Abstract
Purpose of review
Sjögren syndrome (SS) is a chronic autoimmune disease affecting lacrimal and salivary glands and potentially involving extraglandular manifestations. While common in adults, the prevalence and prognosis of childhood SS is unknown, in part due to lack of child-specific diagnostic and classification criteria. This review discusses difficulties in diagnosing childhood SS and highlights recent findings in SS treatment and pathogenesis from studies in adults and animal models over the past 18 months.
Recent findings
Studies of rituximab show some therapeutic potential in adult SS while newer modalities including gene therapy and mesenchymal stem cell transfer are promising. The pathogenesis of SS is emerging, including roles of T and B lymphocytes, autoantibodies, interferons, and glandular epithelial cells. Specific recent notable findings in SS pathogenesis include identification of a type II interferon signature in salivary glands of SS patients, characterization of salivary gland-infiltrating T cell subsets, and characterization of anti-muscarinic acetylcholine receptor type 3 autoantibodies.
Summary
Childhood SS is a poorly defined and underdiagnosed autoimmune disease which requires child-specific criteria in order to study disease burden and prognosis. Studies in adults and animal models continue to elucidate new potential diagnostic and therapeutic targets, which may be relevant for childhood SS.
Keywords: Sjögren syndrome, children, animal models, diagnosis, treatment, pathogenesis
INTRODUCTION
Sjögren syndrome (SS) is a complex autoimmune disease characterized by inflammation of the lacrimal and salivary glands leading to keratoconjunctivitis sicca and xerostomia with up to half of affected adults developing additional extraglandular manifestations. SS is one of the most common autoimmune rheumatic diseases in adults; however, the prevalence in children is unknown owing to the lack of child-specific diagnostic or classification criteria [1, 2]. Over the past 18 months, childhood SS case reports and series have been published highlighting the variable presentation of childhood SS, including recurrent parotitis [3] and renal manifestations [4]. No childhood SS-specific therapy or pathophysiology studies have been conducted. The purpose of this review is to discuss diagnostic issues in childhood SS and to highlight the latest findings in the treatment and pathogenesis of SS based on studies in adults and animal models.
DEFINING CHILDHOOD SS
No adequate childhood SS-specific criteria exist [1, 2]. The Sjögren’s International Collaborative Clinical Alliance (SICCA) recently established classification criteria based on the serologic, salivary histopathologic, and ocular findings commonly detected in adults with SS [5]. Classification of Sjögren syndrome requires at least two of the three criteria in individuals with signs/symptoms suggestive of Sjögren syndrome. Proposed SICCA classification criteria for Sjögren syndrome is as follows:
Positive serum anti-SSA/Ro and/or anti-SSB/La or (positive rheumatoid factor and antinuclear antibody titer at least 1:320).
Labial salivary gland biopsy exhibiting focal lymphocytic sialadenitis with focus score at least 1 focus of at least 50 mononuclear cells per 4mm2 of tissue.
Keratoconjunctivitis sicca with ocular staining score (based on degree of corneal or conjunctival changes noted with fluorescein or lissamine green staining, respectively) at least 3 (assuming no current daily eye drop use for glaucoma and no corneal surgery or cosmetic eyelid surgery in the last 5 years).
These criteria have not been evaluated in childhood SS, but undoubtedly child-specific modifications and/or further study is needed. The serologic criterion may be applicable to childhood SS; however, optimal cutoff for positive antinuclear antibody (ANA) titers in childhood SS needs formal evaluation. The definition of positive focus score (FS) in the histopathologic criterion requires child-specific modification. A focus is an aggregate of at least 50 mononuclear cells (Fig 1). In adults, a salivary gland FS ≥ 1 focus/4 mm2 is necessary to maintain high sensitivity and specificity for SS. In children, we have found that only 50% of childhood SS patients had FS ≥ 1 focus/4 mm2and the presence of at least 1 lymphocytic focus (i.e., FS > 0 foci/4 mm2) was highly sensitive and specific for childhood SS compared to non-SS controls (Yokogawa N, Lieberman SM, Alawi F, Bout-Tabaku S, Guttenberg M, Sherry DD, Vivino FB, unpublished data). Ocular staining scores (OSS) are not routinely reported in the clinical evaluation of children with suspected SS, thus prospective study of OSS in childhood SS is needed. In time, a better understanding of the pathogenesis of SS may provide objective and broadly applicable biomarkers for use in diagnosis. For now, however, we must recognize the limitations of applying adult SS criteria to children. Thus, both researching and managing children suspected of SS is severely hampered by the absence of valid, child-specific criteria.
FIGURE 1.
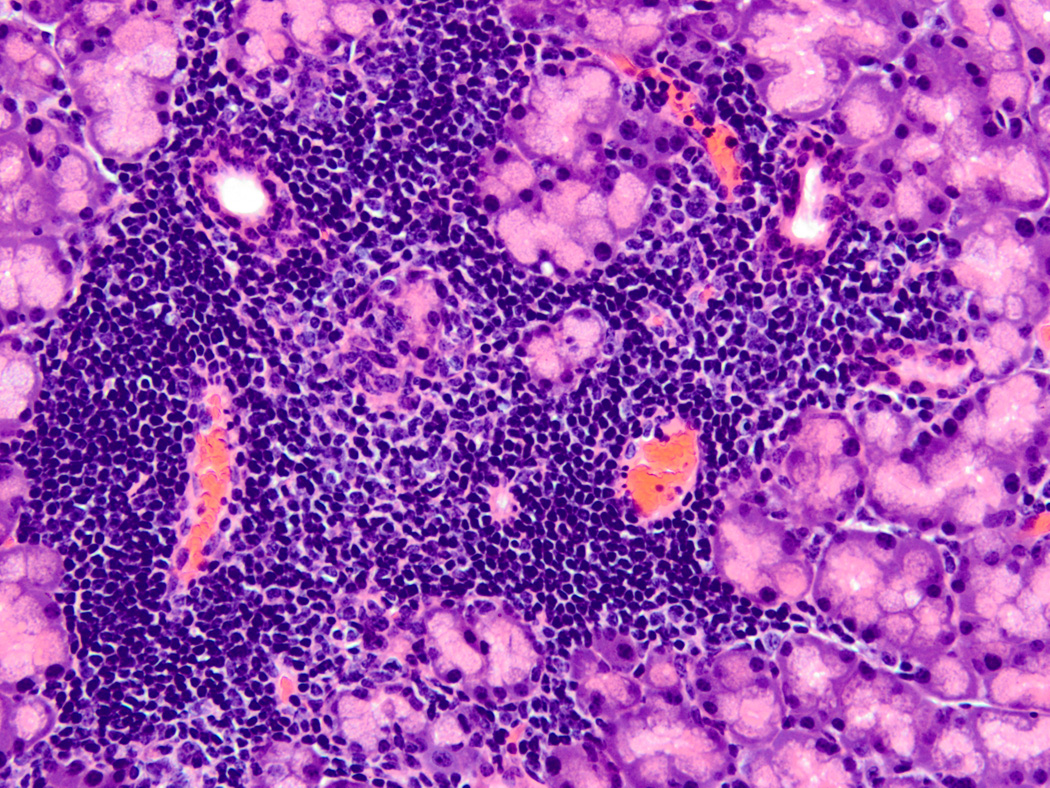
Typical SS focal lymphocytic infiltrate. Hematoxylin and eosin stained section from a formalin-fixed, paraffin embedded lacrimal gland of a 10 week old male nonobese diabetic (NOD) mouse (original magnification 200X). This image depicts a typical focal lymphocytic infiltrate in the periductal and perivascular distribution surrounded by normal acinar tissue. The ducts are present in the upper left and right of the focus, and the blood vessels are in the lower left and right. Similar focal infiltrates are also found in salivary glands of NOD and related mice as well as salivary and lacrimal glands of human SS patients. A focus is defined as a group of at least 50 mononuclear cells, and the focus score (FS) is defined by the number of these lymphocyte-dominant foci per 4 mm2 of tissue section.
TREATMENT
Treatment of SS is largely symptomatic, aimed at improving or replacing exocrine gland function, and includes the use of sialogogues and artificial tears with few systemic therapies demonstrating clear benefit in adults [6]. Systematic study of childhood SS pharmacotherapy has not been done. Treatment studies in adults and animal models published over the past 18 months are highlighted below.
Rituximab
In adults, the most widely studied systemic therapy for SS has been the B cell-depleting antibody rituximab. Rituximab therapy has shown some clinical benefit, specifically in systemic manifestations (arthritis, autoimmune cytopenias, and pulmonary disease) [7] and in global SS-specific disease activity indices: European League Against Rheumatism SS Patient Reported Index (ESSPRI) and Disease Activity Index (ESSDAI) [8]; although, rituximab therapy showed no clear benefit for central nervous system manifestations [9]. Another recent study demonstrated only a mild-moderate benefit of rituximab therapy, with improvement in patient-reported fatigue and oral dryness but no objective improvement in exocrine gland function [10*]. Peripheral B cell depletion was achieved in all rituximab treated patients, but no changes in anti- SSA/B or anti-muscarinic acetylcholine receptor type 3 (anti-M3R) autoantibodies were noted [10*]. While rituximab therapy depletes B cells in circulation, antibody-producing B cells persisted within parotid gland biopsies of SS patients with no change in specific clonal B cell populations after rituximab therapy, suggesting a possible mechanism for incomplete therapeutic response [11*].
Gene Therapy
The use of adenovirus (Ad) to transfer specific genes locally into salivary gland epithelial cells has shown promise in improving salivary gland function. Patients with radiation-induced salivary hypofunction treated with an adenoviral vector expressing human aquaporin 1 showed increased parotid flow and decreased subjective xerostomia [12*]. Studies in the C57BL/6.NOD-Aec1Aec2 mouse model of SS have demonstrated benefits of gene therapy with adeno-associated virus (AAV2) in delivering immunomodulatory proteins either locally or systemically. Administration of CTLA4IgG via retrograde instillation of AAV2-CTLA4IgG into salivary glands led to decreased sialadenitis and increased salivary gland function [13]. Low levels of CTLA4IgG were detected in serum and a trend toward improved lacrimal gland function was noted. Systemic treatment with IL27-expressing AAV2 vector after disease onset resulted in improved salivary gland function with little change in degree of sialadenitis but significantly decreased serum levels of the inflammatory cytokine interleukin (IL)-17 [14].
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are multipotent stem cells with immunomodulatory properties. Recent studies of MSC transfer in the nonobese diabetic (NOD) mouse model of SS showed significant benefit including preserved salivary flow and decreased sialadenitis [15, 16**]. Transfer of MSCs after disease onset also improved salivary gland inflammation and function [16**]. Moreover, this study demonstrated that MSCs from both SS prone NOD mice and human SS patients have defective immunoregulatory function, specifically resulting in decreased in vitro suppression of T cell proliferation, decreased induction of regulatory T cells, and decreased chemokine receptor CXCR4 expression suggesting defective MSC trafficking. Strikingly, treatment of SS patients with allogeneic MSCs resulted in clinical improvement including decreased ESSDAI, increased salivary flow, and improvement of hematologic abnormalities and autoimmune hepatitis. However, no improvements in central or peripheral nervous system disease were noted [16**].
PATHOGENESIS
SS is a lymphocyte-mediated disease characterized by focal lymphocytic infiltrates in salivary and lacrimal glands (Fig 1). Ongoing research is focused on two main themes: the role of lymphocyte subsets and their inflammatory products (e.g., cytokines, autoantibodies) in SS, and genomic and proteomic approaches to identifying new disease-specific biomarkers. Our understanding of the pathogenesis of SS is far from complete, and its continued study is essential to defining better diagnostic and therapeutic modalities.
Genetics and Biomarkers
The genetics of SS is complex and involves polymorphisms in immunoregulatory genes [17]. Newly reported associations of SS with the tumor necrosis factor (TNF)/lymphotoxin α/β locus [18] and the TNF-α promoter [19] suggest dysregulation of apoptosis and systemic inflammatory pathways. In search of SS-specific biomarkers, investigators have studied saliva and tears from SS patients and mouse models. Quantitative proteomic study of parotid saliva identified 529 proteins specific to either primary SS patients or healthy controls, including many proteins with immunologic functions [20]. In tear fluid, immune cell mRNA was detected in autoimmune dacryoadenitis prone mice [21].
The accessibility of salivary gland tissue has also facilitated the study of SS pathogenesis. Novel microRNAs were identified from minor salivary gland (MSG) biopsy specimens from SS patients, and one microRNA was specifically expressed at higher levels in patients with salivary gland dysfunction compared to both SS patients with normal salivary flow and healthy controls [22]. A recent systems analysis identified a highly correlated module of gene expression in salivary glands from SS patients and from C57BL/6.NOD-Aec1Aec2 mice, suggesting common mechanisms of immune dysregulation across mammalian species [23].
Role of Interferons
Members of the interferon (IFN) family of cytokines have variable immunologic effects, and have been associated with a variety of autoimmune diseases. They are divided into type I (IFN-α, -β, -ω), type II (IFN-γ), and type III (IFN-λ). Upregulation of genes induced by type I IFN has previously been shown in primary SS [24]. Recently, the type I IFN signature in peripheral blood monocytes from primary SS patients correlated with increased B cell activating factor expression and increased disease activity [25]. In C57BL/6.NOD-Aec1Aec2 mice intact type I IFN signaling is required for maximal exocrine gland inflammation and salivary gland dysfunction but may not be necessary for autoantibody production [26*]. Another recent study identified a type II IFN signature within MSG tissue of primary SS patients [27**]. Since there is substantial overlap in genes induced by type I and type II IFNs, this study identified genes upregulated specifically by IFN-γ or preferentially by IFN-α, and used the translated proteins as markers to determine specific contributions of type I and type II IFN in target tissues of autoimmune diseases. Including only two candidate proteins from each group, the authors were able to characterize tissue samples as having a type I, type II, or no IFN pattern. Notably, MSG biopsies from 7 of 8 SS patients demonstrated type II IFN-specific patterns, and the remaining SS patient demonstrated a type I IFN pattern [27**]. In contrast, 4 of 4 dermatomyositis muscle biopsy samples demonstrated a type I IFN pattern, and no IFN pattern was detected among the 9 healthy control MSG tissue specimens. This study identifies IFN-γ as a potential new therapeutic target and suggests that optimal treatments may eventually be tailored to SS patients based on their MSG biopsy IFN patterns.
Role of T Lymphocytes
SS is often considered a CD4+ T cell-mediated autoimmune disease with contributions from T helper (Th)1, Th2, and Th17 cells. Immunohistochemical analyses also suggest roles for other T cells, B cells, and innate immune cells. In detailed analyses of MSG specimens from SS patients, increased FS correlated with disease duration, higher B to T cell ratio, and higher CD4+ to CD8+ T cell ratio [28*]. While few differences in cytokine expression were detected between peripheral blood mononuclear cells (PBMC) of SS patients and controls, MSG biopsy specimens from SS patients showed increased Th1, Th2, and Th17 cytokine mRNA [28*]. The finding of differential Th2 cytokine expression was also noted in MSG (and saliva) but not PBMC from SS patients with high versus low FS [28*]. In another study, laser capture microdissection was used to analyze distinct foci from within SS patient MSG tissue. This study demonstrated increased Th1- and Th17-associated mRNA within lesions without germinal centers and increased Th2- and T follicular helper-associated mRNA in lesions with germinal centers [29]. These studies support the hypothesis that SS is mediated by initial T cell infiltration dominated by Th1 and Th17 cells, followed by Th2 and B cell dominant lesions which may progress to germinal center formation; however, whether these differences mark disease progression versus distinct independent mechanisms of disease is unknown. The role of germinal centers in the pathogenesis of SS is not clear, though induction of germinal center-like ectopic lymphoid structures in salivary glands of non-autoimmune prone mice resulted in development of SS-like disease suggesting a potentially pathogenic role [30]. The presence of germinal centers in adult MSG tissue is associated with increased risk of lymphoma development [31], although the relevance of this finding to SS pathogenesis remains unclear. In addition to classical T lymphocytes, recent identification of IL-17+ CD4 and CD8 double negative T cells in peripheral blood and MSG samples from SS patients was reported [32]. These double negative T cells were resistant to dexamethasone-mediated suppression of IL-17 production in vitro and, thus, may serve as a marker for corticosteroid-resistant disease. Overall, these studies underscore the importance of studying target organ tissue to provide disease-relevant information.
Ongoing characterization of the progressive pathogenesis of SS autoimmunity may provide new key therapeutic targets; however, the initiation of autoimmunity remains poorly understood. In humans, the earliest lesions can not be studied since these lesions occur months to years before the onset of symptoms or diagnosis. Thus, the study of animal models of SS-like autoimmunity may provide understanding of the earliest stages of disease. Deficiency in the key endoplasmic reticulum calcium sensors stromal interaction molecule (STIM)1 and STIM2 may play a key role. Mice with T cells that lack both STIM1 and 2 develop anti-SSA/B autoantibodies, sialadenitis, and salivary gland dysfunction resembling SS [33*]. Moreover, decreased STIM1 expression was noted in MSG-infiltrating lymphocytes and decreased STIM1 and STIM2 was noted in PBMCs of SS patients. Functionally, STIM-deficient PBMCs from SS patients released less calcium in response to in vitro stimulation suggesting altered T cell function, though specific relevance to human SS pathogenesis is unknown.
Role of B Lymphocytes and Autoantibodies
In addition to T cells, B cells are found in biopsy specimens with increased numbers in more severe lesions [28, 34]. B cells may play several roles in the pathogenesis of SS. They produce autoantibodies, some of which may have pathological consequences, but they also may directly induce salivary gland epithelial cell apoptosis [35, 36].
The detection of autoantibodies recognizing the ribonucleoproteins SSA/Ro and SSB/La, is a mainstay of SS diagnosis, and this review will not focus on these well-described autoantibodies. A number of additional autoantibodies have recently been detected in SS patients. Autoantibodies recognizing several isoforms of carbonic anhydrase (CA) have been detected in SS patients with anti-CA I and VII recently associated with mucosal dryness, dry cough, and dental decay [37]. Anti-heat shock protein 60 antibodies may correlate with systemic inflammation and MSG FS in SS patients [38]. Anti-tissue kallikrein 11 antibodies were detected at higher levels in SS patients compared to non-SS dry eye disease and healthy controls [39]. Additional autoantibodies, recognizing salivary protein 1, CA VI, and parotid secretory protein were recently reported in SS patients after initial identification early in disease in a mouse model of SS [40*]. These findings illustrate the utility of animal models for studying early events in disease pathogenesis.
The role of anti-M3R autoantibodies in SS has been extensively characterized, and may help explain the glandular dysfunction typical of SS. M3R is a transmembrane protein that regulates calcium entry to promote salivation and lacrimation upon ligation. Anti-M3R autoantibodies affect salivary gland function [41] and have been detected in children with SS [42]. Anti-M3R antibodies recognizing the second extracellular loop decreased calcium influx in a human salivary gland cell line suggesting a pathogenic role in exocrine gland dysfunction [43]. Other proposed mechanisms of anti-M3R autoantibody-mediated exocrine gland dysfunction include induction of M3R internalization and degradation [44], induction of epithelial cell apoptosis [45], and alteration of aquaporin 5 trafficking [46*]. Aquaporin 5 is a water channel in salivary and lacrimal glands that plays a key role in increasing water content in saliva and tears. Upon parasympathetic signals through M3R, aquaporin 5 traffics to the apical plasma membrane to allow water secretion; however, in the presence of anti-M3R autoantibodies, M3R stimulation results in decreased intracellular calcium release and subsequent failure of aquaporin 5 to traffic to the apical plasma membrane [46*]. M3R autoantibodies have also been detected in saliva of SS patients and may correlate with salivary and lacrimal gland dysfunction [47].
Role of Target Epithelial Cells
Organ-specificity of SS for lacrimal and salivary glands can not be easily explained by lymphocyte-intrinsic effects, unless such effects result in exclusive trafficking of pathogenic T cells to the involved organs. Another possibility is an organ-specific defect that leads to aberrant T cell activation and disease initiation. Disruption of epithelial cell-specific STAT3-IκB-ζ signaling in mice led to increased lacrimal gland epithelial cell apoptosis which progressed to dacryoadenitis, anti-SSA/B autoantibody production, and lacrimal gland dysfunction mimicking SS [48**]. Systemic or topical administration of a caspase inhibitor decreased lacrimal gland apoptosis and dacryoadenitis and improved lacrimal gland function in these mice. This study exemplifies how lacrimal gland epithelial cell-specific defects may result in organ-specific autoimmunity despite normal lymphoid and myeloid compartments. Whether defects in the STAT3-IκB-ζ signaling pathway occur in human SS is unknown.
Other target epithelial cell abnormalities in SS have recently been described. Study of human MSG biopsy specimens found alterations in exocytosis machinery in MSG from SS patients including inappropriate localization of apical proteins to the basal surface of acinar cells, resulting in release of mucins into the extracellular space [49]. This creates an inflammatory environment that may initiate autoimmunity. Additionally, decreased sulphotransferase activity was detected in MSG biopsy specimens from SS patients and correlated with degree of inflammation and positive serologies [50]. Finally, lack of appropriate estrogen-dependent immunoregulation was recently demonstrated in cultured salivary gland epithelial cells from SS patients [51]. This finding is intriguing given the female predominance of SS in both children and adults. For further reading on the role of target epithelial cell-specific defects that may promote SS autoimmunity, the reader is directed to a recent comprehensive review [52*].
CONCLUSION
Childhood SS is a poorly defined and underdiagnosed condition. Continued efforts at better understanding the pathophysiology of SS in adults and animal models are underway. Specifically, animal models of SS allow the study of the earliest events in the development of autoimmunity, an effort that is impossible in humans. Additional efforts at defining and evaluating new therapeutic modalities are ongoing, with promising preclinical and initial clinical results. Additional study into the spectrum of SS in children is needed, including the establishment of preliminary childhood SS classification criteria to enable clinical and translational research and assist diagnosis. Nonetheless, new and exciting possibilities in the diagnosis and treatment of childhood SS are on the horizon.
KEY POINTS.
Prevalence, prognosis, and optimal treatment of childhood SS are unknown because no adequate childhood SS specific diagnostic or classification criteria exist.
Rituximab therapy shows some clinical benefit in adults though results are conflicting.
Newer therapies including gene therapy and adoptive transfer of mesenchymal stem cells show promise in preclinical studies and early clinical studies in adults.
Pathogenesis of SS is still unclear, but studies of T lymphocyte subsets and autoantibodies suggest pathogenic roles with additional contribution from salivary and lacrimal gland epithelial cells.
ACKNOWLEDGMENTS
The author gratefully acknowledges Drs. Scott Canna, Jay Mehta, and Bonnie Lieberman for their critical reading of this manuscript. SML is supported by NIH/NEI K08-EY022344 and an Arthritis Foundation Postdoctoral Fellowship.
Disclosure of funding: SML is supported by NIH/NEI K08-EY022344 and an Arthritis Foundation Postdoctoral Fellowship.
Footnotes
The author has no conflicts of interest to declare.
REFERENCES
- 1.Bartunkova J, Sediva A, Vencovsky J, Tesar V. Primary Sjogren's syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol. 1999;17:381–386. [PubMed] [Google Scholar]
- 2.Houghton K, Malleson P, Cabral D, et al. Primary Sjogren's syndrome in children and adolescents: are proposed diagnostic criteria applicable? J Rheumatol. 2005;32:2225–2232. [PubMed] [Google Scholar]
- 3.Baszis K, Toib D, Cooper M, et al. Recurrent parotitis as a presentation of primary pediatric Sjogren syndrome. Pediatrics. 2012;129:e179–e182. doi: 10.1542/peds.2011-0716. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanovic R, Basta-Jovanovic G, Putnik J, et al. Renal involvement in primary Sjogren syndrome of childhood: case report and literature review. Mod Rheumatol. 2013;23:182–189. doi: 10.1007/s10165-012-0633-x. [DOI] [PubMed] [Google Scholar]
- 5.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Tzioufas AG, Stone JH, et al. Treatment of primary Sjogren syndrome: a systematic review. JAMA. 2010;304:452–460. doi: 10.1001/jama.2010.1014. [DOI] [PubMed] [Google Scholar]
- 7.Gottenberg JE, Cinquetti G, Larroche C, et al. Efficacy of rituximab in systemic manifestations of primary Sjogren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis. 2012;72:1026–1031. doi: 10.1136/annrheumdis-2012-202293. [DOI] [PubMed] [Google Scholar]
- 8.Meiners PM, Arends S, Brouwer E, et al. Responsiveness of disease activity indices ESSPRI and ESSDAI in patients with primary Sjogren's syndrome treated with rituximab. Ann Rheum Dis. 2012;71:1297–1302. doi: 10.1136/annrheumdis-2011-200460. [DOI] [PubMed] [Google Scholar]
- 9.Mekinian A, Ravaud P, Larroche C, et al. Rituximab in central nervous system manifestations of patients with primary Sjogren's syndrome: results from the AIR registry. Clin Exp Rheumatol. 2012;30:208–212. [PubMed] [Google Scholar]
- 10. St Clair EW, Levesque MC, Luning Prak ET, et al. Rituximab therapy for primary Sjogren's syndrome: An open-label clinical trial and mechanistic analysis. Arthritis Rheum. 2013;65:1097–1106. doi: 10.1002/art.37850. This study includes a detailed analysis of B cell subsets and B cell-related factors (cytokines, autoantibodies, gene expression profiles) in peripheral blood of SS patients following a single dose of rituximab.
- 11. Hamza N, Bootsma H, Yuvaraj S, et al. Persistence of immunoglobulin-producing cells in parotid salivary glands of patients with primary Sjogren's syndrome after B cell depletion therapy. Ann Rheum Dis. 2012;71:1881–1887. doi: 10.1136/annrheumdis-2011-201189. This study evaluates MSG biopsies before and after rituximab therapy and demonstrates persistence of autoantibody producing cells within MSG of SS patients despite depletion of B cells from circulation.
- 12. Baum BJ, Alevizos I, Zheng C, et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. This study demonstrated that gene therapy using an adenoviral vector to deliver aquaporin 1 to salivary gland epithelium improves salivary gland function and subjective xerostomia in patients with radiation-induced salivary gland dysfunction.
- 13.Yin H, Nguyen CQ, Samuni Y, et al. Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6.NOD-Aec1Aec2 mouse model of Sjogren's syndrome. Arthritis Res Ther. 2012;14:R40. doi: 10.1186/ar3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BH, Carcamo WC, Chiorini JA, et al. Gene therapy using IL-27 ameliorates Sjogren's syndrome-like autoimmune exocrinopathy. Arthritis Res Ther. 2012;14:R172. doi: 10.1186/ar3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili S, Liu Y, Kornete M, et al. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjogren's-like disease. PLoS One. 2012;7:e38615. doi: 10.1371/journal.pone.0038615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Wang D, Liu D, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. This study identifies defects in the immunoregulatory properties of MSCs in SS patients and SS-prone mice. Strikingly, transfer of allogeneic MSCs improved salivary gland inflammation and function in both NOD mice and human SS patients.
- 17.Ice JA, Li H, Adrianto I, et al. Genetics of Sjogren's syndrome in the genome-wide association era. J Autoimmun. 2012;39:57–63. doi: 10.1016/j.jaut.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolstad AI, Le Hellard S, Kristjansdottir G, et al. Association between genetic variants in the tumour necrosis factor/lymphotoxin alpha/lymphotoxin beta locus and primary Sjogren's syndrome in Scandinavian samples. Ann Rheum Dis. 2012;71:981–988. doi: 10.1136/annrheumdis-2011-200446. [DOI] [PubMed] [Google Scholar]
- 19.Cay HF, Sezer I, Dogan S, et al. Polymorphism in the TNF-alpha gene promoter at position - 1031 is associated with increased circulating levels of TNF-alpha, myeloperoxidase and nitrotyrosine in primary Sjogren's syndrome. Clin Exp Rheumatol. 2012;30:843–849. [PubMed] [Google Scholar]
- 20.Ambatipudi KS, Swatkoski S, Moresco JJ, et al. Quantitative proteomics of parotid saliva in primary Sjogren's syndrome. Proteomics. 2012;12:3113–3120. doi: 10.1002/pmic.201200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosenda K, Ichii O, Otsuka S, et al. BXSB/MpJ-Yaa mice develop autoimmune dacryoadenitis with the appearance of inflammatory cell marker mRNAs in the lacrimal fluid. Clin Experiment Ophthalmol. 2013 doi: 10.1111/ceo.12083. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Tandon M, Gallo A, Jang SI, et al. Deep sequencing of short RNAs reveals novel microRNAs in minor salivary glands of patients with Sjogren's syndrome. Oral Dis. 2012;18:127–131. doi: 10.1111/j.1601-0825.2011.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath S, Nazmul-Hossain AN, Pollard RP, et al. Systems analysis of primary Sjogren's syndrome pathogenesis in salivary glands identifies shared pathways in human and a mouse model. Arthritis Res Ther. 2012;14:R238. doi: 10.1186/ar4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, Liu Z, Jallal B, et al. Type I interferons in Sjögren's syndrome. Autoimmun Rev. 2013;12:558–566. doi: 10.1016/j.autrev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Brkic Z, Maria NI, van Helden-Meeuwsen CG, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren's syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis. 2013;72:728–735. doi: 10.1136/annrheumdis-2012-201381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szczerba BM, Rybakowska PD, Dey P, et al. Type I Interferon Receptor Deficiency Prevents Murine Sjogren's Syndrome. J Dent Res. 2013;92:444–449. doi: 10.1177/0022034513483315. This study demonstrates that genetic manipulation disrupting type I IFN signaling in a well-characterized mouse model of SS prevents salivary gland dysfunction and greatly diminishes salivary and lacrimal gland inflammation.
- 27. Hall JC, Casciola-Rosen L, Berger AE, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci U S A. 2012;109:17609–17614. doi: 10.1073/pnas.1209724109. This study identifies protein markers specifically induced only by IFN-γ or preferentially induced by IFN-α and uses these markers to identify the presence of an IFN-γ signature in the majority of MSG from SS patients, whereas no IFN signature was noted in MSG biopsies from non-SS controls. In contrast, an IFN-α signature was noted in muscle from dermatomyositis patients. While the general focus has been on the role of type I IFN in autoimmune diseases, these results suggests that type II IFN may play a key pathogenic role in SS.
- 28. Moriyama M, Hayashida JN, Toyoshima T, et al. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjögren's syndrome. Clin Exp Immunol. 2012;169:17–26. doi: 10.1111/j.1365-2249.2012.04587.x. This study describes detailed analyses of T cell subsets in MSG biopsies demonstrating differences between SS patients and controls as well as between SS patients with high and low focus scores. Notably, these differences were not evident in peripheral blood demonstrating the importance of studying affected target organs to better understand pathogenesis of organspecific autoimmune disease.
- 29.Maehara T, Moriyama M, Hayashida JN, et al. Selective localization of T helper subsets in labial salivary glands from primary Sjogren's syndrome patients. Clin Exp Immunol. 2012;169:89–99. doi: 10.1111/j.1365-2249.2012.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bombardieri M, Barone F, Lucchesi D, et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol. 2012;189:3767–3776. doi: 10.4049/jimmunol.1201216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risselada AP, Looije MF, Kruize AA, et al. The Role of Ectopic Germinal Centers in the Immunopathology of Primary Sjogren's Syndrome: A Systematic Review. Semin Arthritis Rheum. 2013;42:368–376. doi: 10.1016/j.semarthrit.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Alunno A, Bistoni O, Bartoloni E, et al. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren's syndrome. Ann Rheum Dis. 2013;72:286–292. doi: 10.1136/annrheumdis-2012-201511. [DOI] [PubMed] [Google Scholar]
- 33. Cheng KT, Alevizos I, Liu X, et al. STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sjogren's syndrome. Proc Natl Acad Sci U S A. 2012;109:14544–14549. doi: 10.1073/pnas.1207354109. This study identified SS-like autoimmune sialadenitis in a mouse with T cell-specific deficiency of STIM1 and STIM2 calcium sensing molecules leading to identification of STIM-deficient T cells in MSG and peripheral blood of SS patients.
- 34.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun. 2010;34:400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Thabet Y, Le Dantec C, Ghedira I, et al. Epigenetic dysregulation in salivary glands from patients with primary Sjogren's syndrome may be ascribed to infiltrating B cells. J Autoimmun. 2013 doi: 10.1016/j.jaut.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Varin MM, Guerrier T, Devauchelle-Pensec V, et al. In Sjogren's syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun Rev. 2012;11:252–258. doi: 10.1016/j.autrev.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Pertovaara M, Bootorabi F, Kuuslahti M, et al. Carbonic anhydrase autoantibodies and sicca symptoms in primary Sjogren s syndrome. Clin Exp Rheumatol. 2012;30:456–457. [PubMed] [Google Scholar]
- 38.de Jong H, de Jager W, Wenting-van Wijk M, et al. Increased immune reactivity towards human hsp60 in patients with primary Sjogren's syndrome is associated with increased cytokine levels and glandular inflammation. Clin Exp Rheumatol. 2012;30:594–595. [PubMed] [Google Scholar]
- 39.El Annan J, Jiang G, Wang D, et al. Elevated Immunoglobulin to Tissue KLK11 in Patients With Sjogren Syndrome. Cornea. 2013;32:e90–e93. doi: 10.1097/ICO.0b013e31826a1e2e. [DOI] [PubMed] [Google Scholar]
- 40. Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjogren's syndrome. Clin Immunol. 2012;145:251–255. doi: 10.1016/j.clim.2012.09.013. This study demonstrates the use of an animal model to identify autoantibodies present at an early stage of disease leading to subsequent identification of these novel autoantibodies in SS patients.
- 41.Sumida T, Iizuka M, Asashima H, et al. Pathogenic role of anti-M3 muscarinic acetylcholine receptor immune response in Sjogren's syndrome. Presse Med. 2012;41:e461–e466. doi: 10.1016/j.lpm.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Wakamatsu E, Matsumoto I, et al. High prevalence of autoantibodies to muscarinic-3 acetylcholine receptor in patients with juvenile-onset Sjogren syndrome. Ann Rheum Dis. 2008;67:136–137. doi: 10.1136/ard.2007.072421. [DOI] [PubMed] [Google Scholar]
- 43.Tsuboi H, Nakamura Y, Iizuka M, et al. Generation and functional analysis of monoclonal antibodies against the second extracellular loop of human M3 muscarinic acetylcholine receptor. Mod Rheumatol. 2012;22:264–271. doi: 10.1007/s10165-011-0514-8. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Hwang SM, Davies AJ, et al. Autoantibodies in primary Sjogren's syndrome patients induce internalization of muscarinic type 3 receptors. Biochim Biophys Acta. 2012;1822:161–167. doi: 10.1016/j.bbadis.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Reina S, Sterin-Borda L, Borda E. Anti-M(3) peptide IgG from Sjogren's syndrome triggers apoptosis in A253 cells. Cell Immunol. 2012;275:33–41. doi: 10.1016/j.cellimm.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 46. Lee BH, Gauna AE, Perez G, et al. Autoantibodies against Muscarinic Type 3 Receptor in Sjogren's Syndrome Inhibit Aquaporin 5 Trafficking. PLoS One. 2013;8:e53113. doi: 10.1371/journal.pone.0053113. This study eloquently demonstrates the role of M3R signals in mediating appropriate apical localization of aquaporin 5 in salivary gland epithelial cells. Blocking these signals with anti- M3R autoantibodies disrupts this aquaporin 5 localization likely contributing to salivary gland dysfunction in SS.
- 47.He J, Qiang L, Ding Y, et al. The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205-220) antibody in the saliva of patients with primary Sjogren's syndrome. Clin Exp Rheumatol. 2012;30:322–326. [PubMed] [Google Scholar]
- 48. Okuma A, Hoshino K, Ohba T, et al. Enhanced Apoptosis by Disruption of the STAT3-IκB-ζ Signaling Pathway in Epithelial Cells Induces Sjögren’s Syndrome-like Autoimmune Disease. Immunity. 2013;38:450–460. doi: 10.1016/j.immuni.2012.11.016. This study identifies an epithelial-specific signaling alteration that leads to lacrimal gland epithelial cell apoptosis and subsequent autoimmune dacryoadenitis, autoantibodies, and lacrimal gland dysfunction resembling SS autoimmunity. Treatment with a caspase inhibitor decreases apoptosis and improves lacrimal gland function. This study clearly demonstrates that an epithelial cell defect can lead to organ specific autoimmunity.
- 49.Barrera MJ, Sanchez M, Aguilera S, et al. Aberrant localization of fusion receptors involved in regulated exocytosis in salivary glands of Sjogren's syndrome patients is linked to ectopic mucin secretion. J Autoimmun. 2012;39:83–92. doi: 10.1016/j.jaut.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Castro I, Aguilera S, Brockhausen I, et al. Decreased salivary sulphotransferase activity correlated with inflammation and autoimmunity parameters in Sjogren's syndrome patients. Rheumatology (Oxford) 2012;51:482–490. doi: 10.1093/rheumatology/ker351. [DOI] [PubMed] [Google Scholar]
- 51.Manoussakis MN, Tsinti M, Kapsogeorgou EK, Moutsopoulos HM. The salivary gland epithelial cells of patients with primary Sjogren's syndrome manifest significantly reduced responsiveness to 17beta-estradiol. J Autoimmun. 2012;39:64–68. doi: 10.1016/j.jaut.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 52. Barrera MJ, Bahamondes V, Sepulveda D, et al. Sjogren's syndrome and the epithelial target: A comprehensive review. J Autoimmun. 2013;42:7–18. doi: 10.1016/j.jaut.2013.02.001. This is an excellent review of acinar epithelial cell biology and related alterations noted in SS patients.


