Abstract
rhGH was lyophilized with various glass-forming stabilizers, employing cycles that incorporated various freezing and annealing procedures to manipulate glass formation kinetics, associated relaxation processes and glass specific surface areas (SSA’s). The secondary structure in the cake was monitored by IR and in reconstituted samples by CD. The rhGH concentrations on the surface of lyophilized powders were determined from ESCA. Tg, SSA’s and water contents were determined immediately after lyophilization. Lyophilized samples were incubated at 323 K for 16 weeks, and the resulting extents of rhGH aggregation, oxidation and deamidation were determined after rehydration. Water contents and Tg were independent of lyophilization process parameters. Compared to samples lyophilized after rapid freezing, rhGH in samples that had been annealed in frozen solids prior to drying, or annealed in glassy solids after secondary drying retained more native-like protein secondary structure, had a smaller fraction of the protein on the surface of the cake and exhibited lower levels of degradation during incubation. A simple kinetic model suggested that the differences in the extent of rhGH degradation during storage in the dried state between different formulations and processing methods could largely be ascribed to the associated levels of rhGH at the solid-air interface after lyophilization.
Keywords: protein structure, protein formulation, lyophilization, stability, mobility, specific surface area, annealing, surface degradation, growth hormone
Introduction
Lyophilization is widely accepted as an effective method to improve long-term stability of pharmaceuticals, especially therapeutic protein products1. In glassy lyophilized solids, both physical and chemical degradation processes are greatly hindered 1. However, storage of proteins in glassy solid formulations does not always guarantee a desired shelf life2,3. For decades, efforts have been made to choose appropriate formulations and design robust lyophilization cycles to yield stable protein products1. It has been widely documented that disaccharides (sucrose and trehalose) are effective stabilizers during lyophilization and storage, and this stabilizing effect has been ascribed to thermodynamic and/or kinetic stabilization mechanisms4–6. In addition, the lyophilization cycle applied to a given formulation may determine not only the morphology of the cake and physical properties of the glass, but also the stability of the protein during storage in the dried formulation1,4,6–9. For example, the stability of methionyl human growth hormone in dried solid formulations was shown to depend not only on the type of stabilizer included in the formulation, but also on the drying method that was used.9 Despite some insights into mechanisms by which excipients provide stability to proteins, developing a formulation that provides adequate protection against protein damage is still a semi-empirical exercise, as is the design of a lyophilization cycle that provides optimal protein stability for a given formulation.
A conventional lyophilization cycle consists of freezing, primary drying and secondary drying steps. The freezing step is of paramount importance10,11. During freezing, most of the water present in the original liquid (about 80% 10) is crystallized into essentially pure ice, which results in a freeze-concentrated solution for the remaining formulation. The pH of the freeze-concentrated liquid may undergo a shift due to preferential precipitation of buffer components, potentially contributing to protein denaturation.12,13 In addition, the rates of ice nucleation and crystal growth have large impacts on the ice morphology and ice-liquid interfacial area, and consequently on the final solid products’ specific solid-air interfacial area formed after drying 7,10,14,15.
Several strategies10,16 have been developed to manipulate the initial stages of the lyophilization cycle, such as shelf-ramp cooling, annealing, controlled ice nucleation and fast freezing by liquid N2. Shelf-ramp cooling is a standard cooling method in commercial freeze-drying, during which shelf temperature of the lyophilizer is decreased in a roughly linear fashion. In this case the maximum cooling rate is limited by the cooling capacity of lyophilizer17,18. Shelf-ramp cooling typically results in a high degree of supercooling prior to initiation of freezing, followed by a period characterized by rapid nucleation of a large number of ice crystals14. Consequently, many small ice crystals are formed. During the primary drying portion of the lyophilization process, ice crystals are removed by sublimation, and the interface between the glass and the voids left behind contribute the specific surface area (SSA) of the resulting glassy solid15,19,20. The large number of relatively small ice crystals formed after shelf-ramp freezing in turn yields lyophilized cakes with large surface areas10.
Annealing refers to an additional step that may be added after freezing, during which the sample temperature is maintained between the ice melting temperature and the glass transition temperature of the maximally freeze concentrated solution, Tg’11,19 (or the eutectic melting temperature of crystalline excipients, if that temperature is greater than Tg’). Due to both the enhanced mobility of water and the contributions of surface energy to the elevated chemical potential of water in smaller crystals, water is transported from small ice crystals and redeposits onto large ice crystals10. As a result of this Ostwald ripening, larger ice crystals are generated during annealing, which in turn results in lyophilized cakes with reduced specific surface areas11. For the purposes of this report, this type of annealing process will be termed “pre-drying annealing”.
We note that Ostwald ripening also occurs in lyophilization cycles that use shelf ramp cooling without an annealing step, but to a lesser degree, because the length of time available for ripening (the time during which ice crystals are present and the temperature is above Tg’) is much shorter in these cycles. To reduce the extent of Ostwald ripening even further, fast freezing methods may be used to limit the time that samples spend at temperatures between the ice melting temperature and Tg’. One method of fast freezing is to immerse samples contained in vials into liquid N2 (N2-immersion). Fast freezing also may be achieved by spraying liquid droplets of sample directly into liquid N2 (N2-droplet-freezing)13,21–24. In the N2 immersion procedure, the relatively low thermal conductivity of the glass vials limits heat transfer, making the effective cooling rate slower than that which can be achieved using the N2-droplet-freezing method.10 Compared with the standard shelf ramp cooling both of these two fast freezing methods result in smaller ice crystals and larger glassy matrix specific surface areas10.
Another type of annealing (herein termed “post-drying annealing”) may be implemented by briefly incubating dry, glassy samples at a high (but sub-Tg) temperature at the end of the secondary drying step of the lyophilization cycle. Pikal et al. reported that post-drying annealing could enhance protein stability25. The stability increase was presumably a result of relaxation processes that lead to slower motions in the glassy state25.
The current study examined formulations of rhGH in the presence of three glass forming excipients; sucrose, trehalose and hydroxyethyl starch. These formulations were lyophilized using five different methods, which yielded glassy solids with different SSAs, surface protein contents, glassy state mobilities and degrees of retention of native secondary structure. Because we anticipate that protein molecules located on the surface of lyophilized glassy solids will have significantly faster degradation rates, we hypothesize that the extent of rhGH degradation during storage in various dried solid formulations prepared by different processing methods can largely be ascribed to the resulting levels of rhGH found at the solid-air interface after lyophilization.
Materials and methods
Materials
rhGH was expressed in E. coli and purified as described previously 8,26. Hydroxyethyl starch (HES; Viastarch) was purchased from Fresenius (Graz, Austria), and sucrose and trehalose were purchased from Mallinckrodt Baker (Phillipsburg, NJ). All other chemicals were purchased as reagent grade or higher. 5ml lyophilization glass vials (Product Number 68000318) and butyl rubber stoppers (Product Number 19560042) were purchased from West Pharmaceutical Services, Linville, PA.
Methods
Formulation and lyophilization cycle design
rhGH was formulated at a concentration of 1 mg/ml in one of three formulations. In addition to rhGH, each formulation contained 2 mM sodium phosphate at a pH of 7.4, as well as 5% (wt/v) of HES, trehalose or sucrose. Lyophilization was performed using a FTS Lyostar I system. An aliquot (1 ml) of each rhGH formulation was pipetted into vials, and lyophilized with one of five different lyophilization cycles, denoted as standard lyophilization, pre-drying annealing lyophilization, post-drying annealing lyophilization, N2-immersion lyophilization and N2-droplet-freezing lyophilization.
In the standard lyophilization cycle, sample vials were loaded onto the shelf, which was at room temperature. The shelf temperature was reduced to 10 °C, and samples were equilibrated at this temperature for 1 hour. Shelf temperature was then decreased to −5°C at 1°C min−1, kept at −5°C for 20 minutes, and then decreased to −45°C at 1.3°C min−1. Samples were kept frozen at −45°C for 400 minutes. Primary drying was then initiated and performed at a shelf temperature of −20°C and a chamber pressure of 70 mTorr for 1400 minutes. Secondary drying was then started by increasing the shelf temperature to 33 °C at a rate of 0.3°C min−1. Samples were held at 33°C and 70 mTorr for four hours. Finally, vials were sealed in the chamber under dry nitrogen.
For the pre-drying annealing lyophilization cycle, an additional annealing step was added to the standard cycle. After samples were kept frozen at −45°C for 400 minutes, shelf temperature was increased to −5 °C over 30 minutes. Then shelf temperature was kept at −5°C for 6 hours before cooling to −45 °C. The shelf temperature was kept at −45°C for 6 hours, and then the primary and secondary drying steps followed the same protocol as in the standard lyophilization cycle.
Post-drying annealing was performed using the same protocol as standard lyophilization cycle, except that after the standard secondary drying step, shelf temperature was increased up to 50 °C at a rate of 0.3 °C min−1, and held at 50 °C for 6 hours before ending the cycle.
Liquid N2-immersion lyophilization was carried out by immersing the glass vial containing 1ml formulation into a liquid N2 bath for 2 minutes and then putting the vials onto the lyophilizer shelf, which was pre-cooled to −45°C, for 400 minutes. The rest of primary and secondary drying steps were the same as standard lyophilization cycle.
In the liquid N2-droplet-freezing lyophilization cycle, samples were slowly pipetted into the glass vials, which were filled with liquid N2. The sample vials were quickly moved onto the lyophilizer shelf, which was pre-cooled shelf at −45°C, and held at this temperature for 400 minutes. The rest of the primary and secondary drying cycle followed the same protocol as in the standard lyophilization cycle.
Measurement of residual water content
Residual water contents of the lyophilized samples were analyzed using the Karl Fischer method 27. Triplicate samples were prepared in a dry nitrogen-purged box and measured using a Mettler DL37 KF coulometer (Hightstown, NJ), as described previously 8.
Glass transition temperature measurement by differential scanning calorimetry (DSC)
The glass transition temperatures of the maximally freeze-concentrated formulation (Tg’) and the lyophilized formulations (Tg) were measured with a Perkin-Elmer Diamond DSC. For Tg’ measurement, aqueous solutions (20ul) in aluminum pans were cooled from room temperature to −60°C at 10°C min−1. After equilibration at −60°C min−1 for 5 minutes, samples were heated to −5 °C at 5°C/min, and kept at −5°C for 30 minutes before samples were recooled to −60°C again at 10°C/min. The second heating scan was up to 10°C at 5°C/min. In order to eliminate any thermal history effects, Tg’ was determined from the onset of thermal transition measured during the second heating scan. Tg measurement of the lyophilization products followed the same protocol as described previously8. At least triplicate samples were used to determine the Tg and Tg’.
Protein secondary structure by infrared (IR) spectroscopy
In a dry box lyophilized samples (around 0.3 mg rhGH) were mixed with 0.5 g KBr using a mortar and pestle. After being transferred into a stainless steel die, samples were pressed into a disc using a vacuum press. IR spectra were collected on a Bomem MB-series spectrometer (Montreal, PQ, Canada). The spectra of the dried samples and of native aqueous rhGH were obtained as described previously.8 Data were processed to obtain second derivative IR spectra according to a previous publication8. Finally, for the major negative band associated with the α-helix content of rhGH the peak width at half height (w1/2) was computed by subtracting the low wavenumber from the high one at the half peak height8.
Circular dichroism (CD) spectroscopic measurement of rhGH secondary structure after reconstitution
CD spectra of rhGH in aqueous solution before lyophilization and after reconstitution of lyophilized samples were obtained with a Chirascan-Plus (Applied Photophysics, UK) CD spectrometer. For each sample, the CD spectrum was plotted from 200 to 260 nm by averaging spectra from triplicate measurements 8.
Surface area measurement
A Quantachrome Autosorb-1 (Boynton Beach, FL) was employed to measure the SSA’s of lyophilized formulations, using five-point krypton adsorption isotherms. For each formulation, samples from five vials of placebo formulation (no protein) were placed into the cell. The cell with samples was held under vacuum for at least 5 hours at room temperature to remove the moisture prior to initiation of the surface area measurement. Triplicate samples were measured to determine the surface area for each sample.
Electron spectroscopy for chemical analysis (ESCA)
To prevent from moisture uptake by dried formulations, all sample handling and preparation were performed in a glove-bag purged with dry air (RH<5%). Samples of lyophilized powders were deposited using double-sided adhesive tape onto a sample holder (45°) that was covered with a copper tape. The sample holder was transferred to the analysis chamber for ESCA measurements. ESCA was performed with a scanning auger multi probe PHI spectrometer (Model 25-120) equipped with monochromatic Al Kα source (pass energy 100 eV). The C (1s) photoelectron line at 284.6 eV was used as an internal standard for the correction of the charging effect in all samples. The vacuum was maintained at ~10−8 Torr or lower. Spectra were collected by AugerScan (version 3.22) and analyzed using CasaXPS software (version 2.3.12). At least triplicate samples were used to determine elemental composition for each formulation.
Calculation of the mass of rhGH on the surface of lyophilized formulations
ESCA was used to measure elemental compositions of the surface layer of lyophilized powders. ESCA is sensitive to elemental composition in approximately the outermost 100 Å of the lyophilized powders15, and thus was used to measure the mass fraction of rhGH on the surface. The mass of final solid (mt) in each vial after lyophilization is about 52 mg, which is essentially the sum of all the components: rhGH (1mg), sugar (50mg), phosphate buffer (~0.5mg) and residual water (less than 1% of total mass). rhGH contains carbon (C), oxygen (O), nitrogen (N), hydrogen (H) and sulfur (S), and based on its primary sequence, its atomic composition is C995H1541N263O301S8. According to the manufacturer, ESCA is not capable of detecting H or any element whose surface concentration is less than 0.1%. In the lyophilized solids which contain protein, sugar and buffer, the overall S content is diluted down to less than 0.1%, thus all ESCA results reflect only the surface elemental composition of N, C and O. Moreover, because neither sugar nor buffer contain N, the N peak is indicative of rhGH molecules in the outmost 100 Å, i.e. protein on the surface of the dried formulation. The calculated theoretical overall N content of rhGH on a sulfur- and H-free basis is 18.0%. To calculate the mass of protein molecules on the surface, we assume that the surface layer thickness probed by ESCA (l) is equal to the 100 Å.15 Furthermore, following the analysis presented in earlier studies9,15 we assumed that the density of the solid fraction within the cake (ρ) is constant across the cake, with a value of roughly 1.1g cm-3.9 Under these assumptions, the mass fraction of rhGH in the surface layer is:
| (1) |
where mt (52mg) is total lyophilizate mass per vial, SSA is each formulation specific surface area per gram cake (m2/g), l (100 Å) is the surface thickness, and ρ (1.1g cm−3) is the density of the solid within the cake. N% is the surface N percentage measured by ESCA.
rhGH storage stability study
Lyophilized samples were incubated for 16 weeks at 323 K. At each time point (immediately after lyophilization, and after 1, 4, 9 and 16 weeks), after rehydration of samples with water rhGH aggregation, deamidation and oxidation levels were measured by size exclusion chromatography (SEC), ion exchange chromatography (IEC) and reverse phase chromatography (RP), respectively.8 At least triplicate measurements were carried out to determine the quantities of remaining of monomer/native protein at each time point.
Error analysis
Throughout the manuscript, when error bars are presented, they represent the experimental mean ± standard deviation, based on n≥3.
Results
Design of the pre-drying annealing step
A requirement for pre-drying annealing is that the sample temperature be maintained below the freezing point but above the Tg’ (or the eutectic melting temperature if there are any crystalline components). Tg’ values determined from DSC experiments were −15.5±0.3, −37.2±0.2, −38.9±0.2 °C for 5% HES, 5% trehalose and 5% sucrose solutions, respectively. In addition, DSC results showed that ice melt onset temperature of these formulations was −2.5±0.5 °C. Based on these results and previous successful annealing protocols11, a shelf temperature of −5 °C was selected for the annealing temperature for all three formulations.
Cake structures, water content and glass transition temperatures of lyophilized formulations
Formulations prepared by all five lyophilization cycles resulted in visually elegant cake structures 8. The water contents for all lyophilized samples prepared by all of the different cycles were less than 1% wt/wt. The DSC measurements for all three formulations prepared by five different cycles showed single transitions at their respective Tg’s. Tg’s for the three formulations did not show large variations as a function of the lyophilization cycle parameters. Tg’s were 203±3, 98±2 and 64±2 °C for HES, trehalose and sucrose formulations, respectively (table 1) 28,29.
Table 1.
Tg (onset) temperatures measured by DSC, previously reported ln(τβ, h) measured by thermal activity monitor28 and previously reported <u2>−1 measured by neutron scattering29 for lyophilized formulations (without protein) prepared using the standard freeze-drying cycle.
| Tg (onset) (°C) ± SD | ln(τβ, h) (at 323K) | <u2>−1 (Å−2)±SD (at 323K) | |
|---|---|---|---|
| 5% HES | 203±3 | 3.4 | 2.25±0.04 |
| 5% Trehalose | 98±2 | 2.0 | 9.10±0.50 |
| 5% Sucrose | 64±2 | 0.2 | 6.72±0.17 |
Protein structure in lyophilized formulations
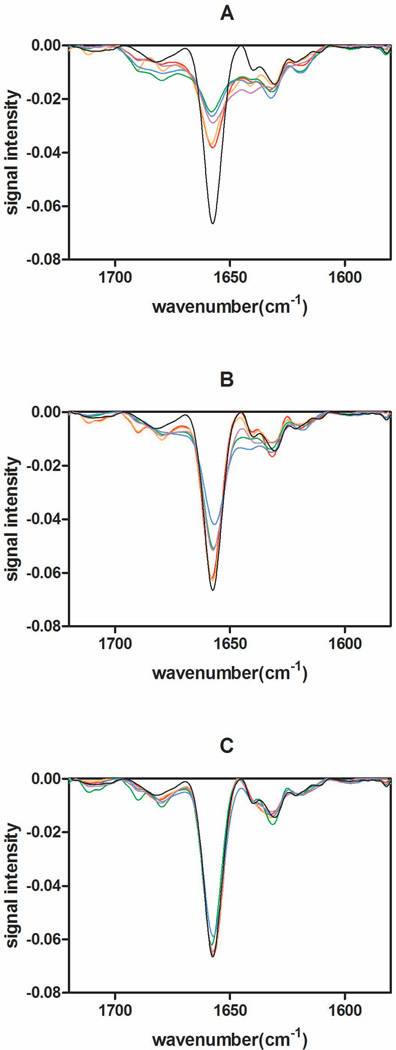
Immediately after lyophilization, samples were analyzed with IR spectroscopy to obtain second derivative spectra. The dominant negative band at 1654 cm−1 corresponds to the α-helical structure of rhGH 8. Figure 1 shows spectra for rhGH lyophilized in the presence of 5% HES (Figure 1A), 5% trehalose (Figure 1B) and 5% sucrose (Figure 1C), for each of the different lyophilization cycles tested. In general, based on the half-height width and the depth of the negative of the α-helix band near 1654 cm−1 measured by IR, rhGH in sucrose and trehalose formulations exhibited greater native-like secondary structural content than rhGH lyophilized in HES formulations; sucrose formulations dried by all cycles led to the most native-like rhGH secondary structure. Most interesting, lyophilization cycles utilizing either pre-drying annealing or post-drying annealing yielded lyophilized formulations with more native-like rhGH secondary structure than those produced using the standard lyophilization cycle. In contrast, fast freezing methods (N2-immersion and N2-droplet-freezing lyophilization) resulted in less native-like structures, as shown in Figure 1.
Figure 1.
2nd-derivative IR spectroscopic analysis of freeze-dried formulations analyzed immediately after lyophilization. (A) 5% HES formulation, (B) 5% trehalose formulation, (C) 5% sucrose formulation. Formulations prepared by pre-drying annealing lyophilization (red), post-drying annealing (orange), standard lyophilization (purple), N2-immersion (green), N2-droplet-freezing (blue), aqueous native control (black).
Protein structure in lyophilized and reconstituted formulations
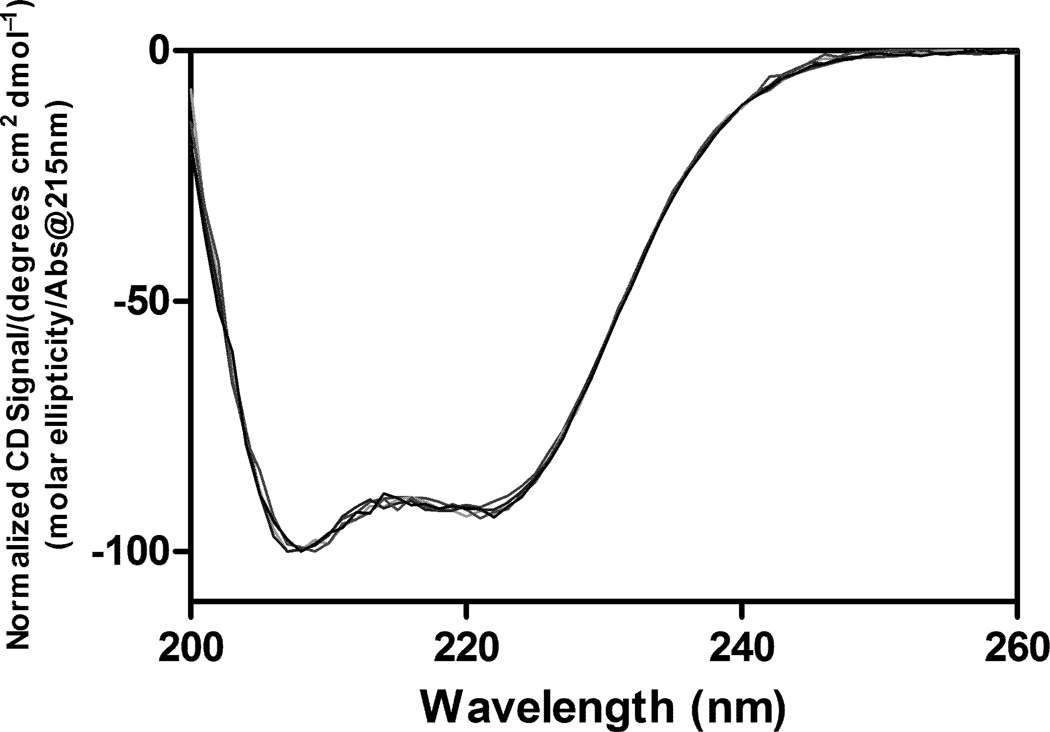
After lyophilization, samples were immediately reconstituted with water, and the secondary structure of rhGH was measured by far-UV (200–260 nm) CD spectroscopy (Figure 2). The spectra for the protein from all the formulations prepared by each of the different lyophilization methods were indistinguishable from that of the aqueous native control protein. The CD data suggested that the secondary structural perturbations observed by IR in lyophilized samples were largely reversible upon reconstitution.
Figure 2.
Protein secondary structures measured by CD spectroscopy. Samples prepared by different lyophilization cycles were reconstituted immediately after lyophilization, and there were no significant variations in the CD spectra between different samples.
Specific surface area, surface N percentage and amount of protein on the surface
SSA’s of the lyophilized formulations were measured using BET krypton adsorption (Table 2). The N2-immersion and N2-droplet-freezing lyophilized samples had much larger SSAs than the respective samples lyophilized using the standard cycle. Post-drying annealing had minimal impact on the SSAs of the lyophilized formulations we studied. On the other hand, pre-drying annealing reduced SSAs up to 2-fold compared to standard lyophilized samples. Furthermore, regardless of which lyophilization method was used, higher SSAs were found for the HES formulation compared to those for the disaccharide formulations.
Table 2.
Specific surface areas of lyophilized formulations, atom percent N in the outmost 100 Å layer of lyophilized formulations and the mass fraction of protein in that layer.
| SSA (m2/g) | N% | Surface rhGH Fraction, % |
||
|---|---|---|---|---|
| Standard lyophilization | 5% HES | 2.3±0.2 | 1.4±0.2 | 10.2 |
| 5% trehalose | 1.6±0.1 | 0.6±0.1 | 3.1 | |
| 5% sucrose | 1.2±0.1 | 0.7±0.1 | 2.7 | |
| Pre-drying annealing | 5% HES | 1.8±0.1 | 1.4±0.1 | 8.0 |
| 5% trehalose | 0.8±0.1 | 0.6±0.1 | 1.5 | |
| 5% sucrose | 0.8±0.1 | 0.7±0.1 | 1.8 | |
| Post-drying annealing | 5% HES | 2.3±0.2 | 1.2±0.2 | 8.8 |
| 5% trehalose | 1.3±0.1 | 0.4±0 | 1.7 | |
| 5% sucrose | 1.2±0.0 | 0.5±0.1 | 1.9 | |
| N2-immersion | 5% HES | 6.0±0.5 | 1.5±0.2 | 28.6 |
| 5% trehalose | 2.6±0.3 | 0.9±0.1 | 7.5 | |
| 5% sucrose | 2.7±0.3 | 0.8±0.1 | 7.0 | |
| N2-droplet-freezing | 5% HES | 6.2±0.5 | 1.6±0.2 | 31.5 |
| 5% trehalose | 3.0±0.2 | 0.9±0.1 | 8.6 | |
| 5% sucrose | 2.9±0.3 | 0.9±0.1 | 8.3 | |
ESCA measurements were first performed on a lyophilized control sample containing a 2:1 weight ratio of rhGH to phosphate buffer salts, without additional excipients. The control measurements yielded 12.6 wt% N (on a sulfur- and H-free basis), which compared well with the expected theoretical value of 12 wt% N. ESCA measurements of the %N on the surface of the lyophilized cakes containing glass-forming excipients are reported in Table 2. If the rhGH were homogeneously distributed throughout the dried solids, a %N value of 0.35% would be expected. However, in all samples tested, the %N values measured in the outermost surface layer probed by ESCA were significantly higher, indicating the presence of surface excesses of rhGH. Compared to results for the standard lyophilization cycle, the two fast freezing methods (N2-immersion and N2-droplet-freezing) both showed slightly higher N% in the outmost 100 Å of the dried formulations. On the other hand, post-drying annealing samples had low N%, and pre-drying annealing samples had N% values equivalent to those for standard lyophilized samples. For all processing methods, samples formulated in HES had higher levels of surface N% than did sucrose or trehalose formulations. Finally, the total amount of rhGH in the outmost 100 Å layer was calculated using the equation (1) (Table 2). Regardless of the type of excipients used, samples prepared by two rapid freezing, liquid N2 treatment methods showed the highest amounts of protein on their surfaces, as a result of both larger SSAs and higher surface N percentages. Likewise, regardless of the processing method that was used, the amounts of rhGH on the surface of lyophilized HES formulations were higher than those for formulations prepared from sucrose or trehalose solutions.
Lyophilization impact on rhGH monomer levels
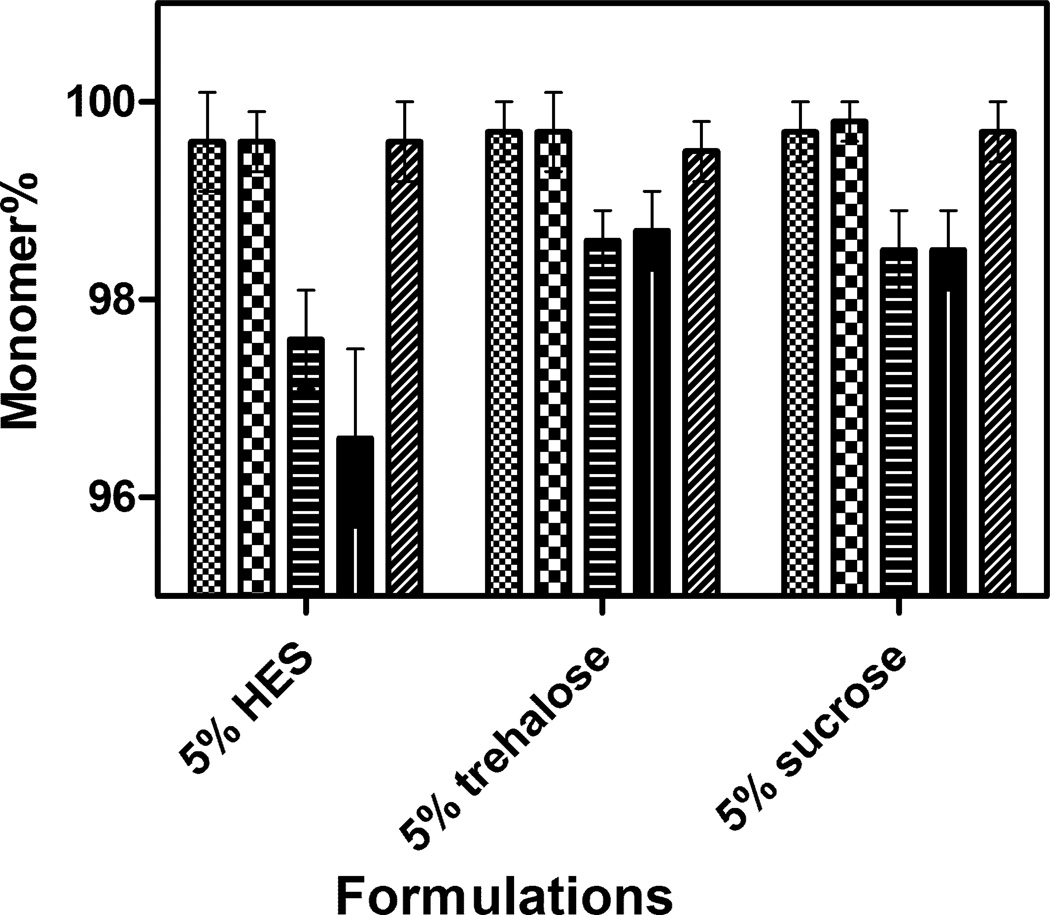
Prior to lyophilization, SEC analysis of rhGH showed that the protein was >99.9% monomeric. Upon reconstitution of samples immediately after lyophilization, about 2–3% monomer loss (compared to control samples) was observed in all formulations prepared by the two faster freezing methods (N2-immersion and N2-droplet-freezing lyophilization cycles) (Figure 3). On the other hand, no significant differences were observed in levels of deamidation or oxidation before and after lyophilization process, regardless of which formulation or lyophilization cycle was used (data not shown).
Figure 3.
Percentage of rhGH monomer detected by SEC analysis after lyophilization with various cycles and immediate reconstitution. ( ) standard lyophilization; (
) standard lyophilization; ( ) pre-drying annealing; (
) pre-drying annealing; ( ) post-drying annealing; (
) post-drying annealing; ( ) N2-immersion; (
) N2-immersion; ( ) N2-droplet-freezing. Relatively higher monomer loss was seen in the samples prepared by N2-immersion and N2-droplet-freezing lyophilization methods.
) N2-droplet-freezing. Relatively higher monomer loss was seen in the samples prepared by N2-immersion and N2-droplet-freezing lyophilization methods.
Storage stability of rhGH lyophilized samples
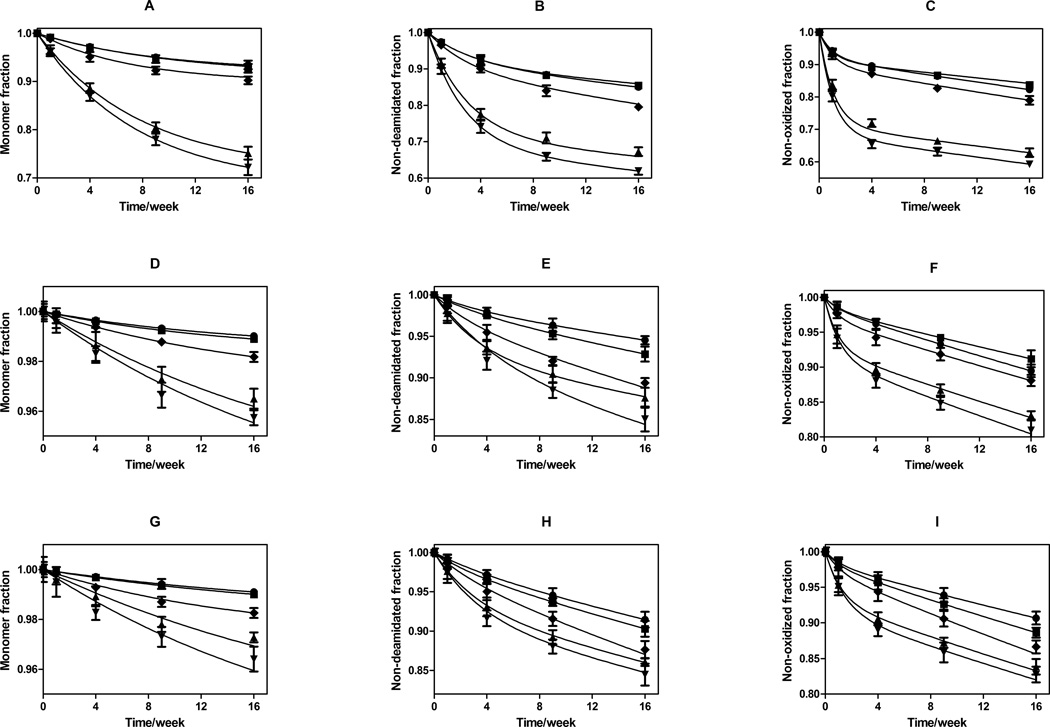
The percentages of remaining monomeric rhGH, unoxidized rhGH and non-deamidated rhGH were determined at each time point throughout the 16-week incubation study (Figure 4). The rate of loss of native protein due to formation of degradation products decreased with increasing time. Some formulations showed plateaus by the end of 16-week incubation study. It is clear that among all three formulations, the highest levels of damage were seen in the samples prepared by fast freezing methods. In contrast, samples treated with annealing (pre-drying or post-dying annealing) showed least damage after the 16-week incubation period. In addition, both deamidation and oxidation were faster than aggregation, and disaccharide formulations (sucrose and trehalose) exhibited slower degradation kinetics for rhGH than HES formulations did.
Figure 4.
Storage stability as a function of incubation time at 323 K for rhGH in formulations lyophilized using various cycle parameters. Panels: A) fraction monomeric rhGH remaining after storage in glassy HES; B) fraction rhGH not deamidated after storage in glassy HES; C) fraction rhGH that is not oxidized after storage in glassy HES; D) fraction monomeric rhGH remaining after storage in glassy trehalose; E) fraction rhGH not deamidated after storage in glassy trehalose; F) fraction rhGH that is not oxidized after storage in glassy trehalose; G) fraction monomeric rhGH remaining after storage in glassy sucrose; H) fraction rhGH not deamidated after storage in glassy sucrose; I) fraction rhGH that is not oxidized after storage in glassy sucrose. For all panels, lyophilization cycle conditions are represented by ♦: standard lyophilization, ●: pre-drying annealing, ■: post-drying annealing, ▲: N2-immersion, ▼: N2-droplet-freezing. Panel A–C represents HES formulation, Panel D–F represents trehalose formulation, and panel G–I represents sucrose formulation. Connected lines are predicted values from two parameter (ks and kb) first-order kinetics model for each individual formulation, using surface protein quantities determined from ESCA and SSA measurements.
Discussion
We hypothesized that the dominant factor that determines the rate of protein degradation observed during storage of lyophilized formulations is the amount of protein found at the solid-air interface after lyophilization. By employing different glass-forming stabilizers and lyophilization methods, samples with a wide range of masses of protein on solid-air interface were generated as a result of modulating both the SSA of the glass and concentration of the protein in the surface layer.
One major factor contributing to differences in the SSAs observed with the various lyophilization processes is Ostwald ripening of ice crystals, which only occurs to a significant extent between the time when freezing is initiated and the time when the samples cool down to the glass transition temperature for the maximally freeze-concentrated solution (Tg’). Samples prepared by the pre-drying annealing method (wherein the temperature was maintained between the freezing temperature and Tg’) had the smallest SSA values of all the methods tested because the time available for Ostwald ripening of ice crystals was longest in that method. In contrast, the fast-freezing methods (liquid N2 immersion or droplet-freezing) cause the solution temperature to rapidly reach Tg’, thus allowing little time for Ostwald ripening and yielding correspondingly high SSA values. Finally, we observed that the SSA values for the formulation processed with the post-drying annealing cycle were equivalent to those for the standard cycle. This can be explained because the freezing portion of the lyophilization cycle was identical for the standard and post-drying annealing methods. Hence, the times available for Ostwald ripening for the two methods were equal, resulting in equal ice crystal size distributions and equivalent SSA values. Also, as long as post-drying annealing is executed well below the system Tg, no change in SSA would be expected during this process.
The N% (surface N percentage) of the various lyophilized formulations determined by ESCA is indicative of protein fraction on the surface, because no other formulation component contains N. For a given formulation, N% was similar for samples prepared by pre-drying annealing, post-drying annealing and standard lyophilization methods, consistent with previous studies on methionyl rhGH9. Samples prepared by the two liquid N2-treated lyophilization protocols resulted in higher N% (Table 2). In addition, the HES formulations had the highest protein fraction on the surface regardless of which lyophilization method was used, again consistent with the earlier study on methionyl rhGH, which demonstrated that surface concentrations of protein were highest in formulations containing polymeric excipients9. A possible explanation for the higher masses of protein found on the surface of lyophilized HES formulations is that, during the freezing step of the lyophilization cycles, the greater viscosity of HES formulations hindered the diffusion of the protein away from growing ice crystal surfaces. The explanation for the high N% found on the surface of formulations prepared with liquid N2 immersion or droplet-freezing may be similar. The rapid ice formation in the process and short time between initiation of freezing and sample glassification at Tg’ limited the time available for protein to diffuse away from growing ice surfaces.
Webb et al. reported that lyophilized formulations of recombinant human interferon-γ with higher SSAs also had higher rates of protein aggregation19. It was also noted, based on electron spectroscopy for chemical analysis (ESCA)9 measurements, that the surfaces of the lyophilized solids were enriched in protein. This enrichment was likely due to a combination protein adsorption at ice-water interfaces, and limited ability of large molecules such as proteins to diffuse away from the freezing front causing them to be trapped at the surface10. Consistent with previous reports15,30,31, we observed substantial enrichment of protein on the solid-air surface of lyophilized powders (Table 2). Proteins found on the solid-air interfaces of glassy lyophilized solids certainly experience an environment that is dramatically different from that inside the glass32. Effective glass transition temperatures are expected to be lower in the interfacial region than in the bulk33,34, allowing greater mobility for any protein molecules found at the surface of glassy lyophilized powders.
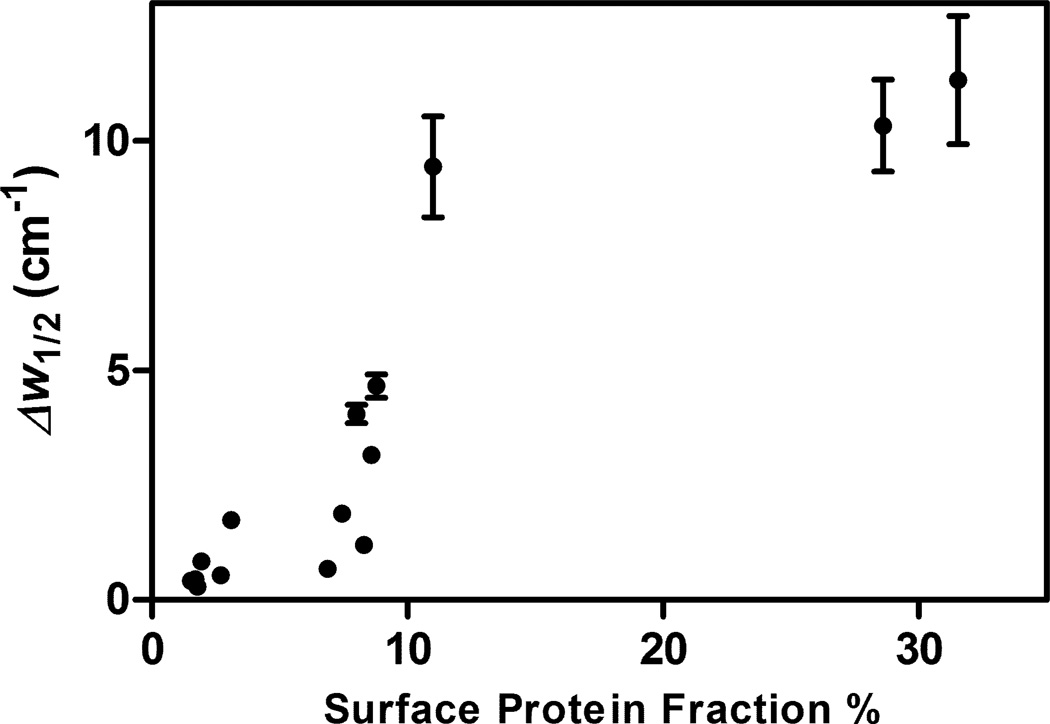
rhGH structures in dried solids prepared using both pre-drying annealing and post-drying annealing were more native-like than in those prepared using the standard lyophilization cycle, whereas rhGH structures were most perturbed in samples that had been rapidly frozen using liquid N2. Webb et al. proposed that annealing could serve to alleviate residual stress and reduce the excess free volume of the glass, thus improving protein structures19. However, another explanation could be that protein molecules on the solid-air surface are more prone to structural perturbation. Hence, those samples with smaller quantities of protein at the solid-air surface (i.e., pre-drying annealed samples) should show more retention of native structure, whereas samples with larger surface protein quantities (e.g., those prepared with liquid-N2 freezing) should show more perturbed structures. This is indeed the case, as can be seen in Figure 5, where formulations exhibiting a larger fraction of rhGH at the interface had less native-like structure as measured by IR, i.e. larger values of Δw1/2 8.
Figure 5.
Change of IR α-helical peak half width measured in lyophilized solids compared to that of native rhGH in aqueous solution (Δw1/2), plotted against the fraction of the total protein found on surface. For some data points, error bars are smaller than the symbols.
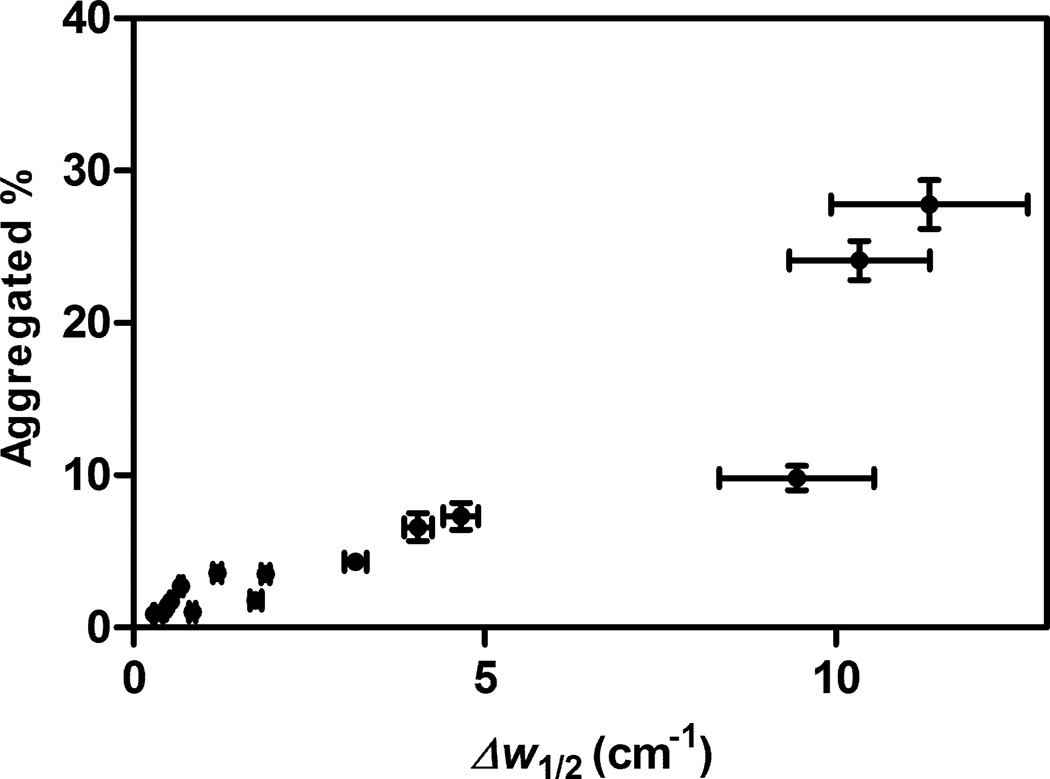
In addition, previously we showed that decreased activation free energies (ΔG†) for aggregation, deamidation and oxidation of rhGH correlated with increasing degrees of protein structural perturbation in lyophilized formulations8. In turn, rhGH degradation (by aggregation, oxidation, and deamidation) was faster in samples wherein the rhGH structure was more perturbed8. A similar result was seen in this study. As shown in Figure 6, the fraction of aggregated protein after 16 weeks incubation at 323 K increases with the degree of rhGH structural perturbation, as reflected in the change after lyophilization of the IR a-helical peak width at half height, Δw1/2.
Figure 6.
Correlation of the percent of rhGH found as aggregates measured after 16 weeks incubation at 323K with the change compared to native rhGH in aqueous solution of the IR α-helical peak half width in the lyophilized solid formulations (Δw1/2). For some data points, error bars are smaller than the symbols.
Kinetic model for rhGH degradation in lyophilized samples
Because populations of protein molecules found at the solid-air interface of lyophilized samples are likely to experience a significantly different environment from those molecules found in the bulk, we analyzed the aggregation, deamidation and oxidation kinetics for rhGH by assuming simple first-order dependence of degradation kinetics, both in the surface layer and in the bulk. Thus, for each of the degradation reactions, we write:
| (2) |
| (3) |
| (4) |
Where Pst and Pbt are the amount of native protein on the surface and in the bulk, respectively, at a certain time t, Pso and Pbo are the initial amount of native protein on the solid surface and in the in the bulk, respectively, and ks,i and kb,i are the apparent first order degradation rate constant for protein on the surface and in bulk, for each of the i reactions (aggregation, deamidation, and oxidation). The sum of Pst and Pbt is equal to Ptot, the amount of remaining native protein at any given time point, which can be measured by size exclusion, ion exchange and reverse phase chromatographic analysis of the samples after reconstitution. For each degradation pathway and each formulation, using initial amounts of protein on the surface and in the bulk determined from the ESCA and SSA measurements for Pso and Pbo, the two parameters ks,i and kb,i were fit to the data from the 16-week incubation study (Table 3), using the evolutionary solving method in the Microsoft Excel® solver package, with constraints of convergence as 10−8, mutation rate as 0.9 and maximum time as 120 seconds. Convergence to the reported optimal values was obtained from multiple initial guess values. Predicted kinetics based upon ks,i and kb,i determined for each formulation are plotted in Figure 4. In general, predicted values from individual two parameter (ks,i and kb,i) first-order kinetics model fit well to the experimental data.
Table 3.
Apparent first-order rate constants for rhGH degradation during incubation at 323 K in the surface layer (ks,i) and in the glassy bulk solid portion (kb,i) for each formulation and lyophilization cycle. The subscript i refers to the type of degradation: aggregation (i=agg), deamidation (i=d) or oxidation (i=ox). Mean absolute percent deviation for these fits are all below 8%.
| Route of Degradation |
Formulations | Apparent First Order Rate Constants (% per week) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggregation | Standard | Pre- annealing |
Post- annealing |
N2- immersion |
N2-droplet- freezing |
||||||
| ks,agg | kb,agg | ks,agg | kb,agg | ks,agg | kb,agg | ks,agg | kb,agg | ks,agg | kb,agg | ||
| 5% HES | 12.1 | 0 | 10.7 | 0 | 9.5 | 0 | 12.7 | 0 | 13.3 | 0 | |
| 5% trehalose | 5.7 | 0 | 6.5 | 0 | 6.6 | 0 | 4.5 | 0 | 4.6 | 0 | |
| 5% sucrose | 6.6 | 0 | 4.4 | 0 | 4.5 | 0 | 3.8 | 0 | 4.2 | 0 | |
| Deamidation | Standard | Pre- annealing |
Post- annealing |
N2- immersion |
N2-droplet- freezing |
||||||
| ks,d | kb,d | ks,d | kb.d | ks.d | kb,d | ks,d | kb,d | ks,d | kb,d | ||
| 5% HES | 32.0 | 0.7 | 30.9 | 0.5 | 29.8 | 0.4 | 33.2 | 0.5 | 36.4 | 0.6 | |
| 5% trehalose | 32.2 | 0.6 | 28.4 | 0.3 | 26.4 | 0.4 | 29.4 | 0.3 | 19.0 | 0.5 | |
| 5% sucrose | 30.6 | 0.7 | 23.4 | 0.4 | 32.6 | 0.5 | 31.2 | 0.5 | 28.6 | 0.5 | |
| Oxidation | Standard | Pre- annealing |
Post- annealing |
N2- immersion |
N2-droplet- freezing |
||||||
| ks,ox | kb,ox | ks,ox | kb,ox | ks,ox | kb,ox | ks,ox | kb,ox | ks,ox | kb,ox | ||
| 5% HES | 92.9 | 0.8 | 99.3 | 0.7 | 97.6 | 0.5 | 92.4 | 0.8 | 93.6 | 0.9 | |
| 5% trehalose | 88.7 | 0.6 | 85.8 | 0.6 | 77.8 | 0.5 | 95.1 | 0.7 | 86.8 | 0.8 | |
| 5% sucrose | 81.5 | 0.8 | 81.5 | 0.5 | 83.3 | 0.6 | 83.7 | 0.7 | 69.2 | 0.7 | |
As expected, values of the surface-layer rate constants ks,i were much higher than the bulk glass rate constants kb,i. For aggregation, the rate constant in the bulk ks,agg was negligible, as expected due to the high viscosity in the glassy state that limits diffusive transport of large molecules such as rhGH and also restricts the relatively large scale motions required for the protein unfolding that is generally associated with aggregation. Oxidation and deamidation reactions, both of which require smaller degrees of molecular motion than does aggregation4 showed rate constants in the bulk glass that were roughly two orders of magnitude smaller than those observed for rhGH in the surface layer.
A striking result of the analysis is that the fitted rate constants do not depend on the composition of the formulations or on the lyophilization cycle that was used to generate the samples. Although parameters that reflect molecular motions within the glassy formulations such as the relaxation time τβ determined from thermal activity monitor measurements28 and the inverse mean square displacement of hydrogen atoms <u2>−1 (see Table 1) are different for the three formulations we examine here, the rate constants for the three reactions are insensitive to these glassy-state relaxation properties*. In fact, the respective surface and bulk rate constants for the various formulations and lyophilization cycle parameters are so similar that the data for each type of rhGH degradation during incubation at 323K could be fit using Equations 2–4 by two global formulation- and lyophilization-cycle independent, apparent first-order rate constants. Table 4 lists the three sets of global rate constants ksg,i and kbg,i, where the subscript i refers to the degradation pathway (aggregation, deamidation or oxidation).
Table 4.
Global first-order degradation rate constants at 323 K for each degradation pathway (aggregation, deamidation and oxidation) for rhGH on the surface and within the bulk solid of all the formulations.
| Degradation Route |
ksg,i (% per week) |
kbg,i (% per week) |
|---|---|---|
| i = Aggregation | 11.6 | 0 (<10−3) |
| i =Deamidation | 35.7 | 0.5 |
| i =Oxidation | 90.8 | 0.7 |
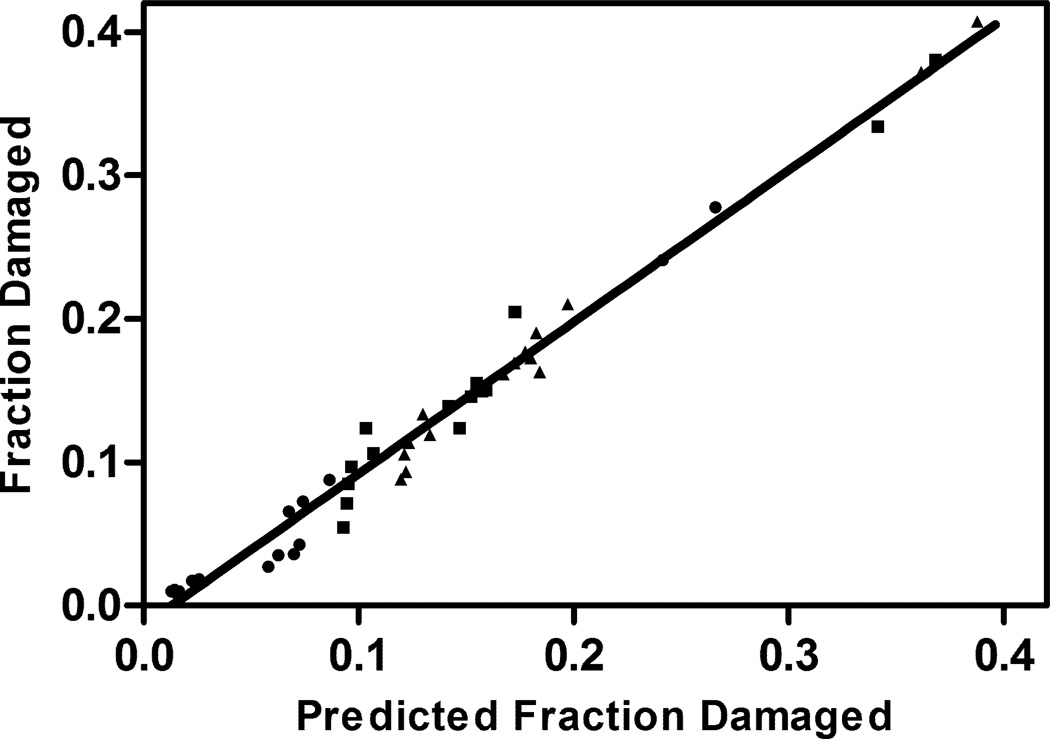
Figure 7 shows the experimentally-determined fractions of the rhGH in the various lyophilized formulations that were damaged by aggregation, oxidation, and deamidation after 16 weeks storage at 323K, plotted against values predicted from Equations 2–4, measured values of the quantity of rhGH in the surface layer and the fitted rate constants presented in Table 4. The simple model correlates the data very well, with a regression line for the plot of actual vs. predicted extent of degradation yielding a slope of 1.07±0.02 and a correlation coefficient R2=0.98.
Figure 7.
Comparison of the predicted fraction protein damaged after 16 weeks incubation at 323K with experimental values measured by liquid chromatography methods (error bars on experimental values are presented in figure 4 and omitted here for clarity). The predicted fraction was computed using pairs of global degradation rate constants ksg,i and kbg,i . The degradation routes are denoted ●: i = aggregation, ■: i = deamidation, ▲: i = oxidation). The linear regression line has a slope of 1.07±0.02, R2=0.98.
Proteins lyophilized in glassy formulations often show degradation as a function of storage time4,8. This observation is perplexing, especially for protein aggregation, because the high viscosities (>1013 Poise 35) found in the glassy state should preclude the relatively large-scale diffusive motions required for proteins to aggregate. One possible explanation to this conundrum is that during storage, protein molecules in glassy solids might accumulate small conformational changes that prime them to become increasingly aggregation-competent upon reconstitution4,5. However, no evidence showing the accumulation of this type of aggregate-competent species during storage has been presented,36 perhaps in part due to the relatively low sensitivity of available optical spectroscopies to examine protein structure in dry solids37. Aggregation of proteins after lyophilization and reconstitution is often correlated with the extent of loss of native protein structure that occurs during the lyophilization process, with those formulations and conditions that yield the greatest loss of native structure resulting in the most aggregation upon reconstitution8. Although numerous studies38–47 have demonstrated the role of unfolded or partially unfolded protein molecules in fostering aggregation, the acute loss of structure during lyophilization does not completely explain why aggregation levels increases during storage.
In current work, by separating the degradation kinetics between surface and bulk of the solid, we find an alternative explanation for protein aggregation during storage. The majority of the degradation occurs in the population of protein molecules found on the solid surface. Adsorption of proteins to interfaces frequently results in conformational perturbations, gelation, and aggregation (e.g. 48–54). Furthermore, in thin films of poly-methylmethacrylate55 and polystyrene56 formed by drying of spin-coated layers, residual stress remaining in the surface layer causes slow relaxation motions and conformational rearrangement of polymer chains. It is plausible that the correlation that we observe between the fraction of the rhGH molecules found in the surface layer of lyophilized powders and the loss of native protein secondary structure (see Figure 5) is due to a similar effect, wherein protein molecules in the glass-ice surface layer experience residual stress upon drying, causing the observed unfolding. Furthermore, the glass transition temperature near the surface of the glasses varies from that of the bulk. For example, glass transition temperatures found in a surface layer approximately 1000Å thick in poly-methylmethacrylate (PMMA) films differ from those of the bulk57. Yu et al. reported that diffusion on the surface of glasses was at least 106 times faster than bulk diffusion, and glass surface diffusion can cause surface evolution at nm to µm scale32,58, which is a length scale that would be expected to be long enough for (conformationally-perturbed) protein molecules to collide with each other in the surface glass layer, causing aggregation.
Conclusion
Pre-drying annealing and post-drying annealing both result more native-like rhGH structure after lyophilization. Fast freezing lyophilization cycle is detrimental for rhGH not only during lyophilization process, but also during storage. The amount of protein on the solid-air interface should be a key factor to consider for formulation and lyophilization design, as protein on the surface degrades much faster than that in the bulk. Finally, due to the substantial mobility differences between proteins on the glass surface and in the bulk, calculating both surface and bulk degradation kinetics is recommended in order to rationally design protein formulation and lyophilization process.
Acknowledgements
We acknowledge funding from NIH/NIBIB under grant R01 EB006398-01A1.
Footnotes
We note that the relaxation properties were measured in protein-free samples and may not reflect motions within the protein molecules themselves.
References
- 1.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: Some practical advice. Pharm Res. 1997;14(8):969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 2.Manning MC, Patel K, Borchardt RT. Stability of Protein Pharmaceuticals. Pharm Res. 1989;6(11):903–918. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 3.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1–2):1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang LQ, Pikal MJ. Mechanisms of Protein Stabilization in the Solid State. J Pharm Sci. 2009;98(9):2886–2908. doi: 10.1002/jps.21825. [DOI] [PubMed] [Google Scholar]
- 5.Cicerone MT, Douglas JF. beta-Relaxation governs protein stability in sugar-glass matrices. Soft Matter. 2012;8(10):2983–2991. [Google Scholar]
- 6.Carpenter JF, Prestrelski SJ, Dong AC. Application of infrared spectroscopy to development of stable lyophilized protein formulations. Eur J Pharm Biopharm. 1998;45(3):231–238. doi: 10.1016/s0939-6411(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 7.Randolph TW. Phase separation of excipients during lyophilization: Effects on protein stability. J Pharm Sci. 1997;86(11):1198–1203. doi: 10.1021/js970135b. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Carpenter JF, Cicerone MT, Randolph TW. Contributions of local mobility and degree of retention of native secondary structure to the stability of recombinant human growth hormone (rhGH) in glassy lyophilized formulations. Soft Matter. 2013;9(32):7855–7865. [Google Scholar]
- 9.Abdul-Fattah AM, Lechuga-Ballesteros D, Kalonia DS, Pikal MJ. The impact of drying method and formulation on the physical properties and stability of methionyl human growth hormone in the amorphous solid state. Journal of Pharmaceutical Sciences. 2008;97(1):163–184. doi: 10.1002/jps.21085. [DOI] [PubMed] [Google Scholar]
- 10.Kasper JC, Friess W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–263. doi: 10.1016/j.ejpb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Searles JA, Carpenter JF, Randolph TW. Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine T-g ' in pharmaceutical lyophilization. J Pharm Sci. 2001;90(7):872–887. doi: 10.1002/jps.1040. [DOI] [PubMed] [Google Scholar]
- 12.Rathore N, Rajan RS. Current perspectives on stability of protein drug products during formulation, fill and finish operations. Biotechnol Prog. 2008;24(3):504–514. doi: 10.1021/bp070462h. [DOI] [PubMed] [Google Scholar]
- 13.Heller MC, Carpenter JF, Randolph TW. Protein formulation and lyophilization cycle design: Prevention of damage due to freeze-concentration induced phase separation. Biotechnol Bioeng. 1999;63(2):166–174. doi: 10.1002/(sici)1097-0290(19990420)63:2<166::aid-bit5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Searles JA, Carpenter JF, Randolph TW. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J Pharm Sci. 2001;90(7):860–871. doi: 10.1002/jps.1039. [DOI] [PubMed] [Google Scholar]
- 15.Webb SD, Golledge SL, Cleland JL, Carpenter JF, Randolph TW. Surface adsorption of recombinant human interferon-gamma in lyophilized and spray-lyophilized formulations. J Pharm Sci. 2002;91(6):1474–1487. doi: 10.1002/jps.10135. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Bhugra C, Pikal M. Reduced Pressure Ice Fog Technique for Controlled Ice Nucleation during Freeze-Drying. Aaps Pharmscitech. 2009;10(4):1406–1411. doi: 10.1208/s12249-009-9338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochran T, Nail SL. Ice Nucleation Temperature Influences Recovery of Activity of a Model Protein after Freeze Drying. J Pharm Sci. 2009;98(9):3495–3498. doi: 10.1002/jps.21815. [DOI] [PubMed] [Google Scholar]
- 18.Rambhatla S, Ramot R, Bhugra C, Pikal MJ. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. AAPS PharmSciTech. 2004;5(4) doi: 10.1208/pt050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb SD, Cleland JL, Carpenter JF, Randolph TW. Effects of annealing lyophilized and spray-lyophilized formulations of recombinant human interferon-gamma. J Pharm Sci. 2003;92(4):715–729. doi: 10.1002/jps.10334. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CC, Nguyen HM, Yeung DA, Brooks DA, Koe GS, Bewley TA, Pearlman R. SURFACE DENATURATION AT SOLID-VOID INTERFACE - A POSSIBLE PATHWAY BY WHICH OPALESCENT PARTICULATES FORM DURING THE STORAGE OF LYOPHILIZED TISSUE-TYPE PLASMINOGEN-ACTIVATOR AT HIGH-TEMPERATURES. Pharm Res. 1995;12(1):69–77. doi: 10.1023/a:1016270103863. [DOI] [PubMed] [Google Scholar]
- 21.Patapoff TW, Overcashier DE. The importance of freezing on lyophilization cycle development. Biopharm-Appl Technol Biopharm Dev. 2002;15(3) 16–+. [Google Scholar]
- 22.Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: Separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12(5):505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 23.Chang BS, Kendrick BS, Carpenter JF. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J Pharm Sci. 1996;85(12):1325–1330. doi: 10.1021/js960080y. [DOI] [PubMed] [Google Scholar]
- 24.Maa YF, Nguyen PA, Sweeney T, Shire SJ, Hsu CC. Protein inhalation powders: Spray drying vs spray freeze drying. Pharm Res. 1999;16(2):249–254. doi: 10.1023/a:1018828425184. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Cicerone MT, Aso Y, Pikal MJ. The Impact of Thermal Treatment on the Stability of Freeze-Dried Amorphous Pharmaceuticals: II. Aggregation in an IgG1 Fusion Protein. J Pharm Sci. 2010;99(2):683–700. doi: 10.1002/jps.21960. [DOI] [PubMed] [Google Scholar]
- 26.Crisman RL, Randolph TW. Crystallization of Recombinant Human Growth Hormone at Elevated Pressures: Pressure Effects on PEG-Induced Volume Exclusion Interactions. Biotechnol Bioeng. 107(4):663–672. doi: 10.1002/bit.22832. [DOI] [PubMed] [Google Scholar]
- 27.May JC, Grim E, Wheeler RM, West J. DETERMINATION OF RESIDUAL MOISTURE IN FREEZE-DRIED VIRAL VACCINES - FISCHER,KARL, GRAVIMETRIC AND THERMOGRAVIMETRIC METHODOLOGIES. Journal of Biological Standardization. 1982;10(3):249–259. doi: 10.1016/s0092-1157(82)80026-7. [DOI] [PubMed] [Google Scholar]
- 28.Chieng N, Mizuno M, Pikal M. Characterization of dynamics in complex lyophilized formulations: I. Comparison of relaxation times measured by isothermal calorimetry with data estimated from the width of the glass transition temperature region. Eur J Pharm Biopharm. 2013;85(2):189–196. doi: 10.1016/j.ejpb.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chieng N, Cicerone MT, Zhong Q, Liu M, Pikal MJ. Characterization of dynamics in complex lyophilized formulations: II. Analysis of density variations in terms of glass dynamics and comparisons with global mobility, fast dynamics, and Positron Annihilation Lifetime Spectroscopy (PALS) Eur J Pharm Biopharm. 2013;85(2):197–206. doi: 10.1016/j.ejpb.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler M, Unger M, Lee G. Surface composition of spray-dried particles of bovine serum albumin/trehalose/surfactant. Pharm Res. 2000;17(7):863–870. doi: 10.1023/a:1007568511399. [DOI] [PubMed] [Google Scholar]
- 31.Millqvist-Fureby A, Malmsten M, Bergenstahl B. Spray-drying of trypsin - surface characterisation and activity preservation. Int J Pharm. 1999;188(2):243–253. doi: 10.1016/s0378-5173(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Brian CW, Swallen SF, Straus PT, Ediger MD, Yu L. Surface Self-Diffusion of an Organic Glass. Physical Review Letters. 2011;106(25):256103. doi: 10.1103/PhysRevLett.106.256103. [DOI] [PubMed] [Google Scholar]
- 33.Zuo B, Liu YJ, Wang L, Zhu YM, Wang YF, Wang XP. Depth profile of the segmental dynamics at a poly(methyl methacrylate) film surface. Soft Matter. 2013;9(39):9376–9384. [Google Scholar]
- 34.Liu D, Orozco RO, Wang T. Deviations of the glass transition temperature in amorphous conjugated polymer thin films. Phys Rev E. 2013;88(2) doi: 10.1103/PhysRevE.88.022601. [DOI] [PubMed] [Google Scholar]
- 35.Angell CA. FORMATION OF GLASSES FROM LIQUIDS AND BIOPOLYMERS. Science. 1995;267(5206):1924–1935. doi: 10.1126/science.267.5206.1924. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski K, Adrjanowicz K, Zakowiecki D, Kaminska E, Wlodarczyk P, Paluch M, Pilch J, Tarnacka M. Dielectric Studies on Molecular Dynamics of Two Important Disaccharides: Sucrose and Trehalose. Mol Pharm. 2012;9(6):1559–1569. doi: 10.1021/mp2004498. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Li C, Nguyen X, Muzammil S, Towers E, Gabrielson J, Narhi L. Qualification of FTIR Spectroscopic Method for Protein Secondary Structural Analysis. Journal of Pharmaceutical Sciences. 2011;100(11):4631–4641. doi: 10.1002/jps.22686. [DOI] [PubMed] [Google Scholar]
- 38.Khurana R, Gillespie JR, Talapatra A, Minert LJ, Ionescu-Zanetti C, Millett I, Fink AL. Partially folded intermediates as critical precursors of light chain amyloid fibrils and amorphous aggregates. Biochemistry. 2001;40(12):3525–3535. doi: 10.1021/bi001782b. [DOI] [PubMed] [Google Scholar]
- 39.Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3(1):R9–R23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 40.Fink AL, Calciano LJ, Goto Y, Kurotsu T, Palleros DR. Classification of acid denaturation of proteins: intermediates and unfolded states. Biochemistry. 1994;33(41):12504–12511. doi: 10.1021/bi00207a018. [DOI] [PubMed] [Google Scholar]
- 41.Carrotta R, Bauer R, Waninge R, Rischel C. Conformational characterization of oligomeric intermediates and aggregates in beta-lactoglobulin heat aggregation. Protein Sci. 2001;10(7):1312–1318. doi: 10.1110/ps.42501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Orellana I, Variano B, Miura-Fraboni J, Milstein S, Paton DR. Thermodynamic characterization of an intermediate state of human growth hormone. Protein Sci. 1998;7(6):1352–1358. doi: 10.1002/pro.5560070611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speed MA, Morshead T, Wang DI, King J. Conformation of P22 tailspike folding and aggregation intermediates probed by monoclonal antibodies. Protein Sci. 1997;6(1):99–108. doi: 10.1002/pro.5560060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King J, Haase-Pettingell C, Robinson AS, Speed M, Mitraki A. Thermolabile folding intermediates: inclusion body precursors and chaperonin substrates. FASEB J. 1996;10(1):57–66. doi: 10.1096/fasebj.10.1.8566549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D, Yu MH. Folding pathway of human alpha 1-antitrypsin: characterization of an intermediate that is active but prone to aggregation. Biochem Biophys Res Commun. 1996;226(2):378–384. doi: 10.1006/bbrc.1996.1364. [DOI] [PubMed] [Google Scholar]
- 46.Grillo AO, Edwards KL, Kashi RS, Shipley KM, Hu L, Besman MJ, Middaugh CR. Conformational origin of the aggregation of recombinant human factor VIII. Biochemistry. 2001;40(2):586–595. doi: 10.1021/bi001547t. [DOI] [PubMed] [Google Scholar]
- 47.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 48.Bantchev GB, Schwartz DK. Surface shear rheology of beta-casein layers at the air/solution interface: Formation of a two-dimensional physical gel. Langmuir. 2003;19(7):2673–2682. [Google Scholar]
- 49.Dickinson E. Adsorbed protein layers at fluid interfaces: interactions, structure and surface rheology. Colloids and Surfaces B: Biointerfacese. 1999;15:161–176. [Google Scholar]
- 50.Krägel J, Derkatch SR, Miller R. Interfacial shear rheology of protein-surfactant layers. Advances in Colloid and Interface Science Designer Molecules for Interfacial Activity, in honour of Alan R Pitt. 2008;144(1–2):38–53. doi: 10.1016/j.cis.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Vessely CR, Carpenter JF, Schwartz DK. Calcium-induced changes to the molecular conformation and aggregate structure of beta-casein at the air-water interface. Biomacromolecules. 2005;6(6):3334–3344. doi: 10.1021/bm050353w. [DOI] [PubMed] [Google Scholar]
- 52.Britt KA, Schwartz DK, Wurth C, Mahler HC, Carpenter JF, Randolph TW. Excipient effects on humanized monoclonal antibody interactions with silicone oil emulsions. Journal of Pharmaceutical Sciences. 2012;101(12):4419–4432. doi: 10.1002/jps.23318. [DOI] [PubMed] [Google Scholar]
- 53.Bee JS, Chiu D, Sawicki S, Stevenson JL, Chatterjee K, Freund E, Carpenter JF, Randolph TW. Monoclonal Antibody Interactions with Micro- and Nanoparticles: Adsorption, Aggregation and Accelerated Stress Stability Studies. Journal of Pharmaceutical Sciences. 2009;98(9):11. doi: 10.1002/jps.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of Surfaces and Leachables on the Stability of Biopharmaceuticals. Journal of Pharmaceutical Sciences. 2011;100(10):4158–4170. doi: 10.1002/jps.22597. [DOI] [PubMed] [Google Scholar]
- 55.Reiter G, Hamieh M, Damman P, Sclavons S, Gabriele S, Vilmin T, Raphael E. Residual stresses in thin polymer films cause rupture and dominate early stages of dewetting. Nature Materials. 2005;4(10):754–758. doi: 10.1038/nmat1484. [DOI] [PubMed] [Google Scholar]
- 56.Gabriele S, Damman P, Sclavons S, Desprez S, Coppee S, Reiter G, Hamieh M, Al Akhrass S, Vilmin T, Raphael E. Viscoelastic dewetting of constrained polymer thin films. Journal of Polymer Science Part B-Polymer Physics. 2006;44(20):3022–3030. [Google Scholar]
- 57.Inoue R, Nakamura M, Matsui K, Kanaya T, Nishida K, Hino M. Distribution of glass transition temperature in multilayered poly(methyl methacrylate) thin film supported on a Si substrate as studied by neutron reflectivity. Physical Review E. 2013;88(3) doi: 10.1103/PhysRevE.88.032601. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y, Zhu L, Wu T, Cai T, Gunn EM, Yu L. Stability of Amorphous Pharmaceutical Solids: Crystal Growth Mechanisms and Effect of Polymer Additives. Aaps J. 2012;14(3):380–388. doi: 10.1208/s12248-012-9345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]