Kim KK, Karunanayaka P, Privitera MD, Holland SK, Szaflarski JP. Semantic association investigated with functional MRI and independent component analysis. Epilepsy & Behavior. 2011;20:613–622. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: Tract-based spatial statistics study of aging. Neuroimage. 2007;35(2):478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport. 2006;17(5):467–470. doi: 10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the berlin aging study. Psychology and Aging. 1997;12(3):410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex (New York, NY : 1991) 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43(1):192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- McDowd J, Hoffman L, Rozek E, Lyons KE, Pahwa R, Burns J, Kemper S. Understanding verbal fluency in healthy aging, alzheimer’s disease, and parkinson’s disease. Neuropsychology. 2011;25(2):210–225. doi: 10.1037/a0021531. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Nicholas M, Obler L, Albert M, Goodglass H. Lexical retrieval in healthy aging. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1985;21(4):595–606. doi: 10.1016/s0010-9452(58)80007-6. [DOI] [PubMed] [Google Scholar]
- Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro A, 3rd, Hyun J, Albert ML. Bilateral brain regions associated with naming in older adults. Brain and Language. 2010;113(3):113–123. doi: 10.1016/j.bandl.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults’ comprehension of spoken sentences: Age differences in resource allocation and connectivity. Cerebral Cortex (New York, NY : 1991) 2010;20(4):773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Munoz Maniega S, Murray C, Gow AJ, Hernandez MC, Clayden JD, Deary IJ. A general factor of brain white matter integrity predicts information processing speed in healthy older people. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26(3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed mediation of adult age differences in cognition. Developmental Psychology. 1993;29(4):722–723. 738. [Google Scholar]
- Schaie KW, Willis SL. Age difference patterns of psychometric intelligence in adulthood: Generalizability within and across ability domains. Psychology and Aging. 1993;8(1):44–55. doi: 10.1037//0882-7974.8.1.44. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the berlin aging study (BASE) Psychology and Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Span MM, Ridderinkhof KR, van der Molen MW. Age-related changes in the efficiency of cognitive processing across the life span. Acta Psychologica. 2004;117(2):155–183. doi: 10.1016/j.actpsy.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Shafto MA, Williams G, Tam P, Tyler LK. White matter changes and word finding failures with increasing age. PloS One. 2011;6(1):e14496. doi: 10.1371/journal.pone.0014496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Thomas JC, Fozard JL, Waugh NC. Age-related differences in naming latency. The American Journal of Psychology. 1977;90(3):499–509. [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51(2):531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische symptomencomplex. Breslau: Cohen and Weigert; 1874. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Fjell AM. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex (New York, NY : 1991) 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nature Neuroscience. 2004;7(7):701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Peelle JE, Grossman M. Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. Aging, Neuropsychology, and Cognition. 2003;10(4):310–322. [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: Automating fiber-tract quantification. PloS One. 2012;7(11):e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec RF, Burkett NR, Markwell SJ, Larsen DL. A cross-sectional study of the effects of age, education, and gender on the boston naming test. The Clinical Neuropsychologist. 2007;21(4):587–616. doi: 10.1080/13854040701220028. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Burnight KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging. 1997;12(3):503–513. doi: 10.1037//0882-7974.12.3.503. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of Aging. 2010;31(11):1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


