Abstract
Annual internal radiation doses resulting from both acute and chronic intakes of all important dose-contributing radionuclides occurring in fallout from nuclear weapons testing at Bikini and Enewetak from 1946 through 1958 have been estimated for the residents living on all atolls and separate reef islands of the Marshall Islands. Internal radiation absorbed doses to the tissues most at risk to cancer induction (red bone marrow, thyroid, stomach, and colon) have been estimated for representative persons of all population communities for all birth years from 1929 through 1968, and for all years of exposure from 1948 through 1970. The acute intake estimates rely on a model using, as its basis, historical urine bioassay data, for members of the Rongelap Island and Ailinginae communities as well as for Rongerik residents. The model also utilizes fallout times of arrival and radionuclide deposition densities estimated for all tests and all atolls. Acute intakes of 63 radionuclides were estimated for the populations of the 20 inhabited atolls and for the communities that were relocated during the testing years for reasons of safety and decontamination. The model used for chronic intake estimates is based on reported whole-body, urine, and blood counting data for residents of Utrik and Rongelap. Dose conversion coefficients relating intake to organ absorbed dose were developed using internationally accepted models but specifically tailored for intakes of particulate fallout by consideration of literature-based evidence to choose the most appropriate alimentary tract absorption fraction (f1) values. Dose estimates were much higher for the thyroid gland than for red marrow, stomach wall, or colon. The highest thyroid doses to adults were about 7,600 mGy for the people exposed on Rongelap; thyroid doses to adults were much lower, by a factor of 100 or more, for the people exposed on the populated atolls of Kwajalein and Majuro. The estimates of radionuclide intake and internal radiation dose to the Marshallese that are presented in this paper are the most complete available anywhere and were used to make projections of lifetime cancer risks to the exposed populations, which are presented in a companion paper in this volume.
Keywords: dose, internal; fallout; Marshall Islands; nuclear weapons
INTRODUCTION
Internal radiation doses to residents of the Marshall Islands during the years of nuclear testing at Bikini and Enewetak (1946–1958), as well as in later years, were a consequence of inadvertent intake of radioactive materials from nuclear tests that were deposited as fallout. Doses were received both from acute intakes, i.e., those intakes occurring at the time of fallout or immediately afterwards, and from chronic intakes of residual radioactivity in the environment, i.e., intakes occurring continuously for many years after deposition. But deriving and understanding the true range of organ doses received by the Marshallese specific to each nuclear test and at each atoll of residence has remained an unmet challenge for many years. Understanding radiation doses to the Marshallese is important for several reasons that include providing to the Marshallese a complete account of the radiation doses they received and the related health consequences, increasing our overall understanding of the health impact of nuclear testing conducted in the past, and increasing our understanding and ability to prepare against fallout events in the future.
A companion paper addresses external doses received by representative persons in the Marshall Islands from nuclear testing (Bouville et al. 2010). This paper addresses internal doses. The sum of the internal and external doses (Simon et al. 2010), when estimated as age-specific annual doses at each atoll, can be used to predict the excess cancer burden that resulted from the exposures. The subject of cancer risks is addressed in a companion paper by Land et al. (2010).
We have attempted to collect and use the available data and information to conduct a dose reconstruction in a manner we believe to be relatively free of intentional biases. To accomplish that, in a companion paper (Beck et al. 2010), we estimated the deposition densities of 63 fallout radionuclides determined to have contributed over 99% of the acute internal dose at all 32 inhabited and uninhabitated atolls of the Marshall Islands, excluding Bikini and Enewetak Atolls where the tests were conducted, and developed a method to estimate acute and chronic intakes of radioactive materials from the nuclear tests for representative persons of various age groups at all inhabited atolls and the related doses to four organs. Acute intakes took place during the period of the time the fallout was being deposited at each atoll (if during the day) or shortly afterwards if the fallout arrived at night. The assumption was made that acute intakes were primarily the result of eating superficially-contaminated food, using contaminated eating utensils, ingesting contamination deposited on the hands and face, and to a lesser degree, drinking contaminated water (Lessard et al. 1985). Following the deposition of radionuclides on the ground, protracted or chronic intakes took place by ingestion but at rates much smaller than those due to the acute intakes. The environmental pathways resulting in chronic intakes are substantially different from the direct deposition of fallout on ground surfaces and materials accounting for acute intakes. Chronic intakes among Marshallese were primarily a result of consumption of seafood and of locally grown terrestrial foodstuffs and, to a lesser degree, inadvertent consumption of soil (Simon 1998 ; NCRP 1999).
Doses estimated in this work are atoll and age-group annual and lifetime radiation absorbed doses (Gy) to four organs, red bone marrow (RBM), thyroid gland, stomach wall, and colon wall, and presented as best estimates and with 90% uncertainty ranges. Doses pertaining to representative persons residing at every inhabited atoll and for all relevant birth years have been estimated for the analysis of cancer risk (Land et al. 2010). In this paper we present the dosimetric findings for four communities (Majuro, Kwajalein, Utrik, and Rongelap) that represent the overall range of doses received across the Marshall Islands as well as represent the populations of the two atolls with the largest number of residents (Majuro, the capital and largest population center, and Kwajalein, home to a U.S. military base and the second largest population center).
As far as we know, there are no publications in the peer-reviewed literature on internal doses to all the Marshallese from fallout on a yearly basis from 1948 through 1970. Previous reports focused primarily on doses to the most exposed populations in the northern Marshall Islands immediately downwind from the 1954 Bravo test (James 1964 ; Lessard et al. 1984, 1985). Much of the earlier work was reported in a special issue of Health Physics (Simon and Vetter 1997) and focused on monitoring of the most impacted islands and people, developing land remediation strategies, and assessing contemporary and possible future doses that might be received by inhabitants of certain atolls of the northern Marshall Islands. However, to our knowledge, no analysis has ever been completed on the intakes and internal doses from all fallout radionuclides, from all tests, and at all inhabited atolls. The primary goal of this publication and the companion papers was to carry out a comprehensive dose assessment and cancer risk projection.
Historical context
Of all the Pacific nuclear tests, the 1954 Castle Bravo test at Bikini Atoll caused the most serious exposures. Following the Bravo detonation on 1 March 1954, heavy early fallout was unexpectedly deposited on nearby atolls in the Marshall Islands to the east of Bikini beginning at about 4 h post-detonation and resulting in moderate to high radiation exposures to small groups of Marshalles e and Americans living or staying on those atolls: 64 Marshallese on Rongelap, 18 Marshallese from Rongelap staying on Sifo Island in Ailinginae Atoll, 159 Marshallese on Utrik Atoll, 28 military weather observers on Rongerik Atoll, and 23 sailors on the Japanese fishing vessel, the Lucky Dragon (see Cronkite et al. 1997 and Simon 1997 for additional history). The magnitudes of internal doses received by the thyroid gland of the Marshallese and American weather servicemen were not completely understood at the time of the Bravo test, primarily because there was little experience at estimating the many factors that are important to the determination of radiation dose, e.g., fission yields, atmospheric dispersion and deposition-r elated factors, quantitative understanding of modes of intake (inhalation vs. ingestion), solubility of different nuclides, doses received per unit activity intake of each radioiodine, etc.
The earliest estimates of internal dose to the highly exposed Rongelap and Ailinginae populations were in a Los Alamos Scientific Laboratory (LASL) memo to the U.S. Atomic Energy Commission (USAEC) (Harris 1954). In that document, a summary of measurements of urinary excretion of 131I and several other nuclides were reported from population pooled urine samples collected from adults at 16, 17, and 19 d post-detonation. Later, James (1964) estimated thyroid doses to Rongelap children based on the LASL excretion data (Harris 1954), though James mistakenly reported that the LASL pooled urine sample contained 20.1% (by volume) from ages 5–16 y and 4.8% from ages <5 y (Harris et al. 2010). Lessard et al. (1985) made the first detailed and methodologically traceable estimates of internal and external doses to the Rongelap and Ailinginae groups using the excretion data of Harris (1954) and other information, in particular, life style information on the Marshallese summarized by Sharp and Chapman (1957). Other investigators, primarily from the medical and health research community, later cited the estimates of Lessard et al. (1985), as that analysis was the most thorough at that time and the best document ed. All of the aforementioned dose assessment reports mistakenly assumed that the LASL pooled urine samples included urine from children (Harris et al. 2010).
In 2004, the National Cancer Institute (NCI) estimated for the first time external and internal doses to residents of all atolls from all nuclear tests conducted in the Marshall Islands (DCEG 2004). However, in that analysis, many simplifying assumptions were made and the dose estimates were conservative so as not to underestimate the cancer risks. This publication and its companion papers (Ibrahim et al. 2010 ; Beck et al. 2010 ; Bouville et al. 2010 ; Moroz et al. 2010 ; Harris et al. 2010 ; Land et al. 2010) provide a comprehensive description of an improved analysis and provide complete descriptions of methodologies used, as well as the findings. Simon et al. (2010) summarizes the main findings of all these papers and also provides tables of relevant data on tests, radionuclides, etc., used in all the papers.
METHODS
The methods described in this section are those used to estimate: (1) the acute intakes that took place during the period of time when fallout was being deposited at each atoll or soon afterwards; (2) the chronic intakes due to the consumption of local aquatic and terrestrial foodstuffs internally contaminated with long-lived radionuclides; (3) the annual and lifetime organ doses per unit acute intake; and (4) the annual and lifetime organ doses per unit chronic intake.
Twenty-six population groups are considered in this work; they include the permanent residents of each of the 20 atolls and reef islands (Ailinglaplap, Ailuk, Arno, Aur, Ebon, Jaluit, Kwajalein, Lae, Lib Island, Likiep, Majuro, Maloelap, Mejit Island, Mili, Namorik, Namu, Ujae, Ujelang, Wotho, and Wotje) that were inhabited during the 1948–1962 testing period as well as six of the seven communities or groups that were evacuated or not resident on their home atoll during at least part of the testing period [Ailinginae, Bikini, Rongelap (two groups), Rongerik, and Utrik]. The seventh population group consists of the people who were evacuated from Enewetak to Ujelang before the testing period; they are considered here to be permanent residents of Ujelang.
As indicated in Beck et al. (2010), it is estimated that 20 nuclear tests deposited fallout of any consequence in the Marshall Islands: Yoke in 1948; Dog and Item in 1951; Mike and King in 1952; Bravo, Romeo, Koon, Union, Yankee, and Nectar in 1954; Zuni, Flathead, and Tewa in 1956; Cactus, Fir, Koa, Maple, Redwood, and Cedar in 1958. Acute intakes and corresponding doses have been estimated for each of the 20 tests, the characteristics of which are presented in Simon et al. (2010, Table 1). For the determination of the internal doses from chronic intakes among atoll population groups that were not evacuated, the cumulative deposition from all tests in each year was used for the intake calculation.
Table 1.
Assumed time-of-intake, TOI (h, post-detonation), of fallout from acute exposure, rounded to nearest whole hour, for the 26 population groups (see Table 2, Simon et al. 2010) and for the 20 tests with measurable deposition (see text)
| Population group | Yoke | Dog | Item | Mike | King | Bravo | Romeo | Koon | Union | Yankee | Nectar | Zuni | Flathead | Tewa | Cactus | Fir | Koa | Maple | Redwood | Cedar |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ailinginaea | - | - | - | - | - | 6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ailinglaplap | 207 | - | - | 56 | 34 | 73 | 154 | 34 | 119 | 56 | 168 | 71 | - | - | 179 | - | - | - | - | |
| Ailuk | 196 | 78 | - | 56 | 34 | 38 | 126 | 34 | 42 | 49 | - | 98 | - | - | - | 84 | - | - | - | - |
| Arno | 189 | - | - | 56 | - | 67 | 168 | 101 | 168 | 67 | - | - | 91 | - | - | 161 | - | - | 101 | - |
| Aur | 190 | 188 | - | 56 | - | 59 | 161 | 91 | 112 | 56 | - | - | 84 | - | - | 158 | - | - | 101 | - |
| Bikini communityb | 50 | - | - | 98 | - | 78 | 202 | 119 | 238 | 154 | 182 | - | - | - | - | 183 | - | - | 101 | - |
| Ebon | 218 | - | - | 56 | - | 76 | 210 | 140 | 175 | 126 | 182 | - | - | - | - | 189 | - | - | 101 | - |
| Enewetak communityc | 17 | 24 | 38 | 17 | 17 | 25 | 59 | 70 | 34 | 31 | 112 | 70 | 31 | 59 | 25 | 76 | 105 | 63 | 91 | 42 |
| Jaluit | 210 | - | - | 56 | 50 | 76 | 196 | 126 | 238 | 154 | 182 | - | 84 | - | - | 176 | - | - | 101 | - |
| Kwajalein | 50 | 91 | - | 56 | 28 | 56 | 140 | 25 | 76 | 42 | 154 | 76 | 70 | - | - | 189 | - | - | 101 | - |
| Lae | 225 | 92 | - | 56 | 28 | 56 | 126 | 34 | 76 | 56 | 126 | 84 | 60 | - | - | 197 | - | - | 101 | - |
| Lib Island | 217 | 102 | - | 56 | 34 | 84 | 154 | 31 | 84 | 56 | 126 | - | 63 | - | - | 192 | - | - | 101 | - |
| Likiep | 202 | 78 | - | 56 | 34 | 36 | 112 | 34 | 39 | 49 | - | 126 | - | - | 174 | - | - | 101 | - | |
| Majuro | 192 | - | - | 56 | 50 | 67 | 140 | 101 | 168 | 67 | - | - | 104 | - | - | 160 | - | - | 101 | - |
| Maloelap | 185 | 178 | - | 56 | 34 | 59 | 154 | 84 | 126 | 56 | - | - | 91 | - | - | 157 | - | - | 101 | - |
| Mejit Island | 175 | 78 | - | 56 | 39 | 42 | 154 | 42 | 49 | 56 | - | - | 98 | - | - | 84 | - | - | 101 | - |
| Mili | 207 | - | - | 56 | 50 | 70 | 168 | 112 | 196 | 168 | - | - | 126 | - | - | 165 | - | - | 101 | - |
| Namorik | 213 | - | - | 56 | 78 | 196 | 140 | 147 | 182 | 182 | - | 98 | - | - | 188 | - | - | 101 | - | |
| Namu | 213 | 195 | - | 56 | 34 | 70 | 154 | 34 | 84 | 56 | 154 | - | 67 | - | - | 185 | - | - | 101 | - |
| Rongelap control groupd |
36 | 63 | - | 56 | 17 | 67e | 140e | 101e | 168e | 67e | - | - | 104e | - | - | 76 | - | - | 102 | 17 |
| Rongelap Island communityd |
36 | 63 | - | 56 | 17 | 8 | 140f | 25f | 76f | 67e | - | - | 104e | - | - | 76 | - | - | 102 | 17 |
| Rongerikg | - | - | - | - | - | 11 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ujae | 227 | 95 | - | 56 | 34 | 52 | 126 | 34 | 34 | 56 | 119 | 84 | 60 | - | - | - | - | - | 101 | - |
| Utrik communityh | 70 | 81 | - | 67 | 35 | 31 | 140i | 25i | 76i | 42i | 154i | 126 | 140 | - | - | 76 | - | - | - | - |
| Wotho | 42 | 88 | - | 56 | 17 | 18 | 126 | 34 | 63 | 49 | 112 | 70 | 92 | - | - | 92 | - | - | - | 17 |
| Wotje | 190 | 85 | - | 56 | 39 | 55 | 140 | 70 | 70 | 56 | - | - | - | - | - | 98 | - | - | 101 | - |
TOI for Rongelap Island community members temporarily on Sifo, Ailinginae.
TOI for Yoke is for Kwajalein, all others for Bikini community are for Kili Island.
All TOIs are for Ujelang.
ALL TOIs are for Rongelap Island except where noted.
TOI at Majuro.
TOI at Kwajalein.
American military weather observers.
TOI at Utrik except where noted.
TOI at Kwajalein.
Sixty-three radionuclides listed in Simon et al. (2010, Table 4) have been considered in the estimation of acute intakes and their corresponding doses. This group of radionuclides was chosen based on screening estimates, using conservative ingestion dose factors, to collectively have contributed at least 98% of the dose to the organs of concern. These screening calculations were based on the relative deposition factors published by Hicks (1981, 1984). Five long-lived radionuclides (55Fe, 60Co, 65Zn, 90Sr, and 137Cs), which were detected in whole-body and bioassay measurements conducted several years after the Bravo test in 1954, were considered for the estimation of chronic intakes and corresponding doses. In addition, acute and chronic intakes of 239+240Pu were crudely estimated based on retrospective measurements of cumulative Pu in soil samples. The depositions of 239Pu and 240Pu for specific tests, relative to 137Cs or any other radionuclide, were not reported by Hicks (1984) as that information is still classified. Intakes of all above radionuclides were estimated for typical (representative) children subdivided into 5 age groups (<1 y, 1–2 y, 3–7 y, 8–12 y, 13–17 y), as well as for representative adults. The estimated radionuclide intakes were used as the basis for estimating organ doses.
Table 4.
Parameter values used to relate the 137Cs deposition density to the initial dietary intake rates after the Bravo test.
| Radionuclide, Z |
a(Z)a (Bq d−1 per kBq m−2) |
a(Z)b (Bq d−1 per kBq m−2) |
k(Z, Bravo, j) |
|||||
|---|---|---|---|---|---|---|---|---|
| Rongelap | Utrik | Ailuk | Likiep | Mejit | Other atolls | |||
| 55Fe | 8.1 | 26 | 4.07 | 2.2 | 2.2 | 1.44 | 1.89 | 1.0 |
| 60CO | 3.2 | 3.0 | 4.07 | 2.2 | 2.2 | 1.44 | 1.89 | 1.0 |
| 65Zn | 290 | 560 | 4.07 | 2.2 | 2.2 | 1.44 | 1.89 | 1.0 |
| 90Sr | 0.013 | 0.013 | 1.45 | 1.1 | 1.2 | 1.0 | 1.05 | 1.0 |
| 137Cs | 3.0 | 7.7 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Applies to fallout from Bravo at all atolls, except for Utrik.
Applies only to fallout from Bravo at Utrik.
Acute intakes
The methods used in this study for estimating acute intakes of fallout radionuclides were based on the following four steps: (1) estimation of the intake of 131I by adults on Rongelap, Ailinginae, and Rongerik following the Bravo test using historical bioassay data, (2) considered in addition to estimation of the intakes of 62 other radionuclides 131I (Simon et al. 2010, Table 4) by adults on Rongelap, Ailinginae, and Rongerik following the Bravo test, (3) estimation of the intakes of the 63 radionuclides by adults on all inhabited atolls following all of the 20 tests that were considered (Simon et al. 2010, Table 1), and (4) estimation of the intakes by children, relative to the intakes by adults.
Estimation of acute intake by adults of 131I at Rongelap, Ailinginae, and Rongerik following the Bravo test
The estimation of 131I intake by the highly exposed populations in this work, as well as previously in Lessard et al. (1985) and NCI (2004), was based on bioassay measurements of urine samples collected within 19 d of the Bravo test originally reported by Harris (1954) and described more fully in Harris et al. (2010). The bioassay data provided direct empirical evidence of the internal contamination following the event to a subset of the Marshall Islands population. Because of the lack of detailed information on the pathways of the acute intakes, the bioassay data were used as the basis for estimating intakes to adults at all atolls. The basic calculation to estimate the average intake of 131I among the adults from whom a 24-h urine sample was collected, is shown in eqn (1):
| (1) |
Where Ǭ = acute intake of 131I intake (Bq, group average);
CR = background adjusted count rate of 131I per mL of urine (cs−1 mL−1);
K =correction factor corresponding to the radioactive decay of 131I between time of sampling and time of counting;
V̄= 24-h urine volume (mL) averaged over sampled population;
EF(t) =urinary excretion fraction for 131I on day of sampling; and
εc = gamma detector counting efficiency (count per decay).
The calculation of radionuclide intakes for this study via eqn (1) depends on having relevant data for the Marshallese population. The data used in our calculations to determine the values of the acute intakes of 131I are described in the Appendix.
Estimation of acute intakes by adults of radionuclides other than 131I at Rongelap, Ailinginae, and Rongerik following the Bravo test
Our estimates of the acute intake of radionuclides other than 131I by adults are based on: (1) an estimate of the time-of-intake (TOI), which is important for short-lived radionuclides due to the rapid change of their activity with time after the detonation, where TOI is derived from the corresponding value at the fallout time-of-arrival (TOA in h, provided in Beck et al. 2010), (2) the calculation of the ground deposition density (Bq m−2) at TOI of the radionuclides considered, and (3) a relationship between intake by adults and ground deposition density for any radionuclide following the Bravo test.
(1) Time-of-intake (TOI)
It is assumed in this work that the acute intake at Rongelap following the Bravo test took place during the period of time that the fallout was being deposited. As a general rule of thumb, based on Nevada Test Site (NTS) fallout data (Quinn 1990), the duration of fallout is approximately equal to the TOA (h). While intake might occur at various times within that period, we made the simplifying assumption that the entire acute intake occurred slightly before midway in the period of deposition, i.e., TOI = TOA + (0.4 × TOA) = 1.4 × TOA. Selection of a point in time less than halfway during the period of fallout is appropriate as a central estimate since the rate of fallout deposition generally decreases with time. The estimated TOA at Rongelap for Bravo was 6 h post-detonation (Beck et al. 2010); the corresponding TOI, rounded to one significant figure, is estimated to be 8 h.
(2) Ground deposition density at TOI
In this work, as in Beck et al. (2010), the model and data reported by Hicks (1982, 1984) to describe the variation of the relative ground deposition densities of all radionuclides deposited in the fallout with time, t, after the detonation, were used to estimate the ground deposition densities at Rongelap, Ailinginae, and Rongerik at the TOIs following the Bravo test. The data of Hicks, termed here as normalized deposition factors or ND factors, relate the ground deposition density of each radionuclide at time t to the activity of a reference radionuclide at some reference time. In this work, we have chosen to use 137Cs activity at 12 h post-detonation as the reference radionuclide and reference time to be consistent with the deposition results discussed in Beck et al. (2010) where it is shown that using 137Cs as the reference allows comparisons of estimated deposition with contemporary soil analyses to validate the fallout estimates. Since the intakes of all radionuclides are based on the intake of 131I at Rongelap, this requires use of the normalized deposition of 131I relative to 137Cs as indicated below in eqn (2).
Hicks (1984) developed the nuclide-specific ND factors only at specific times post-detonation and for a limited set of fractionation ratios. For the purposes of this work, it was necessary to estimate the ND factors at times intermediate to the values Hicks provided (i.e., ~8 h for Rongelap, ~6 h for Ailinginae, and ~11 h for Rongerik). Using 137Cs as the reference radionuclide for ND simplifies the interpolation over t since 137Cs activity varies little with TOA, due to the long half-life of the radionuclide.
As described in Beck et al. (2010), it was also necessary to estimate the degree of fractionation and to modify the reported Hicks (1984) calculations to obtain ND estimates for these estimated fractionation ratios. The estimated fractionation ratios for Bravo for Rongelap, Ailinginae, and Rongerik were 1.4, 1.3, and 1.5, respectively (Beck et al. 2010).
(3) Relationship between ground deposition density and acute intake
The acute intake was assumed to be instantaneous and to be directly proportional to the ground deposition density of each radionuclide. Thus, the ratio of intake to ground deposition density, in all settings, was assumed to be independent of the radionuclide considered. The ratios of the intakes to ground deposition densities for any radionuclide were, thus, derived from the measured intakes of 131I and from the corresponding estimates of ground deposition density at Rongelap, Ailinginae, and Rongerik.
In summary, the average intakes, [Latin capital letter Q with macron above] (Bq), of any radionuclide, Z, other than 131I, by adults at Rongelap, Ailinginae, and Rongerik, were estimated by means of eqn (2):
| (2) |
Estimation of the intakes by adults of any radionuclide on any in habited atoll following any test
The methodology used for Rongelap, Ailinginae, and Rongerik following the Bravo test was also used for all other tests and all other atolls. The intake of any radionuclide at any atoll was assumed to be proportional to the estimated deposition density of that radionuclide at that atoll, i.e., the pathways of acute intake were assumed to similar for all atolls and all tests. This simplifying assumption may not be strictly valid for atolls at large distances from the test site where fallout duration was much longer and particle sizes much smaller than at Rongelap. However, we believe that this model provides reasonable estimates of acute intake without any substantial bias at those atolls, though it is recognized that these estimates are more uncertain than the estimates of 131I intake following deposition of fallout at Rongelap, Ailinginae, and Rongerik from the Bravo test.
(1) Time of intake (TOI)
Here again, we assumed that the acute intake at a given atoll following a given test occurred slightly before midway in the period of deposition, i.e., TOI = 1.4 × TOA. Estimated TOIs for fallout from 20 tests for the 26 population groups residing at 25 atolls are presented in Table 1 as derived from estimated TOAs (Beck et al. 2010, Table 6). TOAs ranged from about 4 h for Bravo test fallout at Ailinginae to about 170 h for the most distant atolls and, thus, intakes there were assumed to have taken place at 6 h and 238 h post-detonation, respectively. As discussed in Beck et al. (2010), the fallout at distant atolls often occurred over extended periods and, therefore, the assumption that all of the intake took place at TOI may, in some cases, result in a slightly conservative estimate of intake for some radionuclides.
Table 6.
Predicted fraction of stable elements transferred to the infant in breast milk following maternal ingestion (prediction based on eqn 10, see Fig. 2).
| Element | f1 (mother) | Fraction transferred from mother to infant through breast milk |
|---|---|---|
| Cu | 5.0×10−1 | 4.06×10−2 |
| As | 5.0×10−1 | 4.02×10−2 |
| Br | 1.0×100 | 8.54×10−2 |
| Rb | 1.0×100 | 8.50×10−2 |
| Y | 1.0×10−4 | 4.05×10−6 |
| Rh | 5.0×10−2 | 3.35×10−3 |
| Pd | 5.0×10−3 | 2.77×10−4 |
| Cd | 5.0×10−2 | 3.33×10−3 |
| In | 2.0×10−2 | 1.24×10−3 |
| Sn | 2.0×10−2 | 1.24×10−3 |
| La | 5.0×10−4 | 2.31×10−5 |
| Pr | 5.0×10−4 | 2.30×10−5 |
| Nd | 5.0×10−4 | 2.30×10−5 |
| Pm | 5.0×10−4 | 2.30×10−5 |
| Sm | 5.0×10−4 | 2.30×10−5 |
(2) Ground deposition density at TOI
As discussed above, in case of the Bravo test, the ND factors were calculated taking into account the degree of fractionation (Beck et al. 2010). The atom ratios of various nuclides released from the detonations of different nuclear weapons varied due to differences in fissile material and device construction (Hicks 1981). As shown in Beck et al. (2010), the 131I to 137Cs ratio was quite insensitive to the particular test, even for non-thermonuclear compared to thermonuclear tests. Although many radionuclide ratios varied only slightly between the types of test (thermonuclear vs. non-thermonuclear), some of the radionuclide ratios differed significantly, reflecting the different fission yields for 239Pu fission compared to 238U fast fission. Most of the fission occurring in the thermonuclear tests was from fast fission of 238U (Glasstone and Dolan 1977). In this work, the radionuclide mixture for the Bravo test was used for deposition-density estimates for all thermonuclear tests, while for non-thermonuclear tests, the radionuclide mixture for the Tesla nuclear test, a typical 239Pu-fueled device tested at the NTS in 1955 (Hicks 1981), was taken to be representative of the non-thermonuclear tests conducted in the Marshall Islands (Beck et al. 2010). Regression equations as a function of time for the ND factors for all nuclides considered were developed and used to interpolate the values to specific times not provided by Hicks (1981, 1984), but needed for the estimated times of intake and for the assumed fractionation ratios. Note that because of the long half-life of 137Cs and the short half-lives of its precursors, the ND values for 137Cs activity can be considered to be constant and equal to unity over the range of TOAs and TOIs that were considered.
The 137Cs deposition densities at TOI that were used to compute deposition from each test at each atoll from equations 3 and 4 described below were, therefore, taken directly from Table 7 in Beck et al. (2010).
Table 7.
Parameters used to estimate 131I and l37Cs intake among adults based on urine bioassay (Harris et al. 2010) following the Bravo test and l37Cs intake per unit 137Cs deposition.
| Group sampled (ID) |
||||
|---|---|---|---|---|
| Date of sampling | Marshallese adults on Rongelap Island (LA316R) 3/16/1954 |
Marshallese adults on Rongelap Island (LA317R) 3/17/1954 |
Marshallese adults on Sifo, Ailinginae (LA319S) 3/19/1954 |
American military weather observers on Rongerik (LA319A) 3/19/1954 |
| Assumed time of intake (H+h) | 8.4 | 8.4 | 5.6 | 11.2 |
| Sampling to counting (d) | 14 | 13 | 11 | 11 |
| cps per 500 mL | 70 | 76 | 33 | 20 |
| Average 24-h urine production (mL d−1) for adults |
427 | 448 | 385 | 1,072 |
| Number of persons sampled for urine in pooled samples |
35 | 31 | 15 | 9 |
| Estimated excretion fraction on day of sampling (see text) |
1.73 × 10−4 | 1.63 × 10−4 | 1.42 × 10−4 | 1.85 × 10−4 |
| Average intake 131I (adult, kBq) |
3,310 | 3,680 | 1,320 | 1,710 |
|
l37Cs deposition from Bravo (kBq m−2) |
100 | 100 | 32 | 67 |
| 137Cs intake (kBq) | 2.9 | 3.2 | 1.2 | 1.4 |
| l37Cs intake per unit | 0.029 | 0.032 | 0.036 | 0.021 |
|
137Cs deposited (kBq per kBq m−2) |
||||
| Uncertainty of 137Cs deposition (GSD) | 1.5 | 1.5 | 1.8 | 2.0 |
|
|
|
|||
| Weighted average 137Cs intake per unit l37Cs depositeda |
0.031(Rongelap and Ailinginae) | 0.021 (Rongerik) | ||
Logarithms of 137Cs intake per unit 137Cs deposition inversely weighted by variance of 137Cs deposition (see text).
(3) Relationship between ground deposition density and intake
As indicated above, the relationship between ground deposition density and intake, for a given test and location, is assumed to be independent of the radionuclide considered because the intake, [Latin capital letter Q with macron above] (Bq), is assumed to be instantaneous and directly proportional to the ground deposition density, Dep (Bq m−2). Also, as discussed earlier, it is assumed in this work that the relationship between ground deposition, Dep, and intake, [Latin capital letter Q with macron above], that was obtained for the Bravo test at Rongelap, holds for all other tests and locations as well. The intakes by adults of 137Cs at atoll i, following test j, are calculated as follows:
| (3) |
Using the results from, eqn (3) the intakes of any radionuclide, Z, other than 137Cs, at atoll i from test j, are calculated as:
| (4) |
Estimating acute radionuclide intakes for younger ages
As described in detail earlier, we have relied upon bioassay data for adults to estimate acute intakes of 131I from Bravo at Rongelap and scaled those intakes to the varying ground deposition of 137Cs from each nuclear test at each atoll to calculate intakes of all other radionuclides by adults. Acute intakes also have been estimated for younger aged persons classified into the five age groups considered by the International Commission on Radiological Protection (ICRP 1993), i.e., 0–1 y, 1–2 y, 3–7 y, 8–12 y, and 13–17 y. For estimating intakes by younger aged persons, we have relied upon a combination of bioassay measurements among persons younger than adult, reported by investigators at the Walter Reed Army Institute (Woodward et al. 1959) and the USAEC (1956), and various age-dependent parameters from the literature that are potentially related to internal contamination of the body. We directly compared the age dependence of the daily excretions (Bq, total beta activity) for young age groups (see Table A2 of Harris et al. 2010) to six different physiologically- and anatomically-related parameters including breathing rates (at rest and during light exercise), body mass, daily water requirements, basal metabolic rate, energy expenditure, and body surface area (ICRP 2002). For the ages younger than adult, we found that the age dependence of body surface area to be most similar to the age dependence of the reported bioassay data.
Our interpretation of body surface area as a surrogate index for scaling adult intakes to younger age groups is related to the concept that particulate contamination of the face and hands (whose area can be considered to be a constant fraction of the body surface at each age) was a major contributor to internal contamination. This would be particularly true for children, for whom hand to mouth contact is frequent. The age-dependent acute intakes, relative to adults, selected in this study are presented in Table 2.
Table 2.
Assumed age dependence of acute radionuclide intake relative to adult intake.
| Age category (y) | Acute intake relative to adult |
|---|---|
| <1 | 0.1 + breastfeeding |
| 1 to <3 | 0.3 |
| 3 to <8 | 0.4 |
| 8 to <13 | 0.6 |
| 13 to <18 | 0.9 |
| ≥18 | 1 |
For the youngest age group (<1 y), we assumed that there are two sources of intake: the consumption of mother’s breast milk and the ingestion of fallout particles. The intake of a given radionuclide via mother’s breast milk is the product of the mother’s radionuclide intake, the fraction of the activity of each nuclide ingested by the mother that is transferred to breast milk (Fbm), and the consumption rate of breast milk by the infant. We discuss the derivation of these factors in a later section. In addition to the intake of radionuclides via breast milk, we assumed infants (0–1 y of age) had direct ingestion of fallout equal to 10% of the adult intake (Table 2), since the body surface area of the infant is about 10% of that of the adult (ICRP 2002).
Chronic intakes
Chronic intakes of radionuclides that persisted in the environment for years after fallout deposition were also assessed. The environmental pathways resulting in chronic intake are substantially different from those of the acute intakes and are primarily related to the consumption of seafood and of locally grown terrestrial foodstuffs internally contaminated with long-lived radionuclides as a result of root uptake, and, to a lesser degree, to the inadvertent consumption of soil (Simon 1998 ; NCRP 1999).
The available whole-body counting and bioassay measurements were used as a basis to estimate the chronic intakes. Those whole-body and bioassay measurements were made on the Rongelap and Utrik evacuees for years after they returned to their respective home atolls (Lessard et al. 1984). Those two atolls had been evacuated within about two days following the detonation of the Castle Bravo test on 1 March 1954. Rongelap and Utrik inhabitants were returned to their home atolls in June 1957 and June 1954, respectively (Simon et al. 2010, Table 3). During the first few weeks after their return and until the 1980’s, a Brookhaven National Laboratory team regularly conducted measurements of whole-body activity of 137Cs, 60Co and 65Zn, as well as urinary concentrations of 90Sr. Measurements of 55Fe in blood were also performed but only once (Lessard et al. 1984).
Table 3.
Values used to estimate chronic intakes for the populations of Rongelap and Utrik. Uncertainties correspond to one standard deviation (based on Lessard et al. 1984).
| Radionuclide, Z |
Atoll, j | Ingestion rate on day of return to the atoll, q(Z, Bravo, j, τ) (Bq d−1) |
Radioactive decay constant, λ(Z, j) (d−1) |
Dietary removal rate, k(Z, j) (d−1) |
Effective half-time of dietary removal, ln 2/[λ(Z,j)+k(Z, j)] (d) |
|---|---|---|---|---|---|
| 55Fe | Rongelap | 1,700 ± 930 | 7.1 × 10−4 | 0a | 980 |
| 60Co | Rongelap | 95 ± 32 | 3.6 × 10−4 | 2.0 × 10−3 | 290 |
| 65Zn | Rongelap | 1,300 ± 940 | 2.8 × 10−3 | 1.3 × 10−3 | 170 |
| 90Sr | Rongelap | 2.1 ± 1.1 | 6.6 × 10−5 | 1.7 × 10−4 | 2,900 |
| 137Cs | Rongelap | 390 ± 130 | 6.3 × 10−5 | 2.0 × 10−4 | 2,600 |
| 55Fe | Utrik | 1,300 ±710 | 7.1 × 10−4 | 0 | 980 |
| 60Co | Utrik | 130 ± 44 | 3.6 × 10−4 | 2.0 × 10−3b | 290 |
| 65Zn | Utrik | 21.000 ± 16,000 | 2.8 × 10−3 | 1.3 × 10−3b | 170 |
| 90Sr | Utrik | 0.40 ± 0.30 | 6.6 × 10−5 | 1.6 × 10−4 | 3,100 |
| 137Cs | Utrik | 210 ± 110 | 6.3 × 10−5 | 1.8 × 10−4 | 2,900 |
Assumed value.
Assumed to be the same as in Rongelap.
The steps used to estimate the chronic intakes of radionuclides were: (1) estimation of the chronic intakes by Rongelap and Utrik adult evacuees due to the Bravo test, (2) estimation of the chronic intakes resulting from the Bravo test by adults of all other atolls, (3) estimation of the chronic intakes by adults resulting from tests other than Bravo, and (4) estimation of the chronic intakes by children.
Estimation of the chronic intakes by Rongelap and Utrik adult evacuees due to the Bravo test
Lessard et al. (1984) summarized the findings of the Brookhaven whole-body counting and bioassay program and estimated the ingestion rates of 55Fe, 60Co, 65Zn, 90Sr, and 137Cs for the adult populations monitored when they returned to their atolls, and also provided data on the variation of the intake rates with time. Assuming implicitly that fallout from the Bravo test at Rongelap and Utrik was much more important than the fallout from all other tests, Lessard et al. (1984) used a single exponential relationship to model the decline of dietary activity intake during the entire period of time in which whole-body and bioassay measurements were made, i.e., from 1957 to 1981. The variation with time of the dietary intake rate, q, of radionuclide, Z, from test Bravo, at atoll, j, with time, t, (assuming no additional fallout) can, thus, be expressed as:
| (5) |
where
q(Z, Bravo, j, [tau]) = the dietary intake rate (Bq d−1) of radionuclide Z from the Bravo test on the day of return to the atoll j;
[tau] = is the time (d) elapsed between the Bravo test and the return to the atoll, and t is greater than, or equal to, [tau];
[lambda](Z) = the radioactive decay constant (d−1) of radionuclide Z; and
k(Z, j) = the dietary removal constant (d−1) of radionuclide Z at atoll j.
The values of q(Z, Bravo, j, [tau]) and k(Z, j) obtained by Lessard et al. (1984) are presented in Table 3. It is worthwhile noting that the uncertainties are large and the values of k for 60Co and 65Zn obtained for Rongelap were used for Utrik by Lessard et al. (1984), as well as in this work because of the paucity of relevant measurements on the Utrik residents. In fact, because many more measurements were made on the Rongelap evacuees than on the Utrik evacuees, only the results obtained for the Ro ngelap evacuees were used as a basis to estimate the chronic intakes for the residents of all other atolls, with the exception of Utrik.
The detection of substantial levels of 65Zn in the bodies of the Rongelap and Utrik evacuees poses a dosimetric estimation problem since normalized deposition factors for 65Zn were not reported by Hicks (1984). We assumed that 65Zn was produced by neutron activation of weapons materials and of entrained sea water, admittedly in small amounts, and was, therefore, present in local and regional fallout. The 65Zn was then apparently absorbed by phytoplankton and zooplankton and further concentrated by fish and other aquatic animals feeding on plankton in ocean and lagoon areas close to each atoll (Donaldson 1963 §; Donaldson et al. 1997). The fact that most of the activity of plankton and fish in the mid-1950’s was due to activation products (55Fe, 57Co, 60Co, 65Zn) seems to indicate the avidity of plankton and seafood for those elements (Welander 1958). On the other hand, 90Sr and 137Cs are mainly found in terrestrial foodstuffs contaminated as a result of root uptake.
Because most of the atolls were not evacuated and their populations not monitored, it is essential to estimate the variation of the dietary intake rate with time after the test. We assumed that the temporal variation of the dietary intake shown in eqn (5) also holds for the initial period of time of approximately three years, during which Rongelap was not inhabited and, therefore, no measurements were made. Eqn (5) can therefore be modified as:
| (6) |
Using eqn (6), the radionuclide intake rates at the time of the Bravo test, q(Z, Bravo, Rongelap, 0), are estimated to be 3,900 Bq d−1 for 55Fe, 1,600 Bq d−1 for 60Co, 164,000 Bq d−1 for 65Zn, 2.8 Bq d−1 for 90Sr, and 540 Bq d−1 for 137Cs. Those “initial” intake rates are theoretical because it would have taken some time for the chronic intake pathways to become established since they involve contamination of the vegetation by root uptake and the contamination of seafood, and the populations of Rongelap and Utrik were evacuated within two days after the Bravo test before any significant chronic intake could occur.
As will be evidenced later, it is essential to establish a relationship between the “initial” intake rates (which are only available for Bravo at Rongelap and Utrik) and the 137Cs deposition densities (which are available for all tests and all atolls). The 137Cs deposition density for Bravo at Rongelap, estimated as 100 kBq m−2 in Beck et al. (2010), cannot be used for that purpose because the results of the bioassay measurements conducted in 1957 among the Rongelap Island community were not only due to Bravo, but also, to some extent, to fallout at Rongelap from all other tests conducted in 1948, 1951, 1952, 1954, and 1956, in addition, to a small degree, to fallout at Kwajalein and Majuro from the tests conducted before or during the periods of residence of the evacuees at those atolls (Table 1, Simon et al. 2010). The environmental inventories of the long-lived radionuclides on Rongelap Atoll in 1957, the year when the whole-body and bioassay measurements were made, include contributions from all tests that resulted in measurable fallout on the atoll before that year. Taking 65Zn as an example, we estimated that the inventory of that radionuclide at Rongelap in 1957 was mainly due to Bravo (73%), with only minor contributions from the other 1954 tests (15%) and from the 1956 tests (12%). Therefore, the 65Zn whole-body contents measured in 1957 could also have been obtained if Bravo had led to a “theoretical” 137Cs deposition density at Rongelap 1.4 times greater than what was estimated (100 kBq m−2; Table 7 of Beck et al. 2010) and if no other test had contributed to the 65Zn whole-body contents measured in 1957 among the Rongelap Island community. In our calculations, we assumed that for each test, the “initial” intake rate of 65Zn was proportional to the deposition density of 137Cs. Taking into account that 65Zn was heavily fractionated at Rongelap, the relationship between the initial intake rate of 65Zn and the theoretical deposition density of 137Cs can be expressed as:
| (7) |
where q(65Zn, Bravo, Rongelap, 0) = 164,000 Bq d−1
a(65Zn) = the ratio of the initial dietary intake of 65Zn, in Bq d−1, and of the deposition density of 137Cs, in kBq m−2, for a reference level of fractionation, R/V, of 0.5;
K(65Zn, Bravo, Rongelap) = 4.07 is the degree of fractionation of 65Zn relative to 137Cs for Bravo at Rongelap;** and
Depthe[137Cs(65Zn), Bravo, Rongelap] = 140 kBq m−2 is the “theoretical” deposition density of 137Cs at Rongelap that would have occurred if only the test Bravo had contributed to the 65Zn inventory in 1957.
Hence, a(65Zn) = 290 Bq d−1 of 65Zn per kBq m−2 of 137Cs. It is important to note that the value of a(65Zn) depends only on the radionuclide that is considered and that it is independent of the nuclear test and of the fallout location.
Similar calculations were carried out to relate the initial dietary intake rates and the theoretical 137Cs deposition densities for the five considered radionuclides at Rongelap and Utrik. Results are presented in Table 4. Values of the dietary intakes at any time after the test Bravo could then be calculated using eqn (6).
Estimation of the chronic intakes resulting from the Bravo test by adults of all other atolls. Whole-body counting and or bioassay data similar to those available for the Rongelap and Utrik evacuees are not available for residents of any of the other 20 inhabited atolls. In this case, there is no need to calculate a modified 137Cs deposition density because the populations were exposed to fallout from all tests at the same location. The general formulation that was used to derive the initial intake rate at atoll j from the 137Cs deposition density at that atoll for the Bravo test is given in eqn (8):
| (8) |
Values of the dietary intakes at each atoll and at any time after the Bravo test were calculated using eqn (6). We assumed that the variation of the dietary intake rates with time estimated for Rongelap held for all other atolls and that the relationship between 137Cs deposition and “initial” intake rates was the same at Rongelap and at all other atolls.
The values of K(Z, Bravo, j) that were used in eqn (8) are shown in Table 4. They reflect the fractionation effects that have been estimated for the Bravo test. Isotopes of Fe, Co, and Zn are highly fractionated in comparison to 90Sr, and even more so in comparison to 137Cs. Consequently, the deposition densities of 55Fe, 60Co, and 65Zn, relative to 137Cs or 90Sr, were much greater on atolls close to the detonation site (Rongelap, Utrik, Ailuk, Likiep, and Mejit), than on more distant atolls where an R/V ratio of 0.5 was systematically used. Estimation of the chronic intakes by adults resulting from tests other than Bravo.
Two of the radionuclides considered (90Sr and 137Cs) are fission products, the other three (55Fe, 60Co, and 65Zn) being activation products. The ND factors for 55Fe and 60Co were derived and reported by Hicks (1984) for only three of six Castle series tests; they show a wide variability from test to test as the activities produced depend on the specific materials used in the construction of each nuclear device. The ND factors for the other activation product, 65Zn, were not reported for any of the tests. In the absence of relevant ND factors, two essential simplifications were made: (1) the variation of the dietary intake rates with time was assumed to be the same for all tests and all atolls as described by eqn (6); and (2) the “initial” intake rates of the long-lived radionuclides were assumed to be proportional to the ground deposition densities of 137Cs as estimated in Beck et al. (2010) for each test and at each inhabited atoll, and were calculated by means of eqn (8) in which K(Z,i,j) is taken to be equal to unity. In that case, we assumed that there was no fractionation of fallout radionuclides for any test other than Bravo at any atoll.
Estimation of the chronic intakes by children
Based on a limited number of whole-body counting measurements on Rongelap evacuees, the ratios of the intake rates of 137Cs by children compared to adults were 1.8 for children aged less than 3 y, 1.4 for children aged 3 to 7 y, and 0.9 for other children. We assumed that the same age dependency was applicable for estimating intakes of 90Sr, which are, as for 137Cs, mainly due to the consumption of internally contaminated terrestrial foodstuffs. However, the intakes of 65Zn, 55Fe, and 60Co were due to the consumption of fish and other seafood. Using the consumption estimates for fish and other seafood provided by Robison and Phillips (1989) and the assumption that the activity intake was proportional to the amounts of seafood consumed, the age-dependent relative intakes of 65Zn, 55Fe, and 60Co were 1 for adults, 0.9 for 15-y-old, 0.8 for 10-y-old, 0.6 for 5-y-old, 0.3 for 1-y-old, and 0.1 for newborn.
Dose calculations
Annual absorbed doses to RBM, thyroid, colon, and stomach wall have been estimated for the time period from 1948 through 1970 for representative individuals who were assumed to be alive in 1970. The methods used to estimate doses resulting from acute intakes and from chronic intakes will be considered in turn.
Annual doses from acute intakes
The method for calculating annual doses from acute intakes is simply the product of the acute average intake, [Latin capital letter Q with macron above] (Bq), of radionuclide i and the dose coefficient (Gy Bq−1) for that radionuclide where the dose coefficient was specific to an interval of time after intake: either the remainder of the calendar year in which the intake occurred, or the full year in successive years:
| (9) |
Where D(o, i, y) = the dose (Gy) for organ o from radionuclide i in a specific year, y, after intake; [Latin capital letter Q with macron above](i) = the average acute intake (Bq) of radionuclide i; and
DC(o, i, age, y) = the annual dose coefficient (mGy Bq−1) for organ o from radionuclide i, for age in a specific year, y, after intake.
The annual dose coefficients, which are the absorbed doses per unit activity intakes (mGy Bq−1), have been estimated for six age groups (<1 y, 1–2 y, 3–7 y, 8–12 y, 13–17 y, 18+ y). Doses to the embryo and fetus have not been calculated as they are expected to have been much smaller than those received during the first year of life. For example, in the case of iodine, which has been relatively well studied, selective uptake of that element by the fetal thyroid does not occur until the end of the 11th week following conception when the fetal thyroid begins to function (ICRP 2001). This implies that the thyroid dose to the fetus per unit intake of 131I by the mother is a small fraction of the dose the infant would receive per unit intake after birth: ~0.001% at 5 wk development, 0.03% at 10 wk, 2% at 15 wk, 6% at 25 wk, and about 10% at 35 wk. In this work, the doses to the embryo and fetus are assumed to be very small and taken to be equal to zero.
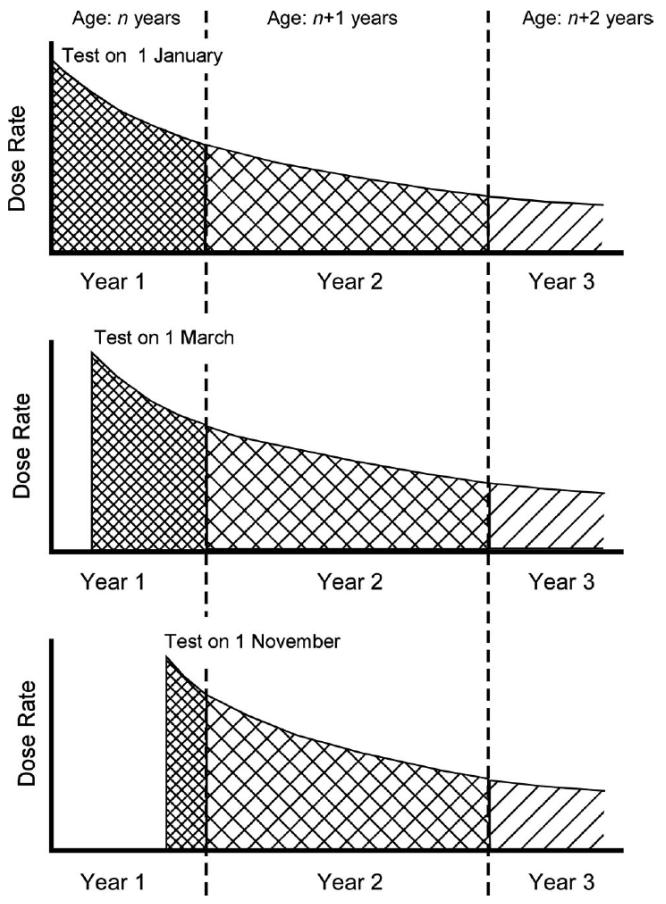
For all age groups and all radionuclides considered, with the exception of the 131I intakes by adults, the dose coefficients are based on the biokinetic models recommended by ICRP (1996, 2004). The only parameter values that have been changed are those of the alimentary tract absorption fractions (f1), which have been taken from the review by Ibrahim et al. (2010), that are specifically related to the intakes of radionuclides in particulate fallout. An established computer code (Eckerman et al. 2006) was used to solve the ICRP biokinetic models and to provide annual dose coefficients for all organs and age groups. For any test, the first year annual dose coefficient was the dose per unit intake received from the date of the intake until the end of the calendar year (e.g., 365 d if the date of intake was 1 January, 306 d if the date of intake was 1 March, and 61 d if the date of intake was 1 November). The annual dose coefficients for the subsequent years were the doses per unit intake received during the full calendar years. This derivation, which influences the first year’s estimated dose as well as estimated doses in subsequent years for radionuclides with long radioactive half-lives, is illustrated in Fig. 1, taking 90Sr as an example. The annual dose for the first year was highest for an intake assumed to have taken place on 1 January, was 15% less if the intake occurred on 1 March, and about 78% less if the intake occurred on 1 November. For the subsequent years, in comparison, the annual dose coefficient for 1 January intake was lower compared to the other dates (4% lower compared to 1 March and about 15% lower compared to 1 November).
Fig 1.
Change of the dose rate as a function of time after a nuclear weapons test and its effect on the dose within a given calender year using 90Sr as an example; for test dates occuring later in the year, the dose delivered from the TOI to the end of the calender year is smaller, while the doses delivered in subsequent years are greater; however, the lifetime dose remains the same.
For intakes of 131I by adults, the dose coefficients were derived based on the parameters of the biokinetic model presented in Fig. A1 for an average of the two preferred data sets of physiologic parameters (Table A1). The set of physiological parameters assigned as 2b (Table A1) assumed a fractional thyroid uptake about one-third greater than is usually assumed for populations with typical western diets (42% compared to 30%). In order to correct the thyroid mass for a greater than typical thyroid uptake (Zvonova 1989), we assumed the thyroid mass to be larger than the usual default assumptions by the same proportion. Hence, for our purposes, we assumed the adult thyroid mass to be 26 g compared to the usual 20 g assumption. These modestly larger thyroid masses were used in the derivation of the thyroid dose coefficients, consistent with findings by Zvonova (1989) and others. The dose coefficients due to ingestion of 131I are presented in Table 5. The dose coefficient derived for thyroid due to ingestion of 131I is about 10% higher for adults in our study, compared to the ICRP default dose coefficients. In addition to the thyroid mass differences, other differences in the kinetic parameters (Table A1) account for the small differences in the dose coefficients.
Table 5.
Absorbed dose per unit intake of 131I used to estimate organ absorbed dose to representative persons of six age groups of Marshallese plus military personnel from acute ingestion of radionuclides. ICRP (1996) values for the public are presented for comparison.
| Dose coefficient for ingestion of 131I (Gy Bq−1) |
|||||
|---|---|---|---|---|---|
| Group | Age (y) | Red marrow | Thyroid | Stomach wall | Colon |
| Marshallese | <1 | 5.3×I0−10a | 3.7×10−6a | 3.5×10−9a | 3.0×10−9a |
| Marshallese | <1 | 1.8×10−10b | 1.2×10−6b | 1.1×10−9b | 9.8×10−10b |
| Marshallese | 1 to <3 | 3.9×10−10 | 3.6×10−6 | 2.0×10−9 | 1.7×10−9 |
| Marshallese | 3 to <8 | 2.4×10−10 | 2.1×10−6 | 9.8×10−10 | 7.0×10−10 |
| Marshallese | 8 to <13 | 1.7×10−10 | 1.0×10−6 | 5.6×10−10 | 2.8×10−10 |
| Marshallese | 13 to <18 | 1.3×10−10 | 6.7×10−7 | 3.8×10−10 | 1.6×10−10 |
| Marshallese | ≥18 (adult) | 1.1×10−10 | 4.7×10−7 | 3.0×10−10 | 1.2×10−10 |
| Military personnelc | ≥18 (adult) | 9.8×10−11 | 4.3×10−7 | 3.0×10−10 | 1.0×10−10 |
| Publicd | ≥18 (adult) | 1.0×10−10 | 4.3×10−7 | 3.0×10−10 | 1.2×10−10 |
Dose coefficient for infants for direct ingestion of fallout (Gy Bq−1 intake).
Dose coefficient for infants for ingestion of breast milk (Gy Bq−1 of mother's intake).
Dose coefficients derived based on the physiological parameters presented in Table A1.
Dose coefficients for ingestion derived for adults in the general public (assuming physiologic and anthropometric characteristics of Western Europeans and North Americans) from ICRP (1996).
Special consideration was given to the calculation of the annual dose coefficients for infants as follows.
- (1) As previously indicated, two sources of exposure were considered for infants: acute intake of deposited fallout and consumption of breast milk, contaminated as the result of acute intake of fallout by the mother. Assumptions we made to complete these calculations included:
-
*The mother’s acute intake was calculated using eqn. (4);
-
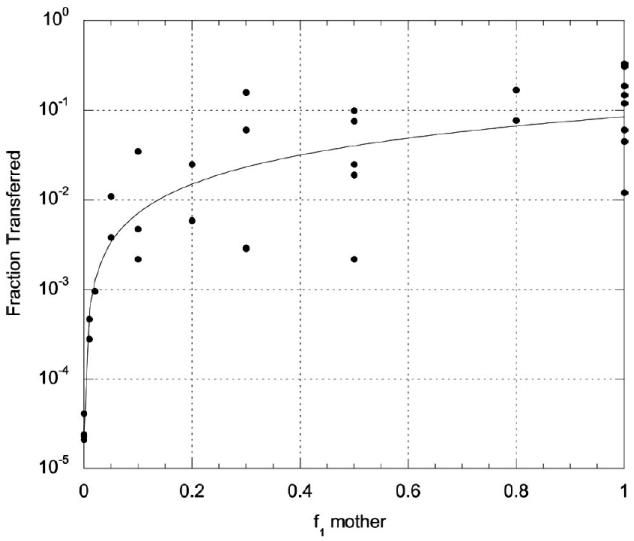
*The fractions of radionuclides ingested by the mother that are transferred to the infant in breast milk were taken from ICRP Publication 95 (2004) for the radionuclides of 35 elements. For the 15 elements that are not considered in the ICRP report, the fractional transfer factors were estimated from a relationship we derived between the reported fractions transferred to the infant in breast milk (ICRP 2004) and the reported alimentary tract absorption fractions (f1) in the mother as adopted by ICRP in its Publication 72 for the 35 elements with available data (ICRP 1996). The relationship, given in eqn (10), is illustrated in Fig. 2:
The estimated values of Fbm for the 15 elements not considered by ICRP (2004) are presented in Table 6.(10) -
*The consumption rate of breast milk by infants was taken to 0.8 L d−1 (ICRP 2004) during the first year of age and to cease afterwards (Levy et al. 1988; WHO 2009).
-
*
Fig. 2.
Relationship between the fractions of elements ingested that are transferred to infants in breast milk (ICRP 2004) and f1 values for the mother (ICRP 1996). Solid line is regression fit of eqn (10): Fbm = 0.0854 × (f1)1.081 (R2 = 0.48).
To eliminate potential bias in doses due to choosing a single DOB, we define a metric of dose to best represent an entire birth cohort, i.e., all persons born within a single year at one atoll. This dose would, in effect, be an average over all the possible birth dates. While a birth-cohort averaged dose would not represent the dose to any single real person, it is the least biased estimator of dose to the cohort as a whole and, hence, is the best single predictor of total cancer risk among that group. Hence, we define a quantity termed the “birth-cohort average dose,” BCAD, for the infant age category (i.e., birth to 1 y of age).
To estimate the BCAD, it is necessary to determine three quantities: (1) the dose (by organ, nuclide, age, location) for a person born before the estimated date of fallout deposition, (2) the proportion of a birth cohort on a single atoll that is born before the date of deposition, and (3) the proportion born afterwards. Assuming that people are equally likely to have been born on every day of the year, the proportions born before and after the date of deposition are easily computed. The proportion born before the date of deposition, termed Pb, can be estimated as equal to the number of days from beginning of the year to the date of deposition divided by 365. Conversely, the proportion born after the date of deposition (termed Pa) would equal 1 − Pb. Using these concepts, the BCAD is simply defined:
| (11) |
It is important to note that for the years following the year of intake after a given test, the age of the representative person increased by increments of one year with each calendar year (for example, a person born at any time in 1954 was considered to be one year old from 1 January to 31 December 1955, two years old during all of 1956, and so on). However, in the calculation of the annual dose coefficients, we assumed that the metabolic and anatomic characteristics of the person did not change with time after intake.
Annual doses from chronic intakes.
In this work, annual doses to RBM, thyroid, stomach, and colon from chronic intakes for each of the 26 population groups considered were calculated as the products of the annual intakes and the annual dose coefficients developed for the purposes of this paper. As the doses result from the consumption of seafood and of terrestrial foodstuffs primarily contaminated through root uptake, the radioactive materials were assumed to be in soluble form and the alimentary tract absorption fractions (f1 values) that we selected for the calculation of the annual dose coefficients were those recommended by the ICRP in its Publication 72 (ICRP 1996) for ingestion by members of the public, rather than for particulate fallout as used for acute intakes. The calculation of the doses takes into account: (1) that for a given test giving rise to a given intake rate of a given radionuclide soon after the test, the annual intake of a person of a given age varies from year to year due to radioactive decay and environmental loss, (2) the dose for a given intake is delivered over several years, and (3) both the intakes and the dose coefficients varied as a function of age. The formulation shown in eqns (12a) through (12c) was used.
For the year of the test, called y1:
| (12a) |
For the following year, called y2:
| (12b) |
For the following year, called y3:
| (12c) |
where D = the absorbed dose (mGy);
i = the radionuclide under consideration;
age = the age at intake;
o = one of the four organs considered (RBM, thyroid, stomach wall, or colon wall);
q = the annual intake (Bq); and
DC = the annual dose coefficient (mGy Bq−1).
Given the large uncertainties in the annual intakes resulting from each test, we judged it sufficient to group the intakes from the tests that occurred in a given year and to assume that the summed intake was due to a single test that was detonated on 1 July of that year. This procedure was used for all population groups and for all years, with the exception of the year 1954 for the population groups that were evacuated as a result of the fallout from the Bravo test, which took place on 1 March 1954. In that case, the chronic dose calculation for the Bravo test was done separately from the calculation of the dose resulting from all other tests that took place in 1954.
FINDINGS
The primary purpose of the models and calculations described here were to estimate: (1) empirically-based acute intakes of 131I by adults among the Marshallese and American military weather observers on Rongerik using urine bioassay data, (2) acute intakes of 131I and 62 other radionuclides by representative Marshallese of six age groups from infancy to adulthood at all inhabited atolls from each of 20 nuclear tests (plus acute intakes by adult military weather observers on Rongerik at the time of Bravo), (3) chronic intakes of residual fallout radioactivity in the environment at all inhabited atolls during the years 1948 through 1970, and (4) internal doses to four tissues or organs (RBM, thyroid, stomach wall and colon wall) from all estimated intakes. The following sections describe findings from the intake models and dose calculations.
Acute intakes of 131I from urine samples
As a necessary step to estimating intakes of all the radionuclides considered in this analysis, by persons of all ages, we first derived empirically-based estimates of the intake of 131I by adults on three atolls where bioassay was conducted (Marshallese on Rongelap Island, Marshallese on Ailinginae, and American military weather observers on Rongerik) using data from Harris (1954) and Harris et al. (2010) via eqn (1). Four different average values of the 131I intake were estimated since urine samples from the Rongelap Island group were collected on two different days. The data used to estimate 131I intake, as well as the results of the calculations, are shown in Table 7. Estimated average intakes of 131I by adults on Rongelap Island and Ailinginae were about 3,600 and 1,300 kBq, respectively. Intakes of 131I by younger ages were assumed to have been smaller as described by the scaling factors discussed in the previous section and presented in Table 2. For the age groups 13–17 y, 8–12 y, 3–7 y, 1–2 y, and <1 y, the estimates of acute intake of 131I on Rongelap were 3,150, 2,100, 1,400, 1,050, and 1,400 Bq, respectively.
Corresponding 131I acute intakes at Ailinginae were about 37% of the intakes at Rongelap. Only adults were on Rongerik at the time of the Bravo test; their intakes of 131I were about 1,700 kBq. Estimates of acute intakes of 131I were converted to 137Cs intakes for the purpose of estimating the intakes of 137Cs per unit of 137Cs deposited. We calculated the intake of 137Cs per unit deposition of 137Cs separately for the pooled samples of adult urine collected from populations exposed to Bravo fallout on Rongelap (groups LA316R and LA317R, Table 7) and Ailinginae (LA319S) and weighted each by the relative precision of our estimates of Bravo 137Cs deposited at the two atolls. For the three urine samplings (LA316R, LA317R, LA319S), our estimates of 137Cs intake per unit deposition of 137Cs were 0.029, 0.032, and 0.036 Bq per Bq m−2 while the estimated uncertainties of the 137Cs deposition at Rongelap and Ailinginae, expressed as geometric standard deviations (GSDs), were 1.5 and 1.8, respectively. Our best estimate of the 137Cs intake per unit 137Cs deposition was derived from a weighted average †† in consideration of the uncertainties of the 137Cs deposition, and was found to be 0.031 Bq per Bq m−2 (Table 7). This indicates that the fallout ingested by adults was approximately equal to the material deposited on 310 cm2. Our evaluation of the likely exposure conditions agrees with those of Lessard et al. (1985) and suggests that particulate contamination of foods, utensils, hands and face, and to a lesser degree, drinking water, led to the internal contamination of adults.
As expected, our average estimate of intake per unit deposition for American military weather observers stationed on Rongerik was less than the average for the Marshallese since their lifestyle was less dependent on outdoor food preparation. Our estimate of intake for the military weather observers stationed on Rongerik was 0.021 Bq per Bq m−2 (also based on bioassay) or about two-thirds of the intake per unit deposition experienced by the Marshallese on Rongelap. We interpret the estimated smaller intake per unit deposition of the Americans to be consistent with our belief that the military personnel took, at least, some precautions against ingestion of fallout particles. According to the records of Sharp and Chapman (1957), some of the military personnel worked indoors during the day though others continued to work outdoors. Hence, the individual weather observers likely received intakes of varied magnitude depending on their work activities during the hours that fallout was deposited.
Acute intakes of any radionuclide resulting from fallout from any test for representative residents of each atoll.
Table 8 presents our estimates of acute intake for the 24 radionuclides contributing the largest doses to adults at four representative communities (Majuro residents, Kwajalein residents, Utrik community, and Rongelap Island community).‡‡ These four communities represent the range of exposures to fallout radioactivity from smallest to largest, both in terms of deposition on the ground (Fig. 2 of Simon et al. 2010), external dose, and internal dose from ingested radioactivity. Intakes presented in Table 8 are from the 1954 Bravo test, from the entire Castle series (all 1954 tests including Bravo), and cumulative over all tests. The intake estimates account for relocations of the Rongelap and Utrik populations. Because of the relocations of the Rongelap and Utrik communities following Bravo to the mid-latitude Kwajalein Atoll and the southern Majuro Atoll (Table 3, Simon et al. 2010), members of those communities did not receive all their intakes at their home atoll.
Table 8.
Estimated acute intakes (kBq) of 24 selected radionuclides by representative adults of four population groups from the Bravo test, the Castle (1954) series, which includes the Bravo test, and over all tests (Total). Doses for Utrik and Rongelap Island communities account for relocations. All nuclides are fission products unless otherwise noted. All values rounded to two significant digits.
| Majuro residents |
Kwajalein residents |
|||||
|---|---|---|---|---|---|---|
| Radionuclide | Bravo | Castle series | Total | Bravo | Castle series | Total |
| 55Fea | 1.0 × 10−3 | 4.8 × 10−3 | 5.6 × 10−3 | 5.1 × 10−4 | 6.5 × 10−3 | 9.7 × 10−3 |
| 89Sr | 1.2 × 100 | 5.7 × 100 | 6.6 × 100 | 6.2 × 10−1 | 7.9 × 100 | 1.1 × 101 |
| 90Sr | 3.9 × 10−3 | 1.9 × 10−2 | 2.2 × 10−2 | 2.0 × 10−3 | 2.5 × 10−2 | 3.8 × 10−2 |
| 92Y | 1.3 × 10−3 | 1.5 × 10−3 | 7.6 × 10−3 | 6.1 × 10−3 | 5.6 × 100 | 5.9 × 100 |
| 93Y | 8.5 × 10−1 | 1.1 × 100 | 1.8 × 100 | 9.2 × 10−1 | 3.3 × 101 | 4.0 × 101 |
| 95Zr | 8.6 × 10−1 | 4.0 × 100 | 4.7 × 100 | 4.3 × 10−1 | 5.5 × 100 | 7.8 × 100 |
| 99Mo | 1.0 × 101 | 3.2 × 101 | 3.8 × 101 | 5.6 × 100 | 7.2 × 101 | 1.1 × 102 |
| 103Ru | 2.6 × 10−3 | 1.2 × 10−2 | 5.5 × 10−2 | 1.3 × 10−3 | 1.7 × 10−2 | 6.1 × 100 |
| 106Ru | 2.3 × 10−1 | 1.1 × 100 | 1.3 × 100 | 1.1 × 10−1 | 1.5 × 100 | 2.2 × 100 |
| l31mTe | 1.5 × 100 | 3.3 × 100 | 4.2 × 100 | 1.0 × 100 | 1.5 × 101 | 2.2 × 101 |
| 131I | 7.6 × 100 | 3.1 × 101 | 3.7 × 101 | 4.0 × 100 | 5.0 × 101 | 7.5 × 101 |
| 132Te | 1.5 × 101 | 4.9 × 101 | 5.9 × 101 | 8.2 × 100 | 1.0 × 102 | 1.6 × 102 |
| 132I | 1.5 × 101 | 5.1 × 101 | 6.1 × 101 | 8.4 × 100 | 1.1 × 102 | 1.6 × 102 |
| 133I | 1.5 × 101 | 2.6 × 101 | 3.4 × 101 | 1.1 × 101 | 1.9 × 102 | 2.7 × 102 |
| 135I | 3.6 × 10−1 | 4.1 × 10−1 | 8.4 × 10−1 | 5.7 × 10−1 | 4.6 × 101 | 5.2 × 101 |
| l37Cs | 1.2 × 10−2 | 5.4 × 10−2 | 6.4 × 10−2 | 5.8 × 10−3 | 7.4 × 10−2 | 1.1 × 10−1 |
| 140Ba | 5.4 × 100 | 2.3 × 101 | 2.7 × 101 | 2.8 × 100 | 3.5 × 101 | 5.2 × 101 |
| 140La | 5.0 × 100 | 2.7 × 101 | 3.1 × 101 | 2.3 × 100 | 2.7 × 101 | 4.0 × 101 |
| 141La | 2.6 × 10−3 | 2.8 × 10−3 | 1.2 × 10−2 | 9.4 × 10−3 | 5.7 × 100 | 6.1 × 100 |
| 141Ce | 2.2 × 100 | 9.8 × 100 | 1.2 × 101 | 1.1 × 100 | 1.4 × 101 | 2.0 × 101 |
| 143Ce | 6.9 × 100 | 1.6 × 101 | 2.0 × 101 | 4.4 × 100 | 6.3 × 101 | 9.1 × 101 |
| 144Ce | 1.1 × 10−1 | 5.3 × 10−1 | 6.2 × 10−1 | 5.6 × 10−2 | 7.2 × 10−1 | 1.1 × 100 |
| 145pr | 4.8 × 10−2 | 5.5 × 10−2 | 1.2 × 10−1 | 8.8 × 10−2 | 9.8 × 100 | 1.1 × 101 |
| 239Npa | 5.6 × 101 | 1.7 × 102 | 2.0 × 102 | 3.2 × 101 | 4.2 × 102 | 6.2 × 102 |
| Utrik community |
Rongelap Island community |
|||||
|---|---|---|---|---|---|---|
| Radionuclide | Bravo | Castle series |
Total | Bravo | Castle series |
Total |
| 55Fea | 1.3 × 10−1 | 1.3 × 10−1 | 1.3 × 10−1 | 1.1 × 100 | 1.1 × 100 | 1.1 × 100 |
| 89Sr | 7.5 × 101 | 8.3 × 101 | 8.4 × 101 | 6.2 × 102 | 6.2 × 102 | 6.3 × 102 |
| 90Sr | 2.5 × 10−1 | 2.7 × 10−1 | 2.8 × 10−1 | 1.6 × 100 | 1.6 × 100 | 1.6 × 100 |
| 92Y | 2.0 × 102 | 2.1 × 102 | 2.1 × 102 | 6.9 × 104 | 6.9 × 104 | 6.9 × 104 |
| 93Y | 1.3 × 103 | 1.3 × 103 | 1.3 × 103 | 5.3 × 104 | 5.3 × 104 | 5.3 × 104 |
| 95Zr | 1.1 × 102 | 1.1 × 102 | 1.1 × 102 | 1.0 × 103 | 1.0 × 103 | 1.0 × 103 |
| 99Mo | 1.8 × 103 | 1.9 × 103 | 1.9 × 103 | 2.0 × 104 | 2.0 × 104 | 2.0 × 104 |
| 103Ru | 1.7 × 10−1 | 1.8 × 10−1 | 8.8 × 10−1 | 1.1 × 100 | 1.1 × 100 | 5.1 × 100 |
| 106Ru | 1.4 × 101 | 1.6 × 101 | 1.6 × 101 | 8.9 × 101 | 9.0 × 101 | 9.1 × 101 |
| 131mTe | 2.3 × 102 | 2.4 × 102 | 2.5 × 102 | 2.5 × 103 | 2.5 × 103 | 2.5 × 103 |
| 131I | 5.4 × 102 | 5.9 × 102 | 5.9 × 102 | 3.6 × 103 | 3.6 × 103| | 3.7 × 103 |
| 132Te | 1.3 × 103 | 1.4 × 103 | 1.4 × 103 | 9.9 × 103 | 9.9 × 103 | 9.9 × 103 |
| 132I | 1.3 × 103 | 1.4 × 103 | 1.4 × 103 | 1.0 × 104 | 1.0 × 104 | 1.0 × 104 |
| 133I | 3.2 × 103 | 3.4 × 103 | 3.4 × 103 | 4.4 × 104 | 4.4 × 104 | 4.4 × 104 |
| 135I | 9.6 × 102 | 1.0 × 103 | 1.0 × 103 | 6.1 × 104 | 6.1 × 104 | 6.1 × 104 |
| 137Cs | 6.6 × 10−1 | 7.3 × 10−1 | 7.4 × 10−1 | 3.1 × 100 | 3.2 × 100 | 3.2 × 100 |
| 140Ba | 4.3 × 102 | 4.6 × 102 | 4.7 × 102 | 3.2 × 103 | 3.2 × 103 | 3.2 × 103 |
| l40La | 2.3 × 102 | 2.6 × 102 | 2.6 × 102 | 5.7 × 102 | 5.8 × 102 | 5.9 × 102 |
| 141La | 1.6 × 102 | 1.6 × 102 | 1.6 × 102 | 6.7 × 104 | 6.7 × 104 | 6.7 × 104 |
| 14lCe | 2.1 × 102 | 2.2 × 102 | 2.2 × 102 | 1.2 × 103 | 1.2 × 103 | 1.2 × 103 |
| 143Ce | 1.9 × 103 | 1.9 × 103 | 1.9 × 103 | 2.7 × 104 | 2.7 × 104 | 2.7 × 104 |
| 144Ce | 1.4 × 101 | 1.5 × 101 | 1.5 × 101 | 1.2 × 102 | 1.2 × 102 | 1.2 × 102 |
| 145Ce | 4.1 × 102 | 4.2 × 102 | 4.2 × 102 | 4.8 × 104 | 4.8 × 104 | 4.8 × 104 |
| 239NPa | 1.1 × 104 | 1.1 × 104 | 1.1 × 104 | 1.3 × 105 | 1.3 × 105 | 1.3 × 105 |
Activation products.
Depending on the half-life of the radionuclide and the transit time of fallout from the test site to the southern atolls, the southern atolls (represented here by Majuro) had acute intakes estimated to be 0.01% to 2% of those received by the more highly exposed Rongelap population. In terms of absolute activity ingested among adult residents of these four atolls, Majuro residents would have had the lowest intake. For example, adult Majuro residents would have had about 6% and 9% of the 131I and 137Cs intake (cumulative over all tests), respectively, of adult Utrik community members, and about 1%, and 2%, respectively, of the intakes of adult Rongelap Island community members.
Chronic intakes
Annual intakes of the five long-lived radionuclides (55Fe, 60Co, 65Zn, 90Sr, 137Cs) giving the largest doses were calculated for the 26 population groups considered in this study for the years 1948 to 1970. Cumulative intakes were obtained as sums of estimated annual intakes. Results of estimation of cumulative intakes by Majuro residents, Kwajalein residents, the Utrik community, and the Rongelap Island community are presented in Table 9. The cumulative intakes of long-lived radionuclides had roughly the same geographic pattern (in terms of relative intakes between atolls) as for the acute intakes. For example, adults on Majuro would have had about 3% and 2% of the chronic 137Cs intakes of those experienced by Utrik and Rongelap community members, respectively.
Table 9.
Estimated cumulative chronic intakes (kBq) of the long-lived radionuclides considered in this study by representative adults of four population groups from the Bravo test (1 March 1954), the Castle (1954) series that includes the Bravo test, and over all tests considered. All values rounded to two significant digits.
| Majuro residents |
Kwajalein residents |
Utrik community |
Rongelap Island community |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radionuclide | Bravo test |
Castle series |
Total over all tests |
Bravo test |
Castle series |
Total over all tests |
Bravo test |
Castle series |
Total over all tests |
Bravo test |
Castle series |
Total over all tests |
| 55Fe | 4.2 | 20 | 23 | 2.1 | 26 | 40 | 1,600 | 1,800 | 1,900 | 1,900 | 2,300 | 2,300 |
| 60Co | 0.50 | 2.3 | 2.7 | 0.26 | 3.1 | 4.9 | 46 | 53 | 55 | 32 | 38 | 42 |
| 65Zn | 27 | 120 | 150 | 14 | 170 | 250 | 4,300 | 5,100 | 5,300 | 230 | 280 | 490 |
| 90Sr | 0.015 | 0.068 | 0.081 | 0.0075 | 0.092 | 0.14 | 0.94 | 1.2 | 1.3 | 3.9 | 15.8 | 5.9 |
| 137Cs | 3.3 | 16 | 18 | 1.7 | 21 | 31 | 460 | 620 | 640 | 540 | 1,020 | 1,040 |
While the geographic pattern of chronic uptakes among atolls was similar to that for acute intakes, the chronic intakes were much greater than the acute intakes of the same radionuclides. This phenomenon is a result of the relatively long residence times of certain radionculides in the environment. For example, at Majuro, the acute intake of 90Sr by adults from all tests was 0.022 kBq (Table 8) compared to the chronic intake of 0.081 kBq (Table 9), indicating chronic intake was close to four times greater. Even more indicative of differences in intake according to the mode of intake was the difference for 137Cs. At Majuro, the acute intake of 137Cs by adults from all tests was 0.064 kBq compared to the chronic intake of 18 kBq, indicating chronic intake was more than 280 times greater than the acute intake. In the case of 137Cs, its continuous movement into coconuts and other fruits via root uptake, as a result of low 40K concentrations in the soil (Simon et al. 2002), leads to much larger intakes over the successive years after fallout.
Absorbed doses
Annual absorbed doses to RBM, thyroid, stomach wall, and colon were estimated for the 26 population groups for the time period from 1948 to 1970. Cumulative doses over that time period were estimated as well. Four population groups have been selected to illustrate the magnitude and the range of dose over the entire territory of the Marshall Islands. Doses from acute and chronic intakes will be discussed in turn.
Doses from acute intakes
Estimation of doses from the acute intakes used dose coefficients as described that were derived from accepted international biokinetic models and adjusted for f1 values specific for radionuclides ingested in fallout particles (see Ibrahim et al. 2010). Annual doses from acute intakes at each atoll varied primarily according to the amount of deposition from the tests conducted in a given year. Fig. 3a to 3d illustrates the annual organ dose (mGy) to two tissues (RBM and thyroid) for three different birth years (1930 or before, 1953, and 1957) at Majuro, Kwajalein, and for the Utrik and Rongelap Island community members (after accounting for their relocations). Similarly, Table 10 presents cumulative doses (mGy) to each of four tissues for all birth years from 1931 (or before) through 1958. Since it is the intent of this work to estimate doses for representative persons, BCAD is reported for the years in which tests occurred.
Figure 3.
(a) Annual doses (mGy) to red bone marrow (RBM) and thyroid due to acute intake of fallout for Majuro residents born in three different years: 1930 or before, 1953, and 1957. Persons born in years intermediate to those shown would have received annual doses intermediate in magnitude to those shown. Note different y-axis scaling of each panel and break in y-axis between 0.0008 and 0.08 (left panel) and between 3 and 12 (right panel); (b) Annual doses (mGy) to RBM and thyroid due to acute intake of fallout for Kwajalein residents born in three different years: 1930 or before, 1953, and 1957. Persons born in years intermediate to those shown would have received annual doses intermediate in magnitude to those shown. Note different y-axis scaling of each panel and break in y-axis between 0.06 and 0.12 (left panel) and between 15 and 40 (right panel); (c) Annual doses (mGy) to RBM and thyroid due to acute intake of fallout for Utrik community members born in three different years: 1930 or before, 1953, and 1957. Persons born in years intermediate to those shown would have received annual doses intermediate in magnitude to those shown. Note different y-axis scaling of each panel and break in y-axis between 0.02 and 2.0 (left panel) and between 8 and 700 (right panel); (d) Annual doses (mGy) to RBM and thyroid due to acute intake of fallout for Rongelap Island community members born in three different years: 1930 or before, 1953, and 1957. Persons born in years intermediate to those shown would have received annual doses intermediate in magnitude to those shown. Note different y-axis scaling of each panel and break in y-axis between 0.03 and 24 (left panel) and between 15 and 3,000 (right panel).
Table 10.
Cumulative radiation absorbed doses (mGy) to four organs of representative persons by birth year (<1931 to 1958) from acute intakes of fallout (all values rounded to two significant digits). Doses for Utrik and Rongelap Island communities account for relocations. Dose in year of tests is birth-cohort averaged dose (BCAD).
| Majuro residents |
Kwajalein residents |
|||||||
|---|---|---|---|---|---|---|---|---|
| Birth year | RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon |
| <1931 | 0.11 | 22 | 0.32 | 4.4 | 0.25 | 66 | 1.1 | 12 |
| 1931 | 0.11 | 22 | 0.32 | 4.4 | 0.27 | 70 | 1.1 | 12 |
| 1932 | 0.11 | 22 | 0.32 | 4.4 | 0.27 | 70 | 1.1 | 12 |
| 1933 | 0.11 | 22 | 0.32 | 4.4 | 0.27 | 70 | 1.1 | 12 |
| 1934 | 0.11 | 22 | 0.32 | 4.4 | 0.27 | 70 | 1.1 | 12 |
| 1935 | 0.11 | 23 | 0.33 | 4.4 | 0.27 | 71 | 1.1 | 13 |
| 1936 | 0.11 | 23 | 0.33 | 4.4 | 0.26 | 71 | 1.1 | 13 |
| 1937 | 0.16 | 28 | 0.37 | 4.8 | 0.34 | 85 | 1.2 | 14 |
| 1938 | 0.16 | 28 | 0.37 | 4.8 | 0.34 | 85 | 1.2 | 14 |
| 1939 | 0.16 | 29 | 0.37 | 4.8 | 0.34 | 86 | 1.2 | 14 |
| 1940 | 0.16 | 29 | 0.37 | 4.9 | 0.34 | 86 | 1.2 | 14 |
| 1941 | 0.16 | 29 | 0.37 | 4.9 | 0.34 | 94 | 1.3 | 15 |
| 1942 | 0.13 | 30 | 0.36 | 5.6 | 0.29 | 96 | 1.3 | 16 |
| 1943 | 0.13 | 30 | 0.36 | 5.6 | 0.29 | 96 | 1.3 | 16 |
| 1944 | 0.13 | 30 | 0.36 | 5.7 | 0.29 | 96 | 1.3 | 16 |
| 1945 | 0.13 | 31 | 0.37 | 5.7 | 0.29 | 98 | 1.3 | 16 |
| 1946 | 0.13 | 31 | 0.37 | 5.7 | 0.30 | 110 | 1.4 | 18 |
| 1947 | 0.11 | 40 | 0.41 | 6.3 | 0.27 | 130 | 1.5 | 20 |
| 1948 | 0.11 | 40 | 0.41 | 6.3 | 0.27 | 120 | 1.3 | 15 |
| 1949 | 0.11 | 41 | 0.41 | 6.3 | 0.20 | 99 | 1.1 | 14 |
| 1950 | 0.11 | 42 | 0.43 | 6.6 | 0.20 | 100 | 1.1 | 14 |
| 1951 | 0.11 | 42 | 0.43 | 6.6 | 0.20 | 100 | 1.1 | 14 |
| 1952 | 0.16 | 55 | 0.60 | 8.6 | 0.28 | 130 | 1.6 | 19 |
| 1953 | 0.13 | 49 | 0.54 | 8.3 | 0.25 | 130 | 1.5 | 19 |
| 1954 | 0.12 | 20 | 0.17 | 1.6 | 0.24 | 64 | 0.63 | 4.2 |
| 1955 | 0.012 | 4.3 | 0.047 | 0.74 | 0.024 | 9.8 | 0.11 | 1.7 |
| 1956 | 0.019 | 3.2 | 0.027 | 0.24 | 0.035 | 6.9 | 0.061 | 0.44 |
| 1957 | 0.0021 | 0.68 | 0.0067 | 0.11 | 0.0028 | 0.91 | 0.0081 | 0.13 |
| 1958 | 0.0030 | 0.37 | 0.0025 | 0.021 | 0.0042 | 0.50 | 0.0031 | 0.026 |
| Utrik community |
Rongelap Island community |
|||||||
|---|---|---|---|---|---|---|---|---|
| Birth Year | RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon |
| <1931 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1931 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1932 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1933 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1934 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1935 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1936 | 2.3 | 740 | 16 | 180 | 25 | 7,600 | 530 | 2,800 |
| 1937 | 2.8 | 870 | 18 | 200 | 30 | 9,700 | 610 | 3,100 |
| 1938 | 2.8 | 870 | 18 | 200 | 30 | 9,700 | 610 | 3,100 |
| 1939 | 2.8 | 870 | 18 | 200 | 30 | 9,700 | 610 | 3,100 |
| 1940 | 2.8 | 870 | 18 | 200 | 30 | 9,700 | 610 | 3,100 |
| 1941 | 2.8 | 870 | 18 | 200 | 30 | 9,700 | 610 | 3,100 |
| 1942 | 2.5 | 900 | 18 | 230 | 27 | 10,000 | 600 | 3,700 |
| 1943 | 2.5 | 900 | 18 | 230 | 27 | 10,000 | 600 | 3,700 |
| 1944 | 2.5 | 900 | 18 | 230 | 27 | 10,000 | 600 | 3,700 |
| 1945 | 2.5 | 900 | 18 | 230 | 27 | 10,000 | 600 | 3,700 |
| 1946 | 2.5 | 900 | 18 | 230 | 27 | 10,000 | 600 | 3,700 |
| 1947 | 2.3 | 1,300 | 21 | 260 | 25 | 15,000 | 690 | 4,100 |
| 1948 | 2.3 | 1.300 | 21 | 260 | 25 | 15,000 | 690 | 4,100 |
| 1949 | 2.3 | 1,300 | 21 | 260 | 25 | 15,000 | 690 | 4,100 |
| 1950 | 2.3 | 1.300 | 21 | 260 | 25 | 15,000 | 690 | 4,100 |
| 1951 | 2.3 | 1.300 | 21 | 260 | 25 | 15,000 | 690 | 4,100 |
| 1952 | 2.9 | 1.800 | 31 | 380 | 31 | 20,000 | 1,100 | 6,100 |
| 1953 | 2.9 | 1.800 | 31 | 380 | 31 | 20,000 | 1,100 | 6,100 |
| 1954 | 1.8 | 470 | 5.7 | 32 | 16 | 5,100 | 150 | 480 |
| 1955 | 0.017 | 6.4 | 0.068 | 1.0 | 0.032 | 12 | 0.14 | 2.0 |
| 1956 | 0.024 | 7.2 | 0.081 | 1.1 | 0.046 | 14 | 0.17 | 2.1 |
| 1957 | 0.015 | 6.1 | 0.073 | 1.1 | 0.029 | 12 | 0.15 | 2.0 |
| 1958 | 0.019 | 3.3 | 0.029 | 0.19 | 0.039 | 7.2 | 0.067 | 0.38 |
The cumulative doses to individual organs are a sum not only over all tests but a sum over all 63 radionuclides. Table 11 presents a summary of the radionuclides which were estimated to be the ten largest contributors to total internal dose from acute intakes for each of the four tissues and for each of the four population groups (Majuro residents, Kwajalein residents, Utrik community, and Rongelap Island community). For the dose to RBM, stomach wall, and colon wall, 239Np was one of the five most important nuclides regardless of the atoll. Other important nuclides for RBM were 132Te, 140Ba and 99Mo. For the stomach wall, the short-lived radioiodines and radiotelluriums 132I, 133I, and 132Te were important at Majuro and Kwajalein while 92Y and 93Y were most important at Rongelap. For the thyroid gland, the radioiodines and radiotelluriums easily gave the largest doses though 133I was the largest contributor at Rongelap and Utrik compared to 131I at Kwajalein and Majuro. All of the radionuclides listed in Table 11 are short lived, the longest half-life being 51 days for 89Sr. Therefore, most of the internal dose resulting from acute intakes was delivered during the year of the test.
Table 11.
Radionuclides giving the largest organ doses (inGy) from the Bravo test (1954) to adults of four population groups (Majuro residents, Kwajalein residents, Utrik community, and Rongelap Island community) from acute intakes of fallout radionuclides and cumulative percentage of total dose resulting from acute intakes of all 63 nuclides considered. Utrik and Rongelap Island community doses account for relocations. All values rounded to two significant digits.
| Majuro residents |
Kwajalein residents |
Utrik community |
Rongelap Island community |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ and ranka |
Nuclide | Dose (mGy) |
Cumulative % of organ doseb |
Nuclide | Dose (mGy) |
Cumulative % of organ dose |
Nuclide | Dose (mGy) |
Cumulative % of organ dose |
Nuclide | Dose (mGy) |
Cumulative % of organ dose |
| Colon | ||||||||||||
| 1 | 239Np | 0.34 | 31 | 239Np | 0.19 | 32 | 239Np | 66 | 39 | 239Np | 764 | 28 |
| 2 | 132Te | 0.21 | 51 | l32Te | 0.11 | 51 | l32Te | 18 | 50 | 93y | 437 | 43 |
| 3 | 140Ba | 0.093 | 59 | 140Ba | 0.048 | 59 | 143Ce | 16 | 59 | l43Ce | 221 | 51 |
| 4 | 99Mo | 0.079 | 67 | 99Mo | 0.044 | 66 | 99Mo | 14 | 68 | 92y | 182 | 58 |
| 5 | 140La | 0.064 | 72 | l43Ce | 0.037 | 72 | 93y | 11 | 74 | 99Mo | 160 | 64 |
| 6 | l43Ce | 0.058 | 78 | 105Rh | 0.033 | 78 | l40Ba | 7.5 | 79 | l32Te | 138 | 69 |
| 7 | l05Rh | 0.053 | 83 | l40La | 0.029 | 83 | 105Rh | 6.8 | 83 | 141La | 136 | 74 |
| 8 | l49Pm | 0.026 | 85 | l49Pm | 0.015 | 85 | l49Pm | 5.1 | 86 | l45Pr | 125 | 78 |
| 9 | 143Pr | 0.025 | 87 | l27Sb | 0.014 | 87 | l40La | 3.0 | 88 | 92Sr | 105 | 82 |
| 10 | l27Sb | 0.025 | 90 | 143pr | 0.011 | 89 | l27Sb | 2.2 | 89 | 105Ru | 83 | 85 |
| RBM | ||||||||||||
| 1 | 132Te | 0.0071 | 31 | l32Te | 0.0039 | 31 | 132Te | 0.62 | 30 | l32Te | 4.7 | 19 |
| 2 | 89Sr | 0.0039 | 48 | l40Ba | 0.0020 | 47 | 239Np | 0.28 | 43 | 239Np | 3.2 | 32 |
| 3 | 140Ba | 0.0038 | 64 | 89Sr | 0.0020 | 63 | 99Mo | 0.28 | 57 | 99Mo | 3.0 | 45 |
| 4 | 99Mo | 0.0015 | 71 | 99Mo | 0.00085 | 70 | l40Ba | 0.19 | 66 | 135I | 2.2 | 54 |
| 5 | 239Np | 0.0014 | 77 | 239Np | 0.00083 | 76 | 133I | 0.15 | 73 | 133I | 1.8 | 61 |
| 6 | 140La | 0.0013 | 83 | 140La | 0.00060 | 81 | l43Ce | 0.064 | 77 | l40Ba | 1.4 | 67 |
| 7 | 131I | 0.00084 | 86 | 133I | 0.00045 | 85 | l40La | 0.061 | 79 | 105R | 1.2 | 72 |
| 8 | l27Sb | 0.00065 | 89 | 131I | 0.00044 | 88 | 89Sr | 0.060 | 82 | 92Sr | 0.91 | 75 |
| 9 | 133I | 0.00061 | 92 | l27Sb | 0.00037 | 91 | l27Sb | 0.058 | 85 | l43Ce | 0.90 | 79 |
| 10 | 132I | 0.00038 | 93 | l31mTe | 0.00023 | 93 | 131mTe | 0.054 | 88 | l29Sb | 0.70 | 82 |
| Stomach | ||||||||||||
| 1 | 239Np | 0.019 | 23 | 239Np | 0.011 | 23 | 239Np | 3.8 | 25 | 92y | 98 | 18 |
| 2 | 132Te | 0.011 | 36 | 132Te | 0.0060 | 35 | 133I | 1.8 | 37 | 93y | 68 | 31 |
| 3 | 132I | 0.010 | 47 | 133I | 0.0059 | 47 | 93Y | 1.7 | 47 | l4lLa | 63 | 43 |
| 4 | 133I | 0.0080 | 57 | 132I | 0.0053 | 58 | l43Ce | 1.1 | 54 | 239Np | 44 | 51 |
| 5 | 99Mo | 0.0055 | 64 | 99Mo | 0.0031 | 64 | 99Mo | 1.0 | 61 | l45Pr | 34 | 58 |
| 6 | l40La | 0.0054 | 70 | 140La | 0.0025 | 69 | l32Te | 0.95 | 67 | l35I | 33 | 64 |
| 7 | l43Ce | 0.0039 | 75 | l43Ce | 0.0025 | 74 | 132I | 0.84 | 72 | 105Rh | 27 | 69 |
| 8 | 105Rh | 0.0038 | 79 | 105Rh | 0.0024 | 79 | 135I | 0.57 | 76 | 133I | 24 | 74 |
| 9 | 140Ba | 0.0032 | 83 | l40Ba | 0.0016 | 82 | 105Rh | 0.48 | 79 | l41La | 17 | 77 |
| 10 | 131I | 0.0023 | 86 | 131I | 0.0012 | 84 | 145Pr | 0.29 | 81 | 92Sr | 17 | 80 |
| Thyroid | ||||||||||||
| 1 | 131I | 3.6 | 66 | 131I | 1.9 | 59 | 133I | 380 | 55 | 133I | 4,200 | 56 |
| 2 | 133I | 1.4 | 92 | 133I | 1.0 | 92 | 131I | 230 | 89 | 131I | 1,700 | 78 |
| 3 | 132Te | 0.34 | 98 | l32Te | 0.19 | 98 | 135I | 36 | 95 | 135I | 1,300 | 96 |
| 4 | 132I | 0.072 | 99 | 132I | 0.040 | 99 | 132Te | 29 | 99 | 132Te | 220 | 99 |
| 5 | 131mTe | 0.022 | 100 | 131mTe | 0.015 | 100 | 132I | 4.5 | 99 | 132I | 47 | 99 |
| 6 | 135I | 0.0078 | 100 | 135I | 0.012 | 100 | 131mTe | 3.4 | 100 | 131mTe | 36 | 100 |
| 7 | 99Mo | 0.00050 | 100 | 99Mo | 0.00028 | 100 | 99Mo | 0.091 | 100 | 133mTe | 4.0 | 100 |
| 8 | 99mTc | 0.00044 | 100 | 99mTc | 0.00025 | 100 | 99mTc | 0.079 | 100 | 99Mo | 1.0 | 100 |
| 9 | l40Ba | 0.00024 | 100 | 140Ba | 0.00012 | 100 | l40Ba | 0.010 | 100 | 99mTc | 0.54 | 100 |
| 10 | 89Sr | 0.00016 | 100 | 89Sr | 0.000081 | 100 | 105Rh | 0.0074 | 100 | 129Sb | 0.11 | 100 |
Rank of 1 indicates radionuclide with highest organ dose; rank of 10 indicates radionuclide with tenth highest organ dose.
Cumulative % is cumulative percentage of total organ dose estimated from all 63 radionuclides considered in acute intake calculations.
Among the radionuclides considered, there were six radionuclides with half-lives of about 1 y or longer. When the biological half-time of residence in the body was longer than 1 y, the dose from acute intake was delivered over several years. This is, for example, the case for 90Sr, with a physical half-life of 29 y and a biological half-time of residence in the body of about 20 y. Tables 12 and 13 present the absorbed dose coefficients to age 70 y for a 1-y-old child and for an adult for the six radionuclides with long radioactive half-lives as derived for the Bravo test (1 March 1954). The tables also present the percentage of the dose delivered in each of the first five years after intake. For both ages, about 100% of the dose is delivered to the colon wall and to the stomach wall in the first year. For the systemic organs, RBM and thyroid, a large fraction of the dose is delivered in the first year, but a significant fraction of the dose is also received in subsequent years due to the biological retention of the radionuclide in the body.
Table 12.
Absorbed dose per unit intake to age 70 y for 1-y-old reference child (acute ingestion) and the percentage of dose delivered in the first 5 y after intake on March 1 (date of Bravo test).
| Radionuclide | f1 | Organ | Absorbed dose per unit intake to age 70 y (Gy By−1) |
Percentage of absorbed dose delivered per year in the first 5 y after intake for 1-y-old reference child |
||||
|---|---|---|---|---|---|---|---|---|
| 1st year | 2nd year | 3rd year | 4th year | 5th year | ||||
| 55Fe | 2.0 × 10−1 | RBMa | 8.1 × 10−9 | 31 | 25 | 15 | 10 | 7 |
| 90Sr | 2.0 × 10−1 | RBM | 2.1 × 10−7 | 52 | 25 | 11 | 5 | 3 |
| 106Ru | 1.0 × 10−2 | RBM | 1.8 × I0−9 | 72 | 19 | 6 | 2 | 1 |
| 125Sb | 5.0 × 10−2 | RBM | 4.1 × 10−9 | 54 | 17 | 10 | 6 | 4 |
| 137Cs | 8.0 × 10−1 | RBM | 8.1 × 10−9 | 100 | — | — | — | — |
| 144Ce | 5.0 × 10−4 | RBM | 2.4 × I0−9 | 63 | 25 | 8 | 3 | 1 |
| 55Fe | 2.0 × 10−1 | Thyroid | 7.5 × IO−10 | 41 | 25 | 13 | 8 | 5 |
| 90Sr | 2.0 × 10−1 | Thyroid | 2.7 × 10−9 | 73 | 10 | 5 | 3 | 2 |
| 106Ru | 1.0 × 10−2 | Thyroid | 1.8 × 10−9 | 70 | 20 | 6 | 2 | 1 |
| 125Sb | 5.0 × 10−2 | Thyroid | 5.0 × 1O−10 | 54 | 16 | 10 | 6 | 4 |
| 137Cs | 8.0 × 10−1 | Thyroid | 8.9 × 10−9 | 100 | — | — | — | — |
| 144Ce | 5.0 × 10−4 | Thyroid | 6.8 × 10−11 | 64 | 24 | 8 | 3 | 1 |
| 55Fe | 2.0 × 10−1 | St wallb | 8.0 × 10−10 | 45 | 23 | 13 | 7 | 4 |
| 90Sr | 2.0 × 10−1 | St wall | 4.4 × 10−9 | 83 | 6 | 3 | 2 | 1 |
| 106RU | 1.0 × 10−2 | St wall | 1.3 × 10−8 | 96 | 3 | 1 | — | — |
| 125Sb | 5.0 × 10−2 | St wall | 1.9 × 10−9 | 89 | 4 | 2 | 2 | 1 |
| 137Cs | 8.0 × 10−1 | St wall | 1.0 × 10−8 | 100 | — | — | — | — |
| 144Ce | 5.0 × 10−4 | St wall | 7.7 × 10−9 | 100 | — | — | — | — |
| 55Fe | 2.0 × 10−1 | Colon wall | 1.9 × 10−9 | 76 | 10 | 6 | 3 | 2 |
| 90Sr | 2.0 × 10−1 | Colon wall | 1.0 × 10−7 | 98 | 1 | <0.01 | <0.01 | <0.01 |
| 106RU | 1.0 × 10−2 | Colon wall | 3.4 × 10−7 | 100 | — | — | — | — |
| 125Sb | 5.0 × 10−2 | Colon wall | 2.8 × 10−8 | 99 | <0.01 | <0.01 | <0.01 | <0.01 |
| 137Cs | 8.0 × 10−1 | Colon wall | 3.6 × 10−8 | 100 | — | — | — | — |
| 144Ce | 5.0 × 10−4 | Colon wall | 3.1 × 10−7 | 100 | — | — | — | — |
Red bone marrow.
Stomach wall.
Table 13.
Absorbed dose per unit intake to age 70 y for reference adult (acute ingestion) and the percentage of dose delivered in the first 5 y after intake on March 1 (date of Bravo test).
| Radionuclide | f 1 | Organ | Absorbed dose per unit intake to age 70 y (Gy Bq−1) |
Percentage of absorbed dose per year delivered in the first 5 y after intake for adult reference person |
||||
|---|---|---|---|---|---|---|---|---|
| 1st year | 2nd year | 3rd year | 4th year | 5th year | ||||
| 55Fe | 1.0 × 10−1 | RBMa | l.1 × 10−9 | 15 | 20 | 17 | 13 | 10 |
| 90Sr | 2.0 × 10−1 | RBM | 1.2 × 10−7 | 17 | 14 | 11 | 9 | 8 |
| 106Ru | 1.0 × 10−2 | RBM | 3.3 × 10−10 | 68 | 20 | 8 | 3 | 1 |
| 125Sb | 5.0 × 10−2 | RBM | 8.0 × 10−10 | 45 | 15 | 10 | 8 | 6 |
| 137Cs | 8.0 × 10−1 | RBM | 1.1 × 10−8 | 86 | 13 | 1 | - | - |
| 144Ce | 5.0 × 10−4 | RBM | 1.9 × 10−10 | 58 | 26 | 10 | 4 | 1 |
| 55Fe | 1.0 × 10−1 | Thyroid | 8.6 × 10−11 | 27 | 22 | 15 | 10 | 7 |
| 90Sr | 2.0 × 10−1 | Thyroid | 4.4 × 10−10 | 63 | 6 | 4 | 4 | 3 |
| 106Ru | 1.0 × 10−2 | Thyroid | 2.9 × 10−10 | 63 | 23 | 9 | 3 | 1 |
| 125Sb | 5.0 × 10−2 | Thyroid | 1.3 × 10−10 | 40 | 17 | 11 | 8 | 6 |
| 137Cs | 8.0 × 10−1 | Thyroid | 1.1 × 10−8 | 86 | 13 | 1 | - | - |
| 144Ce | 5.0 × 10−4 | Thyroid | 1.2 × 10−11 | 56 | 27 | 10 | 4 | 2 |
| 55Fe | 1.0 × 101 | St wallb | 9.2 × 10−11 | 32 | 20 | 14 | 10 | 7 |
| 90Sr | 2.0 × 10−1 | St wall | 6.8 × 10−10 | 76 | 4 | 3 | 2 | 2 |
| 106Ru | 1.0 × 10−2 | St wall | 2.0 × 10−9 | 94 | 4 | 1 | 1 | - |
| 125Sb | 5.0 × 10−2 | St wall | 3.7 × 10−10 | 80 | 5 | 4 | 3 | 2 |
| 137Cs | 8.0 × 10−1 | St wall | 1.1 × 10−8 | 86 | 13 | 1 | - | - |
| 144Ce | 5.0 × 10−4 | St wall | 1.1 × 10−9 | 99 | <0.01 | <0.01 | <0.01 | <0.01 |
| 55Fe | 1.0 × 10−1 | Colon wall | 2.6 × 10−10 | 75 | 7 | 5 | 4 | 3 |
| 90Sr | 2.0 × 10−1 | Colon wall | 1.4 × 10−8 | 97 | <0.01 | <0.01 | <0.01 | <0.01 |
| 106Ru | 1.0 × 10 | Colon wall | 4.6 × 10−8 | 100 | - | - | - | - |
| 125Sb | 5.0 × 10−2 | Colon wall | 4.1 × 10−9 | 98 | 1 | <0.01 | <0.01 | <0.01 |
| 137Cs | 8.0 × 10−1 | Colon wall | 1.4 × 10−8 | 88 | 11 | 1 | - | - |
| 144Ce | 5.0 × 10−4 | Colon wall | 4.2 × 10−8 | 100 | - | - | - | - |
Red bone marrow.
Stomach wall.
Doses from chronic intakes
Annual doses to RBM, thyroid, stomach wall, and colon were calculated for chronic intakes of long-lived radionuclides for the 26 population groups over the years 1948 to 1970. Fig. 4 compares annual doses to thyroid from chronic intakes during the years 1948 through 1958 for three different birth years: 1930 (and earlier), 1953, and 1957. Cumulative doses were obtained as sums of annual doses. Cumulative doses were a function of birth year with the largest cumulative doses estimated for persons born in the years 1950 through 1956. The cumulative dose estimates for Majuro residents, Kwajalein residents, the Utrik community, and the Rongelap Island community are presented in Table 14.
Fig. 4.
Annual doses (mGy) to thyroid due to chronic intake of residual radioactivity in the environment from fallout for Majuro and Kwajalein residents, and for Utrik and Rongelap Island community members, born in three different years: 1930 or before, 1953, and 1957. Doses for Utrik and Rongelap community members account for relocations (see Simon et al. 2010, Table 3). Note different y-axis scaling of each panel.
Table 14.
Cumulative radiation absorbed doses (mGy) to four organs of representative persons by birth year (<1930 through 1968) from chronic intakes of residual radioactivity in the environment from fallout (all values rounded to two significant digits). Doses for Utrik and Rongelap communities account for relocations.
| Majuro residents |
Kwajalein residents |
|||||||
|---|---|---|---|---|---|---|---|---|
| Birth year |
RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon |
| 1929 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1930 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1931 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1932 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1933 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1934 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1935 | 0.98 | 0.76 | 0.75 | 0.99 | 1.7 | 1.3 | 1.3 | 1.7 |
| 1936 | 0.98 | 0.76 | 0.75 | 0.99 | 1.8 | 1.4 | 1.3 | 1.8 |
| 1937 | 1.0 | 0.78 | 0.77 | 1.0 | 1.8 | 1.4 | 1.4 | 1.8 |
| 1938 | 1.0 | 0.78 | 0.78 | 1.0 | 1.8 | 1.4 | 1.4 | 1.8 |
| 1939 | 1.0 | 0.78 | 0.77 | 1.0 | 1.8 | 1.4 | 1.4 | 1.8 |
| 1940 | 1.0 | 0.78 | 0.78 | 1.0 | 1.8 | 1.4 | 1.4 | 1.8 |
| 1941 | 1.0 | 0.78 | 0.78 | 1.0 | 1.9 | 1.4 | 1.4 | 1.8 |
| 1942 | 1.1 | 0.90 | 0.85 | 1.1 | 2.0 | 1.6 | 1.5 | 2.0 |
| 1943 | 1.2 | 0.98 | 0.89 | 1.2 | 2.1 | 1.7 | 1.6 | 2.2 |
| 1944 | 1.3 | 1.0 | 0.90 | 1.3 | 2.2 | 1.7 | 1.6 | 2.2 |
| 1945 | 1.3 | 1.0 | 0.91 | 1.3 | 2.2 | 1.7 | 1.6 | 2.2 |
| 1946 | 1.3 | 1.0 | 0.91 | 1.3 | 2.2 | 1.7 | 1.6 | 2.2 |
| 1947 | 1.3 | 1.1 | 0.97 | 1.3 | 2.2 | 1.8 | 1.6 | 2.3 |
| 1948 | 1.3 | 1.1 | 0.98 | 1.4 | 2.2 | 1.8 | 1.7 | 2.3 |
| 1949 | 1.2 | 1.0 | 0.96 | 1.3 | 1.9 | 1.6 | 1.5 | 2.1 |
| 1950 | 1.2 | 1.0 | 0.95 | 1.3 | 1.8 | 1.5 | 1.4 | 2.0 |
| 1951 | 1.2 | 1.0 | 0.94 | 1.3 | 1.7 | 1.4 | 1.3 | 1.9 |
| 1952 | 1.2 | 1.0 | 0.90 | 1.3 | 1.7 | 1.4 | 1.3 | 1.8 |
| 1953 | 1.1 | 0.93 | 0.83 | 1.2 | 1.6 | 1.3 | 1.2 | 1.8 |
| 1954 | 1.2 | 0.98 | 0.87 | 1.2 | 1.7 | 1.4 | 1.2 | 1.8 |
| 1955 | 0.70 | 0.57 | 0.52 | 0.75 | 1.0 | 0.82 | 0.76 | 1.1 |
| 1956 | 0.40 | 0.31 | 0.30 | 0.48 | 0.61 | 0.47 | 0.47 | 0.74 |
| 1957 | 0.27 | 0.20 | 0.21 | 0.35 | 0.41 | 0.31 | 0.33 | 0.55 |
| 1958 | 0.21 | 0.16 | 0.17 | 0.29 | 0.32 | 0.24 | 0.26 | 0.45 |
| 1959 | 0.17 | 0.13 | 0.14 | 0.25 | 0.26 | 0.20 | 0.22 | 0.38 |
| 1960 | 0.14 | 0.11 | 0.12 | 0.22 | 0.22 | 0.17 | 0.19 | 0.34 |
| 1961 | 0.12 | 0.10 | 0.11 | 0.19 | 0.19 | 0.15 | 0.17 | 0.30 |
| 1962 | 0.11 | 0.087 | 0.10 | 0.18 | 0.17 | 0.14 | 0.15 | 0.28 |
| 1963 | 0.10 | 0.079 | 0.088 | 0.16 | 0.15 | 0.12 | 0.14 | 0.25 |
| 1964 | 0.086 | 0.072 | 0.080 | 0.14 | 0.13 | 0.11 | 0.13 | 0.23 |
| 1965 | 0.076 | 0.065 | 0.073 | 0.13 | 0.12 | 0.10 | 0.11 | 0.21 |
| 1966 | 0.068 | 0.059 | 0.066 | 0.12 | 0.11 | 0.092 | 0.10 | 0.19 |
| 1967 | 0.056 | 0.048 | 0.054 | 0.10 | 0.087 | 0.076 | 0.085 | 0.15 |
| 1968 | 0.045 | 0.039 | 0.044 | 0.081 | 0.070 | 0.061 | 0.069 | 0.13 |
| Utrik community |
Rongelap Island community |
|||||||
|---|---|---|---|---|---|---|---|---|
| Birth year |
RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon |
| 1929 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1930 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1931 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1932 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1933 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1934 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1935 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1936 | 33 | 25 | 24 | 32 | 17 | 14 | 14 | 17 |
| 1937 | 34 | 25 | 25 | 33 | 17 | 14 | 14 | 17 |
| 1938 | 34 | 26 | 25 | 33 | 17 | 14 | 14 | 17 |
| 1939 | 34 | 25 | 25 | 32 | 17 | 14 | 14 | 17 |
| 1940 | 34 | 25 | 25 | 32 | 17 | 14 | 14 | 17 |
| 1941 | 34 | 25 | 25 | 32 | 17 | 14 | 14 | 17 |
| 1942 | 39 | 30 | 28 | 38 | 17 | 14 | 14 | 17 |
| 1943 | 42 | 33 | 30 | 41 | 17 | 14 | 14 | 17 |
| 1944 | 43 | 33 | 30 | 42 | 17 | 14 | 14 | 17 |
| 1945 | 43 | 33 | 30 | 42 | 17 | 13 | 13 | 17 |
| 1946 | 43 | 33 | 30 | 42 | 17 | 13 | 13 | 16 |
| 1947 | 46 | 37 | 33 | 46 | 17 | 13 | 13 | 16 |
| 1948 | 45 | 36 | 33 | 46 | 16 | 12 | 12 | 16 |
| 1949 | 43 | 35 | 32 | 45 | 16 | 12 | 12 | 16 |
| 1950 | 43 | 34 | 32 | 45 | 16 | 12 | 12 | 17 |
| 1951 | 42 | 34 | 32 | 45 | 16 | 12 | 12 | 17 |
| 1952 | 44 | 37 | 33 | 47 | 17 | 12 | 13 | 18 |
| 1953 | 41 | 34 | 31 | 45 | 16 | 12 | 13 | 19 |
| 1954 | 46 | 38 | 34 | 47 | 17 | 13 | 13 | 20 |
| 1955 | 28 | 22 | 20 | 30 | 17 | 13 | 14 | 22 |
| 1956 | 15 | 11 | 11 | 17 | 18 | 14 | 15 | 25 |
| 1957 | 10 | 7.5 | 8.0 | 13 | 23 | 16 | 18 | 30 |
| 1958 | 8.4 | 6.2 | 6.7 | 11 | 21 | 15 | 16 | 28 |
| 1959 | 7.0 | 5.4 | 5.8 | 10 | 18 | 13 | 14 | 25 |
| 1960 | 5.9 | 4.7 | 5.1 | 8.9 | 15 | 12 | 13 | 22 |
| 1961 | 5.1 | 4.1 | 4.5 | 7.8 | 13 | 10 | 11 | 20 |
| 1962 | 4.3 | 3.6 | 3.9 | 6.9 | 11 | 8.9 | 10 | 17 |
| 1963 | 3.7 | 3.1 | 3.5 | 6.1 | 9.8 | 7.8 | 8.6 | 15 |
| 1964 | 3.1 | 2.7 | 3.0 | 5.3 | 8.3 | 6.7 | 7.4 | 13 |
| 1965 | 2.5 | 2.3 | 2.5 | 4.5 | 6.9 | 5.6 | 6.2 | 11 |
| 1966 | 2.1 | 1.9 | 2.1 | 3.8 | 5.7 | 4.7 | 5.2 | 9.4 |
| 1967 | 1.7 | 1.5 | 1.7 | 3.1 | 4.7 | 3.8 | 4.3 | 7.8 |
| 1968 | 1.3 | 1.2 | 1.4 | 2.5 | 3.7 | 3.0 | 3.4 | 6.4 |
The doses from chronic intakes show the same geographical and temporal pattern as the doses resulting from acute intakes. However, because of the absence of short-lived iodine isotopes in the radionuclides that are important to the thyroid doses from acute intakes, the thyroid doses from protracted intakes are not much greater than the doses to other organs and tissues.
Similar to the situation for acute intakes, a few radionuclides contributed most of the organ absorbed dose from chronic intakes. Table 15 presents a ranking of those five radionuclides (55Fe, 60Co, 65Zn, 90Sr, and 137Cs). For all organs and for all four of the atoll and population groups discussed, 137Cs was either the first or second most important contributor to dose. For the Rongelap Island community, 137Cs was the most important contributor to the chronic dose, whereas 65Zn was the largest contributor to dose for the Kwajalein residents, the Majuro residents, and the Utrik community.
Table 15.
Radionuclides giving highest cumulative organ doses (mGy) to adults of four population groups (Majuro residents, Kwajalein residents, Utrik community, and Rongelap Island community) from chronic intakes of long-lived radionuclides. Utrik and Rongelap community doses account for relocations.
| Organ and ranka |
Majuro residents |
Kwajalein residents |
Utrik community |
Rongelap Island community |
||||
|---|---|---|---|---|---|---|---|---|
| Nuclide | Dose | Nuclide | Dose | Nuclide | Dose | Nuclide | Dose | |
| RBM | ||||||||
| 1 | 65Zn | 7.1 × 10−1 | 65Zn | 1.2 × 100 | 65Zn | 2.3 × 101 | 137Cs | 1.4 × 101 |
| 2 | l37Cs | 2.4 × 10−1 | 137Cs | 4.1 × 10−1 | l37Cs | 7.7 × 100 | 55Fe | 2.6 × 100 |
| 3 | 55Fe | 2.6 × 10−2 | 55Fe | 4.5 × 10−2 | 55Fe | 1.9 × 101 | 65Zn | 2.4 × 100 |
| 4 | 90Sr | 1.4 × 10−2 | 90Sr | 2.4 × 10−2 | 90Sr | 2.0 × 10−1 | 90Sr | 1.0 × 100 |
| 5 | 60Co | 5.9 × 10−3 | 60Co | 1.0 × 10−2 | 60Co | 1.1 × 10−1 | 60Co | 9.1 × 10−2 |
| Thyroid | ||||||||
| 1 | 65Zn | 5.2 × 10−1 | 65Zn | 8.9 × 10−1 | 65Zn | 1.8 × 101 | 137Cs | 1.4 × 101 |
| 2 | l37Cs | 2.4 × 10−1 | 137Cs | 4.1 × 10−1 | 137Cs | 7.8 × 100 | 65Zn | 1.8 × 100 |
| 3 | 60Co | 4.7 × 10−3 | 60Co | 8.0 × 10−3 | 55Fe | 1.5 × 10−1 | 55Fe | 2.0 × 10−1 |
| 4 | 55Fe | 2.0 × 10−3 | 55Fe | 3.4 × 10−3 | 60Co | 8.6 × 10−2 | 60Co | 7.2 × 10−2 |
| 5 | 90Sr | 5.2 × 10−5 | 90Sr | 9.0 × 10−5 | 90Sr | 7.6 × 10−4 | 90Sr | 3.8 × 10−3 |
| Stomach | ||||||||
| 1 | 65Zn | 5.1 × 10−1 | 65Zn | 8.7 × 10−1 | 65Zn | 1.6 × 101 | 137Cs | 1.4 × 101 |
| 2 | 137Cs | 2.5 × 10−1 | 137Cs | 4.2 × 10−1 | 137Cs | 7.6 × 100 | 65Zn | 1.7 × 100 |
| 3 | 60Co | 7.1 × 10−3 | 60Co | 1.2 × 10−2 | 55Fe | 1.5 × 10−1 | 55Fe | 2.2 × 10−1 |
| 4 | 55Fe | 2.1 × 10−3 | 55Fe | 3.7 × 10−3 | 60Co | 1.2 × 10−1 | 60Co | 1.1 × 10−1 |
| 5 | 90Sr | 7.2 × 10−5 | 90Sr | 1.2 × 10−4 | 90Sr | 9.8 × 10−4 | 90Sr | 5.2 × 10−3 |
| Colon | ||||||||
| 1 | 65Zn | 6.9 × 10−1 | 65Zn | 1.2 × 100 | 65Zn | 2.2 × 101 | 90Cs | 1.6 × 101 |
| 2 | 137Cs | 2.8 × 10−1 | 137Cs | 4.8 × 10−1 | 137Cs | 8.8 × 100 | 65Zn | 2.3 × 100 |
| 3 | 60Co | 2.4 × 10−2 | 60Co | 4.1 × 10−2 | 60Co | 4.3 × 10−1 | 55Fe | 6.0 × 10−1 |
| 4 | 55Fe | 5.9 × 10−3 | 55Fe | 1.0 × 10−2 | 55Fe | 4.3 × 10−1 | 60Co | 3.7 × 10−1 |
| 5 | 90Sr | 1.0 × 10−3 | 90Sr | 1.8 × 10−3 | 90Sr | 1.4 × 10−2 | 90Sr | 7.5 × 10−2 |
Rank of 1 indicates radionuclide with highest organ dose; Rank of 5 indicates radionuclide with fifth highest organ dose.
DISCUSSION
Comparison of estimated intakes and doses to other published values
There are few estimates in the literature of radiation doses to the Marshallese from nuclear testing that can be compared to the estimates provided here. Lessard et al. (1984, 1985) reported on chronic intakes and doses, and acute intakes and doses, respectively; however, both reports only apply to the Rongelap and Utrik populations and the acute doses were only from the Bravo test. Goetz et al. (1987) reported on acute exposures to the military weather observers on Rongerik exposed to Bravo fallout. No publications known to us have reported acute intakes and doses or chronic intakes and doses for population on atolls other than Rongelap and Utrik. For this reason, the comparisons that can be made with literature data are very limited. Because the intake estimates for 131I and other short-lived radioiodines and radiotelluriums were based on Lessard et al. (1985) and in this work on the same bioassay data (Harris 1954), the estimates are close. Differences in estimated intakes for 131I are a result of different assumptions on the TOIs and the excretion fraction on the day of sampling which must be derived from a specific biokinetic model. For the populations on Rongelap and Ailinginae, our estimates of intake of 131I were similar to those of Lessard et al. (1985), though were about three times greater than those suggested by Goetz et al. (1987) for Rongerik (Table 16; also see Table 6, Harris et al. 2010). Differences in estimates of intakes of radionuclides other than 131I (Table 16) are due to differences in the assumed TOI and in the yield of other nuclides relative to 131I. As discussed, we used ratios of nuclides from the work of Hicks (1984) with small adjustments for fractionation.
Table 16.
Comparison of estimates of average acute intake (MBq) of radioiodines and precursor radionuclides among exposed Marshallese and American groups following deposition of Bravo fallout. All values are rounded to two significant digits.a
| Literature source of estimates | Gender (adults) | 131I (8.02 d) | 132I (2.3 h) | 133I (20.8 h) | 135I (6.6 h) | 131mTe (30 h) | 132Te (3.2 d) |
|---|---|---|---|---|---|---|---|
| Rongelap Island (Marshallese adults) |
|||||||
| Harrisb,c | Male-female average | 2.8 | 9.6 | 30 | 43 | nr | nr |
| Lessard et al. (1985) | Male-female average | 3.4 | 20 | 73 | 120 | 2.8 | 19 |
| This work | Male female average | 3.5 | 9.7 | 40 | 50 | 2.3 | 9.4 |
| Sifo, Ailinginae (Marshallese adults) |
|||||||
| Harrisb,c | Male-female average | 1.3 | 4.4 | 14 | 20 | nr | nr |
| Lessard et al. (1985) | Male-female average | 0.69 | 4.1 | 20 | 41 | 0.84 | 19 |
| This work | Male-female average | 1.2 | 3.5 | 16 | 25 | 0.88 | 3.4 |
| Rongerik (American military, adults) |
|||||||
| Harrisb,c | Male | 0.78 (1.7)d | 2.6 (5.7) | 8.1 (18) | 12 (26) | nr | nr |
| Goetz et al. (1987) | Male | 0.56 (1.2)e | nr | nr | nr | nr | nr |
| This work | Male | 1.7 | 4.6 | 18 | 17 | 1.1 | 4.6 |
nr means not reported.
Harris PS. A summary of the results of urine analysis on Rongelap natives Americans and Japanese fishermen to date. Memorandum to AEC. Los Alamos, NM: Los Alamos Scientific Laboratory; 1954.
Personal communication, P.S. Harris to S.L. Simon, 2005.
500 mL urine volume (same as for Marshallese) was used by Harris; use of 1,100 mL urine volume for LA319A (see Table 7) would have given 1.7 MBq.
500 mL urine volume (same as for Marshallese) was used by Goetz et al. (1987); use of 1,100 mL urine volume for LA319A (see Table 7) would have given 1.2 MBq.
In terms of estimated doses, at Rongelap and Ailinginae, our estimates (Table 17) of absorbed dose to the thyroid from acute intake of 131I from Bravo were similar to those of Lessard et al. (1985), but about four times greater for Rongerik compared to those reported by Goetz et al. (1987). Small differences could have been due to a variety of factors, e.g., the dose conversion coefficients. In our case, the dose conversion coefficients were derived from the same thyroid biokinetic model and sets of parameter values used to derive the excretion fractions.
Table 17.
Comparison of estimates of absorbed dose to the thyroid from the Bravo test, (acute intake) from this work and from Lessard et al. (1985) and Goetz et al. (1987); all entries are in mGy and represent the average for male and female adults (except Rongerik which pertains to adult males only), rounded to two significant digits (entries with dash were not estimated).
| Estimated thyroid absorbed dose (mGy) to adults |
|||||||
|---|---|---|---|---|---|---|---|
| 131I (8.02 d) | 132I (2.3 h) | 133I (20.8 h) | 135I (6.6 h) | 131mTe (30 h) | 132Te (3.2 d) | Total | |
| Rongelap | |||||||
| This work | 1700 | 47 | 4,200 | 1,300 | 41 | 260 | 7,600 |
| Lessard et al. (1985) | 1,400 | 74 | 5,600 | 2,000 | 130 | 1,200 | 11,000 |
| Ailinginae | |||||||
| This work | 500 | 14 | 1,400 | 520 | 13 | 80 | 2,500 |
| Lessard et al. (1985) | 290 | 16 | 1,600 | 670 | 39 | 300 | 2,900 |
| Utrik | |||||||
| This work | 230 | 4.5 | 380 | 36 | 3.8 | 35 | 690 |
| Lessard et al. (1985) | 330 | 15 | 850 | 79 | 27 | 240 | 1,600 |
| Rongerik | |||||||
| This work | 740 | 16 | 2,200 | 820 | 18 | 120 | 4,000 |
| Goetz et al. (1987) | 190 | - | - | - | - | - | 190 |
Contribution of other radionuclides to the internal doses
The 63 radionuclides that have been considered for the estimation of the doses from acute intakes have been selected among those that were systematically reported by Hicks (1984) for all tests, while the five radionuclides considered in the estimation of the doses from chronic intakes are those that were measured in whole-body or from bioassay measurements performed within a few years after the Bravo test. In addition, there are several radionuclides that deserve mention:
-
*
239Pu and 240Pu: The normalized deposition densities of 239Pu and 240Pu for specific tests were not reported by Hicks (1984) as that information is classified. However, 239+240Pu concentrations in the top layer of soil (0–5 cm) were measured in soil samples collected in 1978 by Robison et al. (1982) and in 1991–1993 by Simon and Graham (Simon and Graham 1997; Simon et al. 1999). In order to estimate the 239+240Pu deposition density at the time of fallout, it was assumed that: (1) all of the 239+240Pu fallout occurred at the time of large tests in 1954, (2) the deposited activity migrated relatively rapidly downwards from the top layer of soil during the first year after deposition, then decreased much more gradually with time as the activity became fixed in the soil matrix, (3) the measurements included the contribution from 239+240Pu in global fallout, estimated as 0.24 Bq kg−1, and assumed to have all been deposited in 1962, and (4) the average density of the top layer of coral-based soil was 1.0 g cm−3. The deposition density of 239+240Pu from all Pacific tests, assumed to have occurred in 1954, and the variation with time after fallout of the concentration of 239+240Pu in the top level of soil (0–5 cm) are presented in Table 18 for all atolls and reef islands of the Marshall Islands except the test site atolls. Crude estimates of the doses due to acute intakes were obtained using: (1) the deposition densities presented in Table 18; (2) the relationship of 0.031 Bq intake per Bq m−2 deposited obtained for 137Cs at Rongelap for the test Bravo, and (3) the committed dose coefficients recommended by ICRP (1996). The doses to bone marrow were much greater than those for the other three organs and tissues that we considered. The highest doses to RBM were found for the Rongelap Island community. For example, for adults at the time of the test Bravo, the lifetime equivalent dose to RBM was estimated to be 0.4 mSv, which represents about 2% of the total internal dose to RBM from acute intakes of other nuclides. For the other organs and tissues, the equivalent doses from acute intakes of 239+240Pu represented less than 0.01% of the total internal doses to those organs and tissues from acute intakes. Doses from chronic intakes received during the lifetime of adults at the time of the test Bravo also have been estimated using (1) the concentrations of 239+240Pu in the top level of soil (0–5 cm) presented in Table 18, (2) a daily ingestion intake of soil of 500 mg (Sun et al. 1997), and (3) the committed dose coefficients recommended by ICRP (1990). Here again, the highest doses were those to RBM delivered to the Rongelap Island community. The equivalent dose thus obtained was 0.8 mSv, which represents about 3% of the total internal dose to RBM from chronic intakes. For the other organs and tissues, the equivalent doses from chronic intakes of 239+240Pu represented less than 1% of the total internal doses from chronic intakes; and
-
*
207Bi: Along with 60Co and 137Cs, 207Bi was one of the three radionuclides that were detected with regularity in gamma spectrometry analyses conducted until the mid-1990’s (Noshkin et al. 1997). A summary of all the available data on the concentrations of 207Bi in flesh samples of reef and pelagic fish collected from Bikini and Enewetak Atolls between 1964 and 1995 was published by Noshkin et al. (1997). Their analysis showed that: (1) the highest 207Bi concentrations, by far, were observed in goatfish, which is representative of reef fish, (2) at Enewetak Atoll, 207Bi was lost from the environment with an effective half-time of 5.1 y, whereas at Bikini Atoll, only radioactive decay, with a half-life of 32.2 y, accounted for the rate at which 207Bi was disappearing from the lagoon, and (3) representative concentrations of 207Bi in goatfish flesh in 1978 were 8.1 Bq kg−1 at Bikini and 241.9 Bq kg−1 at Enewetak. Assuming, here again, that all of the radioactive contamination of the lagoons occurred in 1954, the time-integrated concentrations of 207Bi in goatfish flesh from 1954 to infinity were estimated to be 630 Bq y kg−1 at Bikini and 46,000 Bq y kg−1 at Enewetak. Using an assumed daily consumption of 43 g of reef fish (Robison and Sun 1997), these time-integrated concentrations lead to lifetime doses to adults that would be, at most, 5 mGy to the colon walls of persons who would exclusively consume goatfish flesh from the Enewetak lagoon and to doses of less than 1 mGy to the other organs and tissue of consumers of reef fish from Bikini lagoon. It is clear, however, that these doses are vastly overestimated because the residents of Bikini and Enewetak were evacuated in 1946 and 1947, respectively (Table 3 in Simon al. 2010) and because the 207Bi concentrations in fish from lagoons other than Bikini and Enewetak are likely to have been much lower.
Table 18.
Deposition density (kBq m−2) of 239+240Pu at the time of fallout and the variation of surface soil concentration (Bq kg−1 in 0–5 cm) with time (y) after deposition. All values rounded to two significant digits
| Atoll | Deposition density (kBq m−2) |
239+240Pu soil concentration (Bq kg−1) as a function of time (y) after deposition |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 y | 0.5 y | 1 y | 3 y | 5y | 10 y | 20 y | 30 y | 50 y | ||
| Ailinginae | 7.5 | 150 | 130 | 110 | 86 | 78 | 70 | 63 | 59 | 55 |
| Ailinglaplap | 0.055 | 1.1 | 0.90 | 0.77 | 0.61 | 0.56 | 0.50 | 0.45 | 0.42 | 0.40 |
| Ailuk | 0.19 | 3.8 | 3.1 | 2.7 | 2.1 | 2.0 | 1.7 | 1.6 | 1.5 | 1.4 |
| Arno | 0.060 | 1.2 | 1.0 | 0.88 | 0.70 | 0.64 | 0.57 | 0.52 | 0.49 | 0.46 |
| Aur | 0.065 | 1.3 | 1.1 | 0.92 | 0.74 | 0.67 | 0.60 | 0.54 | 0.51 | 0.48 |
| Bikara | 5.7 | 110 | 91 | 79 | 60 | 57 | 49 | 45 | 42 | 42 |
| Ebon | 0.021 | 0.41 | 0.34 | 0.29 | 0.23 | 0.21 | 0.19 | 0.17 | 0.16 | 0.15 |
| Erikub | 0.095 | 0.19 | 0.16 | 0.13 | 0.11 | 0.10 | 0.090 | 0.080 | 0.070 | 0.070 |
| Jabat | 0.033 | 0.65 | 0.54 | 0.46 | 0.37 | 0.34 | 0.30 | 0.27 | 0.25 | 0.24 |
| Jaluitb | 0.085 | 1.7 | 1.4 | 1.2 | 0.97 | 0.88 | 0.78 | 0.71 | 0.67 | 0.63 |
| Jemo | 0.090 | 1.8 | 1.5 | 1.3 | 1.0 | 0.92 | 0.82 | 0.74 | 0.70 | 0.66 |
| Kili | 0.085 | 1.7 | 1.4 | 1.2 | 0.95 | 0.87 | 0.77 | 0.70 | 0.66 | 0.62 |
| Knox | 0.037 | 0.73 | 0.61 | 0.52 | 0.41 | 0.38 | 0.34 | 0.30 | 0.29 | 0.27 |
| Kwajalein | 0.066 | 1.2 | 1.0 | 0.84 | 0.67 | 0.61 | 0.55 | 0.49 | 0.47 | 0.44 |
| Lae | 0.12 | 2.4 | 2.0 | 1.7 | 1.3 | 1.2 | 1.1 | 0.98 | 0.92 | 0.86 |
| Lib | 0.050 | 1.0 | 0.85 | 0.73 | 0.58 | 0.53 | 0.47 | 0.43 | 0.40 | 0.38 |
| Likiep | 0.44 | 8.7 | 7.2 | 6.1 | 4.9 | 4.5 | 4.0 | 3.6 | 3.4 | 3.2 |
| Majuroc | 0.072 | 1.2 | 1.0 | 0.88 | 0.70 | 0.64 | 0.57 | 0.52 | 0.49 | 0.46 |
| Maloelap | 0.055 | 1.1 | 0.94 | 0.81 | 0.64 | 0.59 | 0.52 | 0.47 | 0.45 | 0.42 |
| Mejit | 0.14 | 2.7 | 2.3 | 1.9 | 1.5 | 1.4 | 1.3 | 1.1 | 1.1 | 1.0 |
| Mili | 0.034 | 0.68 | 0.56 | 0.48 | 0.38 | 0.35 | 0.31 | 0.28 | 0.27 | 0.25 |
| Namorik | 0.085 | 1.7 | 1.4 | 1.2 | 0.97 | 0.88 | 0.78 | 0.71 | 0.67 | 0.63 |
| Namu | 0.085 | 1.7 | 1.4 | 1.2 | 0.93 | 0.85 | 0.76 | 0.68 | 0.65 | 0.61 |
| Rongelap Island | 16 | 290 | 240 | 210 | 160 | 150 | 130 | 120 | 110 | 110 |
| Rongerik | 35 | 700 | 580 | 500 | 400 | 360 | 320 | 290 | 270 | 260 |
| Taka | 1.4 | 28 | 23 | 20 | 16 | 14 | 13 | 11 | 11 | 10 |
| Taongi | 0.16 | 3.2 | 2.7 | 2.3 | 1.8 | 1.7 | 1.5 | 1.3 | 1.3 | 1.2 |
| Ujae | 0.075 | 1.5 | 1.3 | 1.1 | 0.87 | 0.80 | 0.71 | 0.64 | 0.60 | 0.57 |
| Ujelang | 0.22 | 4.3 | 3.6 | 3.1 | 2.5 | 2.2 | 2.0 | 1.8 | 1.7 | 1.6 |
| Utrik | 3.5 | 63 | 53 | 45 | 36 | 33 | 29 | 26 | 25 | 23 |
| Wotho | 0.085 | 1.7 | 1.4 | 1.2 | 1.0 | 0.89 | 0.80 | 0.72 | 0.68 | 0.64 |
| Wotje | 0.090 | 1.8 | 1.5 | 1.3 | 1.0 | 0.94 | 0.83 | 0.75 | 0.71 | 0.66 |
Values scaled to those for Rongelap Island, using total 137Cs deposition density (Table 5, Simon et al. 2010) as a guide.
Values from Namorik assumed for Jaluit.
Values from Arno assumed for Majuro.
Comparison of internal doses to external doses
Table 19 compares the estimated acute and chronic doses to an adult for four organs at the four representative atolls with the external doses as reported in Bouville et al. (2010) for those same atolls. Except for doses to the thyroid gland, the external doses were comparable or much greater than the internal doses. As discussed previously, the chronic doses for thyroid were small compared to the acute doses, but the chronic doses to stomach and RBM were comparable or greater than the acute doses (except for the Rongelap community). However, the calculated chronic doses were mainly due to ingestion of 65Zn (Table 15) and are very uncertain since, as discussed previously, they are very dependent on assumptions regarding the intake of 65Zn at atolls other than Rongelap.
Table 19.
Comparison of estimates of acute and chronic internal doses (mGy) with external dose (mGy) for representative adults of four population groups.
| Population groups |
||||
|---|---|---|---|---|
| Organ/Mode of exposure |
Majuro residents |
Kwajalein residents |
Utrik community |
Rongelap Island community |
| Thyroid | ||||
| Acute | 22 | 66 | 740 | 7,600 |
| Chronic | 0.76 | 1.3 | 25 | 14 |
| RBM | ||||
| Acute | 0.11 | 0.25 | 2.3 | 25 |
| Chronic | 0.98 | 1.7 | 33 | 17 |
| Stomach wall | ||||
| Acute | 0.32 | 1.1 | 16 | 530 |
| Chronic | 0.75 | 1.3 | 24 | 14 |
| Colon | ||||
| Acute | 4.4 | 12 | 180 | 2,800 |
| Chronic | 0.99 | 1.7 | 32 | 17 |
| Whole body (external dose) |
9.8 | 22 | 130 | 1,600 |
Comparison of internal doses from fallout to internal doses from natural background radioactivity
It is useful to compare the estimated acute and chronic doses to Marshallese from ingestion of fallout radioactivity with estimates of dose from ingestion of natural radioactivity in the diet. Coral-based soil is low in natural radioactivity, resulting in little natural radioactivity in locally grown foods; hence, seafood provides the largest amount of natural radioactivity to the Marshallese diet (Noshkin et al. 1994). Though the diet of the Marshallese in years past has been difficult to reconstruct precisely (NAP 1994), reasonable estimates of annual intake are possible. Depending on assumptions made about the proportion of the diet from local foods compared to imported foods, the annual intake by adult Marshallese was estimated by Noshkin et al. (1994) to range from 800 Bq (mixture of local and imported food) to 3,000 Bq (local food only) for 210Po, and from 130 Bq to 240 Bq for 210Pb. While Noshkin et al. (1994) used these intakes to estimate effective doses, we used their estimates of intake to calculate organ equivalent doses so that a more direct comparison can be made with our estimated organ absorbed doses resulting from exposure to fallout.
A comparison of the doses from routine ingestion of 210Po and 210Pb with doses from ingestion of fallout radioactivity is complex for several reasons: (1) The types of radiations that give rise to the doses are different: predominantly alpha particles for the doses from 210Pb and 210Po, and electrons and photons for fallout radionuclides from nuclear weapons tests. Consequently, a radiation-weighting factor equal to 20 is necessary to determine the equivalent doses from 210Pb and 210Po, while the factor is equal to 1.0 for the doses from fallout. In this comparison, the doses are expressed in terms of equivalent dose (mSv), as that quantity is generally proportional to the radiation risk; (2) The equivalent doses that result from intakes of the radionuclides considered vary according to age; in this comparison, only the equivalent doses to adults are estimated; (3) The annual equivalent doses from naturally-occurring radionuclides are considered to be constant over time unlike the doses from fallout that were highest in 1954 and generally decreased until 1970. In this analysis, the fallout equivalent doses accumulated from 1948 through 1970 are compared with doses from natural radioactivity in foods for the same number of years. Therefore, the estimated annual equivalent doses from natural radioactivity were summed over 23 y; (4) The equivalent doses from fallout varied substantially among groups of atolls, whereas the doses from naturally-occurring radionuclides were considered to be the same at all atolls of the Marshall Islands. The results of the comparison are presented in Table 20 for representative adults of the four communities discussed throughout this paper. Two general findings emerged, regardless of the atoll: (1) the equivalent dose to RBM of adults from ingestion of fallout was estimated to be substantially less than the equivalent dose from ingestion of naturally-occurring 210Po and 210Pb over an equal number of years of intake; and (2) the equivalent dose to the thyroid of adults from ingestion of fallout was greater than the equivalent doses from naturally-occurring 210Po and 210Pb.
Table 20.
Comparison of equivalent doses (mSv) to four organs of representative adults of four communities from intakes of fallout radioactivity (acute + chronic) with equivalent doses from consumption of naturally-occurring 210Po and 2l0Pb for an equal number of years and according to two different diets (mixture of local and imported foods and local-food-only diets). All values rounded to two significant digits.
| Population group |
||||
|---|---|---|---|---|
| Organ/Source of exposure | Majuro residents |
Kwajalein residents |
Utrik community |
Rongelap Island community |
| Thyroid | ||||
| Fallout (acute + chronic) | 23 | 67 | 760 | 7,600 |
| Natural radioactivity, mixed food diet | 5.4 | 5.4 | 5.4 | 5.4 |
| Natural radioactivity, local-food-only diet | 20 | 20 | 20 | 20 |
| RBM | ||||
| Fallout (acute + chronic) | 1.1 | 1.9 | 35 | 42 |
| Natural radioactivity, mixed food diet | 55 | 55 | 55 | 55 |
| Natural radioactivity, local-food-only diet | 190 | 190 | 190 | 190 |
| Stomach wall | ||||
| Fallout (acute + chronic) | 1.1 | 2.4 | 41 | 550 |
| Natural radioactivity, mixed food diet | 5.4 | 5.4 | 5.4 | 5.4 |
| Natural radioactivity, local-food-only diet | 20 | 20 | 20 | 20 |
| Colon | ||||
| Fallout (acute + chronic) | 5.4 | 14 | 210 | 2,800 |
| Natural radioactivity, mixed food diet | 6.0 | 6.0 | 6.0 | 6.0 |
| Natural radioactivity, local-food-only diet | 22 | 22 | 22 | 22 |
At southern and mid-latitude atolls, best represented by Majuro and Kwajalein, respectively (Fig. 2, Simon et al. 2010), the equivalent doses to the stomach wall and colon from exposure to fallout were smaller than the equivalent doses from ingesting naturally-occurring 210Po and 210Pb. Of the two diets, the local-food-only diet would give a larger dose from natural radioactivity. The thyroid equivalent dose for members of these communities was only slightly greater from fallout than from intakes of naturally-occurring 210Pb and 210Po (20% to 3.3 times larger). These relationships would apply to about 96% of the population alive during the testing years (73% who lived in the southern atolls and 23% who lived in the mid-latitude atolls).
For the Utrik and Rongelap communities, the dose to RBM from fallout, as mentioned, was less than from the dose from natural radioactivity; however, the fallout-related equivalent doses to the other organs (thyroid, stomach wall, and colon) exceeded the diet-related equivalent doses to those organs and tissues from natural radioactivity. In the case of these two population groups, the thyroid equivalent dose was far greater from intakes of fallout radionuclides than from intakes of naturally-occurring 210Pb and 210Po (40 to nearly 400 times larger). The combined Utrik and Rongelap populations composed about 3% of the population alive during the testing years.
Estimation of uncertainties
There are numerous sources of uncertainty in the acute and chronic dose estimates presented here, many of which are difficult to quantify. Because of the various types and sources of data used in the reconstruction, the wide variety of sub-models used, and the many assumptions and interpolations required, numerical determination of the overall uncertainty in doses for each atoll and age group is difficult and involves considerable subjective judgment. In this section, the uncertainties in the total internal dose received by each population group in each year from all tests in that year are crudely quantified. The dosimetric uncertainties for the population groups exposed on the three northern atolls (Rongelap, Ailinginae, Rongerik), for Utrik, and for the mid-latitude and southern latitude atolls (see Fig. 2 in Simon et al. 2010) are considered in turn.
Population groups in the northern latitudes
The doses received by the Rongelap Island community are used here to represent the doses received by the three population groups other than Utrik in the northern atolls (Rongelap Island, Ailinginae, Rongerik). As shown in Table 11, the thyroid doses received by the Rongelap Island community in 1954 were almost entirely due to acute intakes of radioiodines (131I, 133I, 135I) resulting from the Bravo test. These intakes were estimated on the basis of 131I measurements made on samples of pooled urine collected from adults who were at Ailinginae, Rongelap, and Rongerik at the time of fallout from the test. The average thyroid dose to adults from acute intake of 131I from Bravo can be expressed as:
| (13) |
Where C(131I, Bravo, adults) = the measured concentration of 131I in the pooled sample of urine; the average over the two samples taken among the Rongelap people is 0.42 Bq mL−1;
[Latin capital letter Q with macron above] = 3,500 kBq is the estimated intake of 131I averaged over the two urine samples (Table 7); and
D = 1,700 mGy is the estimated thyroid dose due to the intake of 131I (Table 11).
Uncertainties in C
As shown in eqn (A1) (Appendix), C is obtained as the ratio of the background adjusted count rate of 131I, CR (counts s−1 per mL), and of the calibration factor, [varepsilon]C (count per decay). In this analysis, the uncertainties in the estimates of CR and [varepsilon]C are considered to be small in comparison to the uncertainty in [Latin capital letter Q with macron above]/C.
Uncertainties in [Latin capital letter Q with macron above]/C
It follows from eqn (A1) that
where K = the correction factor corresponding to the radioactive decay of 131I between time of sampling and time of counting;
[Latin capital letter V with macron above] = the 24-h urine volume (mL) averaged over the sample population; and
EF(t) = the urinary excretion fraction for 131I on day of sampling, t being the time elapsed between intake and sampling.
Because of the relatively long half-life of 131I (8 d), the uncertainty for K is very small. Uncertainties on [Latin capital letter V with macron above] are discussed in the Appendix and in Harris et al. (2010): the mean 24-h urine volumes averaged over the sample population are 427 and 448 mL for the samplings on March 16 and 17, respectively (Table 7); the distributions of the mean are assumed normal with standard errors of the mean of 42 and 37 mL for the two days of sampling. For this analysis, the mean and the standard error of the mean were taken to be 440 and 40 mL, respectively. The uncertainties in EF(t) are related to those in the parameter values of the biokinetic model. In Appendix A, six possible sets of parameter values for the thyroid biokinetic model were used to quantify the variations of the value of EF(t). Results, presented in Table A1, suggest for the Rongelap Island community a range of values from 0.92 to 2.3 × 10−4 around an arithmetic mean value of 1.7 × 10−4, leading to a GSD of 1.6, assuming that the range of values correspond to one GSD. Using the numerical estimates of the GSDs for [Latin capital letter V with macron above] and for EF(t) indicated above, the GSD for [Latin capital letter Q with macron above]/C for adults of the Rongelap Island community exposed to acute intakes of 131I from the Bravo test is found to be 1.6.
Uncertainties in D/[Latin capital letter Q with macron above]
The uncertainties in the thyroid dose per unit 131I intake, D/[Latin capital letter Q with macron above], are relatively well documented (for example, Dunning and Schwarz 1981; Zvonova 1989; NCI 1997; Apostoaei and Miller 2004). They depend essentially on the uncertainties on the fractional thyroidal uptake and on the thyroid mass, as well as on the degree of correlation between the two parameters. Considering that the quantity of interest is the average thyroid dose per unit intake for adults of the Rongelap Island community, a direct approach was taken: the six possible sets of parameter values for the thyroid biokinetic model that are presented in Table A1 were used to quantify the variations of the value of D/[Latin capital letter Q with macron above] for 131I. The obtained range of values is from 4.3 to 6.1 × 10−7 Gy Bq−1, resulting in a GSD of 1.2.
Using values of 1.0, 1.6, and 1.2 for the GSDs of C, [Latin capital letter Q with macron above]/C, and D/[Latin capital letter Q with macron above], respectively, the GSD for D(131I, Bravo, adults) is estimated to be 1.7.
There are additional uncertainties involved in the estimation of the overall uncertainty in the annual doses received by representative persons of the Rongelap Island community. They include:
-
*
uncertainties in the contributions of 133I (20.8 h) and 135I (6.6 h) to the thyroid dose to adults from the Bravo test, which are estimated to be 4,200 and 1,300 mGy, respectively, and, therefore, of the same order of magnitude as the thyroid dose from 131I (1,700 mGy);
-
*
uncertainties in the estimation of the thyroid dose to children; and
-
*
uncertainties related to small components of the thyroid dose due to acute intakes of 132Te and 132I from the Bravo test, thyroid doses due to chronic intakes from Bravo, and to acute and chronic intakes from other tests of the Castle series.
The contributions of 133I (20.8 h) and 135I (6.6 h) account for about 75% of the thyroid dose from the Bravo test to adults of the Rongelap Island community. As all other radionuclides collectively contribute no more than 25% of the thyroid dose from Bravo, a good approximation of the thyroid dose is:
| (14) |
Because the values of [Latin capital letter Q with macron above](133I) and [Latin capital letter Q with macron above](133I) are correlated to [Latin capital letter Q with macron above](133I), and the values of [D(133I)/[Latin capital letter Q with macron above](133I)] and [D(135I)/[Latin capital letter Q with macron above](135I)] are also correlated to [D(131I)/[Latin capital letter Q with macron above](131I)], the uncertainties in the total thyroid dose to adults from Bravo appear to be close to those of the contribution to the dose due to intake of 131I. However, in the absence of measurements of 133I and 135I in urine, and because of the lack of certainty on the nature of the pathways leading to the acute intakes, the uncertainty in the thyroid dose from the radioiodines was modestly increased to a GSD of 1.9.
The uncertainties in the estimation of the 131I thyroid dose to children are admittedly greater than those to adults, as there was no measurement of 131I in urine from children that could be used to validate them. However, the correction for age dependency seems to be well established for all age groups, with the exception of infants, for whom the contribution to the thyroid dose from breast feeding needs to be added. Also, for infants born in 1954, the dose is averaged over all possible dates of birth, resulting in additional uncertainties related to the estimation of breast feeding and of the doses received in utero (ICRP 2001, 2004). Table 21 provides a comparison of doses to persons born in the year of tests under two assumptions: (1) averaged over all dates of birth (the BCAD), and (2) assumed to have been born on 1 January. In this table, we compare the doses for the same four tissues and atolls as before. Though this study is not concerned with doses to identified persons, this table indicates how much greater a person’s dose might be if they were born in the year of test, but before it took place. As noted earlier, the BCAD is the least biased estimate since the choice of any single DOB cannot be representative of all persons. Because infants represent a very small fraction of the population, the simplifying assumption was made that the uncertainty assigned to adults is also applicable to infants, and to children of any age as well.
Table 21.
A comparison of the birth-cohort averaged dose (BCAD) with the dose to infants who are assumed to have been born on Jan. 1 in the same year as the tests (1948, 1951, 1953, 1954, 1956, 1958). All estimated doses (mGy) are from acute intakes of radionuclides, are truncated to the end of the year, and are rounded to two significant digits.a
| Birth year and year of tests |
Assumption for dose calculation |
Majuro residents |
Kwajalein residents |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon | ||
| 1948 | BCAD | 0.00028 | 0.046 | 0.00027 | 0.0022 | 0.063 | 18 | 0.17 | 0.98 |
| 1948 | Born Jan,l | 0.00080 | 0.13 | 0.00076 | 0.0063 | 0.19 | 53 | 0.51 | 2.9 |
| 1951 | BCAD | - | - | - | - | 0.00056 | 0.11 | 0.00089 | 0.0054 |
| 1951 | Born Jan. 1 | - | - | - | - | 0.0020 | 0.38 | 0.0032 | 0.020 |
| 1952 | BCAD | 0.025 | 6.3 | 0.058 | 0.30 | 0.027 | 7.7 | 0.076 | 0.34 |
| 1952 | Born Jan,1 | 0.030 | 7.3 | 0.067 | 0.35 | 0.031 | 9.0 | 0.088 | 0.39 |
| 1954 | BCAD | 0.10 | 16 | 0.13 | 0.82 | 0.20 | 54 | 0.52 | 2.5 |
| 1954 | Born Jan,1 | 0.42 | 66 | 0.53 | 3.5 | 0.67 | 180 | 1.7 | 8.3 |
| 1956 | BCAD | 0.017 | 2.5 | 0.020 | 0.13 | 0.032 | 6.0 | 0.053 | 0.30 |
| 1956 | Born Jan,1 | 0.036 | 5.4 | 0.044 | 0.29 | 0.072 | 13 | 0.12 | 0.67 |
| 1958 | BCAD | 0.0025 | 0.37 | 0.0025 | 0.021 | 0.0036 | 0.50 | 0.0030 | 0.026 |
| 1958 | Born Jan,1 | 0.0068 | 0.99 | 0.0066 | 0.055 | 0.0094 | 1.3 | 0.0080 | 0.068 |
| Birth year and year of tests |
Utrik community |
Rongelap Island community |
||||||
|---|---|---|---|---|---|---|---|---|
| RBM | Thyroid | Stomach | Colon | RBM | Thyroid | Stomach | Colon | |
| 1948 | 0.00020 | 0.046 | 0.00042 | 0.0026 | 0.033 | 11 | 0.12 | 0.59 |
| 1948 | 0.00058 | 0.14 | 0.0012 | 0.0026 | 0.10 | 33 | 0.35 | 1.8 |
| 1951 | 0.0053 | 1.1 | 0.0093 | 0.055 | 0.010 | 2.2 | 0.020 | 0.11 |
| 1951 | 0.019 | 3.9 | 0.034 | 0.20 | 0.035 | 8.1 | 0.075 | 0.41 |
| 1952 | 0.016 | 3.4 | 0.030 | 0.17 | 0.016 | 4.0 | 0.036 | 0.19 |
| 1952 | 0.019 | 4.0 | 0.036 | 0.20 | 0.020 | 4.7 | 0.043 | 0.23 |
| 1954 | 1.7 | 460 | 5.6 | 31 | 16 | 5,100 | 150 | 480 |
| 1954 | 10 | 2,600 | 33 | 190 | 97 | 32,000 | 920 | 2,900 |
| 1956 | 0.0083 | 1.1 | 0.0085 | 0.058 | 0.017 | 2.5 | 0.020 | 0.13 |
| 1956 | 0.019 | 2.7 | 0.020 | 0.14 | 0.036 | 5.4 | 0.044 | 0.29 |
| 1958 | 0.016 | 3.3 | 0.029 | 0.19 | 0.036 | 7.2 | 0.067 | 0.38 |
| 1958 | 0.045 | 8.9 | 0.078 | 0.52 | 0.087 | 17 | 0.16 | 0.93 |
Note: Table entries with a dash (—) were doses estimated to have been less than 0.001 mGy.
In comparison to the contributions to the thyroid dose due to acute intakes of 131I, 133I, and 135I from the Bravo test, the other components of the thyroid dose received in 1954 by members of the Rongelap Island community (acute intakes of other radionuclides, chronic intakes of long-lived radionuclides, acute and chronic intakes from tests other than Bravo) are very small, so that their levels of uncertainty have little influence on the overall uncertainty. For that reason, we assumed that the uncertainty in the thyroid dose received in 1954 by representative persons of the Rongelap Island community is expressed by a GSD of 2.0, which is only slightly greater than the value used for the thyroid dose from Bravo (GSD of 1.9). We assume that the uncertainty in our estimates of dose to other organs (RBM, stomach wall and colon) is comparable to that which we estimate for the thyroid. The intakes of the nuclides that account for these doses are assumed to be given by the ratio of deposition densities which we assume have relatively small uncertainty. We also assume that the uncertainty in dose per unit intake for these nuclides is comparable to that for the radioiodines. Finally, we assume the same uncertainties for years other than 1954 since, even though the uncertainty in deposition varies somewhat from test to test, the major source of uncertainty is in [Latin capital letter Q with macron above]/D and it should not have varied significantly. The same uncertainty value was assigned to the other population groups (Ailinginae and Rongerik) exposed in the northern group of atolls.
Utrik population group
As is the case for the Rongelap Island community and as shown in Tables 11 and 19, the internal thyroid doses received by the Utrik community in 1954 were, for the most part, due to acute intakes of radioiodines (131I and 133I) resulting from the Bravo test. However, no samples for bioassay of 131I were collected from the members of the Utrik community. Those intakes were estimated from the 137Cs deposition densities provided in Beck et al. (2010) for all tests with measurable fallout. Taking only into consideration the intakes of 131I and 133I from Bravo, the thyroid dose received in 1954 by representative adults of the Utrik community is expressed as:
| (15) |
Uncertainties in Dep(137Cs): As discussed in Beck et al. (2010), an uncertainty estimate was assigned to each estimate of the 137Cs deposition density at each atoll from each test. These uncertainties, expressed in terms of GSDs, ranged from 1.3 to 3.0, depending on the availability and number of measurements of exposure rates and long-lived radionuclides at the atoll for the test under consideration. In the case of Utrik, the 137Cs deposition density resulting from the Bravo test was estimated to be 21 kBq m−2 with an uncertainty (GSD) of 1.5. Uncertainties in [Latin capital letter Q with macron above](131I, Bravo)/Dep(137Cs, Bravo) and [Latin capital letter Q with macron above](133I, Bravo)/Dep(137Cs, Bravo): Taking 131I as an example, [Latin capital letter Q with macron above](131I, Bravo)/Dep(137Cs, Bravo) is, in fact, the product of two terms:
| (16) |
The first term is derived from the tables provided in Hicks (1984) for discrete times of fallout. It is assumed that the deposition ratios have relatively small error (Hicks 1982) and, thus, that the uncertainty in the first term is due primarily to the uncertainty in the TOI estimate of 31 h for Utrik after the Bravo detonation. Estimates of TOI that depend on TOA clearly influence the estimates of intake for short-lived radionuclides due to differences in physical decay. In a simple analysis where TOA (h) was allowed to take on values of the best estimate minus 20% and the best estimate plus 20%, we compared the organ doses at the four atolls discussed. We found that organ doses from acute intakes were 7% to 25% greater at the earlier TOAs (best estimate minus 20%) compared to the best estimates, depending on the organ and population group. Conversely, we found that organ doses were 7% to 17% lower at longer TOAs (best estimate plus 20%) compared to the best estimates depending on the organ and population group. Table 22 presents a summary of the outcome of these calculations and leads to the general conclusion that errors in TOA or TOI potentially lead to errors in dose that are, for the most part, less than ±25% and, more often than not, about ±15%. Considering that the overall uncertainty in internal doses is characterized by a GSD of at least 2, the uncertainty in TOI and, thus, in the first term, is a small component of the overall uncertainty and can be ignored for practical reasons.
Table 22.
Sensitivity of organ doses (mGy) to assumptions in time-of-arrival (TOA). Estimated doses are for adults exposed to Bravo fallout at four atolls and at three TOAs: (i) best estimate (BE) of TOA (Beck et al. 2010), (ii) best estimate of TOA minus 20%, and (iii) best estimate of TOA plus 20%.
| Atoll population | TOA (h) | Organ dose, mGy (% difference from dose based on best estimate TOA) |
|||
|---|---|---|---|---|---|
| RBM | Thyroid | Stomach | Colon | ||
| Majuro residents | 38 (BE −20%) | 0.12 (11.8) | 26 (17.9) | 0.40 (23.6) | 5.2 (18.7) |
| Majuro residents | 48 (BE) | 0.11 (0.0) | 22 (0) | 0.32 (0) | 4.4 (0) |
| Majuro residents | 58 (BE +20%) | 0.10 (−9.4) | 20 (−12.1) | 0.27 (−16.6) | 3.7 (−14.4) |
| Kwajalein residents | 32 (BE −20%) | 0.27 (10.0) | 77 (17.5) | 1.4 (25.2) | 14 (14.5) |
| Kwajalein residents | 40 (BE) | 0.25 (0) | 66 (0) | 1.1 (0) | 12 (0) |
| Kwajalein residents | 48 (BE +20%) | 0.23 (−7.5) | 58 (−12.6) | 0.92 (−16.0) | 1 1 (−10.8) |
| Utrik community | 17.6 (BE −20%) | 2.5 (9.7) | 880 (18.9) | 21 (27.2) | 200 (12.6) |
| Utrik community | 22 (BE) | 2.3 (0) | 740 (0) | 16 (0) | 180 (0) |
| Utrik community | 26 (BE +20%) | 2.1 (−7.1) | 630 (−13.9) | 14 (−16.1) | 160 (−9.1) |
| Rongelap Island community | 4.8 (BE −20%) | 27 (9.7) | 8,100 (6.9) | 660 (23.8) | 3,100 (10.7) |
| Rongelap Island community | 6.0 (BE) | 25 (0) | 7,600 (0) | 530 (0) | 2,800 (0) |
| Rongelap Island community | 7.2 (BE +20%) | 23 (−7.0) | 7.100 (−5.9) | 440 (−17.0) | 2,500 (−8.5) |
The uncertainty in the second term, [Latin capital letter Q with macron above]/Dep, depends on the validity of the assumption that the ratio of the acute intake and of the deposition density of 131I at Utrik for a TOI of 31 h is the same as the ratio that would have been obtained at Rongelap for the same TOI value. Because there are no measurements of 131I from which the intake of the members of the Utrik community can be readily derived, the uncertainty in the best estimate for [Latin capital letter Q with macron above]/Dep at Utrik is greater than that at Rongelap. The fact that, in the case of chronic intakes, the ratios of intake to deposition of 137Cs differ by a factor of about 3 between the two atolls (see Table 4) suggests that there are uncertainties of an unknown nature that should be accounted for. It is worth keeping in mind that the pathways leading to acute intake have not been quantitatively described in an adequate way and that it is assumed that ingestion of fallout deposited on the skin, as well as on cooking utensils and foodstuffs, was the predominant source of internal contamination. The contribution from inhalation is assumed minor, as, based on the meteorological modeling described in a companion paper (Moroz et al. 2010), the particle sizes of fallout from Bravo at Rongelap and at Utrik were very large (>>20 μm). Because the atmospheric conditions, the physical and chemical characteristics of the fallout, and the lifestyle and dietary habits of the populations were similar at Utrik and at Rongelap, the GSD for [Latin capital letter Q with macron above](131I, Bravo)/Dep(131I, Bravo) at Utrik was taken to be 2.0, which is not much greater than the value of 1.6 that was determined for the GSD of [Latin capital letter Q with macron above](131I, Bravo)/C(131I, Bravo) for Rongelap. The same value was used for 133I, which is strongly correlated with 131I. Finally, the uncertainties in the doses per unit intake, D/[Latin capital letter Q with macron above], at Utrik were taken to be characterized by a GSD of 1.4, which is slightly greater than the value of 1.2 at Rongelap; this is due to the fact that there were no measurements of 131I in urine among the members of the Utrik community.
The combination of the assigned uncertainties to the components of the thyroid dose to representative adults of the Utrik community, due to intakes of 131I and 133I from the Bravo test, results in an overall uncertainty (GSD) of 2.4.
As was the case for the members of the Rongelap Island community, the other components of the thyroid dose received in 1954 by members of the Utrik community (acute intakes of other radionuclides, chronic intakes of long-lived radionuclides, acute and chronic intakes from tests other than Bravo) are small, but much more uncertain. Even though these additional components have little influence on the overall uncertainty, the GSD of 2.4 estimated for the thyroid dose from Bravo was modestly increased to 2.5 to represent the uncertainty in the thyroid dose received in 1954 by members of the Utrik community. Again, as for the northern atolls, we assume that the uncertainty in the dose to other organs is comparable to that we estimate for the thyroid. We also assume the same uncertainties for years other than 1954 since, even though the uncertainty in deposition varies from test to test, the uncertainty in [Latin capital letter Q with macron above]/D should not have differed significantly.
Population groups in the mid-latitudes and in the southern latitudes
In the mid-latitudes and in the southern latitudes, the internal doses are much smaller than those for the Rongelap Island and the Utrik communities and the test with the largest contribution to the doses was not Bravo, but rather Romeo, Koon, or Yankee, all of which took place in 1954. Our estimate of uncertainty in internal doses to residents of mid-latitude and southern latitude atolls is again based on the estimated uncertainty in the acute thyroid dose due to intakes of 131I from a particular test in a specific atoll, in this case, from the Romeo test by an adult representative of the Majuro residents.
The thyroid dose to adults from acute intake of 131I from Romeo can be expressed as:
| (17) |
Uncertainties in Dep(137Cs): The 137Cs deposition density resulting from the Romeo test was estimated to be 0.7 kBq m−2 (Beck et al. 2010) with an uncertainty (GSD) of 1.3. Uncertainties in Dep(131I, Romeo)/Dep(137Cs, Romeo): As discussed above for Utrik, the uncertainty in this term is a minor contributor to the overall uncertainty and, thus, can be neglected.
Uncertainties in [Latin capital letter Q with macron above](131I, Romeo)/Dep(131I, Romeo): The uncertainty in [Latin capital letter Q with macron above]/Dep depends on the validity of the assumption that the ratio of the acute intake and of the deposition density of 131I at Majuro for a TOI of 140 h is the same as the ratio that would have been obtained at Rongelap for the same TOI value. Because there were no bioassay measurements of 131I from which the intakes of the Majuro residents can be readily derived, the uncertainty in the best estimate for [Latin capital letter Q with macron above]/Dep at Majuro is clearly greater than that at Rongelap or Utrik. Deposition would have continued for much longer times and been likely influenced by both wet- and dry-deposition processes. Also, for these distant atolls, fallout particles would be considerably smaller, although still, based on the meteorological modeling described in Moroz et al. (2010), generally >10–15 μm in diameter. Thus, there is considerable uncertainty about the magnitude and pathway of the intakes following individual tests. It is likely that, for some tests, much of the fallout took place during the frequent occurrences of heavy rainfall in the south. Consequently, the skin of the residents, as well as the cooking utensils and the foodstuffs, were probably not contaminated to the degree that may have occurred from dry fallout of very large particles at Rongelap. Inhalation doses would, thus, still likely be relatively minor compared to ingestion, particularly when the fallout occurred during rain. Thus, the GSD for [Latin capital letter Q with macron above](131I, Romeo)/Dep(131I, Romeo) at Majuro was taken to be 2.5, which is substantially greater than the values of 1.6 and 2.0 that were determined for the GSD of [Latin capital letter Q with macron above](131I, Bravo)/C(131I, Bravo) for Rongelap and Utrik, respectively. Finally, the uncertainties in the doses per unit intake, D/[Latin capital letter Q with macron above], at Majuro were taken to have the same value of GSD (1.4) as for Utrik.
The combination of the assigned uncertainties to the components of the thyroid dose to representative adults at Majuro due to intakes of 131I from the Romeo test results in an overall uncertainty (GSD) of 2.7. However, contrary to the situation at Rongelap and Utrik, more than one test contributed substantially to the 1954 thyroid dose. The tests Koon and Bravo contributed about as much as Romeo, while Union and Yankee accounted for much smaller 131I intakes (see Table 9, Simon et al. 2010). Because the uncertainty assigned to the deposition of 137Cs from the Romeo test at Majuro was relatively low (GSD = 1.3), the choice of another test could have resulted in an overall uncertainty (GSD) greater than 2.7. For example, an overall uncertainty of 2.9 would have been obtained for Koon, as the uncertainty in the 137Cs deposition density for Koon at Majuro (GSD = 1.5) is greater than that for Romeo. For Yankee, with an even higher uncertainty in the 137Cs deposition density (GSD = 1.8), the overall uncertainty is estimated to be characterized by a GSD of 3.1. For that reason, we assumed that the uncertainty in the thyroid dose received in 1954 by representative persons of the Majuro population and of the populations of other atolls of the mid-latitude and southern regions had the same value (GSD = 3.0), somewhat higher than our estimate for the uncertainty in the thyroid dose from Romeo (GSD of 2.7). Again, as for Rongelap and Utrik, we assume that the uncertainty in the dose to other organs is comparable to that we estimate for the thyroid. Finally, we also assume the same uncertainties for years other than 1954.
In summary, we crudely estimated uncertainties of the population-average age-specific annual doses from internal irradiation that were received from 1948 to 1970 by lognormal probability distributions with GSDs of 2.0 for the population groups of the northern latitudes, 2.5 for the Utrik Community, and 3.0 for the population groups of the mid-latitudes and southern latitudes. Though all dose estimates we have presented for the Marshallese are uncertain, the models and estimation procedures were developed without knowledge of any specific systematic biases that could be corrected.
CONCLUSION
The methods developed in this work and the related dosimetry calculations provide a full accounting and disclosure of the doses received by the Marshallese from regional nuclear testing within the limits of the data known to us. The importance of the bioassay-based approach is clear here, but stands in contrast to methods often used for dose reconstructions relevant to continental nuclear tests sites where intake of fallout activity by the public is usually a consequence of ingestion of contaminated dairy foods (Health Physics 1990) and where suitable and well-characterized pathway models can be used for dose estimation. The pathways leading to acute and chronic intakes by the Marshallese were primarily ingestion of contamination on face and hands, plates, cooking utensils, and foods drying outdoors, and probably less importantly, consumption of contaminated water, and over the long-term, consumption of locally grown fruits. Because there are no pathway models for this lifestyle that have been suitably quantified, the urinary excretion data of Harris (1954) obtained from the highly exposed populations, and, in later years, the whole-body counting data summarized by Lessard et al. (1984, 1985), were of particularly great value to estimating doses.
There were several unusual and interesting aspects of the exposures in the Marshall Islands in addition to the absence of well-known and well-understood exposure pathways. One unusual circumstance was the very limited access to fresh water prior to Bravo exposure. Coupled with a tropical environment that typically leads to significant losses of water through the skin, urine volumes that were obtained for bioassay were smaller than in most temperate climate collections on which radioactivity measurements have been based. In addition, analyses of the contamination from Bravo and the intakes of the highly exposed Marshallese indicated that particles in the environment were large (tens to hundreds of microns in size) and that the large acute intakes were a result, almost exclusively, of ingestion, while inhalation played only a very minor role (Harris 1954; Lessard et al. 1985). In contrast, chronic intakes at more distant atolls, resulting in much lower protracted doses, arise from dietary intakes that occur through ingestion of fruits and crops contaminated by root uptake and the consumption of fish (Robison and Sun 1997). Internal contamination of fruits with 137Cs, e.g., coconuts, whose juice is a common water replacement for native residents, is higher (per unit soil concentration) than in almost every continental location because coral-based soils of the atolls are highly deficient in potassium.
In addition, at least one important conclusion emerged from our analysis of doses on a test-specific basis. For many years, the Bravo test has been assumed to have been the single most important test for all atolls from the point-of-view of exposure. While this is clearly the case for the northern atolls, it is not the case for the mid-latitude and southern latitude atolls (see Table 9, Simon et al. 2010). As a basis for comparison, the proportions of the thyroid dose contributed by Bravo at Rongelap, Utrik, Kwajalein, and Majuro were >99%, 93%, 4.7%, and 24%, respectively. In contrast, among the mid-latitude atolls (Kwajalein and others), the Yankee test was the most important. The contributions from Yankee to the thyroid dose at Rongelap, Utrik, Kwajalein, and Majuro were about <<1%, 3.5%, 37%, and 2.4%. Among the southern atolls, the Koon test was the most important contributor to thyroid dose. The contributions to the thyroid dose from Koon at Rongelap, Utrik, Kwajalein, and Majuro were about 0.2%, 2%, 19%, and 28%, respectively.
One over-arching finding from our dose assessment was a distinctive geographic pattern of internal doses received by residents of the atolls, which, as discussed in Simon et al. (2010), was the same as for deposition, external dose, and projected cancer risk. Our data (see Fig. 2, Simon et al. 2010) clearly illustrate an overall decreasing trend in internal doses received from more northern latitude atolls to southern latitude atolls (see Table 5 of Simon et al. 2010). Moreover, we found that our best estimates of internal dose varied less than two-fold within the southern atolls and within the mid-latitude atolls, suggesting that the doses within each of those groups of atolls were relatively consistent.
Uncertainty in estimated doses has been assessed based on some simplifications, and while uncertainties are relatively large, as expected, our estimates of intakes of fallout radionuclides by the Marshallese, and their related doses, contain no known biases that require correction or that might unduly influence the estimates of cancer risk provided by Land et al. (2010). Our estimates of radiation dose to the Marshallese living on all inhabited atolls should add considerably to our understanding of the cancer risks to the Marshallese from nuclear testing at Bikini and Enewetak during the years 1946–1958. In addition, these estimates of intakes and related doses add to our understanding in more general ways about the consequences of exposure to radioactive fallout from nuclear detonations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intra-Agency Agreement between the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, NIAID agreement #Y2-Al-5077 and NCI agreement #Y3-CO-5117.
The authors are indebted to several individuals whose analyses and research have made substantial contributions to this work. They include Payne Harris, who collected and analyzed the original urine samples of the exposed Marshallese, William Robison for various data and publications on radionuclide measurements made in the Marshall Islands, Shawki Ibrahim for analysis of f1 data on fallout radionuclides, Brian Moroz for meteorological analysis and graphic support, Jack Robbins (deceased) for discussions about thyroid exposures and tissue damage seen among the Marshallese, as well as other scientists who have added to our understanding of the contamination and consequences in the Marshall Islands through their scientific publications, many of which are cited here.
REFERENCES
- Apostoaei AI, Miller LF. Uncertainties in dose coefficients from ingestion of 131I, 137Cs, and 90Sr. Health Phys. 2004;86:460–482. doi: 10.1097/00004032-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Beck HL, Bouville A, Moroz BE, Simon SL. Fallout deposition in the Marshall Islands from Bikini and Enewetak nuclear weapons tests. Health Phys. 2010;99:124–142. doi: 10.1097/HP.0b013e3181bbbfbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli L, Melo DR, Lipsztein J, Cruz-Suarez R. AIDE: internal dosimetry software. Radiat Protect Dosim. 2008;130:358–367. doi: 10.1093/rpd/ncn059. [DOI] [PubMed] [Google Scholar]
- Bevington PR. Data reduction and error analysis for the physical sciences. McGraw Hill; New York: 1969. [Google Scholar]
- Bouville A, Beck HL, Simon SL. Doses from external irradiation to Marshall Islanders from nuclear weapons testing. Health Phys. 2010;99:143–156. doi: 10.1097/HP.0b013e3181dc521d. [DOI] [PubMed] [Google Scholar]
- Chilean Iodine Educational Bureau . Iodine content of foods. Lange, Maxwell & Springer; London: 1952. [Google Scholar]
- Comroe JH. Physiology of perspiration. Year Book Medical Publishers; Chicago: 1965. [Google Scholar]
- Cronkite EP, Conard RA, Bond VP. Historical events associated with fallout from BRAVO Shot—Operation Castle and 25 years of medical findings. Health Phys. 1997;73:176–186. doi: 10.1097/00004032-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Division of Cancer Epidemiology and Genetics. National Cancer Institute. National Institutes of Health . Estimation of the baseline number of cancers among Marshallese and the number of cancers attributable to exposure to fallout from nuclear weapons testing conducted in the Marshall Islands. NCI; Bethesda, MD: 2004. [Google Scholar]
- Donaldson LR, Seymour AH, Nevissi AH. University of Washington’s radioecological studies in the Marshall Islands, 1946-1977. Health Phy. 1997;73:214–222. doi: 10.1097/00004032-199707000-00018. [DOI] [PubMed] [Google Scholar]
- Dore C, Weiner JS, Wheeler EF, El-Neil H. Water balance and body weight: studies in a tropical climate. Annals Human Biol. 1975;2:25–33. doi: 10.1080/03014467500000531. [DOI] [PubMed] [Google Scholar]
- Dosios T, Billis A, Skalkeas G. Evaporative water loss of adult surgical patients in Greece. The Am J Surgery. 1974;128:15–18. doi: 10.1016/0002-9610(74)90227-x. [DOI] [PubMed] [Google Scholar]
- Dunning DE, Jr, Schwarz G. Variability of human thyroid characteristics and estimates of dose from ingested I-131. Health Phys. 1981;40:661–675. doi: 10.1097/00004032-198105000-00005. [DOI] [PubMed] [Google Scholar]
- Eckerman KF, Leggett RW, Cristy M, Nelson CB, Ryman JC, Sjoreen AL, Ward RC. Oak Ridge National Laboratory; Oak Ridge, TN: 2006. Dose and risk calculation software (DCAL) ORNL/TM-2001/190. [Google Scholar]
- Elebute EA. Determinants of evaporative fluid loss in a tropical environment. Bulletin de la Societé Internationale de Chirurge (Surgery) 1973;2:2–6-213. [PubMed] [Google Scholar]
- Food and Agriculture Organization . Leaflet No. 8: Coconut [online] South Pacific Commission; Noumea, New Calcedonia: [Accessed May 2010]. 1983. Available at: http://www.fao.org/WAIRdocs/x5425e/x5425e08.htm. [Google Scholar]
- Fuge R, Johnson CJ. The geochemistry of iodine: a review. Environ Geochem and Health. 1986;8:31–54. doi: 10.1007/BF02311063. [DOI] [PubMed] [Google Scholar]
- Glasstone S, Dolan PJ. The effects of nuclear weapons. United States Department of Defense and Energy Research and Development Administration; Washington, DC: 1977. [Google Scholar]
- Goetz J, Klemm J, Phillips J, Thomas C. Defense Nuclear Agency; Washington, DC: 1987. Analysis of radiation exposure—service personnel on Rongerik Atoll, Operation Castle—Shot Bravo. SAIC-86/1608. [Google Scholar]
- Haldimann M, Alt A, Blanc A, Blondeau K. Iodine content of food groups. J Food Composition Analysis. 2005;18:461–471. [Google Scholar]
- Harris PS. Memo to Atomic Energy Commission. Los Alamos Scientific Laboratory; Los Alamos, NM: 1954. Summary of the results of urine analysis on Rongelap natives Americans and Japanese fisherman to date. [Google Scholar]
- Harris PS, Simon SL, Ibrahim SA. Urinary excretion of radionuclides from Marshallese exposed to fallout from the Bravo nuclear test. Health Phys. 2010;99:217–232. doi: 10.1097/HP.0b013e3181dc50a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MT, McFarlane S, Harden RM, Wayne E. Nature and availability of iodine in fish. Am J Clinical Nutrition. 1965;17:73–33. doi: 10.1093/ajcn/17.2.73. [DOI] [PubMed] [Google Scholar]
- Health Physics Special issue: Evaluation of environmental radiation exposures from nuclear testing in Nevada. Health Phys. 1990;59(5) [PubMed] [Google Scholar]
- Hicks HG. Rates from fallout and the related radionuclide composition. Lawrence Livermore National Laboratory; Livermore, CA: 1981. Report UCRL-53152, Parts 1-7. [Google Scholar]
- Hicks HG. Calculation of the concentration of any radionuclide deposited on the ground by offsite fallout from a nuclear detonation. Health Phys. 1982;42:585–600. doi: 10.1097/00004032-198205000-00003. [DOI] [PubMed] [Google Scholar]
- Hicks HG. Results of calculations of external gamma radiation exposure rates from local fallout and the related radionuclide compositions of selected U.S. Pacific events. Lawrence Livermore National Laboratory; Livermore, CA: 1984. UCRL-53505. [Google Scholar]
- Ibrahim SA, Simon SL, Bouville A, Melo D, Beck HL. Alimentary tract absorption (f1 values) for radionuclides in local and regional fallout from nuclear tests. Health Phys. 2010;99:233–251. doi: 10.1097/HP.0b013e3181b186ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Commission on Radiological Protection . Agedependent doses from intakes of radionuclides. 2. Vol. 20. Pergamon Press; Oxford: 1990. ICRP Publication 56, Ann ICRP. [Google Scholar]
- International Commission on Radiological Protection . Age dependent doses to members of the public from intake of radionuclides: Part 2 ingestion dose coefficients. Elsevier; New York: 1993. ICRP Publication 67. [PubMed] [Google Scholar]
- International Commission on Radiological Protection . Doses to members of the public from intake of radionuclides: Part 5 Compilation of ingestion and inhalation dose coefficients. Elsevier; New York: 1996. ICRP Publication 72. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection . Doses to the embryo and fetus from intakes of radionuclides by the mother. 103. Vol. 31. Elsevier; New York: 2001. ICRP Publication 88, Ann ICRP. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection . Basic anatomical and physiological data for use in radiological protection: reference values. Elsevier; New York: 2002. ICRP Publication 89. [Google Scholar]
- International Commission on Radiological Protection . Doses to infants from radionuclides ingested in mother’s milk. Elsevier; New York: 2004. ICRP Publication 95. [Google Scholar]
- James RA. Estimate of radiation dose to thyroids of the Rongelap children following the BRAVO event. Lawrence Radiation Laboratory; Livermore, CA: 1964. UCRL-12273. [Google Scholar]
- Johnson LR. Essential medical physiology. Lippincott Williams & Wilkins; Baltimore: 1998. [Google Scholar]
- Kuno Y. Human perspiration. Thomas; Springfield, IL: 1956. [Google Scholar]
- Land CE, Bouville A, Apostoaei I, Simon SL. Projected lifetime cancer risks from exposure to regional radioactive fallout in the Marshall Islands. Health Phys. 2010;99:201–215. doi: 10.1097/HP.0b013e3181dc4e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham MC. Human nutrition in the developing world. Food and Agriculture Organization of the United Nations; Rome: 1997. FAO Food and Nutrition Series, No. 29. [Google Scholar]
- Leithead CS, Pallister MA. Observations on dehydration and sweating. The Lancet. 1960;2:114–117. doi: 10.1016/s0140-6736(60)91263-0. [DOI] [PubMed] [Google Scholar]
- Lessard ET, Miltenburger RP, Cohn SH, Musolino SV, Conard RA. Protracted exposure to fallout: the Rongelap and Utirik experience. Health Phys. 1984;46:511–527. doi: 10.1097/00004032-198403000-00002. [DOI] [PubMed] [Google Scholar]
- Lessard E, Miltenberger R, Conard R, Musolino S, Naidu J, Moorthy A, Schopfer C. Thyroid absorbed dose for people at Rongelap, Utrik and Sifo on March 1, 1954. Brookhaven National Laboratory, Safety and Environmental Protection Division; Upton, NY: 1985. BNL 51882. [Google Scholar]
- Levy SJ, Taylor R, Higgins IL, Grafton-Wasserman DA. Fertility and contraception in the Marshall Islands. Studies Family Planning. 1988;19:179–185. [PubMed] [Google Scholar]
- Mao IF, Ko YC, Chen ML. The stability of iodine in human sweat. Japanese J Physiol. 1990;40:693–700. doi: 10.2170/jjphysiol.40.693. [DOI] [PubMed] [Google Scholar]
- Mao IF, Chen ML, Ko YC. Electrolyte loss in sweat and iodine deficiency in a hot environment. Archives of Environmental Health. 2001;56:271–277. doi: 10.1080/00039890109604453. [DOI] [PubMed] [Google Scholar]
- Moroz BE, Beck HL, Bouville A, Simon SL. Predictions of dispersion and deposition of fallout using the NOAAHYSPLIT meteorological model. Health Phys. 2010;99:252–269. doi: 10.1097/HP.0b013e3181b43697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy Press, National Academy of Sciences . Predicting decrements in military performance due to inadequate nutrition. The National Academies Press; Washington, DC: 1986. [Google Scholar]
- National Academy Press, National Academy of Sciences . Personal needs in hot environments: applications for military personnel in field operations. The National Academies Press; Washington, DC: 1993. [PubMed] [Google Scholar]
- National Academy Press, National Academy of Sciences . Radiological assessments for resettlement of Rongelap in the Republic of the Marshall Islands. The National Academies Press; Washington, DC: 1994. [PubMed] [Google Scholar]
- National Cancer Institute . Estimated exposures and thyroid doses received by the American people from Iodine-131 in fallout following the Nevada atmospheric nuclear bomb tests (U.S. Department of Health and Human Services, Washington) NCI; Bethesda, MD: 1997. [Google Scholar]
- National Council on Radiological Protection and Measurements . Recommended screening levels for contaminated surface soil and review of factors relevant to site-specific studies. NCRP; Bethesda, MD: 1999. NCRP Report 129. [Google Scholar]
- Noshkin VE, Robison WL, Wong KM. Concentration of Po-210 and Pb-210 in the diet at the Marshall Islands. Sci Total Environ. 1994;155:87–104. doi: 10.1016/0048-9697(94)90364-6. [DOI] [PubMed] [Google Scholar]
- Noshkin VE, Robison WL, Wong KM, Brunk JL, Eagle R, Jones HE. Past and present levels of some radionuclides in fish from Bikini and Enewetak Atolls. Health Phys. 1997;73:49–65. doi: 10.1097/00004032-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Pennington JA, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of core foods of the U.S. food supply 1982-1991. J Food Composition Analysis. 1995;8:171–217. [Google Scholar]
- Quinn VE. Analysis of meteorological and radiological data for selected fallout episodes. Health Phys. 1990;59:577–592. doi: 10.1097/00004032-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Rall JE, Conard RA. Elevation in the serum protein-bound iodine level in inhabitants of the Marshall Islands. Am J Med. 1966;40:883–886. doi: 10.1016/0002-9343(66)90203-8. [DOI] [PubMed] [Google Scholar]
- Riggs DS. Quantitative aspects of iodine metabolism in man. Pharmacol Rev. 1952;4:284–370. [PubMed] [Google Scholar]
- Robison WR, Sun C. The use of comparative 137Cs body burden estimates from environmental data/models and whole body counting to evaluate diet models for the ingestion pathway. Health Phys. 1997;73:152–166. doi: 10.1097/00004032-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Robison WL, Mount ME, Phillips WA, Conrado C, Stuart ML, Stoker CE. The Northern Marshall Islands radiological survey: terrestrial food chain and total doses. Pt 4. Lawrence Livermore National Laboratory; Livermore, CA: 1982. UCRL-52853. [Google Scholar]
- Robison WL, Phillips WA. Estimates of the radiological dose from ingestion of 137Cs and 90Sr to infants, children, and adults in the Marshall Islands. Lawrence Livermore National Laboratory; Livermore, CA: 1989. UCRL-53917. [Google Scholar]
- Sharp R, Chapman WH. Report to the scientific director: Exposure of Marshall Islanders and American military personnel to fallout, WT-938, Operation Castle—Project 4.1 Addendum. Naval Medical Research Institute; Bethesda, MD: 1957. Extract version prepared for Defense Nuclear Agency; 1980. [Google Scholar]
- Simon SL. A brief history of people and events related to atomic weapons testing in the Marshall Islands. Health Phys. 1997;73:5–20. doi: 10.1097/00004032-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Simon SL. Soil ingestion by humans: a review of data, history, and etiology with application to risk assessment of radioactively contaminated soil. Health Phys. 1998;74:647–672. doi: 10.1097/00004032-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Simon SL, Graham JC. Findings of the first comprehensive radiological monitoring program of the Republic of the Marshall Islands. Health Phys. 1997;64:66–85. doi: 10.1097/00004032-199707000-00006. [DOI] [PubMed] [Google Scholar]
- Simon SL, Vetter RJ. Consequences of nuclear testing in the Marshall Islands. Health Phys. 1997;73:66–85. [Google Scholar]
- Simon SL, Borchert A, Graham JC. Concentrations and spatial distribution of plutonium in the terrestrial environment of the Republic of the Marshall Islands. Science Total Environment. 1999;229(1-2):21–39. [Google Scholar]
- Simon SL, Graham JC, Terp SD. Uptake of 40K and 137Cs in native plants of the Marshall Islands. J Environmental Radioact. 2002;59:223–243. doi: 10.1016/s0265-931x(01)00138-2. [DOI] [PubMed] [Google Scholar]
- Simon SL, Bouville A, Land CE, Beck HL. Radiation doses and cancer risks in the Marshall Islands associated with exposure to radioactive fallout from Bikini and Enewetak nuclear weapons tests: summary. Health Phys. 2010;99:105–123. doi: 10.1097/HP.0b013e3181dc523c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stather JW, Greenhalgh JR. The metabolism of iodine in children and adults. National Radiological Protection Board; Chilton, Didcot, UK: 1983. NRPB-R140. [Google Scholar]
- Sun LC, Meinhold CB, Moorthy AR, Kaplan E, Baum JW. Assessment of plutonium exposure in the Enewetak population by urinalysis. Health Phys. 1997;73:127–132. doi: 10.1097/00004032-199707000-00010. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fujimori K, Simon SL, Bechtner E, Trott KR. Thyroid nodules, thyroid function and dietary iodine in the Marshall Islands. International J Epidemiol. 1999;28:742–749. doi: 10.1093/ije/28.4.742. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Trott KR, Fujimori K, Nakashima N, Ohtomo H, Minouk J, Schoemaker MJ, Simon SL. Thyroid disease in the Marshall Islands, findings from 10 years of study. Tohoku University Press; Sendai, Japan: 2001. [Google Scholar]
- U.S. Atomic Energy Commission . A report of the Marhallese and Americans accidentally exposed to radiation from fallout and a discussion of radiation injury in the human being. U.S. AEC; Washington, DC: 1956. Some effects of ionizing radiation on human beings. TID 5358. [Google Scholar]
- Varo P, Saari E, Paaso A, Koivistoinen P. Iodine in Finnish foods. International J Vit Nutr Res. 1982;52:80–89. [PubMed] [Google Scholar]
- Welander AD. Radiobiological studies of the fish collected at Rongelap and Ailinginae atolls, July 1957. United States Atomic Energy Commission; Washington, DC: 1958. Report UWFL-55. [Google Scholar]
- Wenlock RW, Buss DH, Moxon RE, Bunton NG. Trace nutrients, iodine in British food. British J Nutrition. 1982;47:381–390. doi: 10.1079/bjn19820049. [DOI] [PubMed] [Google Scholar]
- Woodward KT, Schrodt AG, Anderson JE, Claypool HA, Hartgering JB. The determination of internally deposited radioactive isotopes in the Marshallese people by excretion analysis. Division of Nuclear Medicine, Walter Reed Army Institute of Research; Washington, DC: 1959. [Google Scholar]
- World Health Organization . Draft report: Water requirements, impinging factors, and recommended intakes [online] WHO; Geneva: [Accessed 2008]. 2004. Available at: http://www.who.int/water_sanitation_health/dwq/nutwaterrequir.pdf. [Google Scholar]
- World Health Organization [Accessed 2 June 2010];The WHO global data bank on breastfeeding and complementary feeding [online] 2009 Available at: http://apps.who.int/research/iycf/bfcf/
- Wright S. Applied physiology. Oxford University; London: 1956. [Google Scholar]
- Zvonova IA. Dietary intake of stable iodine and some aspects of radioiodine dosimetry. Health Phys. 1989;57:471–475. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.